Abstract
Purpose
The systemic inflammation response index (SIRI) and the systemic immune inflammation index (SII) are indicators that reflect the body’s overall systemic inflammatory response. Inflammation plays an important role in the pathogenesis of in-stent restenosis (ISR). The aim of this study was to investigate the predictive value of preoperative SIRI and SII for the occurrence of ISR in patients undergoing coronary stent implantation.
Materials and Methods
We retrospectively analyzed the clinical, hematological, and angiographic data of 387 patients who underwent coronary angiography for recurrent angina after coronary stent implantation at Qilu Hospital of Shandong University. Receiver operating characteristic curve (ROC) analysis was used to determine the optimal cutoff values for SIRI and SII to predict ISR. Based on the optimal cutoff values for SIRI and SII, patients were categorized into high-SIRI, low-SIRI, high-SII, and low-SII groups. Multivariate logistic regression models were constructed to assess the predictive value of SIRI and SII for ISR >50% and ISR >70%.
Results
This study included a total of 387 patients who underwent coronary angiography and follow-up at Qilu Hospital of Shandong University. Patients in the high-SIRI group had a higher incidence of ISR than those in the low-SIRI group (ISR >50%: 44.8% vs 30.7%, p = 0.018; ISR >70%: 41.5% vs 4.5%, p < 0.001). In addition, ISR occurred more frequently in patients with a higher SII than in patients with a lower SII (ISR >50%: 52.6% vs 35.7%, p = 0.001; ISR >70%: 51.9% vs 23%, p < 0.001). In multivariate logistic regression analysis, SIRI and SII were found to be independent predictive factors for ISR, both as continuous and categorical variables. In the ROC analysis, the optimal cutoff value for SIRI was set at 0.54 (sensitivity: 84.5%, specificity: 27%), and the optimal cutoff value for SII was set at 545.29 (sensitivity: 44.1%, specificity: 71.7%).
Conclusion
Elevated preoperative SIRI and SII values help predict ISR and may serve as a useful screening tool to perform interventional procedures based on the patient’s risk of ISR after stent implantation.
Introduction
In recent decades, significant advances in percutaneous coronary intervention (PCI) have led to safe and effective treatment of the vast majority of patients requiring PCI, including those with unstable manifestations, complex disease patterns, and multiple complications. However, restenosis after stent implantation has always been considered the major problem with PCI.Citation1–3
The underlying mechanisms and predisposing factors of in-stent restenosis (ISR) are complex and not fully understood.Citation3–5 In general, the inflammatory response to vessel wall injury during PCI plays a central role in restenosis after stent implantation by promoting fibroblast growth and smooth muscle cell proliferation.Citation6,Citation7 Using biomarkers to predict ISR risk, a number of studies have investigated the association between inflammatory biomarkers during stent implantation and subsequent restenosis. Routine blood tests, including white blood cell counts and subpopulation counts, are commonly used as surrogate markers of systemic inflammation in clinical practice.Citation8,Citation9 Several blood cell parameters such as monocyte to high-density lipoprotein cholesterol (HDL-C) ratio,Citation10–12 neutrophil to lymphocyte ratioCitation13 and red blood cell distribution widthCitation14 have been identified as valuable predictors of ISR. Recently, the systemic inflammatory response index (SIRI) and the systemic immune-inflammation index (SII) have emerged as two novel inflammatory indices consisting of three independent subsets of white blood cells.Citation15,Citation16 SII and SIRI were first proposed as predictors of unfavorable prognosis in cancer. These indices have shown promising predictive value for the prognosis of cardiovascular disease. Jin et al concluded that higher SII and SIRI were associated with a higher risk of developing cardiovascular disease during a median follow-up of 10 years.Citation17 A cohort study also showed that the dynamic status of SII and SIRI was significantly associated with the risk of cardiovascular disease.Citation18
These indicators may reflect the degree of systemic inflammatory response and have certain predictive value for the occurrence and prognosis of ISR. Two recent studies have shown that a higher SII is independently associated with an increased risk of ISR after PCI in patients with acute coronary syndrome, and may be a predictive indicator of ISR.Citation19,Citation20 There is still a lack of effective evidence for the association between SIRI and ISR. The aim of this study was to investigate the associations of SIRI and SII with ISR in patients with coronary artery disease undergoing PCI and to evaluate the predictive value of these two indicators for ISR. By investigating the relationships between these inflammatory markers and ISR, we hope to better understand the pathogenesis of ISR and provide more accurate information for the treatment and management of PCI patients.
Method
Study Population
We conducted a retrospective analysis of patients who underwent PCI and repeat coronary angiography at Qilu Hospital of Shandong University between March 2022 and March 2023. The inclusion criteria for the patients were as follows: (1) age ≥18 years; (2) a history of PCI in the past 6 to 60 months; (3) repeated coronary angiography; and (4) willingness and ability to provide written informed consent. The main exclusion criteria were as follows: (1) history of coronary artery bypass grafting; (2) lesions treated only with balloon angioplasty without stent implantation; (3) symptomatic heart failure, cardiomyopathy, congenital heart disease, or severe valvular heart disease; (4) severe hepatic or renal dysfunction (estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m²); (5) acute or chronic inflammation, malignancies, hematologic disorders or autoimmune diseases. The sample size required for the study was analyzed using the Power Analysis and Sample Size (PASS) software. According to the results from literature,Citation20 the mean SII in the ISR and non-ISR groups were 1885 and 1086, with standard deviations of 1211 and 762, respectively. The statistical test power β and significance level α were set at 0.10 and 0.05, respectively. The calculated result showed that the ISR >50% group and the ISR ≤50% group each required 35 patients. With a dropout rate of 20%, each group should include 42 patients. The available sample size exceeded the calculated sample size. This retrospective study conformed to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Qilu Hospital of Shandong University [No.KYLL-2022(ZM)-1344]. Written or verbal informed consent was obtained from all participants.
Data Collection and processing
Patients’ clinical and demographic characteristics such as age, sex, history of hypertension, diabetes or stroke, tobacco or alcohol use, systolic blood pressure (SBP), diastolic blood pressure (DBP), left ventricular ejection fraction (LVEF) and medications taken were recorded from the electronic medical record system by trained physicians. Meanwhile, we recorded platelet, white blood cell, neutrophil, lymphocyte and monocyte counts, fasting blood glucose (FBG), uric acid, creatinine and blood lipid profile consisting of triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C) and HDL-C, which were determined in the biochemistry and outpatient laboratory of Qilu Hospital of Shandong University. Body mass index (BMI) was calculated as weight (kg)/height squared (m2). SII was defined as (neutrophils × platelets)/lymphocytes, and SIRI was defined as (neutrophils × monocytes)/lymphocytes (peripheral blood count).
Evaluation of the ISR
All patients included in the analysis underwent follow-up angiography using the standard Judkin technique after successful PCI. ISR was previously defined as the presence of significant diameter stenosis (>50%) in the segment within the stent or at the 5-mm margins.Citation21 Due to lack of data on coronary functional outcomes and quantitative coronary angiography, patients were categorized into the ISR >70% and ISR ≤70% groups after comparing the ISR >50% and ISR ≤50% groups based on angiographic follow-up results.
Statistical Analysis
All statistical analyses were performed using SPSS version 25.0 (SPSS, Chicago, IL, United States) and R Programming Language 4.0.2. Continuous variables with a normal distribution were presented as mean ± standard deviation (SD), while continuous variables with a non-normal distribution were presented as median with the 25th and 75th percentiles. Categorical variables were presented as frequencies (%). The Student t test was used to assess differences between two groups for normally distributed continuous variables, and the Mann–Whitney U-test was used to assess differences between two groups for non-normally distributed continuous variables. The chi-square test was used to analyze categorical variables. Potential associations were analyzed using the Pearson correlation test or Spearman’s rank test where appropriate.
Univariate logistic regression analyses were performed to identify the determinants of ISR in patients. Variables with statistical significance (p-value <0.05) in the univariate analyses were included in the multivariate logistic regression analysis. Finally, three models were created to control confounding variables and to assess the association between two indices (modulated as continuous or categorical variables) and ISR. In addition, to evaluate the predictive values of SIRI and SII, the area under the curve (AUC) and the optimal cutoff value were determined by analyzing the receiver operating characteristic (ROC) curves. A p-value < 0.05 was considered statistically significant.
Result
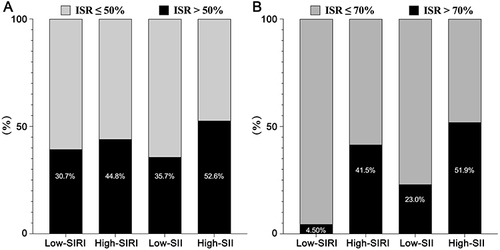
A total of 387 patients with a mean age of 61.73 ± 9.44 years were included in this study. Based on the optimal cutoff values for SIRI and SII, baseline demographic and clinical data are shown in . Patients with higher SIRI were predominantly male and had higher BMI, uric acid, creatinine, FBG, baseline Syntax score and SII, longer stent length, lower LVEF and HDL-C, and shorter coronary angiography interval. The proportion of drug-eluting stents was lower in the high-SIRI group than in the low-SIRI group. There was no significant difference in the proportion of oral antiplatelet agents and chest pain after PCI between the high-SIRI group and the low-SIRI group. Both the proportion of patients with diabetes and the proportion of patients with hypertension were higher in the high-SIRI group than in the low-SIRI group. In addition, the proportion of patients with an ISR >50% and with an ISR >70% was higher in the group with an SIRI above the optimal threshold than in the group with an SIRI below the optimal threshold ( and ). When patients were grouped according to the optimal threshold for the SII, there were no significant differences in sex, age, BMI, smoking, alcohol consumption, stent diameter, baseline Syntax score, and the proportion of oral antiplatelet agents and chest pain after PCI in patients with a higher SII compared with patients with a lower SII, as shown in . The proportion of drug-eluting stents was lower in the high-SII group than in the low-SII group. However, patients with a higher SII had lower hemoglobin, mean corpuscular volume, mean platelet volume, HDL-C levels, and shorter interval time of coronary angiography. The incidence of ISR >50% and ISR >70% was higher in patients with an SII above the optimal threshold than in patients with a lower SII ( and ).
Table 1 Baseline Characteristics of the Study Population Grouped According to the SIRI and SII
Figure 1 The effects of SIRI and SII on the prevalence of patients with an ISR >50% (A) and an ISR >70% (B).
Participants were further divided into the ISR >50% group (n = 161) and the ISR ≤50% group (n = 226), as shown in . There were no significant differences in sex, age, BMI, SBP, DBP, LVEF, proportion of hypertensives, previous stroke and chest pain after PCI between the 226 patients with ISR ≤50% and the 161 patients with ISR >50%. However, the proportion of smokers and the proportion of drinkers were both higher in the patients with an ISR >50% than in the patients with an ISR ≤50%. Most biochemical parameters were similar between the two groups. The white blood cell, neutrophil, monocyte and platelet counts, stent length, stent diameter, Syntax score at baseline and coronary angiography interval were higher in patients with an ISR >50% than in patients with an ISR ≤50%. The proportion of diabetic patients was higher in the group with an ISR >50% than in the group with an ISR ≤50% at borderline significance (p = 0.097). Compared with the group with an ISR ≤50%, the group with an ISR >50% showed a significant increase in the use of bare-metal stents and a decrease in the proportion of oral dual antiplatelet agents and drug-eluting stents. In addition, it is noteworthy that the group with an ISR >50% had significantly higher SIRI and SII than the group with an ISR ≤50% ().
Table 2 Baseline Characteristics of the Patients with ISR >50% versus ISR ≤50% and ISR >70% versus ISR ≤70%
Figure 2 Comparison of SIRI and SII values between the patients with an ISR >50% and the patients with an ISR ≤50% (A) and comparison of SIRI and SII values between the patients with an ISR >70% and the patients with an ISR ≤70% (B).
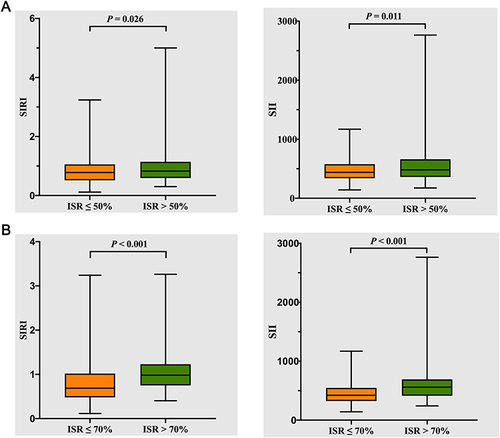
In , the participants were also categorized into the group with an ISR >70% (n = 161) and the group with an ISR ≤70%. No significant differences in sex, age, BMI, SBP, DBP, LVEF, prevalence of hypertension, history of stroke and proportion of chest pain after PCI were found between the group with an ISR ≤70% and the group with an ISR >70%. However, compared to the ISR ≤70% group, the ISR >70% group had a significantly longer stent length and coronary angiography interval, a larger stent diameter, a higher Syntax score at baseline and a higher proportion of smokers, diabetes. A decrease in HDL-C levels was observed in the ISR >70% group. In addition, SIRI and SII levels were significantly increased in the group with an ISR >70% compared to those in the group with an ISR ≤70% ().
Associations of Preoperative SIRI and SII with Other Cardiovascular Risk Factors
A Spearman correlation analysis was performed to examine the relationships between preoperative SIRI and SII and other cardiovascular risk factors. The results are shown in . SIRI showed a positive correlation with BMI, white blood cell count (r = 0.534, p < 0.001), platelet count (r = 0.117, p = 0.022), homocysteine (r = 0.143, p = 0.005), uric acid (r = 0.107, p = 0.035) and creatinine (r = 0.120, p = 0.018). Conversely, SIRI showed a negative correlation with HDL-C (r = −0.304, p < 0.001) and LVEF (r = −0.175, p = 0.001). SII showed a positive correlation with white blood cell count (r = 0.380, p < 0.001), platelet count (r = 0.534, p < 0.001) and FBG (r = 0.140, p = 0.006), and a negative correlation with HDL-C (r = −0.190, p < 0.001), hemoglobin (r = −0.140, p = 0.006), mean corpuscular volume (r = −0.198, p < 0.001), and mean platelet volume (r = −0.135, p = 0.008).
Table 3 The Associations of Preoperative SIRI and SII with Other Cardiovascular Risk Factors
Associations of SIRI and SII with the Risk of ISR in Univariate and Multivariate Analyses
Logistic regression analyzes were performed to further investigate the association between preoperative SIRI and SII and the risk of ISR >50% and ISR >70%. Univariate logistic regression analyzes revealed that a 1-unit increase in SIRI and SII, when treated as a continuous variable, was significantly associated with an increased incidence of ISR >50% (SIRI: odds ratio [OR] = 1.832, 95% confidence interval [CI] 1.165 to 2.819, p = 0.009; SII: OR = 1.002, 95% CI 1.001 to 1.003, p = 0.001). The incidence of ISR >70% also increased significantly (SIRI: OR = 4.573, 95% CI 2.636 to 7.935, p < 0.001; SII: OR = 1.003, 95% CI 1.002 to 1.005, p < 0.001). When SIRI and SII were categorized by cutoff values, patients with higher SIRI or SII had a higher risk of ISR >50% (SIRI >0.54: OR = 1.835, 95% CI 1.105 to 3.047, p = 0.019; SII >545.29: OR = 1.997, 95% CI 1.306 to 3.054, p = 0.001). In addition, current smoking, current alcohol consumption, homocysteine and creatinine levels were all risk factors for ISR >50% in univariate analyses (). When SIRI and SII were used as categorical variables, the incidence of ISR >70% was higher in patients with high SIRI or SII (SIRI >0.54: OR = 14.880, 95% CI 5.317 to 41.642, p < 0.001; SII >545.29: OR = 3.602, 95% CI 2.303 to 5634, p < 0.001). Meanwhile, in the univariate analysis, current smoking, diabetes, HDL-C level, creatinine level and clopidogrel use were all risk factors for ISR >70% (). In the multivariate logistic regression models, SIRI was initially assessed as a continuous variable. As shown in , the increase of SIRI was an independent risk factor for ISR >50% in Model 1 (OR = 1.764, 95% CI: 1.119 to 2.783, p = 0.015), Model 2 (OR = 1.902, 95% CI: 1.161 to 3.117, p = 0.011) and Model 3 (OR = 1.865, 95% CI: 1.102 to 3.159, p = 0.020). When SIRI was treated as a categorical variable, it remained an independent predictor of ISR >50% in all three models. When SII was evaluated as a categorical variable, patients above the cutoff value showed a significantly increased risk of ISR compared to patients below the optimal cutoff value in Model 1 (OR = 2.029, 95% CI: 1.332 to 3.115, p = 0.001), Model 2 (OR = 2.254, 95% CI: 1.420 to 3.579, p = 0.001) and Model 3 (OR = 2.183, 95% CI: 1.353 to 3.522, p = 0.001). This relationship remained significant in all three models when SII was analyzed as a continuous variable. In the multivariate logistic regression models, both SIRI and SII were independent predictors of ISR >70% when categorical variables and continuous variables were involved ().
Table 4 Significant Predictors of ISR >50% and ISR >70% in Univariate Logistic Regression Analyses
Table 5 Significant Predictors of ISR >50% in Multivariate Logistic Regression Analyses
Table 6 Significant Predictors of ISR >70% in Multivariate Logistic Regression Analyses
Receiver Operating Characteristic Curves
ROC were used to assess the predictive values of SIRI and SII for ISR >50%. SIRI could provide a mild predictive value for ISR >50%, with an AUC of 0.57 (95% CI: 0.51 to 0.62, p = 0.026). The optimal cutoff value for SIRI was set at 0.54, yielding a sensitivity of 84.5% and a specificity of 27% in predicting ISR >50% ( and ). As shown in and , the ROC analysis revealed that the SII could provide a mild predictive value for ISR >50% with an AUC of 0.58 (95% CI: 0.52 to 0.64, p = 0.011). The optimal cutoff value was 545.29 (sensitivity: 44.1%, specificity: 71.7%).
Table 7 Predictive Value of SIRI and SII for ISR
Figure 3 Receiver operating characteristic (ROC) curve analysis of SIRI and SII for prediction of ISR >50% (A) and ISR >70% (B) in all patients undergoing percutaneous coronary intervention, and ROC curve analysis of SIRI and SII for prediction of ISR >50% (C) and ISR >70% (D) in patients with high SIRI and SII.
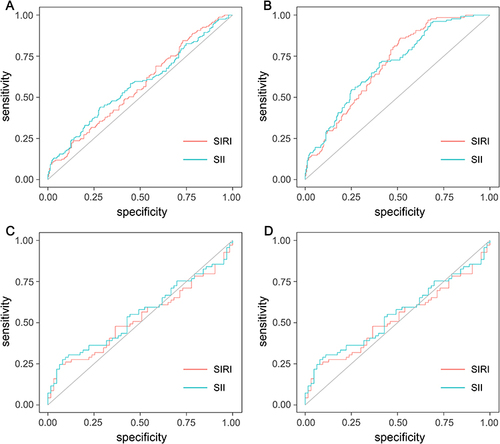
Similarly, ROC analysis was performed to assess the predictive ability of SIRI and SII for ISR >70%. The optimal cutoff value for SIRI was 0.67, with a sensitivity of 85.9%, specificity of 48.6% and an AUC of 0.70 (95% CI: 0.65 to 0.75, p <0.001). The optimal cutoff value for SII was determined to be 454.88 with a sensitivity of 71.1%, a specificity of 59.8% and an AUC of 0.70 (95% CI: 0.64 to 0.75, p <0.001) ( and ).
We analyzed the predictive values of SIRI and SII for ISR >50% and ISR >70% in patients with high SIRI and SII using ROC analysis. and show that SIRI and SII could not provide predictive value for ISR >50% in patients with high SIRI and SII. The optimal cutoff value for SIRI was 1.05, with a sensitivity of 24.6%, a specificity of 93.7%, and an AUC of 0.53 (95% CI: 0.43 to 0.62, p = 0.62). The optimal cutoff value for SII was 476.40, with a sensitivity of 27.5%, a specificity of 92.1%, and an AUC of 0.55 (95% CI: 0.45 to 0.65, p = 0.32). ROC analysis was performed to assess the predictive power of SIRI and SII for ISR >70% ( and ). The optimal cutoff value for SIRI was 1.63, with a sensitivity of 21.6%, specificity of 81.7%, and an AUC of 0.53 (95% CI: 0.43 to 0.62, p = 0.62). The optimal cutoff value for SII was determined to be 885.42 with a sensitivity of 27.5%, a specificity of 92.1%, and an AUC of 0.55 (95% CI: 0.45 to 0.65, p = 0.32).
In addition, we analyzed the predictive values of SIRI and SII for ISR >50% and ISR >70% in patients with unstable angina using ROC analysis. The optimal cutoff value for SIRI was set at 0.54, yielding a sensitivity of 88.1%, a specificity of 19.1%, and an AUC of 0.51 (95% CI: 0.45 to 0.58, p = 0.702) in predicting ISR >50%. SII also failed to provide a predictive value for ISR >50% with an AUC of 0.53 (95% CI: 0.46 to 0.60, p = 0.392). The optimal cutoff value was 543.19 (sensitivity: 45.8%, specificity: 64.2%) ( and ). SIRI could provide a mild predictive value for ISR >70%, with an AUC of 0.65 (95% CI: 0.59 to 0.72, p < 0.001). The optimal cutoff value for SIRI was set at 0.67, yielding a sensitivity of 89.1% and a specificity of 40.2% in predicting ISR >70%. The optimal cutoff value for SII was set at 447.78, yielding a sensitivity of 75%, a specificity of 52.8%, and an AUC of 0.66 (95% CI: 0.60 to 0.73, p < 0.001) in predicting ISR >70% ( and ).
Figure 4 Receiver operating characteristic (ROC) curve analysis of SIRI and SII for prediction of ISR >50% (A) and ISR >70% (B) in patients with unstable angina, and ROC curve analysis of SIRI and SII for prediction of ISR >50% (C) and ISR >70% (D) in patients with acute myocardial infarction.
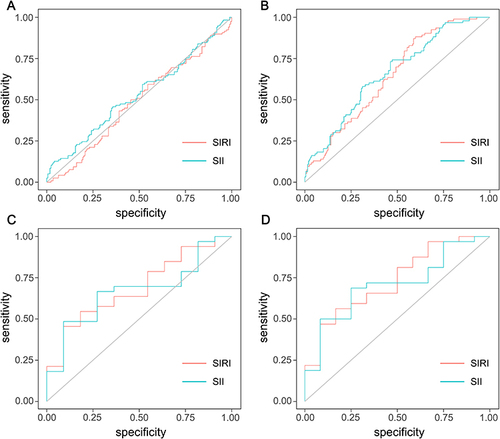
We then analyzed the predictive values of SIRI and SII for ISR >50% and ISR >70% in patients with acute myocardial infarction using ROC analysis. The results showed that at an optimal cutoff value of 1.05 for SIRI, the sensitivity for predicting ISR >50% was 45.5%, the specificity was 90.9% and the AUC was 0.69 (95% CI: 0.52 to 0.86, p = 0.063). When SII had an optimal cutoff value of 476.40, the sensitivity was 62.7%, the specificity was 72.7%, and the AUC was 0.67 (95% CI: 0.50 to 0.84, p = 0.096) ( and ). As shown in and , SIRI and SII could provide predictive values for ISR >70% in patients with acute myocardial infarction. The optimal cutoff value for SIRI was 1.00, with a sensitivity of 56.3%, a specificity of 83.3%, and an AUC of 0.73 (95% CI: 0.57 to 0.90, p = 0.019). The optimal cutoff value for SII was 476.40, with a sensitivity of 68.8%, a specificity of 75% and an AUC of 0.71 (95% CI: 0.54 to 0.87, p = 0.035).
Discussion
This study provided several interesting results. First, patients with an ISR >50% or >70% had higher SIRI and SII values than patients with an ISR ≤50% or ≤70%. Second, there was a significant association between SIRI and SII and several cardiovascular disease risk factors. Third, in the adjusted model, both continuous and categorical SIRI and SII variables were independently associated with an increased risk of ISR. Finally, an SIRI value above 0.54 predicted ISR >50% with a sensitivity of 84.5% and a specificity of 27%, and an SIRI above 0.67 predicted ISR >70% with a sensitivity of 85.9% and a specificity of 48.6%. Accordingly, an SII above 545.29 predicted an ISR >50% with a sensitivity of 44.1% and a specificity of 71.7%, and an SII above 454.88 predicted an ISR >70% with a sensitivity of 71.1% and a specificity of 59.8%. Previous studies have emphasized the importance of SIRI and SII in various cardiovascular diseases. To our knowledge, this study is the first to demonstrate a strong association between SIRI and ISR.
Previous studies have shown that neutrophil granulocytes play a crucial role in the inflammatory response in cardiovascular disease via several mechanisms. They can secrete various cytokines, chemokines and proteolytic enzymes, leading to endothelial cell injury, leukocyte aggregation, infarct enlargement and increased tissue ischemia.Citation22–25 The activation of monocytes and their transformation into lipid-laden macrophages is a necessary process in the development of atherosclerotic lesions.Citation26 Conversely, lymphocytes have a regulatory function in inflammation and may have an inhibitory effect on atherosclerosis.Citation25–27 A relatively low lymphocyte count in patients with coronary artery disease is independently associated with a poorer prognosis.Citation28,Citation29 Therefore, the SIRI, calculated as (monocyte count × neutrophil count) / lymphocyte count, is closely related to the occurrence and prognosis of cardiovascular disease. When the functionality of platelets, neutrophils and lymphocytes is taken into account, SII can be associated with the risk of coagulation and inflammation in cardiovascular events. Several studies have demonstrated a link between SII, SIRI and cardiovascular disease. Jin’s study, with an average follow-up of 10 years, indicated an increased incidence of myocardial infarction associated with elevated SIRI. In addition, the study showed that elevated SIRI and SII were associated with an increased risk of hemorrhagic and ischemic stroke subtypes and an increased risk of all-cause mortality.Citation17 Another cohort study with a follow-up of 20 years discovered a strong association between SIRI as a novel composite inflammatory index and cardiovascular mortality and all-cause mortality.Citation30 Zhang et al concluded that SIRI performed better than neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, lymphocyte-monocyte ratio and red blood cell distribution width in predicting stroke prognosis.Citation31 Candemir et al found that SII is a risk factor for atherosclerosis and suggested that SII may be a better predictor of coronary artery disease severity than measures such as neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio.Citation32 In addition, SIRI has been shown to predict the presence of atrial fibrillation in patients with ischemic stroke and is also associated with poor short-term prognosis in patients with ischemic stroke who have atrial fibrillation.Citation33,Citation34
As a comprehensive index, SIRI and SII are less influenced by factors such as change in body fluids. Compared to traditional individual blood cell counts, they better reflect the body’s systemic inflammatory response. Few studies have investigated the relationship between these two measures and the ISR, but the relationship between various components such as neutrophils, monocytes, lymphocytes, the lymphocyte-monocyte ratio and the neutrophil-lymphocyte ratio and the ISR has been widely discussed.Citation35,Citation36 The mechanism of ISR is multifactorial, with inflammation and endothelial dysfunction playing an important role.Citation6,Citation7,Citation37,Citation38 Two recent studies on the relationship between SII and ISR suggest that SII is independently associated with an increased risk of ISR after PCI in patients with acute coronary syndrome and may serve as an independent predictive indicator of ISR.Citation19,Citation20 In our study, we found a strong association between high SIRI and SII levels and ISR, further confirming the possible link between systemic inflammation and the occurrence and development of ISR. We hypothesize that SIRI and SII may trigger inflammation and oxidative stress, leading to endothelial dysfunction and subsequent ISR. This suggests that by assessing the systemic inflammatory response, we can identify patients at higher risk of ISR before surgery, which has important clinical implications for deciding whether more aggressive interventional measures are needed to prevent ISR. In our study, diabetes had a borderline significant effect on ISR >50% (OR = 1.41, 95% CI: 0.94 to 2.12, p = 0.097). Meanwhile, diabetes is a risk factor for ISR>70% (OR = 1.589, 95% CI: 1.036 to 2.438, p = 0.034). We hypothesize that this result may be related to the standard use of medications to protect against atherosclerosis and the tight control of blood glucose in diabetic patients. We also found that SIRI and SII have some predictive value for predicting ISR >50%. Using ROC curve analysis, we determined an AUC of 0.57 and 0.58 for SIRI and SII, respectively. We increased the criteria for restenosis to 70% and found that the AUC for SIRI and SII to predict ISR >70% was 0.70 and 0.71, respectively.
Although the diagnostic power is relatively low, we believe this is due to the limited sample size in our study. Further studies with a larger number of patients are needed to validate our results and further evaluate the potential clinical application of SIRI and SII in practice.
Conclusion
The preoperative SIRI and SII values can be used as independent predictors of ISR. An increase in preoperative SIRI and SII levels can significantly predict ISR and may serve as a useful screening tool to implement interventional measures based on the patient’s risk of ISR after stent implantation.
Ethics Approval and Consent to Participate
This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Qilu Hospital of Shandong University. Additionally, informed consent was obtained by us from the participants.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We are grateful to all the subjects who participated in the study.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? the Andreas Grüntzig Lecture ESC 2014. Eur Heart J. 2015;36(47):3320–3331. doi:10.1093/eurheartj/ehv511
- Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63(24):2659–2673. doi:10.1016/j.jacc.2014.02.545
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897–1907. doi:10.1016/j.jacc.2010.07.028
- Cassese S, Byrne RA, Tada T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart. 2014;100(2):153–159. doi:10.1136/heartjnl-2013-304933
- Liu S, Yang Y, Jiang S, et al. Understanding the role of non-coding RNA (ncRNA) in stent restenosis. Atherosclerosis. 2018;272:153–161. doi:10.1016/j.atherosclerosis.2018.03.036
- Inoue T, Croce K, Morooka T, Sakuma M, Node K, Simon DI. Vascular inflammation and repair: implications for re-endothelialization, restenosis, and stent thrombosis. JACC. 2011;4(10):1057–1066. doi:10.1016/j.jcin.2011.05.025
- Lee MS, Banka G. In-stent Restenosis. Interv Cardiol Clin. 2016;5(2):211–220. doi:10.1016/j.iccl.2015.12.006
- Mo X, Li T, Ji G, Lu W, Hu Z. Peripheral polymorphonuclear leukocyte activation as a systemic inflammatory response in ischemic stroke. Neurol Sci. 2013;34(9):1509–1516. doi:10.1007/s10072-013-1447-0
- Mrdjen D, Hartmann FJ, Becher B. High Dimensional Cytometry Of Central Nervous System Leukocytes During Neuroinflammation. Methods Mol Biol. 2017;1559:321–332.
- Yilmaz S, Akboga MK, Sen F, et al. Usefulness of the monocyte-to-high-density lipoprotein cholesterol ratio to predict bare metal stent restenosis. Biomarker Med. 2016;10(9):959–966. doi:10.2217/bmm-2016-0069
- Ucar FM. A potential marker of bare metal stent restenosis: monocyte count - to- HDL cholesterol ratio. BMC Cardiovasc Disord. 2016;16(1):186. doi:10.1186/s12872-016-0367-3
- Tok D, Turak O, Ç Y, Ozcan F, Tok D, Çağlı K. Monocyte to HDL ratio in prediction of BMS restenosis in subjects with stable and unstable angina pectoris. Biomarker Med. 2016;10(8):853–860. doi:10.2217/bmm-2016-0071
- Turak O, Ozcan F, Isleyen A, et al. Usefulness of the neutrophil-to-lymphocyte ratio to predict bare-metal stent restenosis. Am J Cardiol. 2012;110(10):1405–1410. doi:10.1016/j.amjcard.2012.07.003
- Yildiz A, Tekiner F, Karakurt A, Sirin G, Duman D. Preprocedural red blood cell distribution width predicts bare metal stent restenosis. Coron Artery Dis. 2014;25(6):469–473. doi:10.1097/MCA.0000000000000105
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
- Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122(14):2158–2167. doi:10.1002/cncr.30057
- Jin Z, Wu Q, Chen S, et al. The associations of two novel inflammation indexes, SII and SIRI with the risks for cardiovascular diseases and all-cause mortality: a ten-year follow-up study in 85,154 individuals. J Inflamm Res. 2021;14:131–140. doi:10.2147/JIR.S283835
- Li J, He D, Yu J, et al. Dynamic status of SII and SIRI alters the risk of cardiovascular diseases: evidence from kailuan cohort study. J Inflamm Res. 2022;15:5945–5957. doi:10.2147/JIR.S378309
- Xie F, Yu Z, Xiong Y, Wu Z, Wu Y. Systemic immune-inflammation index and in-stent restenosis in patients with acute coronary syndrome: a single-center retrospective study. Eur J Med Res. 2024;29(1):145. doi:10.1186/s40001-024-01736-4
- Ösken A, Polat F, Çakir B, et al. Systemic immune inflammation index and its implication on in-stent restenosis among patients with acute coronary syndrome. Coron Artery Dis. 2024;35(3):209–214. doi:10.1097/MCA.0000000000001325
- Ryan TJ, Bauman WB, Kennedy JW, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American heart association/American college of cardiology task force on assessment of diagnostic and therapeutic cardiovascular procedures (Committee on Percutaneous Transluminal Coronary Angioplasty). Circulation. 1993;88(6):2987–3007. doi:10.1161/01.cir.88.6.2987
- Huh JY, Ross GW, Chen R, et al. Total and differential white blood cell counts in late life predict 8-year incident stroke: the Honolulu heart program. J Am Geriatr Soc. 2015;63(3):439–446. doi:10.1111/jgs.13298
- Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: a CALIBER cohort study. J Am Coll Cardiol. 2017;69(9):1160–1169. doi:10.1016/j.jacc.2016.12.022
- Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J. 2004;25(15):1287–1292. doi:10.1016/j.ehj.2004.05.002
- Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk. J Am Coll Cardiol. 2005;45(10):1638–1643. doi:10.1016/j.jacc.2005.02.054
- Kim JH, Lee YJ, Park B. Higher monocyte count with normal white blood cell count is positively associated with 10-year cardiovascular disease risk determined by Framingham risk score among community-dwelling Korean individuals. Medicine. 2019;98(17):e15340. doi:10.1097/MD.0000000000015340
- Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106(4):470–476. doi:10.1016/j.amjcard.2010.03.062
- Bian C, Wu Y, Shi Y, et al. Predictive value of the relative lymphocyte count in coronary heart disease. Heart Vessels. 2010;25(6):469–473. doi:10.1007/s00380-010-0010-7
- Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79(6):812–814. doi:10.1016/S0002-9149(96)00878-8
- Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. 2023;12(3):1128. doi:10.3390/jcm12031128
- Zhang Y, Xing Z, Zhou K, Jiang S. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. 2021;16:1997–2007. doi:10.2147/CIA.S339221
- Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship Between Systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology. 2021;72(6):575–581. doi:10.1177/0003319720987743
- Lin KB, Fan FH, Cai MQ, et al. Systemic immune inflammation index and system inflammation response index are potential biomarkers of atrial fibrillation among the patients presenting with ischemic stroke. Eur J Med Res. 2022;27(1):106. doi:10.1186/s40001-022-00733-9
- Bağcı A, Aksoy F. Systemic immune-inflammation index predicts new-onset atrial fibrillation after ST elevation myocardial infarction. Biomarker Med. 2021;15(10):731–739. doi:10.2217/bmm-2020-0838
- Gençbay M. Do pre-procedural laboratory parameters predict drug-eluting stent restenosis. Turk Kardiyol Dern Ars. 2015;43(5):417–419. doi:10.5543/tkda.2015.71340
- Wang Z, Liu C, Fang H. Blood cell parameters and predicting coronary in-stent restenosis. Angiology. 2019;70(8):711–718. doi:10.1177/0003319719830495
- Lafont A, Durand E, Samuel JL, et al. Endothelial dysfunction and collagen accumulation: two independent factors for restenosis and constrictive remodeling after experimental angioplasty. Circulation. 1999;100(10):1109–1115. doi:10.1161/01.CIR.100.10.1109
- Kitta Y, Nakamura T, Kodama Y, et al. Endothelial vasomotor dysfunction in the brachial artery is associated with late in-stent coronary restenosis. J Am Coll Cardiol. 2005;46(4):648–655. doi:10.1016/j.jacc.2005.04.055
