Abstract
Background and Aims
The objective of this study was to investigate the effect of neutrophil-to-lymphocyte ratio (NLR) on the survival of cirrhotic patients with esophagogastric variceal bleeding (EGVB) treated with transjugular intrahepatic portosystemic shunt (TIPS).
Methods
A total of 293 patients treated with TIPS were included. The receiver operator characteristic curve (ROC) was used to calculate the optimal cut-off values of parameters such as NLR. The Kaplan-Meier curve and Cox proportional risk model were used to evaluate the effects of NLR and other variables on 2-year all-cause mortality.
Results
The area under the ROC for NLR was 0.634, with an optimal cutoff value of 4.9. Two-year mortality rates for patients with high (≥4.9) and low (<4.9) NLR were 22.1% and 9.3%, respectively (Log rank test: P = 0.002). After correcting for confounders, multivariate analysis demonstrated that NLR ≥ 4.9 (HR = 2.741, 95% CI 1.467–5.121, P = 0.002), age ≥ 63 (HR = 3.403, 95% CI 1.835–6.310, P < 0.001), and gender (male) (HR = 2.842, 95% CI 1.366–5.912, P = 0.001) were independent risk factors for the mortality outcome. Considering the stratification of early and selective TIPS treatment, high NLR still significantly increased the risk of mortality for patients (Log rank test: P = 0.007, HR = 2.317, 95% CI 1.232–4.356).
Conclusion
NLR can help to predict survival in EGVB patients after TIPS, and the type of TIPS should also be considered in practical applications.
Introduction
Portal hypertension is a prevalent manifestation of decompensated cirrhosis, leading to esophageal and gastric variceal bleeding (EGVB), refractory ascites, or hepatic dysfunction. EGVB is a prominent indication for transjugular intrahepatic portosystemic shunts (TIPS), including specific patients with acute variceal bleeding or patients who have failed after standard of care.Citation1 Despite the exact efficacy, the 2-year mortality rate after TIPS remains high, ranging from 14.6% to 38.0%.Citation2–5 Therefore, identifying risk factors and developing predictive markers to screen patients who may benefit is critical to improving survival.
Several imaging features, such as sarcopenia markers (eg, skeletal muscle and fat content, etc.)Citation6,Citation7 and the distance between the distal end of the TIPS stent and the hepatic junction,Citation8 have recently been used to predict survival after TIPS. However, the complexity of these radiologic characteristics makes them difficult to obtain routinely.
In contrast, relatively simple parameters available pre-TIPS are particularly crucial in the clinical examinations, including age, bilirubin, albumin, creatinine, and gender. The capability of systems that were developed based on these factors for the prediction of prognoses, such as model for end-stage liver disease (MELD),Citation9 MELD-Na,Citation10 Freiburg’s indexCitation11 and MELD 3.0,Citation4 has been demonstrated in previous researches.
With the intensive study of chronic liver disease, systemic inflammatory markers have also been revealed to be associated with the prognosis of cirrhotic patients.Citation12 Neutrophil-to-lymphocyte ratio (NLR) is a clinically accessible and cost-effective marker of inflammation that has been widely used to assess the outcomes of patients with cirrhosis,Citation13 hepatocellular carcinoma,Citation14 and liver transplantation.Citation15 The cut-off value of NLR has not been standardized in these studies, as the level of NLR may be affected by the patient’s age,Citation16 diabetic status,Citation17 or the etiology of cirrhosis.Citation12 There is an emerging consensus that higher NLR tends to be associated with poorer survival in patients with chronic liver disease. However, the predictive value of NLR in EGVB patients undergoing TIPS implantation is currently unknown.
Therefore, the purpose of our study was to evaluate the correlation of NLR, a facile indicator of inflammation, with all-cause mortality at 2 years post-TIPS in EGVB patients with cirrhosis and to determine the appropriate level of NLR truncation relevant to prognosis.
Materials and Methods
Patients
Clinical data of patients treated with TIPS for cirrhosis EGVB from November 2015 to August 2021 at two medical centers were retrospectively collected. Inclusion criteria included the following: age older than 18 years, cirrhosis diagnosed by images, or liver biopsies. Exclusion criteria included the following: previous treatments with TIPS or surgical shunt; primary liver cancer or extrahepatic tumors; the presence of definite active inflammatory or connective tissue diseases such as pneumonia, spontaneous peritonitis, acute hepatitis, and rheumatoid arthritis; and incomplete baseline data or loss to follow-up.
This study was authorized by the Institutional Ethics Review Board of Wuxi People’s Hospital, affiliated with Nanjing Medical University (Wuxi, Jiangsu Province, China; No. KY23126), and was conducted in accordance with the principles of the Declaration of Helsinki. Due to the retrospective nature of this study, the requirement for written informed consent was waived by the Institutional Ethics Review Board of Wuxi People’s Hospital affiliated with Nanjing Medical University. We declare that patient information was kept strictly confidential.
Data Collection and Outcomes
The electronic medical record system was used to collect the basic information of patients, as well as laboratory tests and imaging results within 72 h before surgery, allowing for the calculation of liver function and immune indicators, including Child-Pugh, MELD, and NLR. The NLR was derived from the absolute peripheral blood neutrophil count (109/L) divided by the absolute lymphocyte count (109/L). The primary outcome was the 2-year all-cause mortality according to the pre-TIPS NLR score.
TIPS Procedure and Follow-Up
Patients included in this study all received an 8 mm diameter VIATORR stent (Gore, Phoenix, USA). All TIPS placements were performed by at least one interventionalist with at least 10 years of clinical experience in liver disease. The specific TIPS procedures have been described previously.Citation18 The treatment of EGVB patients after admission and the definition of early TIPS referred to the Baveno VII consensus.Citation1 Hepatitis B virus (HBV), hepatitis C virus (HCV), and autoimmune hepatitis (AIH) patients, all of whom were evaluated at the hepatology specialty after the procedure, received regular antiviral or nonspecific immunosuppressive therapy, among others.
Patients were followed-up through inpatient hospitalization, outpatient services, or by telephone, and the occurrence of the 2-year all-cause mortality outcome was recorded. Follow-up time was defined as the interval from initial TIPS placement to death, liver transplantation, or the end of the study in August 2023 (last patient enrolled with 2 years of follow-up).
Statistical Analysis
Categorical variables are expressed as frequencies (percentages) and continuous variables as medians and interquartile range (IQR). Clinical data were analyzed for differences using the Mann–Whitney U-test, chi-square test, or Kruskal–Wallis H and Wilcox tests. The optimal cutoff values for NLR or other variables in predicting mortality at 2 years post-TIPS were determined by plotting the receiver operating characteristic curve (ROC). Kaplan-Meier analysis was used to explore the effects of parameters such as NLR on 2-year mortality, and Log rank tests were used to test for differences between groups. A univariate Cox proportional risk analysis was performed to identify variables that might be associated with 2-year mortality, and continuous variables (NLR, age, and creatinine) were converted to categorical variables (NLR ≥4.9, age ≥63.0, and creatinine ≥71.9) based on cutoff values. Significant predictors (P < 0.1) from the univariate analysis were used to determine independent risk factors for 2-year mortality. The contribution of each parameter to the risk of an outcome is recorded as the hazard ratio (HR) with a 95% confidence interval (CI). Subgroup stratification analysis was conducted to explore potential effect modifiers of the association between NLR and patient prognosis following TIPS. The distribution of NLR at various time points is presented using box plots, and the correlation between NLR values and the risk of mortality is illustrated using a heatmap of Spearman correlation coefficients.
All tests were two-sided and P < 0.05 was considered significant. Statistical analysis was performed using the SPSS 26.0 (IBM Corporation, Somers, New York) or R 3.6.3 (http://www.R-project.org/) software packages.
Results
Patient Characteristics
A total of 293 patients were included in the study (). Their baseline demographic and clinical characteristics are detailed in . The main causes of cirrhosis were HBV (152/293, 51.9%), AIH (43/293, 14.7%), schistosomiasis (41/293, 14.0%), and others (57/293, 19.5%). The median age was 59.0 years (IQR 49.0–67.0) and the median Child-Pugh and MELD scores were 7.0 (IQR 6.0–9.0) and 11.0 (IQR 9.0–13.0), respectively. A total of 148 (50.5%) patients received early TIPS to control acute variceal bleeding and 145 (49.5%) patients received TIPS to prevent variceal rebleeding after failure of standard care. The portal pressure gradient (PPG) was 26.0 mmHg (IQR 22.0–32.0) pre-TIPS and 13.0 mmHg (IQR 9.6–16.0) post-TIPS. Forty-four (15.0%) patients died within 2 years with a median follow-up of 39.0 (IQR 25.0–54.5) months. There were statistically significant differences between survivors and non-survivors in the following pre-TIPS parameters: Age, gender, neutrophil count and NLR ().
Table 1 Baseline Characteristics of Surviving and Non-Surviving Patients
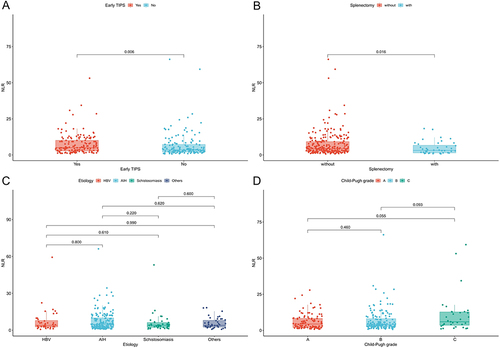
The median NLR was 4.4 (IQR 2.5–9.2). As shown in and , higher NLR levels were observed in patients receiving early TIPS (5.0 vs 3.5, P = 0.006) and in those without splenectomy (4.5 vs 3.2, P = 0.016). In contrast, the distribution of NLR was not statistically significant in patients with different cirrhosis etiologies (P = 0.690) and liver function grades (P = 0.110), as shown in and . Supplementary Figure 1A–D demonstrates the allocation of NLR in the subgroups of age ≥ 63 years, diabetes mellitus, ascites, and portal vein thrombosis (PVT) (all P values > 0.05).
Optimal Cutoff Value
The ROC analysis for NLR is shown in . The optimal cutoff value of NLR for predicting death at 2 years post-TIPS was 4.9, and the area under the curve was 0.634 (95% CI 0.541–0.727), corresponding to a sensitivity and specificity of 68.2% and 59.0%, respectively. Based on this cutoff value, 131 (44.7%) and 162 (55.3%) patients were categorized into high NLR (≥4.9) and low NLR (<4.9) groups, respectively. Supplementary Table 1 provides a comparison of the clinical characteristics of these two NLR status groups. Overall, patients with high NLR had elevated White blood cell count (6.6 vs 3.9, P < 0.001) and neutrophil count (5.3 vs 2.0, P < 0.001), decreased lymphocyte count (0.5 vs 0.8, P < 0.001), and a prolonged prothrombin time (16.1 vs 15.4, P = 0.016).
Figure 3 ROC curve to determine the cut-off value of the NLR for the prediction of 2-year mortality in EGVB patients undergoing TIPS. The optimal cut-off values for NLR (A), age (B), and creatinine (B) are 4.9, 63.0 and 71.9 respectively.
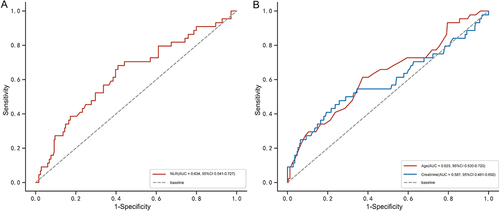
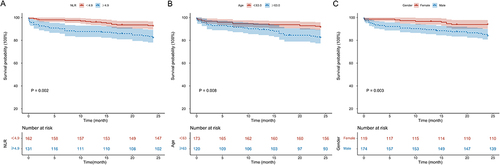
Kaplan-Meier analysis () indicated that the 2-year mortality rate was significantly higher in the high NLR group than in the low NLR group (22.1% vs 9.3%, HR = 2.608, 95% CI 1.398–4.865, Log rank test: P = 0.002). The ROCs for age and creatinine are shown in , with optimal cut-off values of 63.0 and 71.9, area under the curve of 0.625 (95% CI 0.530–0.720) and 0.587 (95% CI 0.481–0.692), and sensitivities and specificities of 61.4% and 62.7% and 47.7% and 74.3%, respectively.
Two-Year All-Cause Mortality
The results of univariate analysis () showed that NLR ≥ 4.9, age ≥ 63.0, creatinine ≥ 71.9, and gender were influential factors for 2-year mortality. These parameters were introduced into the Cox model, and the results demonstrated that NLR ≥ 4.9 (HR = 2.741, 95% CI 1.467–5.121, P = 0.002), age ≥ 63.0 (HR = 3.403, 95% CI 1.835–6.310, P < 0.001) and gender (male) (HR = 2.842, 95% CI 1.366–5.912, P = 0.001) were independent influences on 2-year mortality.
Table 2 Univariate and Multivariate Analysis of Risk Factors for 2-Year Mortality After TIPS
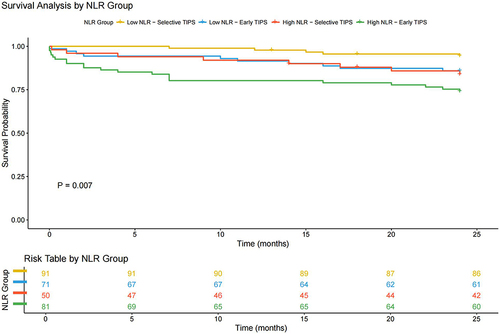
As shown in , patients of advanced age (≥63.0) had a significantly higher 2-year mortality rate (21.9% vs 10.6%, HR = 2.450, 95% CI 1.335–4.495, Log rank test: P = 0.003). Moreover, compared with males, 2-year mortality was significantly reduced in female patients (7.6% vs 20.1%, HR = 0.352, 95% CI 0.169–0.732, Log rank test: P = 0.003) (). The subgroup stratification analysis results () show that patients with low NLR who underwent selective TIPS had a better prognosis, whereas patients with high NLR who received early TIPS had a worse prognosis (Log rank test: P = 0.007, HR = 2.317, 95% CI 1.232–4.356).
The Changes in NLR After TIPS Placement
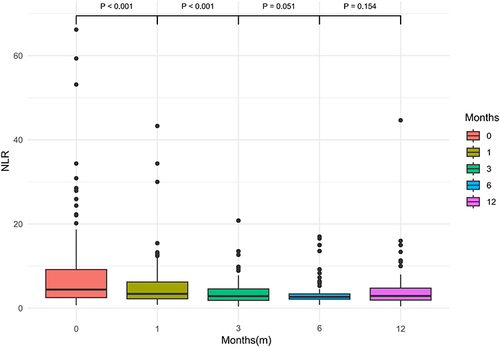
The changes in NLR values at 1 month, 3 months, 6 months, and 12 months post-TIPS are shown in . The box plot demonstrates an overall decreasing trend in NLR after TIPS. The decrease in NLR was statistically significant when comparing 1 month post-TIPS to pre-TIPS levels (P < 0.001), and 3 months post-TIPS to 1 month post-TIPS (P < 0.001). Furthermore, the change in NLR from 3 months to 6 months post-TIPS showed marginal significance (P = 0.051).
Figure 6 Box plots of NLR values at different time points post-TIPS placement. Comparing NLR values at 1 month, 3 months, 6 months, and 12 months post-TIPS to the previous time point yielded P values of < 0.001, < 0.001, 0.051, and 0.154, respectively.
Supplementary Figure 2 presents a heatmap of the Spearman correlation coefficients between NLR and the risk of mortality. At 1 month, 3 months, 6 months, and 12 months post-TIPS, the correlation coefficients between NLR values and mortality risk were 0.107, 0.016, −0.021, and 0.036, respectively. These results indicate a weak correlations between NLR at different post-TIPS time points and the risk of mortality.
Discussion
In cirrhotic patients with EGVB suffering from predominantly viral hepatitis, we observed significantly increased 2-year mortality after TIPS in the high NLR group. The appropriate cutoff value for NLR, as an objective and easily accessible indicator, for predicting 2-year mortality was 4.9. In addition, age and sex were also independent risk factors for 2-year survival post-TIPS.
There is growing evidence that NLR is associated with multiple outcomes in the context of chronic liver disease. In cirrhotic patients awaiting liver transplantation, NLR predicts the risk of death in patients that are stable (without acute decompensation)Citation15,Citation19 or MELD scores below 20.Citation20 Moreover, its predictive ability was independent of Child-Pugh and MELD scores, and, even, cirrhosis stage. These findings are useful for prioritization and organ allocation decisions in liver transplantation. In addition, the prediction of 90-day short-term mortality by NLR in cirrhotic patients with acute-on-chronic liver failureCitation21 or acute decompensationCitation22 also showed satisfactory outcomes. Additionally, high NLR has also been shown to be associated with post-operative recurrence, as well as poorer overall survival and progression-free survival, in patients with primary hepatocellular carcinoma treated with surgical resection,Citation23 transarterial chemoembolization,Citation14 or targeted and immuneCitation24 therapies. Similar results were detected in our study, where the risk of death in patients with high NLR was 2.6 times higher than that in patients with low NLR in a TIPS cohort with definitive 2-year follow-up outcomes. Notably, a clear cutoff for NLR is not easy to determine, which may be related to the baseline situation of patients included in different studies, in which the cutoff value for NLR to predict prognosis generally ranges from 3 to 9. Furthermore, we found that NLR demonstrated an overall gradual decrease following TIPS placement, with this change becoming more pronounced within the first six months post-procedure. This may be associated with reduced portal vein pressure and improved inflammatory status.
Changes in neutrophil and lymphocyte counts are associated with the activation of both congenital and adaptive cellular immune responses, with NLR representing (to some extent) the balance between the two immune responses.Citation25 The frequency of neutrophil subpopulations with pro-inflammatory properties-such as low-density granulocytes and monocytes-is higher, while circulating natural killer cells are less frequent in patients with high NLR.Citation20 This evidence laterally supports the hypothesis that NLR serves as a surrogate marker of systemic inflammatory response and immune dysregulation. As we have described, patients with high NLR usually have higher neutrophil, leukocyte, and lower lymphocyte levels, and have deviant coagulation. This may be due to the inflammatory state leading to activation of the coagulation system.Citation26
In addition, NLR was higher in patients whose bleeding was less than 72 hours from operation (ie, early TIPS). This may be due to the fact that, in patients with acute bleeding, the inflammatory response is activated, and the levels of inflammatory factors are higher than in patients in a relatively stable state with selective TIPS. Therefore, based on the results of the stratified analysis, we recommend that, when using NLR to predict TIPS prognosis in practice, it is essential to differentiate between TIPS types. In particular, patients with high NLR undergoing early TIPS require closer follow-up and timely intervention. Another notable finding was that NLR was lower in patients with splenectomy. The spleen is the host and main source of circulating immune cells and, as such, myeloid cells, neutrophils, macrophages, dendritic cells, and monocytes are reduced after splenectomy. Alterations in peripheral blood immune cells may further lead to changes in immune infiltration at the primary tumor or metastatic site, which may be helpful in suppressing tumor metastasis.Citation27 However, the effect of splenectomy on the immune microenvironment of cirrhotic patients deserves further exploration.
Zhang et alCitation28 have recently reported that NLR could independently predict 30- and 90-day mortality after TIPS. However, their study included patients with predominantly refractory ascites (48%), in addition to a small amount of EGVB (29%) and hepatic pleural fluid (5%) patients. The composition of cirrhosis etiology also differed from that of Chinese patients. In contrast, an advantage of our study is that only EGVB patients were included, minimizing heterogeneity in the baseline dimension. The follow-up of endpoint events was also extended to 2 years, without limiting to short-term mortality. Earlier attention was also given to the prognostic impact of NLR in a Chinese population with predominantly HBV, providing potentially informative parameters for clinical trial design, risk factor stratification, or predictive modeling in the Chinese cirrhosis population. However, it is important to recognize that the presence of HBV infection may also lead to a higher inflammatory state or NLR level in the body. Future research should involve in-depth studies on inflammatory cytokines, pathological tissues, inflammation-related genes, and/or metabolic products.
The impact of age on death after TIPS has been well-documented. Whether in alcoholic hepatitis, and hepatitis C or hepatitis B, ascites, or EGVB-dominated cohorts, Saad,Citation29 Vizzutti,Citation30 and our study have identified that early or mid- to long-term mortality after TIPS increases with age. Age is a more robust predictor of prognosis because as advanced age is often accompanied by frailty, impaired muscle contractile function, or reduced skeletal muscle, which are known to be associated with outcomes in cirrhotic patients.Citation31 However, advanced age is not a contraindication to TIPS, and survival in these patients can still be improved through reducing the risk of further decompensatory events and improving nutritional status through TIPS implantation.Citation30 Interestingly, we found that there is a survival benefit for female patients compared to males, which is comparable to the results of a recent study.Citation32 It is worth noting that the reduced risk of death may not be due to gender, per se, but may be influenced through gender-related attributes such as glomerular filtration rate and muscle mass. Future research in this area is warranted.
There are several limitations to this study. First, due to the retrospective character of the research, selection bias is unavoidable. Second, the harmonization of baseline indications can help to reduce confounding factors, but also poses a challenge regarding generalization of the results, which are to be interpreted with caution in ascites or other populations. Third, we did not observe a correlation between postoperative NLR values and prognosis. This may be influenced by factors such as the timing of NLR measurements, concurrent viral infections, or even complex biological mechanisms. However, this does not imply that NLR lacks value in postoperative management. To the contrary, it underscores the need for further research to explore the roles of NLR in the context of different disease states. Finally, the study lacked mechanistic exploration of NLR and the inflammatory state, cellular immunity, and so on. Exploration of the targeted gut-liver-immunity axis involving bacterial DNA or intestinal floraCitation33 may provide novel insights for mechanistic analysis and treatment.
Conclusion
In summary, our retrospective study revealed that, in patients with EGVB who underwent TIPS implantation, the NLR can assist in predicting post-operative survival. In particular, patients with a low NLR who received selective TIPS had a better prognosis. However, larger-scale, prospective, and multicenter studies are still needed to provide higher-level evidence.
Abbreviations
NLR, neutrophil-to-lymphocyte ratio; EGVB, esophagogastric variceal bleeding; TIPS, transjugular intrahepatic portosystemic shunt; ROC, receiver operator characteristic curve; MELD, model for end-stage liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; AIH, autoimmune hepatitis; IQR, interquartile range; HR, hazard ratio; CI, confidence interval; PVT, portal vein thrombosis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflicts of interest in this work.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author (Wei-Dong Wang).
Additional information
Funding
References
- de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII faculty. Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–974. doi:10.1016/j.jhep.2021.12.022
- Lv Y, Chen H, Luo B, et al. Concurrent large spontaneous portosystemic shunt embolization for the prevention of overt hepatic encephalopathy after TIPS: a randomized controlled trial. Hepatology. 2022;76(3):676–688. doi:10.1002/hep.32453
- Lv Y, Chen H, Luo B, et al. Transjugular intrahepatic portosystemic shunt with or without gastro-oesophageal variceal embolisation for the prevention of variceal rebleeding: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7(8):736–746. doi:10.1016/S2468-1253(22)00087-5
- Song J, Wang X, Yan Y, et al. MELD 3.0 score for predicting survival in patients with cirrhosis after transjugular intrahepatic portosystemic shunt creation. Dig Dis Sci. 2023;68(7):3185–3192. doi:10.1007/s10620-023-07834-3
- Li WC, Zhong BY, Zhang S, et al. Emergent transjugular intrahepatic portosystemic shunt as a first-line therapy in patients with cirrhosis with acute gastroesophageal variceal hemorrhage. J Vasc Interv Radiol. 2023;34(3):344–350. doi:10.1016/j.jvir.2022.11.015
- Liu J, Ma J, Yang C, et al. Sarcopenia in patients with cirrhosis after transjugular intrahepatic portosystemic shunt placement. Radiology. 2022;303(3):711–719. doi:10.1148/radiol.211172
- Alatzides GL, Haubold J, Steinberg HL, et al. Adipopenia in body composition analysis: a promising imaging biomarker and potential predictive factor for patients undergoing transjugular intrahepatic portosystemic shunt placement. Br J Radiol. 2023;96(1146):20220863. doi:10.1259/bjr.20220863
- Sun SH, Eche T, Dorczynski C, et al. Predicting death or recurrence of portal hypertension symptoms after TIPS procedures. Eur Radiol. 2022;32(5):3346–3357. doi:10.1007/s00330-021-08437-0
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871. doi:10.1053/he.2000.5852
- Leise MD, Kim WR, Kremers WK, et al. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology. 2011;140(7):1952–1960. doi:10.1053/j.gastro.2011.02.017
- Bettinger D, Sturm L, Pfaff L, et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J Hepatol. 2021;74(6):1362–1372. doi:10.1016/j.jhep.2021.01.023
- Peng Y, Li Y, He Y, et al. The role of neutrophil to lymphocyte ratio for the assessment of liver fibrosis and cirrhosis: a systematic review. Expert Rev Gastroenterol Hepatol. 2018;12(5):503–513. doi:10.1080/17474124.2018.1463158
- Rice J, Dodge JL, Bambha KM, et al. Neutrophil-to-lymphocyte ratio associates independently with mortality in hospitalized patients with cirrhosis. Clin Gastroenterol Hepatol. 2018;16(11):1786–1791. doi:10.1016/j.cgh.2018.04.045
- Wang S, Zhang X, Chen Q, et al. A novel neutrophil-to-lymphocyte ratio and sarcopenia based TACE-predict model of hepatocellular carcinoma patients. J Hepatocell Carcinoma. 2023;10:659–671. doi:10.2147/JHC.S407646
- Leithead JA, Rajoriya N, Gunson BK, et al. Neutrophil-to-lymphocyte ratio predicts mortality in patients listed for liver transplantation. Liver Int. 2015;35(2):502–509. doi:10.1111/liv.12688
- Li J, Chen Q, Luo X, et al. Neutrophil-to-lymphocyte ratio positively correlates to age in healthy population. J Clin Lab Anal. 2015;29(6):437–443. doi:10.1002/jcla.21791
- Liu S, Zheng H, Zhu X, et al. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabet Res Clin Pract. 2017;130:90–97. doi:10.1016/j.diabres.2017.05.008
- Tang HH, Zhang ZC, Zhao ZL, et al. Large paraumbilical vein shunts increase the risk of overt hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. J Clin Med. 2022;12(1):158. doi:10.3390/jcm12010158
- Biyik M, Ucar R, Solak Y, et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2013;25(4):435–441. doi:10.1097/MEG.0b013e32835c2af3
- Kalra A, Wedd JP, Bambha KM, et al. Neutrophil-to-lymphocyte ratio correlates with proinflammatory neutrophils and predicts death in low model for end-stage liver disease patients with cirrhosis. Liver Transpl. 2017;23(2):155–165. doi:10.1002/lt.24702
- Sun J, Guo H, Yu X, et al. A neutrophil-to-lymphocyte ratio-based prognostic model to predict mortality in patients with HBV-related acute-on-chronic liver failure. BMC Gastroenterol. 2021;21(1):422. doi:10.1186/s12876-021-02007-w
- Janka T, Tornai D, Papp M, et al. The value of neutrophil-to-lymphocyte ratio to identify bacterial infection and predict short-term mortality in patients with acutely decompensated cirrhosis. Diagnostics. 2023;13(18):2954. doi:10.3390/diagnostics13182954
- Qu Z, Lu YJ, Feng JW, et al. Preoperative prognostic nutritional index and neutrophil-to-lymphocyte ratio predict survival outcomes of patients with hepatocellular carcinoma after curative resection. Front Oncol. 2021;11:823054. doi:10.3389/fonc.2021.823054
- Ochi H, Kurosaki M, Joko K, et al. Usefulness of neutrophil-to-lymphocyte ratio in predicting progression and survival outcomes after atezolizumab-bevacizumab treatment for hepatocellular carcinoma. Hepatol Res. 2023;53(1):61–71. doi:10.1111/hepr.13836
- Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 2021;122(7):474–488. doi:10.4149/BLL_2021_078
- Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26–S34. doi:10.1097/CCM.0b013e3181c98d21
- Stöth M, Freire Valls A, Chen M, et al. Splenectomy reduces lung metastases and tumoral and metastatic niche inflammation. Int, J, Cancer. 2019;145(9):2509–2520. doi:10.1002/ijc.32378
- Zhang W, Aryan M, Chen Z, et al. Prognostic value of neutrophil-to-lymphocyte ratio in cirrhosis patients undergoing transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2022;34(4):435–442. doi:10.1097/MEG.0000000000002295
- Saad N, Rude MK, Darcy M, et al. Older age is associated with increased early mortality after transjugular intrahepatic portosystemic shunt. Ann Hepatol. 2016;15(2):215–221. doi:10.5604/16652681.1193716
- Vizzutti F, Celsa C, Calvaruso V, et al. Mortality after transjugular intrahepatic portosystemic shunt in older adult patients with cirrhosis: a validated prediction model. Hepatology. 2023;77(2):476–488. doi:10.1002/hep.32704
- Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74(3):1611–1644. doi:10.1002/hep.32049
- Mazumder NR, Celaj S, Atiemo K, et al. Liver-related mortality is similar among men and women with cirrhosis. J Hepatol. 2020;73(5):1072–1081. doi:10.1016/j.jhep.2020.04.022
- Tranah TH, Edwards LA, Schnabl B, et al. Targeting the gut-liver-immune axis to treat cirrhosis. Gut. 2021;70(5):982–994. doi:10.1136/gutjnl-2020-320786