Abstract
ALI(acute lung injury) is a severe respiratory dysfunction caused by various intrapulmonary and extrapulmonary factors. It is primarily characterized by oxidative stress and affects the integrity of the pulmonary barrier. In severe cases, ALI can progress to ARDS(acute respiratory distress syndrome), a condition that poses a serious threat to the lives of affected patients. To date, the etiological mechanisms underlying ALI remain elusive, and available therapeutic options are quite limited. AMPK(AMP-activated protein kinase), an essential serine/threonine protein kinase, performs a pivotal function in the regulation of cellular energy levels and cellular regulatory mechanisms, including the detection of redox signals and mitigating oxidative stress. Meanwhile, Nrf2(nuclear factor erythroid 2-related factor 2), a critical transcription factor, alleviates inflammation and oxidative responses by interacting with multiple signaling pathways and contributing to the modulation of oxidative enzymes associated with inflammation and programmed cell death. Indeed, AMPK induces the dissociation of Nrf2 from Keap1(kelch-like ECH-associated protein-1) and facilitates its translocation into the nucleus to trigger the transcription of downstream antioxidant genes, ultimately suppressing the expression of inflammatory cells in the lungs. Given their roles, AMPK and Nrf2 hold promise as novel treatment targets for ALI. This study aimed to summarise the current status of research on the AMPK/Nrf2 signaling pathway in ALI, encompassing recently reported natural compounds and drugs that can activate the AMPK/Nrf2 signaling pathway to alleviate lung injury, and provide a theoretical reference for early intervention in lung injury and future research on lung protection.
Introduction
As is well documented, acute lung injury (ALI) refers to hypoxaemic respiratory insufficiency resulting from various intrapulmonary and extrapulmonary causes. It is characterized by apoptotic damage driven by excessive inflammation of the alveolar endothelium and epithelial cells, which leads to dysfunction of the alveolar-capillary barrier and, in severe cases, progresses to acute respiratory failure and acute respiratory distress syndrome (ARDS).Citation1 At present, ALI/ARDS has a high morbidity and mortality rate. A multicenter study enrolling 459 ARDS patients in intensive care units (ICUs) from 50 countries, published in <JAMA> in 2016, documented that among 29,144 patients admitted to ICUs, the prevalence of ARDS was 10.4%, with an in-hospital mortality rate of roughly 40%.Citation2 Currently, its pathogenesis remains underexplored and encompasses several factors, including various pro-inflammatory cytokines such as tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and monocyte chemotactic protein-1 (MCP-1). Additionally, imbalances in coagulation and fibrinolytic systems, apoptosis, disturbances in oxidative and antioxidant homeostasis, and impaired water channel protein are also implicated in the development of ALI.
Increasing evidence suggests that ALI is linked to the prolonged accumulation of reactive oxygen species (ROS) due to excessive inflammation.Citation3 ROS are integral to the organism’s fundamental biochemical activities, helping to sustain the redox balance within tissues and cells. However, excess ROS have been established to lower cell membrane fluidity and damage epithelial and microvascular barriers, eventually causing pulmonary edema and vasodilation.Citation4 ROS function as inflammatory signaling molecules that can intensify pulmonary inflammation by activating nuclear transcription factor κB (NF-κB), nucleotide-binding oligomeric structural domain-like protein 3 (NLRP3), and other inflammatory pathways.Citation5 Moreover, infections by pathogens such as bacteria, viruses, and fungi are all capable of rapidly increasing ROS levels, potentially triggering a systemic cytokine storm due to local ROS accumulation and exacerbating the inflammation.Citation6
In summary, the stimulation and oxidative stress induction by various noxious substances can disrupt the balance between the oxidative and antioxidant systems in vivo, thereby contributing to ALI/ARDS progression. Currently, no consistently effective medications for ALI treatment have been identified.Citation7
AMP-activated protein kinase (AMPK) is a protein kinase involved in the regulation of cellular energy states. It is activated under ischemic and hypoxic conditions as a result of multiple upstream kinase phosphorylations. Activated AMPK acts on downstream target proteins and signal transduction pathways to regulate cellular catabolism. AMPK has been demonstrated to relieve several diseases associated with inflammation and oxidative stress, with a particularly significant impact on ALI.Citation8
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that attenuates oxidative damage and inhibits the inflammatory response. It regulates the expression of Phase II detoxifying enzymes, like reduced coenzyme II (NADPH), quinone oxidoreductase 1 (NQO-1), and heme oxygenase-1 (HO-1). Moreover, it is involved in triggering the activity of antioxidant enzymes, including glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD).Citation9,Citation10 These enzymes play a decisive role in exerting anti-inflammatory effects, scavenging oxygen free radicals, and protecting cells from various forms of damage.
As an upstream regulator of Nrf2, the AMPK pathway participates in the direct modulation of pulmonary inflammation and activates the Nrf2 pathway via its phosphorylation. This activation leads to an increase in nuclear transcription accumulation, boosts anti-inflammatory and antioxidant effects, and reduces ALI.Citation11 In the past, the AMPK/Nrf2 pathway has often served as a target for the treatment of hepatic lipid infiltration and inflammation and has been mostly reported in mediating oxidative stress and lipid metabolism.Citation12,Citation13 However, nowadays, more and more studies suggest that activation of the AMPK/Nrf2 signaling pathway is likely to be an effective strategy for the prevention and treatment of ALI. Current research reveals that signaling pathways such as PI3K/Akt, NF-κB, MAPK, VEGF, and JAK/STAT independently or collectively regulate the inflammatory processes of ALI. The AMPK/Nrf2 pathway occupies a central position among them, intricately connecting with each of these pathways.
The PI3K-AKT pathway is an intracellular signaling mechanism that enhances metabolism, proliferation, cell survival, growth, and angiogenesis. Activation of AMPK regulates cellular metabolism and inflammation by stimulating PI3K/Akt activity, while Akt influences Nrf2 transcription in the nucleus.Citation14 AMPK activation suppresses the NF-κB and MAPK pathways, thereby decreasing pro-inflammatory cytokine production.Citation15 Nrf2 indirectly inhibits oxidative stress from the NF-κB and MAPK pathways by regulating antioxidant gene expression.Citation16,Citation17 VEGF, a crucial regulator of angiogenesis and permeability, is affected by AMPK, which modulates endothelial cell metabolism and survival, thereby influencing VEGF expression and function to control vascular permeability and inflammation.Citation18 Nrf2, through its antioxidant actions, mitigates VEGF-mediated oxidative stress, protecting vascular endothelial cells.Citation19 The JAK/STAT pathway is essential for cytokine signaling and immune responses. AMPK activation inhibits JAK/STAT pathway phosphorylation, thereby reducing pro-inflammatory cytokine production.Citation20
This review outlined advancements in investigating the AMPK/Nrf2 signaling pathway concerning ALI, intending to present novel insights into the treatment of lung injury and the development of effective drugs.
AMPK
AMPK, acting as an energy sensor, plays an instrumental role in both generating and conserving energy in eukaryotic cells. Indeed, the enzyme directly senses the energy available within the cell by binding to adenosine triphosphate (ATP), a key molecule in energy metabolism.Citation21 Maintaining adequate levels of ATP is essential for the physiological functioning of all living cells, given that ATP hydrolysis to adenosine diphosphate (ADP) yields the necessary energy to drive cellular processes.Citation22 Furthermore, ADP can be further converted to adenosine monophosphate (AMP). Cells must maintain a balance between the supply and demand of ATP to ensure an adequate supply. Oxidative stress can activate AMPK by altering the ratio of AMP/ATP or ADP/ATP.Citation23 A decrease in ATP production leading to a relative increase in AMP or ADP activates AMPK, resulting in the phosphorylation of specific enzymes and growth control nodes. This activation ultimately maximizes ATP production and concomitantly minimizes consumption, thereby maintaining energy homeostasis.
AMPK Structure and Activation
First discovered in 1973,Citation24 AMPK is a heterotrimeric protein complex consisting of a catalytic subunit α and two regulatory subunits β and γ. Each subunit comprises multiple isoforms, classified as α1, α2, β1, β2, γ1, γ2, and γ3, resulting in twelve distinct AMPK complexes.Citation25 These complexes are expressed in almost all eukaryotic cells, including plants, fungi, and animals.Citation26
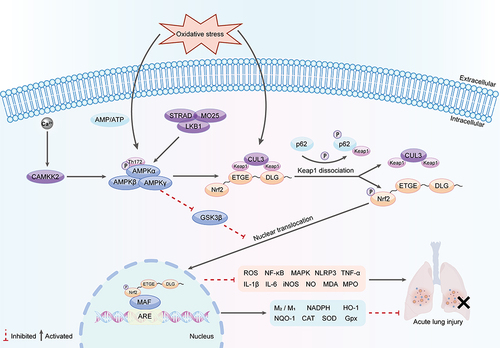
Under ischemic and hypoxic states, AMPK is activated by upstream kinases phosphorylating the intracyclic threonine residue (Thr172) on its α-subunit. This activation triggers a cascade effect wherein AMPK acts on downstream target proteins, thereby modulating intracellular catabolic processes. Notably, the β-subunit features a carbohydrate-binding site, facilitating the binding of AMPK to glycogen. On the other hand, the γ-subunit serves as a sensor for alterations in the ratio of AMP/ATP.Citation27 Furthermore, AMPK plays a central role in inhibiting the dephosphorylation of Thr172 and safeguarding it from phosphatases.Citation28
The liver kinase B1 (LKB1, also known as STK11) complex, a heterotrimer consisting of LKB1, STRAD, and MO25, is one of the upstream kinases that activate AMPK by responding to an increase in AMP levels.Citation29 Specifically, it is activated by either stress signals or activators. LKB1 is abundantly expressed in various tissues of the body and is typically upregulated in the pancreas, liver, and skeletal muscle. During energetic stress, AMP stimulates axonal proteins to interact with it, leading to LKB1 simultaneously phosphorylating Thr172 and activating AMPK. Interestingly, AMPK phosphorylates Thr172 not only through its dependence on LKB1 but also via the activation by Ca2+/calmodulin-dependent protein kinase 2 (CAMKK2, also referred to as CAMKKβ) as a response to calcium fluxes. AMPK is hypothesized to serve as a crucial link connecting calcium signaling with energy metabolism. CAMKK2 is highly abundant in neural tissue in response to neuronal depolarization.Citation30 It is noteworthy that CAMKK2 kinase and LKB1 are two mammalian kinases displaying the highest degree of sequence homology.Citation31
In addition to the aforementioned methods, various allosteric activators can activate AMPK. These activators are involved in most of the catabolic and anabolic processes of AMPK, and so far the exact mechanism by which most drugs and compounds activate AMPK remains unclear. It is undeniable that AMPK has the potential to mitigate numerous diseases induced by inflammation and oxidative stress, especially ALI.Citation32 Therefore, we postulate that AMPK likely plays a compelling role in preserving intracellular oxidative homeostasis.
AMPK Functions
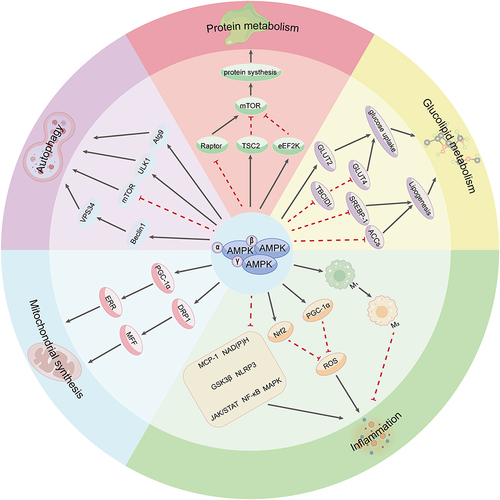
AMPK is involved in mediating several crucial metabolic processes within cells, such as glucose metabolism, lipid metabolism, and protein metabolism. Additionally, it plays a role in governing cellular autophagy, maintaining mitochondrial homeostasis, and exerting anti-inflammatory effects.Citation33 These functions ultimately encompass nearly all physiological activities observed in living organisms ().
Glucolipid Metabolism
AMPK is a key target for the management of metabolic diseases, including diabetes, obesity, and fatty liver. One way in which AMPK enhances glucose uptake is by driving the translocation and governing the expression of glucose transporter protein 2 (GLUT2) from intracellular storage vesicles to the plasma membrane of cells.Citation34 GLUT2, the predominant isoform in hepatocytes, plays a key role in glucose uptake.Citation35 Phosphorylation of AMPK regulates GLUT4-mediated glucose uptake in muscle by inhibiting TBC1D1, a Rab-GAP protein that under basal conditions prevents fusion of GLUT4-containing vesicles with the plasma membrane.Citation36 In lipid metabolism, AMPK reduces fatty acid synthase expression by decreasing the phosphorylation level of the transcription factor SREBP-1.Citation37 Numerous studies have highlighted the significant impact of metformin on glucose and lipid metabolism, with many attributing AMPK as a vital element in its physiological effects.Citation38 Metformin activates AMPK by elevating the cytoplasmic ADP/ATP and AMP/ATP ratios. Besides, the phosphorylation of AMPK by acetyl-CoA carboxylases (ACCs) contributes to its ability to induce lipid synthesis, while metformin reciprocally influences insulin sensitivity and muscle glucose uptake.Citation39 Intriguingly, retrospective studies have reported lower cancer rates in type 2 diabetic patients using metformin.Citation40 The activation of AMPK has been suggested to enhance potential tumor-suppressive and anti-tumorigenic properties, presenting encouraging avenues for forthcoming cancer investigation and therapeutic approaches.
Protein Metabolism
AMPK, a key regulator of protein metabolic processes, plays a pivotal role in modulating the growth master regulator mTOR. Under nutrient-rich conditions, mTOR activity increases with decreasing AMPK activity. Conversely, under energy-poor conditions, activated AMPK suppresses mTOR activity, leading to inhibition of cell growth and protein synthesis.Citation41 This regulatory mechanism is crucial in various physiological processes, including aging, inflammation, and lipid and glucose metabolism. mTOR complex-1 (TORC1) is a key target of AMPK. It is inhibited through the direct phosphorylation of its upstream regulator TSC2 and the subunit Raptor, thereby suppressing protein synthesis.Citation42,Citation43 Furthermore, AMPK directly regulates protein synthesis by phosphorylating eEF2K, which acts as a negative regulator of protein elongation and is a downstream target of the mTOR pathway.Citation44
Autophagy
AMPK activation also plays an equally important role in cellular autophagy. Autophagy refers to the physiological process of engulfing and degrading cytoplasmic proteins or organelles through the formation of autophagic lysosomes and is an indispensable step in the regulation of cellular metabolism.Citation45 ATG is an indispensable gene implicated in autophagy, whilst the UNC-51-like kinase 1 (ULK1) complex is its mammalian homolog.Citation46 AMPK can regulate mitochondrial autophagy and maintain intracellular homeostasis by phosphorylating ULK1 and promoting its activity.Citation47 In addition, AMPK can promote autophagy by phosphorylating Beclin1, which activates vacuolar protein sorting 34 complex (VPS34),Citation48 or by phosphorylating autophagy-associated gene (Atg9) protein, which induces autophagic vesicle formation.Citation49 AMPK phosphorylation inhibits the downstream activation and phosphorylation of mTOR, thereby regulating autophagy.Citation50
Mitochondrial Synthesis
AMPK is regarded as a key regulator of mitochondrial biogenesis, created to boost ATP production in response to increased energy demands. Under energy stress conditions, AMPK optimizes the formation of new mitochondria by modulating the interaction between peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) and the estrogen-related receptor (ERR).Citation51 Moreover, AMPK regulates mitochondrial division by phosphorylating mitochondrial fission factor (MFF) through dynamin-related protein 1 (DRP1).Citation52 This not only enables the regulation of mitochondrial fragmentation but also facilitates the replacement of defective mitochondria with new functional ones, thereby serving a mitochondrial purification function.Citation53
Inflammation
AMPK is considered an interesting target involved in the regulation of immune homeostasis as well as in the treatment of chronic inflammatory diseases. AMPK activation significantly induces the transition of macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype, thus exerting anti-inflammatory effects.Citation54 Additionally, AMPK inhibits pro-inflammatory signaling pathways by suppressing the expression of MCP-1, an essential chemokine involved in macrophage recruitment.Citation55 By up-regulating PGC-1α activity, AMPK also impedes mitochondrial ROS production,Citation56 while down-regulating NAD(P)H gene expression limits cytosolic ROS production during mitochondrial regulation.Citation57 Furthermore, AMPK activation alleviates ischemia-induced endoplasmic reticulum (ER) stress. Severe ER stress can trigger apoptosis of alveolar epithelial cells, whilst phosphorylated AMPK has been shown to inhibit inflammatory vesicle activity and attenuate rat ER stress,Citation58 which is crucial for the regulation of post-ischemic injury, survival, and remodeling. AMPK also exerts its anti-inflammatory effects by activating PI3K/Akt and Nrf2 and inhibiting NF-κB, MAPK, and JAK/STAT pathway activation.
In summary, AMPK plays a dual role in balancing energy supply with demand and acts as a critical coordinator of vital intracellular pathways, mediating redox states, relieving inflammation, and regulating autophagy.
Nrf2
In recent years, Nrf2 has emerged as a major target for the treatment of lung diseases. It is an essential transcription factor that participates in the primary cellular antioxidant mechanism, suppressing inflammatory reactions and minimizing oxidative injury.Citation59 As a key regulator of redox balance, Nrf2 not only maintains the phenotype of type 2 alveolar epithelial cells but also enhances their defense against inflammatory injury.Citation60 It interacts with numerous signaling pathways to regulate the activity of oxidative enzymes (including NOX, NOS, XO, and CYP) implicated in inflammation and cellular death. In ALI, Nrf2 confers antioxidative and anti-inflammatory effects by regulating gene expression through the Antioxidant Response Element (ARE), a specific DNA promoter binding site.Citation61 The ARE is located in the presence of protective genes such as SOD, GST, and others. These protective genes can be activated by several electrophilic and oxidative compounds to promote the expression of phase II antioxidant and detoxification enzyme genes. Lastly, this activation enhances the ability of cell tissues to effectively inhibit stress, thus playing a protective role.Citation62
Nrf2 Structure
Nrf2, a member of the Cap’n’Collar (CNC) subfamily, was initially isolated from a human leukemia cell line (K562) and is composed of seven functional domains spanning from Nrf2-ECH homology (Neh)1 to Neh7.Citation63 The Neh2 segment includes two crucial motifs known as DLG and ETGE, indispensable for facilitating the interaction of Nrf2 with its cytoplasmic inhibitor, the kelch-like ECH-associated protein 1 (Keap1).Citation64 These motifs, DLG and ETGE, represent the areas within the Nrf2-Neh2 structural domains that attach to the kelch structural domains within Keap1’s homodimers. This attachment crucially impacts Nrf2’s transcriptional activities by negatively regulating them and encouraging their breakdown.Citation65 The ETGE motif, notable for its higher affinity, is termed the hinge, whereas the DLG motif functions as the latch.Citation66 This binding phenomenon is recognized as the “Hinge-and-latch model”, demonstrating the structural basis by which Nrf2 maintains stability in the cytoplasm.
Under homeostatic conditions, Keap1 acts as a substrate articulating protein for cullin3 (CUL3)-dependent E3 ubiquitin ligase, mediating Nrf2 ubiquitination. It subsequently suppresses Nrf2 transcriptional activity via proteasomal degradation.Citation67 When the ubiquitin chain reaches a length of four ubiquitins, the polyubiquitinated protein becomes a new substrate for 26S proteasome degradation.Citation68
Nrf2 Functions and Effects
Under physiological conditions, Nrf2 is inactivated by binding to its negative regulator, Keap1. Upon stimulation by endogenous or exogenous oxidative stress, the sensing cysteine within Keap1 dissociates the binding of Nrf2 from Keap1. As a result, Nrf2 escapes ubiquitination and translocates from the cytoplasm into the nucleus.Citation69 In the nucleus, Nrf2 forms a heterodimer with a small Maf protein termed Nrf2-Maf. This complex then binds to the downstream ARE promoter region, activating the transcription of antioxidant genes such as HO-1, NQO1, SOD, and CAT.Citation68 Furthermore, the Nrf2-Maf complex inhibits the expression and release of pro-inflammatory factors associated with NF-κB-mediated inflammatory responses, thus attenuating pulmonary inflammation.Citation70 Conversely, the excess ROS generated by oxidative stress leads to the accumulation of unfolded proteins (UPR) in the endoplasmic reticulum, which aids in the separation of the Nrf2-Keap1 complex and facilitates the transport of Nrf2 into the nucleus through the endoplasmic reticulum kinase (PERK). Taken together, PERK-mediated nuclear movement of Nrf2 assists in sustaining redox balance,Citation71 allowing Nrf2 to play a dual positive regulatory role.
Another unconventional pathway activated by Nrf2, which holds considerable implications in mitigating oxidative stress, is the autophagy-lysosomal pathway alongside the Nrf2-Keap1 axis. p62/SQSTM1, alternatively referred to as p62, is a typical receptor for selective autophagy that is responsible for the breakdown of ubiquitinated substrates. Autophagy dysfunction, encompassing Atg5 or Atg7 deficiency and exposure to arsenic, conduces to the accumulation of p62 autophagy bridging proteins.Citation72 As a multidomain protein capable of interacting with various proteins, excessive p62 accumulation interferes with the functions of multiple binding proteins, including Keap1. Consequently, p62 competes with Nrf2 for Keap1 binding and sequesters it into the autophagosome, thereby impeding Keap1-mediated degradation of Nrf2 and ultimately leading to the liberation of Nrf2 and the subsequent transcriptional activation of its target genes.Citation73,Citation74 Phosphorylating p62 enhances its ability to bind more tightly to Keap1.Citation75 It is found that p-Akt mediates Nrf2 nuclear localization, leading to the upregulation of HO-1 and NQO1, which alleviates lung inflammation, oxidative stress, and apoptosis.Citation76 Conversely, Nrf2 deficiency decreases p-Akt activity, leading to cell death.Citation77
Nrf2 has traditionally been considered a tumor suppressor owing to its cytoprotective role, recognized as the first-line cellular defense against both external and internal stimuli. Notwithstanding, recent studies evinced that excessive activation of the Nrf2 pathway may create a cellular microenvironment conducive to the survival of both healthy and cancerous cells, collectively protecting against oxidative stress and conferring resistance to chemotherapeutic drugs and radiotherapy.Citation78 These findings insinuate that Nrf2 may play a dual role as both a suppressor and a promoter in tumorigenesis. Considering that the application of Nrf2 in the field of oncology is in its early phases, there is an urgent need to identify genes linked with this pathway and its regulatory framework for an in-depth understanding.
AMPK/Nrf2 Signaling Pathway
AMPK/Nrf2
A functional interconnection exists between AMPK and Nrf2. AMPK, an upstream enzyme of Nrf2, enhances the expression of antioxidant genes, thereby inhibiting oxidative damage.Citation79 Additionally, AMPK can trigger the phosphorylation of residue Ser558 (Ser550 in mice) on Nrf2, influencing the nuclear accumulation of Nrf2.Citation80 Notably, following the mutation of this residue, the activation of AMPK is unable to promote the nuclear import of Nrf2. In cells, AMPK activation prompts the release of Nrf2 from Keap1, facilitating its nuclear translocation and accumulation. Nrf2 then binds to the ARE, initiating the expression of multiple antioxidant and detoxification enzymes, which restores oxidative equilibrium in the cell ().Citation81,Citation82
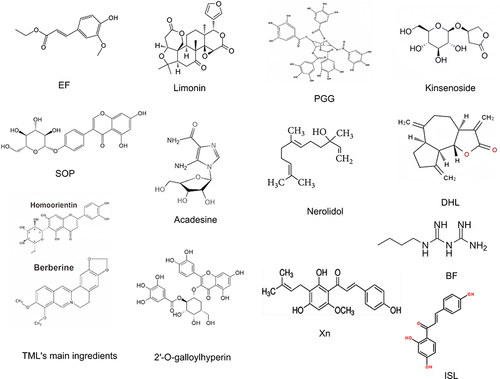
Compounds such as Ethyl ferulate (EF),Citation83 Limonin,Citation84 1,2,3,4,6-O-Pentagalloylglucose(PGG),Citation14 KinsenosideCitation85 and Sophoricoside(SOP)Citation86 have been shown to play a key role in multiple biological processes. In other words, they are involved in activating the phosphorylation of AMPK and up-regulating the level of Nrf2 protein. Additionally, they promote the nuclear translocation of Nrf2, inhibit the synthesis and release of iNOS, NO, and inflammatory cytokines (TNF-α, IL-1β, and IL-6), lower intracellular malondialdehyde (MDA) and myeloperoxidase (MPO) levels, and enhance the activity of antioxidant enzymes such as SOD, GSH, and Gpx. Furthermore, these compounds mitigate the increase in permeability of mouse lung tissues and inflammatory lung injury and oxidative stress. Acadesine mitigates inflammation and tissue damage linked to severe acute pancreatitis-induced acute lung injury (SAP-ALI) by activating AMPK, which regulates the Nrf2-dependent antioxidant pathway. This action ameliorates endothelial dysfunction, decreases oxidative stress, and suppresses apoptosis.Citation87
AMPK/Nrf2/HO-1 Signaling Pathway
Acting under the regulation of Nrf2, Heme oxygenase-1 (HO-1) serves as a crucial enzyme in the cellular defense mechanism against oxidative stress and is essential for the preservation of endothelial homeostasis, operating as a signaling molecule in the pathway downstream of Nrf2.Citation88 HO-1 initiates the production of biliverdin-IXα (BV) through the oxidative degradation of heme and further processes it into bilirubin-IXα (BR) with the help of biliverdin reductase (BVR).Citation89 These substances, known for their strong systemic antioxidant properties, contribute to the removal of ROS and offer protection at the cellular level.
Liang et al determined that berberine is effective in preventing acute lung inflammation caused by cigarette smoke by mitigating lipopolysaccharide-induced ALI via the PERK-mediated Nrf2/HO-1 signaling pathway.Citation90 These observations suggest that the activation of the Nrf2/HO-1 pathway plays a decisive role in mitigating lung inflammation. Additionally, 5-aminoimidazole-4-carboxamide ribonucleoside(AICAR), an AMPK activator, has been found to up-regulate HO-1 expression by activating Nrf2 in endothelial cells, thereby exerting a protective effect against cytokine-mediated apoptosis. Moreover, AICAR can restore AMPK phosphorylation and increase HO-1 levels in bromine-exposed cells and pulmonary tissues, consequently attenuating lung injury and enhancing lung function.Citation91,Citation92 Meanwhile, Wang et al demonstrated that the AMPK/Nrf2/HO-1 signaling pathway enhances the phenotype of M2 macrophages and exerts anti-inflammatory effects.Citation54 Taken together, these studies indicate that up-regulation of the Nrf2/HO-1 signaling pathway can inhibit the inflammatory response, highlighting its pulmonoprotective role against various insults.
Of note, NerolidolCitation93 and Lycium barbarum polysaccharide (LBP)Citation94 can mitigate cellular oxidative stress and lung injury in mice by inducing the phosphorylation of AMPK, activating the AMPK/Nrf2/HO-1 signaling axis, and enhancing the activities of Nrf2 and Gpx, thereby inhibiting the levels of MPO, MDA, and IL-1β, IL-6.
Macrophages and AMPK/Nrf2
Macrophages play a vital role as effector cells in the exudative, proliferative, and fibrotic phases of ALI/ARDS. There are two primary phenotypes into which macrophages can be polarized, namely the classically activated (M1) phenotype and the alternatively activated (M2) phenotype.Citation95 During the initial exudative stage of pneumonia, mediators at the area of injury stimulate macrophages, inducing their differentiation into M1 macrophages through polarization. These M1 macrophages subsequently release pro-inflammatory factors, such as TNF-α, IL-1β, IL-6, and iNOS, and recruit neutrophils, thereby increasing levels of oxidative stress-induced products and causing tissue damage. Following the transition from the exudative phase to the proliferative repair phase, pulmonary macrophages shift from an M1 to an M2 phenotype. Thereafter, these M2 macrophages activate the anti-inflammatory signaling pathway and boost the production of anti-inflammatory agents such as IL-10 and TGF-β. This process aids in managing pulmonary inflammation and promotes the repair of lung tissue.Citation96,Citation97
AMPK is essential for macrophage metabolism and immune function. Situated at the crossroads of metabolism and inflammation in macrophages, AMPK regulates polarization towards the anti-inflammatory M2 phenotype via signal transduction.Citation98 This process decreases pro-inflammatory cytokine production and increases IL-10 secretion.Citation99 Additionally, AMPK modulates macrophage phagocytic activity. Upon oxidative stress, Nrf2 activation enhances macrophage antioxidant capacity, reducing ROS levels and mitigating oxidative damage.Citation100 These findings portray a potential therapeutic strategy for modulating macrophage polarization and alleviating pulmonary inflammation.
In a mouse and cellular model of MRSA-induced ALI, Wu et al noted that Dehydrocostus lactone (DHL) attenuates p38 MAPK and NF-κB phosphorylation, promotes the activation of the AMPK/Nrf2 signaling pathway and AMPK phosphorylation, up-regulates the protein levels and gene expression levels of Nrf2 and HO-1, shift macrophage polarization from the M1 phenotype to the M2 phenotype, and inhibits neutrophil infiltration and the production of TNF-α, IL-1β, and IL-6, thus alleviating ALI in mice.Citation101
NF-κB, MAPK and AMPK/Nrf2
The NF-κB and Mitogen-activated protein kinase(MAPK) signaling pathways are closely associated with oxidative inflammatory diseases. The NF-κB, a dimeric transcription factor localized in B lymphocytes, regulates the levels of inflammatory factors, playing a significant role in the development of lung diseases.Citation102 Identified in nearly all cell types via the inhibitor protein IκB-α, the p65 and p50 subunits of NF-κB are acetylated at several lysine sites.Citation103 It plays a role in mediating cellular inflammation, apoptosis, and proliferationCitation104 and is triggered by diverse extrinsic factors, including cytokines, free radicals, and microbial or viral antigens. Activation of Nrf2 has been evinced to trigger anti-inflammatory cascades associated with NF-κB-mediated inflammatory responses. This process impairs the phosphorylation of IκB-α kinase (IKK) and prevents the NF-κB p65 subunit from translocating into the nucleus, which results in decreased levels of MPO, MDA, and ROS. This also down-regulates the expression of inflammatory cytokines and relieves both inflammation and oxidative stress in ALI.Citation16 Furthermore, AMPK contributes to inflammatory reactions by decreasing NF-κB nuclear translocation and inhibiting the transcription of factors related to innate immunity and inflammation through the suppression of the NF-κB signaling pathway.Citation105 The Nrf2/Maf/ARE antioxidant pathway, which is activated following the translocation of Nrf2 into the cell nucleus, partially inhibits the expression of the NF-κB signaling pathway, thereby regulating oxidative homeostasis.Citation70
The mitogen-activated protein kinase (MAPK) pathway is crucial in eukaryotic signal transduction, regulating cell proliferation, differentiation, apoptosis, and stress responses. It controls pro-inflammatory mediator synthesis and release primarily through macrophage activation,Citation106 playing a role in inflammation and oxidative stress in ALI.Citation107 MAPK has three main subfamilies: ERK, p38, and JNK. p38 activation triggers significant inflammation, ERK regulates cytokines like TNF-α, IL-1β, and IL-6, and JNK controls apoptosis and inflammation.Citation108 Activation of any MAPK subfamily can accelerate ALI progression. Inhibition of the MAPK pathway alleviates LPS-induced ALI via the NF-κB pathway,Citation109,Citation110 showing that NF-κB activation is regulated by MAPK.Toll-like receptors (TLRs) are essential in innate immunity. TLR4 initiates ALI inflammatory responses through MyD88 (myeloid differentiation factor 88)-dependent and independent pathways.Citation111 High mobility group box 1 (HMGB1), a nuclear protein, is released from damaged cells and induces pro-inflammatory cytokine release as a primary TLR4 ligand.Citation112 The HMGB1–TLR4 pathway activates MAPK and NF-κB, promoting inflammation.Citation113 AMPK reduces ALI severity by inhibiting HMGB1 and TLR4 activity, thereby suppressing MAPK and NF-κB pathway activation.Citation15
Polygonatum, a traditional Chinese medicine with a history spanning nearly a thousand years, is extensively utilized in clinical settings to treat lung diseases, fatigue, weakness, and indigestion. Gan et al isolated and extracted polysaccharides from raw Polygonatum cyrtonema Hua (PCP), honey-processed Polygonatum cyrtonema Hua (HPCP), and revealed that PCP and HPCP inhibited NF-κB phosphorylation, activated the AMPK/Nrf2 signaling pathway, and enhanced downstream HO-1 and NQO-1 activities, thereby lowering the levels of pro-inflammatory mediators and regulating pulmonary oxidative homeostasis in an LPS-induced ALI model of mice and that HPCP appeared to have a superior effect to PCP.Citation114
The aqueous extract of velvet antler (AVA) can elevate HO-1 activity by activating the AMPK/Nrf2 pathway, significantly decreasing the expression level of IκB-α and NF-κB, and the levels of ERK, JNK, and p38 protein phosphorylation in the MAPK pathway. Furthermore, it suppressed excessive neutrophil activation and the release of MDA, MPO, TNF-α, IL-1β, and IL-6 in mouse lung tissue, as well as upregulated the expression of IL-10 and antioxidant enzymes. Importantly, it plays a role in alleviating pulmonary edema and anti-oxidative stress.Citation115 Compounds that exert comparable effects include 2′-O-galloylhyperin,Citation116 Ethyl acetate fractions of Adenostemma Lavenia(EAAL),Citation117 and so on.
NLRP3 Inflammasome and AMPK/Nrf2
The nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3) is an integral component of a multi-protein intracellular inflammation pathway that plays an essential role in cellular antioxidant reactions and acts as a primary intracellular innate immune signaling receptor. This protein detects alterations in the cellular environment and responds to internal danger signals, leading to the assembly and activation of the NLRP3 inflammasome.Citation118 The NLRP3 inflammasome consists of NLRP3, the apoptosis-associated speck-like protein (ASC) containing the caspase recruitment domain, and caspase-1, forming a complex oligomer.Citation119 This complex is activated in response to ROS and a wide array of endogenous and exogenous stimuli, resulting in lysosomal and mitochondrial damage and disturbances in ionic balance. Upon activation, the NLRP3 inflammasome facilitates the cleavage of caspase-1 into caspase-1 p20, which subsequently promotes the cleavage of pro-IL-1β and pro-IL-18 into their bioactive mature forms.Citation120 The increased activity of the NLRP3 inflammasome correlates with lung injury induced by bacterial pathogens and culminates in ventilator-associated ALI, along with the progression of chronic respiratory conditions like asthma and chronic obstructive pulmonary disease (COPD).Citation121
In animal models of acute pancreatitis (AP), was discovered to enhance the nuclear translocation of Nrf2, suppress the activation of NLRP3 inflammasome and the NF-κB inflammatory pathway, and lower the expression of TNF-α, IL-1β, and IL-6, thereby conferring protection against AP-induced lung injury in rats.Citation122 According to a previous study, activating AMPK down-regulated the expression of thioredoxin interacting protein (TXNIP), affecting the production of inflammatory vesicles. Additionally, it suppresses the expression of NLRP3 by inhibiting the phosphorylation of the NF-κB p65 subunit,Citation123,Citation124 thereby alleviating ALI.
In another study on PM-induced ALI mice, Badamjav et al observed that Thalictrum minus L (TML) effectively down-regulated NLRP3 and Keap expression, enhanced p-AMPK and Nrf2 activity, and reduced the effects of inflammatory factors in vivo.Citation125 These actions significantly ameliorated lung injury and prevented apoptosis in mice. Furthermore, Galactose lectin 1 (Gal-1), a natural β‐galactoside‐binding protein, was found to inhibit NLRP3-mediated inflammatory vesicle generation and suppress the release of ROS, MDA, iNOS, COX2, and other inflammatory factors in LPS-induced lung tissues of mice. This outcome was achieved by increasing AMPK phosphorylation and elevating Nrf2 protein levels, thereby further mitigating pathological lung injury.Citation82
GSK3β and AMPK/Nrf2
Glycogen synthase kinase-3 (GSK3β) is a protein kinase B and one of the Akt substrates involved in Keap1-independent Nrf2 stabilization and regulation. Under physiological conditions, GSK3β within the cytoplasm phosphorylates two segments of Nrf2, leading to the ubiquitination of β-TRCP and initiating the nuclear degradation of Nrf2.Citation126 Likewise, AMPK can mediate the phosphorylation and inactivation of GSK3β, consequently boosting the nuclear accumulation of Nrf2.Citation127 Furthermore, ROS can directly promote the nuclear translocation of Nrf2 by enhancing the phosphorylation of GSK3β. These processes conjointly contribute to the upregulation of downstream antioxidant factors and the inhibition of the NF-κB inflammatory pathway. However, following excessive ROS accumulation, the Nrf2/ARE antioxidant pathway becomes overwhelmed and is thus unable to maintain the balance between oxidation and antioxidation, thus leading to pulmonary inflammation.
In an ALI model, LPS stimulation disrupted the AMPK/Nrf2/GSK3β signaling pathway in macrophages. Xanthohumol (Xn) can alleviate lung oxidative damage and apoptosis by stimulating the phosphorylation of AMPK and GSK3β. This activation triggers the AMPK/Nrf2/GSK3β axis in macrophages, leading to the translocation of Nrf2 into the nucleus. This event boosts the production of antioxidant enzymes such as SOD and GSH, simultaneously decreasing the production of ROS and HMGB1.Citation32 This pulmonoprotective mechanism involving AMPK/Nrf2 activation, GSK3β phosphorylation and inactivation, NLRP3 inflammasome inhibition is also shared between Buformin (BF)Citation128 and Isoliquiritigenin (ISL),Citation129 as evidenced in both mouse and cellular models.
Clinical Trials and AMPK/Nrf2
Current research indicates that certain pharmacological activators (such as metformin, phenformin, thiazolidinediones, berberine, and resveratrol) can indirectly activate AMPK by inhibiting mitochondrial respiration, thereby increasing AMP levels.Citation130 A randomized clinical trial from Iran, published in 2023, involved 189 patients diagnosed with COVID-19, divided into a metformin group (85 patients) and a control group (104 patients). Results showed that diabetic patients with prior metformin treatment had lower ICU admission rates and mortality compared to non-diabetic patients. Metformin improved COVID-19 outcomes in diabetic patients and significantly reduced intubation rates.Citation131
Another study demonstrated that vitamin D might protect the respiratory system by reducing pulmonary inflammation through antimicrobial peptide cathelicidins. Metformin may enhance vitamin D receptor sensitivity by improving AMPK signaling, thereby augmenting its lung-protective effects.Citation132 Sorafenib, commonly used in chemotherapy for non-small cell lung cancer (NSCLC), showed in a phase II clinical trial involving KRAS-mutant advanced NSCLC that combining sorafenib with metformin enhanced AMPK activity, which improved the inhibition of cancer cell proliferation and had a beneficial impact on tumor regression.Citation133
Resveratrol (RESV), a natural polyphenol and AMPK activator, exhibits significant antiviral and antioxidant properties. A study on 190 patients with SARS-CoV-2 hospitalized at Hospital das Clínicas in Brazil found that RESV reduced neutrophil activation and free DNA release, suggesting that RESV adjunctive therapy might help mitigate inflammation and improve patient outcomes.Citation134 Interestingly, resveratrol is also an Nrf2 pathway activator.Citation135
Curcumin, another natural polyphenol, and Nrf2 activator, targets the Nrf2 pathway to protect cells from oxidative damage.Citation136 In a double-blind, placebo-controlled randomized clinical trial involving 60 patients with stage 3 and 4 COPD, participants were assigned to either an 80 mg nanocurcumin group (n=30) or a placebo group (n=30) for 3 months. The effect of nanocurcumin on lung function was assessed using the FEV1/FVC ratio. Results indicated that patients in the nanocurcumin group had significantly lower IL-6 levels and improved respiratory function compared to the placebo group. Nanocurcumin supplementation appeared to benefit the inflammatory state and respiratory indices in severe COPD patients.Citation137
Historically, the AMPK/Nrf2 pathway was well-known for treating hepatic lipid infiltration and inflammation. Recently, it has gained attention as a potential therapy for lung injury. However, clinical evidence supporting compounds and drugs that simultaneously activate the AMPK/Nrf2 pathway remains insufficient, and their therapeutic potential requires further exploration.
Summary and Perspective
ALI is a complex clinical syndrome involving inflammatory responses, endothelial barrier dysfunction, and abnormal macrophage migration. Activated AMPK senses redox signals and has anti-inflammatory effects. In respiratory inflammation, pulmonary AMPK is significantly inhibited, and its activation can prevent lung injury. Recent research reveals that gut microbiota influences ALI, with certain short-chain fatty acids alleviating SAP-ALI via AMPK activation, offering new therapeutic insights.Citation138 Nrf2 is a key antioxidant pathway. Under oxidative stress, Nrf2 moves from the cytoplasm to the nucleus, promoting antioxidant enzyme expression and reducing ALI-induced oxidative damage. AMPK, as an upstream kinase of Nrf2, enhances Nrf2 nuclear accumulation and GSK3β phosphorylation, upregulating HO-1 and NQO-1. This inhibits MAPK and NF-κB inflammatory pathways and NLRP3 inflammasome activation, reducing oxidative and inflammatory mediators (MDA, MPO, TNF-α, IL-6, IL-1β) and increasing antioxidant enzymes (SOD, GSH), improving pulmonary inflammation ().Despite advances in understanding ALI pathogenesis, its mechanisms remain unclear, and treatment is challenging. New therapeutic methods are urgently needed. AMPK/Nrf2 is emerging as a key target for lung injury research, with promising prospects. This review summarizes drugs or compounds that simultaneously activate AMPK and Nrf2 to treat ALI ( and ). However, these studies are still preclinical and lack definitive clinical evidence. Further research on the combinations and clinical use of these activators is needed, offering new directions for ALI/ARDS treatment strategies.
Table 1 Activation of the AMPK/Nrf2 Signaling Pathway by Natural Compounds and Drugs to Attenuate ALI
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors gratefully acknowledge the financial support by the National Natural Science Foundation of China (82160022).
References
- Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–350. doi:10.5858/arpa.2015-0519-RA
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi:10.1001/jama.2016.0291
- Ivanov AV, Valuev-Elliston VT, Tyurina DA, et al. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget. 2017;8(3):3895–3932. doi:10.18632/oncotarget.13904
- Zhang H, Wang Z, Liu R, et al. Reactive oxygen species stimulated pulmonary epithelial cells mediate the alveolar recruitment of FasL+ killer B cells in LPS-induced acute lung injuries. J Leukoc Biol. 2018;104(6):1187–1198. doi:10.1002/JLB.3A0218-075R
- Yang H, Lv H, Li H, Ci X, Peng L. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun Signal. 2019;17(1):62. doi:10.1186/s12964-019-0366-y
- Agita A, Alsagaff MT. Inflammation, immunity, and hypertension. Acta Med Indones. 2017;49(2):158–165.
- Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–637. doi:10.1016/S0140-6736(21)00439-6
- Su ZQ, Mo ZZ, Liao JB, et al. Usnic acid protects LPS-induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. Int Immunopharmacol. 2014;22(2):371–378. doi:10.1016/j.intimp.2014.06.043
- Tosi ME, Bocanegra V, Manucha W, et al. The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO). Cell Stress Chaperones. 2011;16(1):1. doi:10.1007/s12192-010-0221-y
- Li H, Wu S, Shi N, Lian S, Lin W. Nrf2/HO-1 pathway activation by manganese is associated with reactive oxygen species and ubiquitin-proteasome pathway, not MAPKs signaling. J Appl Toxicol. 2011;31(7):690–697. doi:10.1002/jat.1654
- Chan YL, Wang B, Chen H, et al. Pulmonary inflammation induced by low-dose particulate matter exposure in mice. Am J Physiol Lung Cell Mol Physiol. 2019;317(3). doi:10.1152/ajplung.00232.2019
- Wang D, Yang L, Liu Y. Targeting AMPK signaling in the liver: implications for obesity and type 2 diabetes mellitus. Curr Drug Targets. 2022;23(11):1057–1071. doi:10.2174/1389450123666220429082702
- Xu W, Zhao T, Xiao H. The implication of oxidative stress and AMPK-Nrf2 antioxidative signaling in pneumonia pathogenesis. Front Endocrinol. 2020;11:400. doi:10.3389/fendo.2020.00400
- Zhang Q, Cheng S, Xin Z, et al. 1,2,3,4,6-O-Pentagalloylglucose Protects against Acute Lung Injury by Activating the AMPK/PI3K/Akt/Nrf2 Pathway. Int J Mol Sci. 2022;23(22):14423. doi:10.3390/ijms232214423
- Tao L, Cao F, Xu G, Xie H, Zhang M, Zhang C. Mogroside IIIE Attenuates LPS‐Induced acute lung injury in mice partly through regulation of the TLR4/MAPK/NF‐κB Axis via AMPK Activation. Phytother Res. 2017;31(7):1097–1106. doi:10.1002/ptr.5833
- Tang Y, Ding F, Wu C, Liu B. hucMSC conditioned medium ameliorate lipopolysaccharide-induced acute lung injury by suppressing oxidative stress and inflammation via Nrf2/NF-κB signaling pathway. Anal Cell Pathol. 2021;2021:6653681. doi:10.1155/2021/6653681
- Vitucci EC, Simmons AE, Martin EM, et al. Epithelial MAPK signaling directs endothelial NRF2 signaling and IL-8 secretion in a tri-culture model of the alveolar-microvascular interface following diesel exhaust particulate (DEP) exposure. Partic Fibre Toxicol. 2024;21(1). doi:10.1186/s12989-024-00576-8
- Wang JC, Chen SY, Wang M, et al. Nickel-induced VEGF expression via regulation of Akt, ERK1/2, NFκB, and AMPK pathways in H460 cells. Environ Toxicol. 2019;34(5). doi:10.1002/tox.22731
- Fan J, Lv H, Li J, et al. Roles of Nrf2/HO-1 and HIF-1α/VEGF in lung tissue injury and repair following cerebral ischemia/reperfusion injury. J Cell Physiol. 2019;234(6). doi:10.1002/jcp.27767
- Rutherford C, Speirs C, Williams JJ, et al. Phosphorylation of Janus kinase 1 (JAK1) by AMP-activated protein kinase (AMPK) links energy sensing to anti-inflammatory signaling. Sci Signaling. 2016;9(453). doi:10.1126/scisignal.aaf8566
- Crozet P, Margalha L, Confraria A, et al. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front Plant Sci. 2014;5:190. doi:10.3389/fpls.2014.00190
- Boyer PD, Chance B, Ernster L, et al. Oxidative phosphorylation and photophosphorylation. Annu Rev Biochem. 1977;46(1):955–966. doi:10.1146/annurev.bi.46.070177.004515
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi:10.1038/nrm3311
- Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme a carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973;248(1):378–380. doi:10.1016/S0021-9258(19)44486-4
- Viollet B, Athea Y, Mounier R, et al. AMPK: lessons from transgenic and knockout animals. Front Biosci. 2009;14(1):19–44. doi:10.2741/3229
- Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582(1):81–89. doi:10.1016/j.febslet.2007.11.018
- Xiao B, Sanders MJ, Carmena D, et al. Structural basis of AMPK regulation by small molecule activators. Nat Commun. 2013;4:3017. doi:10.1038/ncomms4017
- Davies SP, Helps NR, Cohen PT, Hardie DG. 5’-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377(3):421–425. doi:10.1016/0014-5793(95)01368-7
- Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101(10):3329–3335. doi:10.1073/pnas.0308061100
- Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi:10.1016/j.cmet.2005.05.009
- Fogarty S, Hawley SA, Green KA, Saner N, Mustard KJ, Hardie DG. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem J. 2010;426(1):109–118. doi:10.1042/BJ20091372
- Lv H, Liu Q, Wen Z, et al. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi:10.1016/j.redox.2017.03.001
- Višnjić D, Lalić H, Dembitz V, et al. AICAr, a widely used AMPK Activator with Important AMPK-independent effects: a systematic review. Cells. 2021;10(5):1095. doi:10.3390/cells10051095
- Zhu D, Yan Q, Li Y, et al. Effect of konjac mannan oligosaccharides on glucose homeostasis via the improvement of insulin and leptin resistance in vitro and in vivo. Nutrients. 2019;11(8). doi:10.3390/nu11081705
- Guillam MT, Burcelin R, Thorens B. Normal hepatic glucose production in the absence of GLUT2 reveals an alternative pathway for glucose release from hepatocytes. Proc Natl Acad Sci U S A. 1998;95(21):12317–12321. doi:10.1073/pnas.95.21.12317
- Frøsig C, Pehmøller C, Birk JB, Richter EA, Wojtaszewski JFP. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol. 2010;588(Pt 22):4539–4548. doi:10.1113/jphysiol.2010.194811
- Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13(4):376–388. doi:10.1016/j.cmet.2011.03.009
- Howell JJ, Hellberg K, Turner M, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25(2). doi:10.1016/j.cmet.2016.12.009
- Fullerton MD, Galic S, Marcinko K, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19(12):1649–1654. doi:10.1038/nm.3372
- Quinn BJ, Kitagawa H, Memmott RM, et al. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24(9):469–480. doi:10.1016/j.tem.2013.05.004
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi:10.1038/nrm.2017.95
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi:10.1016/s0092-8674(03)00929-2
- Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi:10.1016/j.molcel.2008.03.003
- Leprivier G, Remke M, Rotblat B, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell. 2013;153(5):1064–1079. doi:10.1016/j.cell.2013.04.055
- Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14(3). doi:10.1038/nrgastro.2016.185
- Chan EYW, Kir S, Tooze SA. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J Biol Chem. 2007;282(35):25464–25474. doi:10.1074/jbc.M703663200
- Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:6016):456–461. doi:10.1126/science.1196371
- Zhao Y, Wang Q, Qiu G, et al. RACK1 promotes autophagy by enhancing the Atg14L-Beclin 1-Vps34-Vps15 complex formation upon phosphorylation by AMPK. Cell Rep. 2015;13(7):1407–1417. doi:10.1016/j.celrep.2015.10.011
- Masouminia M, Samadzadeh S, Mendoza AS, et al. Upregulation of autophagy components in alcoholic hepatitis and nonalcoholic steatohepatitis. Exp Mol Pathol. 2016;101(1):81–88. doi:10.1016/j.yexmp.2016.07.002
- Shao BZ, Wang SL, Fang J, et al. Alpha7 nicotinic acetylcholine receptor alleviates inflammatory bowel disease through induction of AMPK-mTOR-p70S6K-mediated autophagy. Inflammation. 2019;42(5). doi:10.1007/s10753-019-01027-9
- Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi:10.1016/S0092-8674(00)80611-X
- Wang C, Youle R. Cell biology: form follows function for mitochondria. Nature. 2016;530(7590):288–289. doi:10.1038/530288a
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi:10.1038/ncb2329
- Wang Y, Huang Y, Xu Y, et al. A Dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid. Redox Signaling. 2018;28(2):141–163. doi:10.1089/ars.2017.7003
- Zhao P, Wong KI, Sun X, et al. TBK1 at the Crossroads of Inflammation and Energy Homeostasis in Adipose Tissue. Cell. 2018;172(4):731–743.e12. doi:10.1016/j.cell.2018.01.007
- Rabinovitch RC, Samborska B, Faubert B, et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21(1). doi:10.1016/j.celrep.2017.09.026
- Song P, Zou MH. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic Biol Med. 2012;52(9):1607–1619. doi:10.1016/j.freeradbiomed.2012.01.025
- Bai A, Ma AG, Yong M, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80(11):1708–1717. doi:10.1016/j.bcp.2010.08.009
- Ahmed SMU, Luo L, Namani A, et al. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi:10.1016/j.bbadis.2016.11.005
- Traver G, Mont S, Gius D, et al. Loss of Nrf2 promotes alveolar type 2 cell loss in irradiated, fibrotic lung. Free Radic Biol Med. 2017;112. doi:10.1016/j.freeradbiomed.2017.08.026
- Luan R, Ding D, Yang J. The protective effect of natural medicines against excessive inflammation and oxidative stress in acute lung injury by regulating the Nrf2 signaling pathway. Front Pharmacol. 2022;13:1039022. doi:10.3389/fphar.2022.1039022
- Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–1309. doi:10.1016/j.freeradbiomed.2009.07.035
- Namani A, Li Y, Wang XJ, Tang X. Modulation of NRF2 signaling pathway by nuclear receptors: implications for cancer. Biochim Biophys Acta. 2014;1843(9):1875–1885. doi:10.1016/j.bbamcr.2014.05.003
- Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88(Pt B):101–107. doi:10.1016/j.freeradbiomed.2015.05.034
- Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199–218. doi:10.1016/j.tibs.2014.02.002
- Tong KI, Padmanabhan B, Kobayashi A, et al. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27(21):7511–7521. doi:10.1128/MCB.00753-07
- Pierce NW, Kleiger G, Shan S, Deshaies RJ. Detection of sequential polyubiquitylation on a millisecond timescale. Nature. 2009;462:7273):615–619. doi:10.1038/nature08595
- Baird L, Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 Pathway. Mol Cell Biol. 2020;40(13):e00099–20. doi:10.1128/MCB.00099-20
- Strom J, Xu B, Tian X, Chen QM. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016;30(1):66–80. doi:10.1096/fj.14-268904
- Mohamed GA, Ibrahim SRM, El-Agamy DS, et al. Terretonin as a new protective agent against sepsis-induced acute lung injury: impact on SIRT1/Nrf2/NF-κBp65/NLRP3 signaling. Biology. 2021;10(11):1219. doi:10.3390/biology10111219
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23(20):7198–7209. doi:10.1128/MCB.23.20.7198-7209.2003
- Jiang T, Harder B, Rojo de la Vega M, et al. p62 links autophagy and Nrf2 signaling. Free Radic Biol Med. 2015;88(Pt B):199–204. doi:10.1016/j.freeradbiomed.2015.06.014
- Komatsu M, Kurokawa H, Waguri S, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12(3):213–223. doi:10.1038/ncb2021
- Lau A, Wang XJ, Zhao F, et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30(13):3275–3285. doi:10.1128/MCB.00248-10
- Kwon J, Han E, Bui CB, et al. Assurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascade. EMBO Rep. 2012;13(2):150–156. doi:10.1038/embor.2011.246
- Fu Z, Jiang Z, Guo G, et al. rhKGF-2 attenuates smoke inhalation lung injury of rats via Activating PI3K/Akt/Nrf2 and Repressing FoxO1-NLRP3 Inflammasome. Front Pharmacol. 2021;12:641308. doi:10.3389/fphar.2021.641308
- Yan J, Li J, Zhang L, et al. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic Biol Med. 2018;121:78–85. doi:10.1016/j.freeradbiomed.2018.04.557
- Menegon S, Columbano A, Giordano S. The dual roles of NRF2 in cancer. Trends Mol Med. 2016;22(7):578–593. doi:10.1016/j.molmed.2016.05.002
- An H, Hu Z, Chen Y, et al. Angiotensin II-mediated improvement of renal mitochondrial function via the AMPK/PGC-1α/NRF-2 pathway is superior to norepinephrine in a rat model of septic shock associated with acute renal injury. Ann Translat Med. 2021;9(6). doi:10.21037/atm-21-621
- Loboda A, Damulewicz M, Pyza E, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cellul Molecul Life Sci. 2016;73(17). doi:10.1007/s00018-016-2223-0
- Badamjav R, Sonom D, Wu Y, et al. The protective effects of Thalictrum minus L. on lipopolysaccharide-induced acute lung injury. J Ethnopharmacol. 2020;248:112355. doi:10.1016/j.jep.2019.112355
- Huang XT, Liu W, Zhou Y, et al. Galectin-1 ameliorates lipopolysaccharide-induced acute lung injury via AMPK-Nrf2 pathway in mice. Free Radic Biol Med. 2020;146:222–233. doi:10.1016/j.freeradbiomed.2019.11.011
- Wu YX, Wang YY, Gao ZQ, et al. Ethyl ferulate protects against lipopolysaccharide-induced acute lung injury by activating AMPK/Nrf2 signaling pathway. Acta Pharmacol Sin. 2021;42(12):2069–2081. doi:10.1038/s41401-021-00742-0
- Liang H, Liu G, Fan Q, Nie Z, Xie S, Zhang R. Limonin, a novel AMPK activator, protects against LPS-induced acute lung injury. Int Immunopharmacol. 2023;122:110678. doi:10.1016/j.intimp.2023.110678
- Yang Y, Zhong ZT, Xiao YG, Chen H-B. The Activation of AMPK/NRF2 pathway in lung epithelial cells is involved in the protective effects of kinsenoside on lipopolysaccharide-induced acute lung injury. Oxid Med Cell Longev. 2022;2022:3589277. doi:10.1155/2022/3589277
- Wu YX, Zeng S, Wan BB, et al. Sophoricoside attenuates lipopolysaccharide-induced acute lung injury by activating the AMPK/Nrf2 signaling axis. Int Immunopharmacol. 2021;90:107187. doi:10.1016/j.intimp.2020.107187
- Zhu X, Duan F, Zhang Y, et al. Acadesine alleviates acute pancreatitis-related lung injury by mediating the barrier protective function of pulmonary microvascular endothelial cells. Int Immunopharmacol. 2022;111:109165. doi:10.1016/j.intimp.2022.109165
- Lei L, Chai Y, Lin H, et al. Dihydroquercetin Activates AMPK/Nrf2/HO-1 signaling in macrophages and attenuates inflammation in LPS-induced endotoxemic mice. Front Pharmacol. 2020;11:662. doi:10.3389/fphar.2020.00662
- Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244(23):6388–6394. doi:10.1016/S0021-9258(18)63477-5
- Liang Y, Fan C, Yan X, et al. Berberine ameliorates lipopolysaccharide-induced acute lung injury via the PERK-mediated Nrf2/HO-1 signaling axis. Phytother Res. 2019;33(1):130–148. doi:10.1002/ptr.6206
- ming LX, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol. 2011;300(1):H84–93. doi:10.1152/ajpheart.00749.2010
- Ahmad I, Molyvdas A, Jian MY, et al. AICAR decreases acute lung injury by phosphorylating AMPK and upregulating heme oxygenase-1. Eur Respir J. 2021;58(6):2003694. doi:10.1183/13993003.03694-2020
- Ni YL, Shen HT, Su CH, et al. Nerolidol suppresses the inflammatory response during lipopolysaccharide-induced acute lung injury via the modulation of antioxidant enzymes and the AMPK/Nrf-2/HO-1 pathway. Oxid Med Cell Longev. 2019;2019:9605980. doi:10.1155/2019/9605980
- Zheng G, Ren H, Li H, et al. Lycium barbarum polysaccharide reduces hyperoxic acute lung injury in mice through Nrf2 pathway. Biomed Pharmacother. 2019;111:733–739. doi:10.1016/j.biopha.2018.12.073
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi:10.1172/JCI59643
- Huang X, Xiu H, Zhang S, Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018:1264913. doi:10.1155/2018/1264913
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
- Juban G, Saclier M, Yacoub-Youssef H, et al. AMPK activation regulates LTBP4-Dependent TGF-β1 secretion by pro-inflammatory macrophages and controls fibrosis in Duchenne muscular dystrophy. Cell Rep. 2018;25(8):2163–2176.e6. doi:10.1016/j.celrep.2018.10.077
- Antonioli L, Colucci R, Pellegrini C, et al. The AMPK enzyme-complex: from the regulation of cellular energy homeostasis to a possible new molecular target in the management of chronic inflammatory disorders. Expert Opin Ther Targets. 2016;20(2):179–191. doi:10.1517/14728222.2016.1086752
- Luo J, Li P, Dong M, et al. SLC15A3 plays a crucial role in pulmonary fibrosis by regulating macrophage oxidative stress. Cell Death Differ. 2024;31(4). doi:10.1038/s41418-024-01266-w
- Wu YX, Jiang FJ, Liu G, et al. Dehydrocostus lactone attenuates methicillin-resistant staphylococcus aureus-induced inflammation and acute lung injury via modulating macrophage polarization. IJMS. 2021;22(18):9754. doi:10.3390/ijms22189754
- Sun CY, Xu LQ, Zhang ZB, et al. Protective effects of pogostone against LPS-induced acute lung injury in mice via regulation of Keap1-Nrf2/NF-κB signaling pathways. Int Immunopharmacol. 2016;32:55–61. doi:10.1016/j.intimp.2016.01.007
- Wang X, Gao Y, Tian N, et al. Astragaloside IV represses high glucose-induced mesangial cells activation by enhancing autophagy via SIRT1 deacetylation of NF-κB p65 subunit. Drug Des Devel Ther. 2018;12:2971–2980. doi:10.2147/DDDT.S174058
- Song SY, Zhou B, Yang SM, et al. Preventive effects of sevoflurane treatment on lung inflammation in rats. Asian Pac J Trop Med. 2013;6(1):53–56. doi:10.1016/S1995-7645(12)60200-4
- Li W, Qiu X, Jiang H, et al. Ulinastatin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of AMPK/NF-κB pathway. Int Immunopharmacol. 2015;29(2):560–567. doi:10.1016/j.intimp.2015.09.028
- Lee CY, Yang JJ, Lee SS, et al. Protective effect of Ginkgo biloba leaves extract, EGb761, on endotoxin-induced acute lung injury via a JNK- and Akt-dependent NFκB pathway. J Agric Food Chem. 2014;62(27):6337–6344. doi:10.1021/jf501913b
- Yeh CH, Yang JJ, Yang ML, et al. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-κB pathway. Free Radic Biol Med. 2014;69:249–257. doi:10.1016/j.freeradbiomed.2014.01.028
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600). doi:10.1126/science.1072682
- Liu S, Feng G, Wang GL, et al. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol. 2008;584(1). doi:10.1016/j.ejphar.2008.02.009
- Lee HS, Kim HJ, Moon CS, et al. Inhibition of c-Jun NH2-terminal kinase or extracellular signal-regulated kinase improves lung injury. Respir Res. 2004;5(1). doi:10.1186/1465-9921-5-23
- Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2). doi:10.1016/j.cell.2008.02.043
- Yang H, Hreggvidsdottir HS, Palmblad K, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA. 2010;107(26). doi:10.1073/pnas.1003893107
- Chen Z, Chen Y, Pan L, et al. Dachengqi decoction attenuates inflammatory response via Inhibiting HMGB1 Mediated NF-κB and P38 MAPK signaling pathways in severe acute pancreatitis. Cell Physiol Biochem. 2015;37(4). doi:10.1159/000430403
- Gan Q, Wang X, Cao M, et al. NF-κB and AMPK-Nrf2 pathways support the protective effect of polysaccharides from Polygonatum cyrtonema Hua in lipopolysaccharide-induced acute lung injury. J Ethnopharmacol. 2022;291:115153. doi:10.1016/j.jep.2022.115153
- Chang JS, Lin HJ, Deng JS, et al. Preventive effects of velvet antler (Cervus elaphus) against lipopolysaccharide-induced acute lung injury in mice by inhibiting MAPK/NF-kB Activation and Inducing AMPK/Nrf2 Pathways. Evid Based Complement Alternat Med. 2018;2018:1–13. doi:10.1155/2018/2870503
- Zhang SD, Wang P, Zhang J, et al. 2′O-galloylhyperin attenuates LPS-induced acute lung injury via up-regulation antioxidation and inhibition of inflammatory responses in vivo. Chem Biol Interact. 2019;304:20–27. doi:10.1016/j.cbi.2019.02.029
- Chen JJ, Deng JS, Huang CC, et al. p-coumaric-acid-containing adenostemma lavenia ameliorates acute lung injury by activating AMPK/Nrf2/HO-1 signaling and improving the anti-oxidant response. Am J Chin Med. 2019;47(7):1483–1506. doi:10.1142/S0192415X19500769
- Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–489. doi:10.1038/s41577-019-0165-0
- Mangan MSJ, Olhava EJ, Roush WR, et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(9):688. doi:10.1038/nrd.2018.149
- Saber S, Abd El-Fattah EE, Yahya G, et al. A novel combination therapy using rosuvastatin and lactobacillus combats dextran sodium sulfate-induced colitis in high-fat diet-fed rats by targeting the TXNIP/NLRP3 interaction and influencing gut microbiome composition. Pharmaceuticals. 2021;14(4):341. doi:10.3390/ph14040341
- McVey MJ, Steinberg BE, Goldenberg NM. Inflammasome activation in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2021;320(2):L165–L178. doi:10.1152/ajplung.00303.2020
- Gao Z, Sui J, Fan R, et al. Emodin protects against acute pancreatitis-associated lung injury by inhibiting NLPR3 inflammasome activation via Nrf2/HO-1 signaling. Drug Des Devel Ther. 2020;14:1971–1982. doi:10.2147/DDDT.S247103
- Wei H, Bu R, Yang Q, et al. Exendin-4 protects against hyperglycemia-induced cardiomyocyte pyroptosis via the AMPK-TXNIP pathway. J Diabetes Res. 2019;2019:8905917. doi:10.1155/2019/8905917
- Li F, Chen Y, Li Y, Huang M, Zhao W. Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur J Pharmacol. 2020;886:173449. doi:10.1016/j.ejphar.2020.173449
- Badamjav R, Zhang L, Sonom D, et al. Thalictrum minus L. ameliorates particulate matter-induced acute lung injury in mice. J Ethnopharmacol. 2021;264:113379. doi:10.1016/j.jep.2020.113379
- Mathur A, Pandey VK, Kakkar P. Activation of GSK3β/β-TrCP axis via PHLPP1 exacerbates Nrf2 degradation leading to impairment in cell survival pathway during diabetic nephropathy. Free Radic Biol Med. 2018;120:414–424. doi:10.1016/j.freeradbiomed.2018.04.550
- Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016;36(14):1931–1942. doi:10.1128/MCB.00118-16
- Liu B, Wang Z, He R, et al. Buformin alleviates sepsis-induced acute lung injury via inhibiting NLRP3-mediated pyroptosis through an AMPK-dependent pathway. Clin Sci. 2022;136(4):273–289. doi:10.1042/CS20211156
- Liu Q, Lv H, Wen Z, Ci X, Peng L. Isoliquiritigenin activates nuclear factor erythroid-2 related factor 2 to Suppress the NOD-like receptor protein 3 inflammasome and inhibits the NF-κB pathway in macrophages and in acute lung injury. Front Immunol. 2017;8:1518. doi:10.3389/fimmu.2017.01518
- Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing γ subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11(6):554–565. doi:10.1016/j.cmet.2010.04.001
- Shaseb E, Ghaffary S, Garjani A, et al. Long and short-term metformin consumption as a potential therapy to prevent complications of COVID-19. Adv Pharm Bull. 2023;13(3):621–626. doi:10.34172/apb.2023.066
- Gomaa AA, Abdel-Wadood YA, Thabet RH, Gomaa GA. Pharmacological evaluation of vitamin D in COVID-19 and long COVID-19: recent studies confirm clinical validation and highlight metformin to improve VDR sensitivity and efficacy. Inflammopharmacology. 2024;32(1):249–271. doi:10.1007/s10787-023-01383-x
- Groenendijk FH, Mellema WW, van der Burg E, et al. Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation. Int, J, Cancer. 2015;136(6):1434–1444. doi:10.1002/ijc.29113
- de Souza Andrade MM, Leal VNC, Fernandes IG, et al. Resveratrol Downmodulates Neutrophil Extracellular Trap (NET) generation by neutrophils in patients with severe COVID-19. Antioxidants. 2022;11(9):1690. doi:10.3390/antiox11091690
- Lian N, Zhang S, Huang J, Lin T, Lin Q. Resveratrol Attenuates intermittent hypoxia-induced lung injury by activating the Nrf2/ARE pathway. Lung. 2020;198(2):323–331. doi:10.1007/s00408-020-00321-w
- Ashrafizadeh M, Ahmadi Z, Mohammadinejad R, Farkhondeh T, Samarghandian S. Curcumin Activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr Mol Med. 2020;20(2):116–133. doi:10.2174/1566524019666191016150757
- Zare’i M, Rabieepour M, Ghareaghaji R, et al. Nanocurcumin supplementation improves pulmonary function in severe COPD patients: a randomized, double blind, and placebo-controlled clinical trial. Phytoth Res. 2024;38(3). doi:10.1002/ptr.8114
- Wang Z, Liu J, Li F, et al. Mechanisms of qingyi decoction in severe acute pancreatitis-associated acute lung injury via gut microbiota: targeting the short-chain fatty acids-mediated AMPK/NF-κB/NLRP3 pathway. Microbiol Spectr. 2023;11(4):e03664–22. doi:10.1128/spectrum.03664-22