Abstract
Inflammatory cytokines, interleukin-36 (IL-36), IL-37, IL-38 belong to IL-1 family. The IL-36 subfamily obtains pro- and anti-inflammatory effects on various immune responses. Cytokine IL-37, has anti-inflammatory functions in immunity, and the recently identified IL-38 negatively associated with disease pathogenesis. To date, expression of IL-36, IL-37, IL-38 is reported dysregulated in osteoarthritis (OA) and rheumatoid arthritis (RA), and may be disease markers for arthritis-related diseases. Interestingly, expression of IL-38 was different either in OA patients or animal models, and expression of IL-36Ra in synovium was different in OA and RA patients. Moreover, functional studies have demonstrated significant role of these cytokines in OA and RA progress. These processes were related to immune cells and non-immune cells, where the cytokines IL-36, IL-37, IL-38 may regulate downstream signalings in the cells, and then involve in OA, RA development. In this review, we comprehensively discuss recent advancements in cytokines and the development of OA, RA. We hope that targeting these cytokines will become a potential treatment option for OA and RA in the future.
Introduction
Cytokines in IL-1 family consist of 7 agonists (such as IL-36α/β/γ), four cytokines having anti-inflammatory activities (such as IL-36Ra, IL-37, IL-38).Citation1–6 In recent years, the IL-36 subfamily, IL-37, IL-38 are recognized important in various immune response. Interleukin-36 subfamily includes IL-36α/β/γ, IL-36Ra, IL-36R, and IL-1RAcP.Citation1,Citation2 IL-36 was previously considered as an IL-1 family cytokine that is able to induce generation of pro-inflammatory cytokines and activate MAPK and NF-κB signalings. This cytokine was mainly discussed in psoriasis in the past years.Citation7 IL-36 subfamily has sequence homology with IL-1α/β of 21–37%. Interluekin-36Ra shows 52% sequence homology with IL-1Ra. Interleukin-36α/β/γ bind to IL-36R, and then, may play pro-inflammatory functions. Interleukin-36Ra bound to IL-36R, may play anti-inflammatory functions. The IL-36 subfamily is expressed in various non-immune cells, including epithelial cells, keratinocytes, and in immune cells, including monocytes and T cells. Interleukin-37 (IL-37) was formerly recognized as the IL-1 family member 7. It was a new member of IL-1 family.Citation8 Its molecular weight is approximately 17–25 kDa. It contains five splice variants (IL-37a/b/c/d/e). Interleukin-37b/c has exon 1, exon 2. Interleukin-37b is the most abundant isoform. Interleukin-37 was widely expressed, including the thymus, bone marrow, monocytes.Citation3,Citation4 Interleukin-37 is considered to have anti-inflammatory functions. Interleukin-38 (IL-38) was initially cloned as an IL-1 family cytokine as well, and it was called IL-1HY2 in year 2001.Citation9 The human IL-38 gene is located at chromosome 2p13, having 4 exons. Interleukin-38 is a 17–18 kDa protein. Interleukin-38 binds to IL-36R, showing anti-inflammatory functions among different immune responses.Citation5,Citation6 Interestingly, these cytokines were reported to involve in arthritic diseases pathogenesis.
Osteoarthritis (OA) and rheumatoid arthritis (RA) were two important arthritic disorders.Citation10,Citation11 Inflammation is a marker of OA and RA pathogenesis. OA is the most common chronic degenerative joint disease.Citation12 Subchondral bone remodeling dysfunction featured by overactivated osteoclastogenesis may result in articular cartilage degeneration, OA progression. This process may relate to overactivated osteoclasts in subchondral bone, which will lead to type-H vessels and increased oxygen concentrations, and finally result in cartilage degeneration.Citation13 Treatment by regenerative methods including tissue engineering methods was established recently, which is potential for early treatment of cartilage degeneration in OA joints.Citation14 It is known that plant extracellular vesicles are nanoscale particles encapsulated by phospholipid bilayers, and can promote intercellular communication via transporting various bioactive molecules. Owing to their safety, abundant sources, good biocompatibility, they were recognized to be potential platform for treatment of RA.Citation15 Moreover, development of engineered microorganism-based delivery systems may also be used for these diseases treatment.Citation16 However, treatment of OA and RA is limited. To date, several studies have discussed the expression profile of IL-36, IL-37, IL-38 in OA and RA. Functional studies have also discussed the effects of these cytokines on the progress of OA, RA. In the current study, we comprehensively discussed association of these IL-1 family cytokines with risk of OA, RA. Understanding roles of cytokines in OA, RA will improve the targeting potential of cytokines for OA and RA treatment in the future.
Effects of the Cytokines in Innate and Acquired Immunity
Immune response is required for host defense either in humans or in animal models. It is well known that cytokines are important proteins in regulating different pro-inflammatory or anti-inflammatory immune responses. Similarly, different innate and adaptive immune cells are involved in inflammation and diseases development. To date, the IL-1 family cytokines IL-36, IL-37 and IL-38 are widely discussed in different immune cells, either in regulating inflammatory cytokines, chemokines production or in regulating immune cells differentiation, proliferation, apoptosis. We comprehensively discussed role of the IL-1 family cytokines in the immune cells based on available evidence up to date.
Roles of IL-36 in Immune Cells
IL-36 subfamily was critical for immunity. For IL-36 subfamily in macrophages, bone marrow-derived macrophages (BMDMs) from wild-type (WT) mice were treated with oxidized low-density lipoprotein (oxLDL), showing increased expression of IL-36γ.Citation17 Addition of IL-36γ in BMDMs and RAW264.7 cell lines led to more foam cell formation, oxLDL uptake, up-regulated expression of CD36 in the presence of oxLDL. In contrast, CD36 expression in BMDMs was inhibited by stimulation with phosphoinositide 3-kinase (PI3K) inhibitor.Citation17 For monocyte-induced macrophages (MIMs) from healthy volunteers, after bacillus Calmette-Guérin (BCG) infection in the presence IL-36γ stimulation, there was elevated uptake of BCG in macrophages.Citation18 However, in the presence of IL-36Ra, there was an increase in the intracellular survival of macrophages after infection with the virulent Mycobacterium tuberculosis (M. tuberculosis) virulent strain H37Rv. In H37Rv-infected MIMs, IL-36γ stimulation up-regulated the conversion of LC3-I to LC3-II (autophagosome formation-related factors), expression of autophagy-related 5 (ATG5), Wingless-type Family Member 5A (WNT5A), and reduced expression of p62, and the effects can be inhibited by autophagy inhibitor. In H37Rv-infected MIMs cultured with ERK, NF-κB P65 inhibitors, expression of IL-36γ-induced WNT5A was inhibited.Citation18 Thus, IL-36γ promotes foam cell formation and killing of M. tuberculosis. Dendritic cells from WT mice treated with IL-36α, or IL-36β, or IL-36γ had high expression of IL-12, TNFɑ, CD80, CD86, MHCII.Citation19 In contrast, stimulatory function of IL-36α/β/γ on bone marrow-derived dendritic cells (BMDCs) is inhibited after addition of IL-36Ra in IL-36α/β/γ-treated BMDCs. Interleukin-36γ-stimulated WT neutrophils generated high expression of ROS, granzyme B, whereas IL-36R gene deficient (IL-36R−/−) neutrophils did not induce ROS production.Citation20 When IL-36γ-stimulated WT neutrophils were co-cultured with irradiated tumor cells, there was increased production of ROS. Interestingly, upon blocking ROS generation by NADPH oxidase inhibitor, IL-36γ-mediated cytotoxicity was inhibited.Citation20 Interleukin-36Ra−/− mice had elevated contact hypersensitivity (CHS) response, more infiltration of neutrophils, and neutrophil extracellular traps (NETs) formation, as well as increased expression of IL-1β, CXCL1.Citation21 In contrast, blockade of NETs formation downregulated neutrophils infiltration and IL-1β, CXCL1 expression. These findings suggested that knockdown of IL-36Ra promoted CHS responses caused by neutrophil recruitment and NETs formation, and that IL-36 signaling-activated neutrophils were able to kill tumor cells ().Citation20,Citation21 Thus, IL-36 subfamily differently regulates function of macrophages, DCs, and neutrophils.
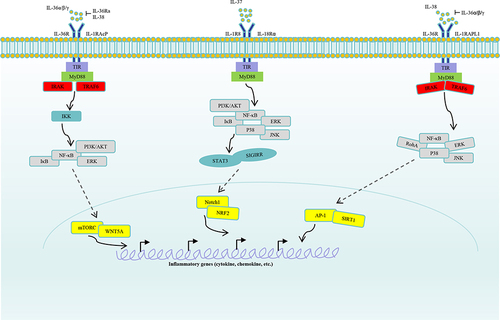
Figure 1 Signaling of IL-36, IL-37 and IL-38. IL-36α, IL-36β and IL-36γ counteract with IL-36Ra, IL-38, and then bind to IL-36R, IL-1RAcP. Moreover, activating signalings were transported to intracellular cascades, including IKK, which will activate signalings such as IκB, NF-κB, PI3K/AKT, ERK. Subsequently, transcription factors mTORC, WNT5A in nucleus will regulate inflammatory cytokines, chemokines, growth factors production. IL-37 binds to receptors IL-1R8, IL-18Rɑ, and then activate signalings such as PI3K/ATK, ERK, JNK, P38. Activated cascades will regulate Notch1, NRF2 activation by STAT3, SIGIRR. Finally, expression of pro-inflammatory or anti-inflammatory components in nucleus were regulated by the IL-37 axis. IL-38 binds to receptors IL-36R or IL-1RAPL1 by competing with the IL-36α/β/γ. Then, the activating signalings were transmitted to cascades RohA, ERK, JNK, P38, and subsequently, transcription factors AP-1, SIRT1 were activated. Finally, inflammatory factors in nucleus were regulated by the IL-38 axis.
For IL-36 subfamily and T cells, stimulation of CD4+ T cells from healthy volunteers with IL-36α/β/γ promoted levels of IFN-γ, and up-regulated cellular proliferation and activation.Citation19,Citation22 Additionally, stimulation with IL-36Ra revealed low expression of T-bet, IFN-γ, IL-17.Citation19,Citation23 CD4+ T cells from patients with ventilator-associated pneumonia cultured with IL-36Ra, showing suppressed expression of perforin, TNFα.Citation23 Similarly, CD8+ T cells from patients with HIV-1 infection cultured with IL-36γ showed high expression of perforin, granzyme B, granulysin.Citation24 CD8+ T cells from WT mice under IL-36β stimulation promote levels of CD69, CD25, IFN-γ, granzyme B, phosphorylated ribosomal protein S6 (P-S6), accelerate degradation of IκB, and enlarge CD8+ T cells.Citation25 Addition of mTORC1 inhibitor, or PI3K/AKT inhibitor, or IkappaB kinase (IKK) inhibitor suppressed IL-36β-induced elevation of P-S6, and inhibited CD8+ T cellular proliferation. Therefore, IL-36Ra suppressed peripheral CD4+ and CD8+ T cellular responses, and IL-36β may up-regulate CD8+ T cellular activation. CD4+ T cells co-culturing with BMDCs from WT mice under IL-36β stimulation reported elevated levels of IFN-γ.Citation26 Co-culturing CD4+ T cells with IL-12p35−/− BMDCs under IL-36β stimulation did not induce IFN-γ expression. However, Rag1−/− mice transferred with IL-36R−/− T cells showed an elevated proportion of CD4+Foxp3+ T cells.Citation27 In lamina propria lymphocytes (LPL) of IL-36β-injected mice, number of Foxp3+ T cells was reduced.Citation28 CD4+ T cells treated with Th17-polarizing condition and IL-36α, showing elevated expression of IL-17, RORγt.Citation29 However, the addition of a PI3K/AKT or ERK signaling inhibitor downregulated the expression of inflammatory components and inhibited Th17 cell differentiation. Together, IL-36α/β may promote Th1, Th2, and Th17 cellular differentiation and inhibit Treg cellular differentiation.
Impacts of IL-37 on Immune Responses
Macrophages are important in innate immunity. Lipopolysaccharide (LPS)+IFN-γ stimulation on THP-1 cells induced differentiation of resting macrophages, whereas addition of IL-37 inhibited levels of iNOS, NF-κB P65, Notch1 signalings, up-regulated expression of CD206, IL-10.Citation30 Similarly, THP-1-derived macrophages treated with monosodium urate (MSU) promoted expression of intracellular ROS, closed mitochondrial permeability transition pore (mPTP).Citation31 Administration of MSU-treated macrophages in the presence of IL-37 protected mitochondrial function, evidenced by reduced expression of ROS, caspase-1, caspase-3, caspase-5, gasdermin D (GsdmD), ratio of iNOS+/Arg-1+ macrophages, and improved mPTP closure degree. Inhibition of IL-37 in THP-1-derived macrophages in the presence of MSU significantly downregulated percentage of macrophages phagocytosing MSU, up-regulated levels of GsdmD, IL-6.Citation31 High glucose (HG)/ox‑LDL stimulation and IL‑37 stimulation inhibited HG/ox‑LDL‑induced ferroptosis in macrophages, including improved cell membrane oxidation, decreased malondialdehyde expression, up-regulated glutathione peroxidase 4 (GPX4) expression and nuclear translocation of NRF2.Citation32 On the contrary, addition of NRF2 inhibitor suppressed effects of IL‑37 on macrophages ferroptosis. When WT mice were infected with A/California/07/2009 (H1N1) and then were injected with IL-37, expression of macrophage-related pro-inflammatory cytokines MCP-1, IL-1β, MIP-1α, MIP-1β, IFN-γ, RANTES was reduced in the lung.Citation33 RAW264.7 macrophages infected with H1N1 under IL-37 stimulation revealed low levels of TNFα, IL-1β, MIP-1β, P-ERK, P-P38, NLRP3 ( and ). Thus, IL-37 may polarize macrophage to M2 features and related functions.
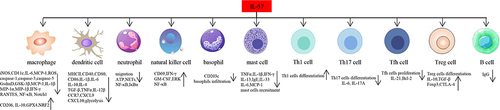
Figure 2 Function of IL-37 in different immune cells. IL-37 inhibits expression of inflammatory components such as iNOS, IL-6, MCP-1, ROS in macrophages, and promotes expression of IL-10, GPX4, NRF2, CD206. IL-37 inhibits dendritic cells maturation, including downregulation of expression of MHCII, CD40, CD86, CD80. IL-37 suppresses infiltration of basophils, and Th17, Tfh cells differentiation, proliferation, whereas it promotes Th1, Treg cells differentiation. IL-37 is able to inhibit B cells production of IgG.
Murine cytomegalovirus infected-mice treated with IL-37 showed low proportion of MHCII+ CD40+, CD80+, CD86+ DCs in liver and low expression IL-1β, IL-6, TNFα.Citation34 In carcinogenic 7.12-dimethylbenzoanthracene/12-o-tetradecylphorbol-13-acetate-induced skin cancer mice, overexpressing IL-37 (IL-37tg mice) downregulated expression of CD80, CD86, CD40, CC chemokine receptor 7 (CCR7), CXCL9, CXCL10 in CD103+ DCs.Citation35 When CD103+ DCs from IL-37tg mice were cultured with poly(I:C), there were reduced levels CD40. Addition of IL-37 inhibited extracellular acidification rate, decreased glycolysis. However, the suppressive effects were rescued by SIGIRR knockdown in CD103+ DCs,Citation35 indicating that IL-37 may suppress glycolysis of CD103+ DCs by SIGIRR signaling. In mice with endometriosis, injection of IL-37 up-regulated number of CD40+, CD86+ DCs.Citation36 CD4+ T cells co-cultured with IL-37-stimulated DCs strongly suppressed Th2 cells differentiation, up-regulated Th1/Th2 cell ratio, expression of IFN-γ, TNFα, STAT3.Citation36 Moreover, degree of CD1a+ DCs infiltration was related to IL-37 expression in tumor tissues of hepatocellular carcinoma (HCC) patients,Citation37 and DCs treated with supernatant from IL-37 overexpressed human HCC cell line Hep3B showed more recruitment of DCs, increased expression of CCL3, CCL20. In IL-37tg ApoE−/− (apolipoprotein E gene deficient) mice, there were reduced CD11c+ DCs infiltration, low expression of CD86, MHCII, TLR-4.Citation38 BMDCs from ApoE−/− mice treated with IL-37+oxLDL displayed low levels of CD86, MHCII, IL-1β, IL-12β. Interestingly, in TLR-4−/− mice, IL-1R8 was highly expressed.Citation38 Together, IL-37 may not only suppress maturation of DCs by IL-1R8-TLR-4-NF-κB signaling, but also suppress Th2 response via inducing DCs maturation.
IL-37 isoform IL-37d was expressed in nucleus of neutrophils from Lewis lung carcinoma (LLC) mice, where injection of IL-37d inhibited bone marrow neutrophils (BMNs) migration, cellular ATP production in BMNs.Citation39 Co-culturing LLC cells with BMNs from WT mice led to more BMNs migration, increased ATP production, whereas addition of IL-37d inhibited LLC-induced BMNs migration and ATP generation.Citation39 Injection of coxsackievirus B3 (CVB3) into WT mice resulted in acute viral myocarditis (VMC), by which there was much production of NETs in the heart.Citation40 Administration of IL-37 inhibited generation of NETs, and downregulated levels of P-NF-κB P65 in heart. When neutrophils from healthy volunteers were stimulated with PMA (phorbol-12-myristat-13-acetate), there were much NETs formation. However, generation of NETs was suppressed after addition of IL-37 or NF-κB inhibitor.Citation40 For role of IL-37 in NK cells, NK cells stimulated with IL-15+IL-37 promote levels of CD69, IFN-γ, GM-CSF, P-ERK, P-NF-κB P65, and promote NK cells cytotoxic activity against tumors, such as colon carcinoma cell lines HT-29, SW480 than those in IL-15-treated NK cells, revealing improvement of NK cells-mediated anti-tumor response.Citation41 Co-culturing human basophils with keratinocyte HaCaT cells in the presence of IL-37b downregulated thymic stromal lymphopoietin (TSLP), and lowered expression of basophil activation marker CD203c.Citation42 Atopic dermatitis mice injected with IL-37b showed less basophils infiltration in ear. This was similar to role of mast cells in allergic contact dermatitis (ACD), where application of IL-37 in ACD rats downregulated expression of TNFα, IL-13, IgE, IL-33, suppressed mast cells recruitment.Citation43 Peritoneal mast cells from ACD rats cultured with IL-37 displayed low expression of TNFα, and restrained NF-κB, P38 activation.Citation43 It is suggested that IL-37 may target basophils, mast cells to alleviate AD, ACD.
T cells and B cells involve in acquired immune responses. IL-37tg mice infected with M. tuberculosis showed high proportion of Th1 cells, low proportion of Th17 cells in spleen.Citation44 Interestingly, mice with coxsackievirus B3-induced viral myocarditis were injected with IL-37, revealing less proportion of Th17 cells in spleen and more proportion of Treg cells in spleen and high expression of IL-10.Citation45 These were similar to effects of IL-37 in hand, foot, and mouth disease (HFMD), by which stimulation of PBMCs from HFMD patients with IL-37 up-regulated number of Treg cells, downregulated number of Th17 cells.Citation46 In healthy volunteers, knockdown IL-37 in Treg cells will inhibit expression of IL-10, CTLA-4.Citation47 There was low expression of IL-37 and high proportion of follicular Th (Tfh), B cells in myasthenia gravis (MG) patients.Citation48 IL-37 can bind to SIGIRR, and then suppress proliferation of Tfh cells, IL-21, Bcl-2 expression in Tfh cells by inhibiting STAT3 signaling or suppress IgG production in B cells through suppressing STAT3 signaling.Citation48 Collective, IL-37 may inhibit differentiation/function of Th17, Tfh cells, B cells, and promote function of Th1, Treg cells.
Inflammatory Roles of IL-38 in Immune Cells
Association between IL-38 and immune cells mainly involves macrophages, T cells, and B cells. High levels of IL-38 were observed in apoptotic A549 lung cancer cells, and the co-culture of macrophages from healthy volunteers with IL-38-overexpressing A549 cells led to reduced IL-6 and IL-8.Citation49 In contrast, co-culturing macrophages from healthy volunteers with IL-38−/− A549 cells reported elevated IL-6 and IL-8 and activated adaptor protein complex-1 (AP1) signaling in macrophages. Naive CD4+ T cells co-cultured with macrophages that were pre-treated with IL-38−/− A549 cells, showing elevated expression of IL-17, reduced the expression of IL-10. Naive CD4+ T cells co-cultured with macrophages pre-treated with IL-38-overexpressing A549 cells showed reduced IL-17 expression and elevated IL-10 expression.Citation49 In peritoneal macrophages from WT mice, LPS stimulation up-regulates IL-38 expression, and LPS-stimulated macrophages treated with IL-38 shift from M1 to M2 phenotype.Citation50 The LPS-stimulated macrophages were cultured with IL-38, which showed inhibition of macrophage apoptosis, decreased expression of TNFα.Citation50,Citation51 Similarly, IL‐38 is highly expressed in infiltrating macrophages from mice with myocardial ischemia/reperfusion injury (MIRI), and injection of IL‐38 in MIRI mice inhibits macrophage infiltration.Citation51 Cardiomyocytes co-cultured with IL‐38‐stimulated macrophages inhibits apoptosis of the cells. Interleukin-36R+ macrophages accumulate in mice with abdominal aortic aneurysm (AAA), and MMP-2 and MMP-9 are highly expressed in these macrophages.Citation52 Injection of IL-38 into AAA mice downregulates M1 macrophage accumulation and MMP-2 and MMP-9 expression. Macrophages from AAA mice stimulated with IL-38 show low MMP-2 and MMP-9 expression, and these effects are reversed by a P38 inhibitor.Citation52 Thus, IL-38 negatively regulates macrophage function and inhibits apoptosis ().
Stimulating CD4+CD25+ Treg cells with LPS significantly promotes IL-38 expression, and administration of IL-38 in Treg cells promotes expression of CD152.Citation53 Moreover, stimulation of PBMCs from healthy volunteers with IL-38 suppressed levels of RORγt, whereas IL-38-mediated Th17 cellular differentiation was abrogated by addition of a NF-κB inhibitor.Citation54 In patients with allergic rhinitis, PBMCs stimulated with IL-38 showed low proportion of Th17 cells.Citation55 Similarly, injection of IL-38 into mice with experimental autoimmune uveitis (EAU) downregulated number of Th17, Th1 cells and expression of colony stimulating factor 2 (CSF2) and IL-23R in the retina.Citation56 CD4+ T cells from EAU mice were treated under Th17-polarizing condition+IL-38, revealing less levels of IL-23R. When Th17 cells were co-cultured with IL-38-treated CD11C+ antigen-presenting cells, IL-23R expression was inhibited.Citation57 Regarding the role of IL-38 in B cells, IL-38−/− mice have an increased number of plasma cells in lymphoid organs and low expression of IgA and IgM.Citation58 During B cell differentiation, IL-38 knockdown up-regulates levels of CD38, IL-6 in CD27+ B cells, and downregulates the expression of IgM, IgA and IgG.Citation58 Collectively, IL-38 may inhibit Th1, Th17, and B cells differentiation and up-regulate Treg and Th2 cells functions.
Association of the Cytokines with OA, RA
Previous studies have discussed the expression of the IL-1 family cytokines in OA and RA. Different samples from patients with OA and animal models, or patients with RA and animal models may have partly different findings. It would be interesting to discuss the potential of inflammatory cytokines as biomarkers of OA and RA. Moreover, functional studies have widely discussed the role of cytokines in OA and RA development in vivo, and in vitro findings have evaluated the effects of cytokines on the production of disease-related components. In this part, we widely discussed expression profile of the cytokines, association with OA, RA, and markedly discussed the potential mechanisms of the cytokines involved in OA and RA development either by regulating different immune cells or by regulating distinct non-immune cells.
OA
Osteoarthritis can lead to many clinical symptoms, such as joint tenderness, joint stiffness, joint pain, crepitus, and effusion. The treatment for OA includes changes in lifestyle, use of medicine, and selection of surgery, which alters the rate of disease progression. To date, several genetic, mechanical, and biochemical factors have been correlated with OA pathogenesis. A combination of these factors results in inflammation during the disease progression. For example, joint inflammation may be caused by macrophages and inflammatory cytokines produced by immune cells. It has been found that the cytokines IL-36, IL-37 and IL-38 are significantly related to OA risk.
IL-36
In patients with OA, articular cartilage has higher expression of IL-36α, IL-36R, and MMP-13 and lower expression of IL-36Ra, which were significantly related to severity of cartilage degeneration ().Citation59 In a Tgfbr2−/− mouse model, a spontaneous OA-like disease model, there were severe disease clinical features, including loss of proteoglycan, synovitis, elevated expression of collagen X and OARSI score ().Citation59 Interestingly, the Tgfbr2−/− mice had highly expressed IL-36α, IL-36R and low expression of IL-36Ra. In WT mice treated with destabilization of the medial meniscus (DMM), there was lower expression of TGFBR2, IL-36Ra and higher expression of IL-36α, IL-36R, and MMP-13.Citation59 Injection of IL-36Ra into Tgfbr2−/− mice inhibited OA development, as evidenced by downregulated expression of MMP-13, OARSI score. Injection of IL-36α in Tgfbr2−/− mice promoted OA development. Similarly, injection of IL-36Ra in DMM-induced OA mice inhibited OA process, along with reduced degeneration of articular cartilage; however, injection of IL-36α exacerbated OA disease.Citation59 Hydrogels are biocompatible hydrophilic polymers that can sustain large amounts of water. They are occasionally used in drug delivery systems. In a study that discussed temperature-sensitive poly(lactic-co-glycolic acid)-poly(ethyleneglycol)-poly(lactic-co-glycolic acid) (PLGA-PEG-PLGA) hydrogel system incorporating IL-36Ra (IL-36Ra@Gel), stimulating chondrocytes with IL-36Ra@Gel reduced expression of ADAMTS-5, but increased the expression of aggrecan and collagen X.Citation52 When mice with DMM were injected with IL-36Ra@Gel in the joint cavity, the severity of cartilage tissue destruction was lower than that in control groups.Citation60 Moreover, cartilage damage, chondrocytes apoptosis were downregulated after overexpressing H19 in the OA mice, whereas inflammatory cytokines were up-regulated and cartilage damage and chondrocytes apoptosis were increased after overexpressing H19 and inhibiting IL-36R in the OA mice.Citation61 Therefore, expression of IL-36α and IL-36R was elevated and IL-36Ra was reduced in OA, and targeting IL-36 subfamily was possible in OA, such as treatment with IL-36Ra and PLGA-PLEG-PLGA hydrogel ().
Table 1 Expression of IL-36, IL-37 and IL-38 in Osteoarthritis and Rheumatoid Arthritis
Figure 3 In vivo effects of IL-36, IL-37 and IL-38 on OA development. Injection of IL-36Ra inhibited Tgfbr2−/− spontaneous OA mice synovitis, whereas injection of IL-36α promoted OA development. In DMM-induced OA mice, injection of IL-36Ra or IL-36Ra@Gel suppressed severity of cartilage tissue destruction. On the contrary, injection of IL-36α exacerbated OA progress. For IL-37, injection of IL-37 into TMJOA rats suppressed swelling and hyperplasia in tissues around the cartilage. Regarding role of IL-38, DMM-induced OA mice injected with overexpressed IL-38 significantly improved cartilage damage, downregulated OARSI score.
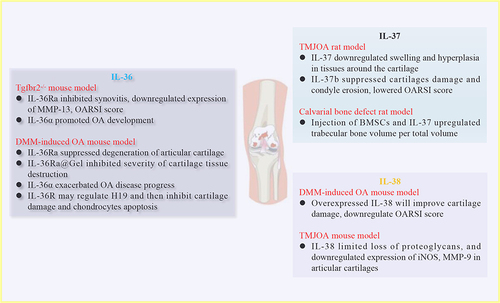
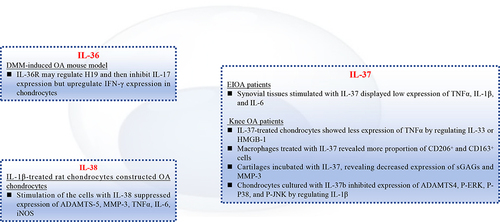
Figure 4 In vitro role of the IL-1 family cytokines in OA chondrocytes and macrophages. IL-36R may regulate H19, then, inhibit expression of IL-17 in chondrocytes from DMM-induced OA mice. Synovial tissues from EIOA patients stimulated with IL-37 will reveal low expression of TNFα, IL-1β, and IL-6. Macrophages from knee OA patients were treated with IL-37, showing more CD206+ and CD163+ cells. IL-1β-treated rat chondrocytes constructed OA chondrocytes, evidenced by high expression of ADAMTS-5, MMP-3, TNFα. However, addition of IL-38 suppressed the pro-inflammatory cytokines.
IL-37
Interleukin-37 expression was elevated in chondrocytes from patients with OA, and there was higher expression of IL-37 in the hip joint cartilage in patients with OA ().Citation62 Similarly, IL-37 was expressed in patients with temporomandibular joint OA (TMJOA), and IL-37 expression in the synovial fluid is positively related to visual analog scale (VAS) score.Citation85 In patients with erosive inflammatory OA (EIOA), IL-37, TNFα, IL-1β, and IL-6 expression in PBMCs and serum levels of IL-37, TNFα, IL-1β, and IL-6 were higher.Citation63 Serum levels of IL-37 in patients with EIOA were positively correlated with the VAS score, CRP, and ESR. Interleukin-37, TNFα expression in synovial fluid and synovial cells from patients with EIOA was elevated, and IL-37 expression was related to TNFα expression in synovial cells. Therefore, IL-37 is highly expressed in patients with OA, may correlate with OA risk.
Regarding the role of IL-37 in OA development, expression of pro-inflammatory cytokines was suppressed after PBMCs from patients with EIOA were stimulated with IL-37. Synovial tissues from patients with EIOA were stimulated with IL-37, showing reduced expression of pro-inflammatory cytokines as well ().Citation63 Interleukin-33- or HMGB-1-treated chondrocytes from patients with OA revealed elevated expression of TNFα.Citation62 Interleukin-37-treated chondrocytes from patients with OA revealed less expression of TNFα. When the chondrocytes were treated with IL-33 or HMGB-1 under IL-37 stimulation, there was attenuated expression of TNFα, and macrophages from patients with OA treated with IL-37 showed more proportion of CD206+ and CD163+ cells.Citation62 Macrophages from patients with OA were stimulated with IL-37, showing up-regulated CD206 expression,Citation86 suggesting that IL-37 may counteract ongoing inflammation and favor M2 macrophage phenotype. Furthermore, the cartilage of patients with OA was incubated with IL-37, revealing decreased expression of sulfated glycosaminoglycans (sGAGs) and MMP-3.Citation87 When the cartilage was pre-labeled withCitation35 S-sulphate and then stimulated with IL-37, the number ofCitation35 S-labeled sGAGs decreased. Interleukin-37-incubated cartilages treated with MMP-3 suppressor showed a decrease in sGAG release, suggesting that IL-37 may interact with MMP-3 and diminish proteoglycan loss in OA cartilage.Citation87 Interestingly, chondrocytes were cultured with IL-1β, showing increased expression of ADAMTS4, P‐MAPK signalings such as P‐JNK.Citation55 In contrast, addition of a member the IL‐37 subfamily, IL-37b, inhibited expression of pro-inflammatory components induced by IL-1β.Citation85 Moreover, human bone mesenchymal stem cells (BMSCs) cultured under osteogenic differentiation condition in the presence of IL-37 show increased expression of alkaline phosphatase (ALP), osteocalcin (OCN), COL1A1 (collagen type I 1 gene (COL1A1)), P-AKT and calcium deposits.Citation88 However, the elevated expression of COL1A1, OCN induced by IL-37 was abolished after the addition of the PI3K/AKT suppressor, revealing that IL-37 regulates PI3K/AKT signaling and contributes to osteogenic differentiation.Citation88 Rats with TMJOA exhibit swelling, joint hyperplasia, and thinner and flatter cartilages.Citation85 Injection of IL-37 into TMJOA rats inhibited swelling and hyperplasia in tissues around the cartilage, limited inflammatory cellular infiltration into synovial tissues, suppressed proteoglycan loss, decreased the expression of MMP-9, and MMP-13 in the synovial lining ().Citation86 After injection of IL‐37b, there were limited cartilages damage and condyle erosion, less proteoglycan loss, lower OARSI score, and expression of COL1A1 in the cartilage layer.Citation85 In a rat calvarial bone defect model, injection of BMSCs and IL-37 increased trabecular bone volume per total volume and trabecular thickness values compared to those in a BMSCs group.Citation88 Rats injected with BMSCs and IL-37 also showed thick calli consisting of newly formed bone tissue in a defect area.Citation88 Therefore, IL-37 may serve as a potential therapeutic target in OA.
IL-38
Patients with knee OA from a population in Turkey (without treatment) showed lower serum levels of IL-38 ().Citation64 Another study showed that there was elevated IL-38, IL-23, and TNFα expression in serum and synovial fluid from Chinese patients with OA (without treatment), and IL-38 expression was positively related to IL-23, and TNFα expression in patients with OA.Citation65 This was similar to a study of patients with OA (without treatment) in Iran, where there were higher serum IL-38 levels in patients with OA.Citation66 Interestingly, DMM-induced OA mice showed much higher synovial fluid expression of IL-38, and IL-38 expression increased after chondrocytes were stimulated with IL-1β.Citation61 When IL-1β-treated rat chondrocytes constructed OA chondrocytes, there was higher expression of ADAMTS-5, MMP-3, cyclooxygenase-2, iNOS, NLRP3, caspase-1, IL-18, P-P38, P-ERK, P-P65, P-JNK, and RhoA. In contrast, addition of IL-38 inhibited the expression of the inflammatory components in IL-1β-induced OA chondrocytes ().Citation65,Citation89 When OA mice were injected with overexpressed lentivirus-IL-38, there was improved cartilage damage, downregulated OARSI score, and fewer apoptotic chondrocytes in the knee joints.Citation61 In TMJOA mice that the TMJ joints were synovial joints, injection of IL-38 into the TMJ articular cavity significantly limited loss of proteoglycans, and downregulated the expression of iNOS in articular cartilages ().Citation89 Taken together, IL-38 may protect against OA pathogenesis, and the expression of IL-38 is abnormal in OA. Considering the differences in the expression of IL-38, this may be related to different ethnicities and sample sizes.
RA
Rheumatoid arthritis is mainly characterized by IgG and citrullinated protein-specific autoantibodies that induce synovial inflammation and hyperplasia, resulting in the progressive destruction of joints and various extra-articular manifestations (such as cardiovascular and pulmonary dysfunction). The reasons for RA risk are complex, and RA is associated with the interaction of genes, sex, environmental factors, and dysregulated immune responses. Fibroblast-like synoviocytes (FLSs) are involved in RA progression, which produce pro-inflammatory cytokines, and promote joint destruction and attack the cartilage. In turn, pro-inflammatory cytokines will promote downstream signalings activation, leading to more pro-inflammatory cytokines, chemokines production, and inflammatory immune cells dysfunction. To date, association of the IL-1 family cytokines and RA risk was considered in many studies.
Il-36
Plasma levels of IL-36α and IL-36γ were higher in RA patients with interstitial lung disease (RA-ILD) compared to those in healthy controls or RA patients without ILD ().Citation67 Rheumatoid arthritis-ILD patients with usual interstitial pneumonia pattern (UIP) showed more plasma levels of IL-36γ than that in RA-ILD patients without UIP. Rheumatoid arthritis patients complicated with systemic lupus erythematosus (SLE) revealed elevated serum levels of IL-36α and IL-36γ than those in patients with SLE.Citation68 In collagen-induced arthritis (CIA) mice and patients with RA, expression of IL-36α, IL-36β, IL-36γ, and IL-36Ra in the synovium was up-regulated.Citation69,Citation70 Taken together, expression of IL-36 subfamily is abnormal in RA, and may serve as a marker for RA.
Considering the role of IL-36 subfamily in RA, several studies have discussed different roles of IL-36 in arthritis pathogenesis and development. Injection of IL-36 overexpression plasmids into RA-FLSs suppressed synoviocyte proliferation and sequestered RA-FLSs migration and invasion.Citation90 Rheumatoid arthritis-FLSs were stimulated with IL-36α, showing induced expression of IL-6 and IL-8.Citation70 When CIA and antigen-induced arthritis (AIA) were induced in WT mice, injection of anti-IL-36R antibody did not significantly modify the incidence and severity of CIA and AIA, or articular inflammation, and structural damage was similar to that in mice treated as controls.Citation91 Similarly, when AIA was induced in IL-36R−/− mice, the severity of arthritis was similar to that in WT mice, and IL-36R−/− mice injected with K/BxN serum had a similar incidence and severity of the disease as the WT mice. Thus, arthritis severity may be independent of IL-36 receptor signaling. This was confirmed in a previous study that discussed the role of anti-IL-36R antibodies in human TNF-transgenic (hTNFtg) mice. The hTNFtg mouse model has been widely used to study RA. This mouse model spontaneously develops clinical symptoms of arthritis. Injection of hTNFtg mice with an anti-IL-36R antibody did not significantly affect weight, grip strength loss, cartilage erosion, or the proportion of osteoclasts recruited to inflammatory sites.Citation92 Interestingly, expression of IL-6, trabecular number, and trabecular thickness were not changed before and after injection of anti-IL-36R antibody. Both osteoclast precursors from patients with RA and osteoclast precursors from CIA mice differentiate into mature osteoclast after stimulation with recombinant human IL-36α.Citation92 These findings suggest that the blockade of IL-36 signaling in different arthritis models has no significant effect on arthritis. However, another study showed that injection of recombinant IL-36 into the articular cavity of CIA mice resulted in lower weight and larger plantar thickness, whereas mice treated with controls displayed significant inflammatory cellular infiltration, indicating that IL-36 treatment may improve the symptoms of arthritis.Citation90 These differences can be attributed to several factors. First, different arthritic models may yield different results. As discussed above, IL-36 signaling did not modify arthritis in AIA and K/BxN serum transfer-induced arthritis mice or hTNFtg mice. In CIA mice, the different results may be related to different treatments. One study discussed the anti-IL-36R antibody, and another discussed the recombinant IL-36 antibody. Second, the IL-36 subfamily includes three activating ligands (IL-36α, IL-36β, and IL-36γ), one receptor antagonist (IL-36Ra in mouse, and IL-36RN in humans), one homoreceptor (IL-36R), and one accessory protein (IL-1RAcP). These studies discussed the in vivo and in vitro roles of the IL-36 subfamily in arthritis did not confirm the same subfamily of IL-36. For example, the role of the IL-36 subfamily in CIA mice was different, as discussed for the anti-IL-36R and recombinant IL-36 antibodies. Therefore, in the future, the role of the IL-36 subfamily needs to be further validated in RA.
IL-37
Higher serum levels of IL-37 were observed in patients with RA without treatment,Citation71–73 or in RA patients with unknown treatment,Citation74–76 or in patients with RA treated with prednisolone, methotrexate, hydroxychloroquine, leflunomide, etanercept, and infliximab ()Citation77 Serum IL-37 levels were higher in RA patients with radiographic bone erosion than those in patients without bone erosion, and were elevated in RA patients with osteopenia and osteoporosis than in those with normal bone mineral density.Citation76 Interestingly, serum IL-37 levels are associated with DAS28, CRP, and ESR levels.Citation72 The serum levels of IL-17, neutrophil/lymphocyte ratio, and platelet/lymphocyte ratio are higher in patients with RA.Citation71 Proportion of CD3+CD26+ T cells is higher in patients with RA.Citation72 Serum levels of IL-37 were related to serum levels of IL-17, rheumatoid factor, TNFα, neutrophil/lymphocyte ratio, and proportion of CD3+CD26+ T cells in patients with RA.Citation71–73,Citation75 Similarly, plasma levels of IL-37 are higher in untreated patients with RA or in RA patients with unknown treatment than those in healthy controls.Citation78–80 Plasma levels of IL‐37 were correlated with DAS28, IL-4, IL-7, IL-12, and IL-13 expression in patients with RA.Citation79,Citation80 Moreover, synovial fluid levels of IL-37 are elevated compared to IL-37 in serum of patients with RA.Citation73 Furthermore, the expression of IL-37 in PBMCs, synovial cells, and CD4+ T cells of patients with RA was higher than that in healthy controls, which correlated with DAS28 and CRP expression.Citation74,Citation79,Citation81 After treatment (leflunomide+tripterygium wilfordii+iguratimod), patients with RA revealed lower serum/plasma levels of IL-37.Citation71,Citation78 Collectively, expression of IL-37 was increased in patients with RA and may be correlated with RA pathogenesis.
With regard to IL-37 gene polymorphisms and RA susceptibility, RA patients from Egypt showed no association of rs3811047 and RA.Citation93 Interestingly, the rs3811047 GG genotype was associated with higher DAS28 score in patients with RA. Interleukin-37 gene polymorphisms (rs2723186, rs3811046, rs4241122) in northern Chinese Han patients with RA were not associated with RA risk.Citation94 In the Iraqi population, the IL-37 gene rs2723176 polymorphism was not related to RA susceptibility.Citation77 Thus, IL-37 gene polymorphisms may not be correlated with the risk of RA.
Fibroblast-like synoviocytes are involved in progress of arthritis. In TNFα-stimulated RA-FLSs, NLRP3, IL-1β, caspase-1, GsdmD, and P-P65 expression was elevated, and cellular proliferation was elevated and apoptosis was reduced.Citation95 However, addition of IL-37 decreased expression of inflammatory components and cellular proliferation and enhanced RA-FLSs apoptosis. Transfecting NF-κB P65 overexpressed plasmids into TNFα+IL-37-treated RA-FLSs significantly up-regulated the expression of caspase-1 and GsdmD.Citation95 Growth, migration, invasion of RA-FLSs, and expression of TNFα, IL-6, and IL-8 in RA-FLSs was suppressed when RA-FLSs were transfected with miR-300 inhibitor; however, IL-37 expression was elevated.Citation96 When RA-FLSs were transfected with both an miR-300 inhibitor and IL-37 siRNA, IL-37 deficiency counteracted the inhibitory effects caused by the miR-300 inhibitor.Citation96 Injection of IL-37 into CIA rats strongly downregulated joint swelling and erythema in the hind paw, clinical score, and plasma levels of IL-18, and GsdmD in synovial tissues.Citation95 Injection of adenovirus encoding IL-37 into knee joints of CIA mice downregulated the incidence and symptoms of arthritis, inhibited synovial hyperplasia, pannus formation, cartilages damage, and bone erosion, and decreased the expression of CII-specific IgG2a in synovial fluid.Citation97 In Streptococcus pyogenes-induced arthritis mice, injection of recombinant human IL-37 reduced the severity of arthritis, downregulated synovial inflammation and influx of pro-inflammatory cells into the joint space, and lowered the expression of CCL3 in synovial tissues and G-CSF in plasma.Citation98 Therefore, IL-37 may regulate TNFα and miR-300, and then, inhibit arthritis development.
IL-38
RA patients showed elevated serum levels of IL-38, TNFα, and IL-38 expression was related to the inflammatory cytokine TNFɑ, disease duration ().Citation82 Plasma levels of IL-38 and IL-38 in PBMCs were elevated in patients with RA as well. After treatment, patients with RA had lower serum levels of IL-38.Citation83 Interleukin-38 expression is higher in the synovial lining of patients with RA than that in the synovial lining of patients with OA.Citation84 In the joints of K/BxN-induced arthritis mice, IL-38 expression was elevated. Thus, IL-38 expression was increased in both patients with RA and in mouse models, and may correlate with RA pathogenesis.
Several studies have found a critical role for IL-38 in arthritis-related inflammation in vitro. Rheumatoid arthritis-FLSs treated with full-length IL-38 promoted the activation of ERK, JNK, and P38, and accelerated RA-FLSs migration and invasion.Citation99 In contrast, RA-FLSs treated with IL-1β showed less expression of IL-6 and IL-8, inhibited activation of ERK, JNK, and P38, and suppressed RA-FLSs migration and invasion. Addition of full-length IL-38 suppressed IL-1β-induced effects on RA-FLSs, suggesting that full-length IL-38 may act as a promoter in RA by regulating IL-1β.Citation99 Overexpression of IL-38 gene in synoviocytes promotes cellular proliferation, migration, and invasion and inhibits LC3-II accumulation.Citation90 Autophagy inhibitor-stimulated cells showed greater migration and invasion, whereas autophagy inducer-stimulated cells showed less migration and invasion. When synovial cells were stimulated with an autophagy inducer in the presence of IL-38 overexpression, IL-38 mitigated LC3-II accumulation. When synovial cells were stimulated with an autophagy inhibitor in the presence of IL-38, cellular proliferation increased. Overexpression of IL-38 gene in autophagy inhibitor-stimulated cells promotes cell invasion and migration and also up-regulates the number of transmembrane cells, indicating that IL-38 may promote synoviocyte proliferation, migration, and invasion via autophagy.Citation90 In contrast, when the synovial cells of RA patients were stimulated with truncated IL-38, the expression of IL-1β, and IL-13 was reduced. Addition of silent information regulator 1 (SIRT1) inhibitor suppressed the inhibitory effects of truncated IL-38, indicating that truncated IL-38 may suppress inflammatory responses in CIA rats by regulating SIRT1.Citation100 Moreover, compared to those in CIA rats with foot redness/heat, ankle joint structure disordered, synovial cells proliferated, administration of recombinant IL-38 in CIA rats induced less swelling of the foot, and there were less serum levels of IL-13, RANKL (receptor activator nuclear factor kappa-B ligand), RANK, VEGFR1 (vascular endothelial growth factor receptor 1), VEGFR2, VEGF, HIF-1α (hypoxia-inducible factor 1 alpha), and TLR-4, and elevated serum levels of osteoprotegerin (OPG) and SIRT1.Citation100 During IL-38−/− mice injection with K/BxN serum, the mice showed exacerbation of clinical score, increased inflammation in joint tissues and bone erosion score.Citation84 Similarly, articular injection of an adeno-associated virus encoding IL-38 (AAV-IL-38) into mice with CIA, AIA, and K/BxN-induced arthritis decreased arthritis incidence, clinical score, and proportion of Iba1+ monocytes/macrophages, and downregulated the expression of IL-23p19, IL-22, CXCL1, and RANKL in the paws and serum.Citation101 The above findings show that IL-38 inhibits arthritis development in vivo, but IL-38 may have different effects on arthritis-related inflammation in vitro. This may correlate with several reasons. First, pro-inflammatory effect of IL-38 is full-length IL-38, whereas the anti-inflammatory effect of IL-38 is truncated IL-38 in in vitro studies. The truncated and full-length forms of IL-38 can bind to IL-1RAPL, which may have different effects on downstream signaling and immune responses. However, this finding needs to be validated by further in vitro functional studies. In contrast, full-length IL-38 interacts with IL-1β, showing pro-inflammatory effect, and truncated IL-38 interacts with SIRT1, showing anti-inflammatory effect. Thus, IL-38 interacts with different downstream signaling pathways and may play different roles. Second, different cell IL-38 has effects at different doses and times, and IL-38 that has effects on targeted cells may show different results.
Conclusion
Although a lot remains to be discussed regarding expression profiles of IL-36, IL-37, and IL-38 in OA, RA, and functional role of these inflammatory cytokines in OA- or RA-related inflammatory components and their significant effects on OA and RA development, it is not negligible that IL-36, IL-37, and IL-38 are abnormally expressed in OA and RA, either in patients or animal models. It is demonstrated that the expression of IL-36α, IL-36β, IL-36γ, IL-36R, and IL-37 was elevated in OA and RA. However, IL-38 expression has been reported to be either elevated or reduced in OA. Interleukin-37 gene polymorphisms were not associated with RA risk. The expression of IL-36Ra is decreased in OA, whereas IL-36Ra expression was increased in RA. Thus, several points need to be clarified regarding the association of IL-36, IL-37, and IL-38 with the risk of OA and RA. First, IL-38 expression differed in patients with OA from different populations and sample sizes. In the future, multiple centers with larger sample sizes and different ethnicities are needed to validate the expression profile of IL-38 in OA. Second, one study showed that IL-36Ra expression is increased in RA. Interleukin-36Ra is a receptor antagonist in IL-36 signaling. Thus, whether increased IL-36Ra expression inhibits RA pathogenesis requires further clarification. Third, few studies have evaluated the association between IL-37 gene polymorphisms and RA susceptibility. Future studies with different ethnicities and larger sample sizes are necessary to determine the potential relationship between IL-37 gene polymorphisms and RA risk. To date, the role of the IL-36 subfamily is unclear in RA pathogenesis, of which there are six members (IL-36α, IL-36β, IL-36γ, IL-36Ra, IL-36R, and IL-1RAcP). More studies on the role of the IL-36 subfamily in RA pathogenesis require functional discussions in vitro and in vivo. Similarly, although IL-37 limited the pro-inflammatory effects of IL-33, HMGB-1, and MMP-3 on chondrocytes, it is important to discuss how IL-37 interacts with IL-33, HMGB-1, and MMP-3 and participates in OA development in vivo. As discussed above, the IL-1 family of cytokines IL-36, IL-37, and IL-38 play significant roles in OA and RA, and targeting these cytokines may be a potential treatment for OA and RA.
It is interesting to discuss more about therapeutic potential of targeting IL-36, IL-37, and IL-38, where potential therapies and ongoing clinical trials would be beneficial in the future. Moreover, identifying gaps in the current knowledge and proposing specific studies will address the gaps, and enhance more knowledge about the role of IL-36, IL-37, and IL-38 in OA and RA. To date, low-dose IL-2 has been used in clinical treatment of lupus and RA patients, and showed meaning, useful responses.Citation102 Moreover, several TNFα-, IL-17- related biologics were used in clinical treatment of RA patients.Citation103,Citation104 Therefore, the IL-1 family cytokines are also potential for treatment of OA and RA in clinical practice in the future. However, several important questions need further clarification. First, current studies discussed role of the IL-1 family cytokines in OA, RA development are all animal studies. Studies about the cytokines in OA, RA patients are needed. Second, effects of the cytokines in OA, RA are not clearly clarified to date. For example, effects of the IL-36 subfamily on RA development are not consistent. Similarly, the truncated and full-length forms of IL-38 have different roles in regulating RA-related inflammatory components production. Thus, with respect to the truncated and full-length forms of IL-38, in vivo studies in animal models and in vitro studies in RA synovial cells either from patients or different animal models are still needed. Third, orthobiologics may be potential in OA, RA treatment because of the ability to influence articular cells and regulate the inflammatory environment in OA, RA. For instance, a multi-centre study discussed effects of single injectable polyacrylamide hydrogel on knee OA patients, showing strong reduction in the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) pain score from baseline to 52 weeks’ of treatment.Citation105 Another study discussed effectiveness and safety of hydrogel-based, matrix-associated autologous chondrocyte implantation (M-ACI) in treatment of patients with knee cartilage defects.Citation106 This study found marked clinical benefit in pain and symptoms improvement after 2 years’ follow-up. Interestingly, a study evaluated the effectiveness of the hyaluronic-acid-based hydrogel (Hymovis®) in treatment of knee OA patients.Citation107 This study showed significant improvement of pain, and reduced VAS score after 6 months’ treatment. However, because of the complex mechanisms of action of the different orthobiologics, construction of reliable platform is seriously considered and precisely finding out suitable platform for individual is needed regarding the IL-1 family cytokines in the future. Furthermore, RA is a complex disease. There is persistent inflammatory environment in RA joints, which will result in much angiogenesis and damage of bone tissues. To date, many types of hydrogel used for RA treatment only delivered one type of drugs such as methotrexate, anti-TNF-α drugs.Citation108 This is mainly due to properties of hydrogels, and which will lead to poor treatment effects. Thus, challenges and difficulties are still existed in application of hydrogels in treatment of RA. For the potential of hydrogel encapsulated by IL-36, IL-37, and IL-38 in RA treatment, it is a long way to discuss in the future, especially used in clinical trials.
Disclosure
The authors have declared that no competing interests exist.
Acknowledgments
This work was supported by grants from the Sichuan Tourism University (2023SCTUZD04, 2023SCTUBSZK07).
References
- Cao J, Liu JH, Wise SG, Fan J, Bao S, Zheng GS. The role of IL-36 and 37 in hepatocellular carcinoma. Front Immunol. 2024;15:1281121. doi:10.3389/fimmu.2024.1281121
- Yuan ZC, Xu WD, Liu XY, Liu XY, Huang AF, Su LC. Biology of IL-36 signaling and its role in systemic inflammatory diseases. Front Immunol. 2019;10:2532. doi:10.3389/fimmu.2019.02532
- Gu M, Jin Y, Gao X, Xia W, Xu T, Pan S. Novel insights into IL-37: an anti-inflammatory cytokine with emerging roles in anti-cancer process. Front Immunol. 2023;14:1278521. doi:10.3389/fimmu.2023.1278521
- Wu Q, Zhou J, Yuan ZC, Lan YY, Xu WD, Huang AF. Association between IL-37 and systemic lupus erythematosus risk. Immunol Invest. 2022;51(4):727–738. doi:10.1080/08820139.2020.1869254
- de Graaf DM, Teufel LU, Joosten LAB, Dinarello CA. Interleukin-38 in health and disease. Cytokine. 2022;152:155824. doi:10.1016/j.cyto.2022.155824
- Xu WD, Su LC, Fu L, et al. IL-38, a potential therapeutic agent for lupus, inhibits lupus progression. Inflamm Res. 2022;71(7–8):963–975. doi:10.1007/s00011-022-01581-3
- Shaik Y, Sabatino G, Maccauro G, et al. IL-36 receptor antagonist with special emphasis on IL-38. Int J Immunopathol Pharmacol. 2013;26(1):27–36. doi:10.1177/039463201302600103
- Xu WD, Zhao Y, Liu Y. Insights into IL-37, the role in autoimmune diseases. Autoimmun Rev. 2015;14(12):1170–1175. doi:10.1016/j.autrev.2015.08.006
- Lin H, Ho AS, Haley-Vicente D, et al. Cloning and characterization of IL-1HY2, a novel interleukin-1 family member. J Biol Chem. 2001;276(23):20597–20602. doi:10.1074/jbc.M010095200
- Wong AY, Samartzis D, Maher C. The global burden of osteoarthritis: past and future perspectives. Lancet Rheumatol. 2023;5(9):e496–e497. doi:10.1016/S2665-9913(23)00207-2
- Rech J, Tascilar K, Hagen M, et al. Abatacept inhibits inflammation and onset of rheumatoid arthritis in individuals at high risk (ARIAA): a randomised, international, multicentre, double-blind, placebo-controlled trial. Lancet. 2024;403(10429):850–859. doi:10.1016/S0140-6736(23)02650-8
- Donate R, Tamaddon M, Ribeiro V, Monzón M, Oliveira JM, Liu C. Translation through collaboration: practice applied in BAMOS project in in vivo testing of innovative osteochondral scaffolds. Biomat Translat. 2022;3(2):102–104. doi:10.12336/biomatertransl.2022.02.003
- Zhang H, Wang L, Cui J, et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci Adv. 2023;9(14):eabo7868. doi:10.1126/sciadv.abo7868
- Tamaddon M, Gilja H, Wang L, et al. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic. Biomat Translat. 2020;1(1):3–17. doi:10.3877/cma.j.issn.2096-112X.2020.01.002
- Han R, Wu Y, Han Y, Liu X, Liu H, Su J. Engineered plant extracellular vesicles for autoimmune diseases therapy. Nano Res. 2024;17(4):2857–2873. doi:10.1007/s12274-023-6112-1
- Huang X, Guo H, Wang L, Shao Z. Engineered microorganism–based delivery systems for targeted cancer therapy: a narrative review. Biomat Translat. 2022;3(3):201–212. doi:10.12336/biomatertransl.2022.03.004
- Zhang M, Liu J, Gao R, et al. Interleukin-36γ aggravates macrophage foam cell formation and atherosclerosis progression in ApoE knockout mice. Cytokine. 2021;146:155630. doi:10.1016/j.cyto.2021.155630
- Gao Y, Wen Q, Hu S, et al. IL-36γ promotes killing of mycobacterium tuberculosis by macrophages via WNT5A-induced noncanonical WNT signaling. J Immunol. 2019;203(4):922–935. doi:10.4049/jimmunol.1900169
- Bhutani T, Hawkes JE. Exploring the clinical features, immunopathogenesis and approach to diagnosis for generalized pustular psoriasis (Podcast). Clin Cosmet Invest Dermatol. 2023;16:1553–1558. doi:10.2147/CCID.S424073
- Roy S, Fitzgerald K, Lalani A, et al. Autonomous IL-36R signaling in neutrophils activates potent antitumor effector functions. J Clin Invest. 2023;133(12):e162088. doi:10.1172/JCI162088
- Hasegawa Y, Iwata Y, Fukushima H, et al. Neutrophil extracellular traps are involved in enhanced contact hypersensitivity response in IL-36 receptor antagonist-deficient mice. Sci Rep. 2022;12(1):13384. doi:10.1038/s41598-022-16449-z
- Maarouf M, Kuczma M, Denning TL. IL-36/IL-36R signaling promotes CD4+ T cell-dependent colitis via pro-inflammatory cytokine production. bioRxiv. 2023;2023. doi:10.1101/2023.05.24.542162
- Xin T, Xing R, Jiang H, Jin F, Li M. Interleukin-36 receptor antagonist stimulation in vitro inhibits peripheral and lung-resident T cell response isolated from patients with ventilator-associated pneumonia. Int Immunopharmacol. 2024;129:111513. doi:10.1016/j.intimp.2024.111513
- Zhou Y, Chen J, Bai S, et al. Interleukin-36gamma mediates the in vitro activation of CD8+ T cells from patients living with chronic human immunodeficiency virus-1 infection. Viral Immunol. 2024;37(1):24–35. doi:10.1089/vim.2023.0080
- Zhao X, Chen X, Shen X, et al. IL-36β promotes CD8+ T cell activation and antitumor immune responses by activating mTORC1. Front Immunol. 2019;10:1803. doi:10.3389/fimmu.2019.01803
- Vigne S, Palmer G, Martin P, et al. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood. 2012;120(17):3478–3487. doi:10.1182/blood-2012-06-439026
- Leon G, Hernandez Santana YE, Irwin N, et al. IL-36 cytokines imprint a colitogenic phenotype on CD4(+) T helper cells. Mucosal Immunol. 2022;15(3):491–503. doi:10.1038/s41385-022-00488-w
- Zhu J, Xu Y, Li Z, Liu S, Fu W, Wei Y. Interleukin-36β exacerbates DSS-induce acute colitis via inhibiting Foxp3+ regulatory T cell response and increasing Th2 cell response. Int Immunopharmacol. 2022;108:108762. doi:10.1016/j.intimp.2022.108762
- Qin X, Zhang T, Wang C, Li H, Liu M, Sun Y. IL-36α contributes to enhanced T helper 17 type responses in allergic rhinitis. Cytokine. 2020;128:154992. doi:10.1016/j.cyto.2020.154992
- Zhou P, Li Q, Su S, et al. Interleukin 37 suppresses M1 macrophage polarization through inhibition of the notch1 and nuclear factor kappa B pathways. Front Cell Dev Biol. 2020;8:56. doi:10.3389/fcell.2020.00056
- Zhao L, Zhao T, Yang X, et al. IL-37 blocks gouty inflammation by shaping macrophages into a non-inflammatory phagocytic phenotype. Rheumatology. 2022;61(9):3841–3853. doi:10.1093/rheumatology/keac009
- Xu J, Han X, Xia N, Zhao Q, Cheng Z. IL-37 suppresses macrophage ferroptosis to attenuate diabetic atherosclerosis via the NRF2 pathway. Exp Ther Med. 2023;25(6):289. doi:10.3892/etm.2023.11988
- Qi F, Liu M, Li F, et al. Interleukin-37 ameliorates influenza pneumonia by attenuating macrophage cytokine production in a MAPK-dependent manner. Front Microbiol. 2019;10:2482. doi:10.3389/fmicb.2019.02482
- Ruan Y, Wen Z, Chen K, et al. Exogenous Interleukin-37 alleviates hepatitis with reduced dendritic cells and induced regulatory T cells in acute murine cytomegalovirus infection. J Immunol Res. 2023;2023:1462048. doi:10.1155/2023/1462048
- Zeng FL, Wang XY, Hu YW, et al. Interleukin-37 promotes DMBA/TPA skin cancer through SIGIRR-mediated inhibition of glycolysis in CD103+DC cells. MedComm. 2023;4(2):e229. doi:10.1002/mco2.229
- Li L, Liao Z, Ye M, Jiang J. Recombinant human IL-37 inhibited endometriosis development in a mouse model through increasing Th1/Th2 ratio by inducing the maturation of dendritic cells. Reprod Biol Endocrinol. 2021;19(1):128. doi:10.1186/s12958-021-00811-3
- Liu Y, Zhao JJ, Zhou ZQ, et al. IL-37 induces anti-tumor immunity by indirectly promoting dendritic cell recruitment and activation in hepatocellular carcinoma. Cancer Manag Res. 2019;11:6691–6702. doi:10.2147/CMAR.S200627
- Liu T, Liu J, Lin Y, et al. IL-37 inhibits the maturation of dendritic cells through the IL-1R8-TLR4-NF-kappaB pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(10):1338–1349. doi:10.1016/j.bbalip.2019.05.009
- Guo Y, Zhang Y, Guan Y, et al. IL-37d enhances COP1-mediated C/EBPβ degradation to suppress spontaneous neutrophil migration and tumor progression. Cell Rep. 2024;43(2):113787. doi:10.1016/j.celrep.2024.113787
- Li B, Cao X, Ai G, et al. Interleukin-37 alleviates myocardial injury induced by coxsackievirus B3 via inhibiting neutrophil extracellular traps formation. Int Immunopharmacol. 2022;113(Pt A):109343. doi:10.1016/j.intimp.2022.109343
- Landolina N, Mariotti FR, Pelosi A, et al. The anti-inflammatory cytokine IL-37 improves the NK cell-mediated anti-tumor response. Oncoimmunology. 2023;13(1):2297504. doi:10.1080/2162402X.2023.2297504
- Hou T, Tsang MS, Kan LL, et al. IL-37 targets TSLP-primed basophils to alleviate atopic dermatitis. Int J Mol Sci. 2021;22(14):7393. doi:10.3390/ijms22147393
- Li W, Ding F, Zhai Y, et al. IL-37 is protective in allergic contact dermatitis through mast cell inhibition. Int Immunopharmacol. 2020;83:106476. doi:10.1016/j.intimp.2020.106476
- Liu H, Zheng R, Wang P, et al. IL-37 confers protection against mycobacterial infection involving suppressing inflammation and modulating T cell activation. PLoS One. 2017;12(1):e0169922. doi:10.1371/journal.pone.0169922
- An B, Liu X, Li G, Yuan H. Interleukin-37 ameliorates coxsackievirus b3-induced viral myocarditis by modulating the Th17/regulatory T cell immune response. J Cardiovasc Pharmacol. 2017;69(5):305–313. doi:10.1097/FJC.0000000000000476
- Lv Y, Wang X. Interleukin-37 inhibits the imbalance between T Helper 17 cells and regulatory T cells in hand, foot, and mouth disease. J Interferon Cytokine Res. 2019;39(7):421–427. doi:10.1089/jir.2019.0005
- Shuai X, Wei-min L, Tong YL, Dong N, Sheng ZY, Yao YM. Expression of IL-37 contributes to the immunosuppressive property of human CD4+CD25+ regulatory T cells. Sci Rep. 2015;5:14478. doi:10.1038/srep14478
- Liu Z, Zhu L, Lu Z, et al. IL-37 represses the autoimmunity in myasthenia gravis via directly targeting follicular Th and B cells. J Immunol. 2020;204(7):1736–1745. doi:10.4049/jimmunol.1901176
- Mora J, Schlemmer A, Wittig I, et al. Interleukin-38 is released from apoptotic cells to limit inflammatory macrophage responses. J Mol Cell Biol. 2016;8(5):426–438. doi:10.1093/jmcb/mjw006
- Ge Y, Chen J, Hu Y, Chen X, Huang M. IL-38 alleviates inflammation in sepsis in mice by inhibiting macrophage apoptosis and activation of the NLRP3 inflammasome. Mediators Inflamm. 2021;2021:6370911. doi:10.1155/2021/6370911
- Wei Y, Xing J, Su X, et al. IL-38 attenuates myocardial ischemia-reperfusion injury by inhibiting macrophage inflammation. Immun Inflamm Dis. 2023;11(6):e898. doi:10.1002/iid3.898
- Kurose S, Matsubara Y, Yoshino S, et al. Interleukin-38 suppresses abdominal aortic aneurysm formation in mice by regulating macrophages in an IL1RL2-p38 pathway-dependent manner. Physiol Rep. 2023;11(2):e15581. doi:10.14814/phy2.15581
- Ge Y, Huang M, Wu Y, Dong N, Yao YM. Interleukin-38 protects against sepsis by augmenting immunosuppressive activity of CD4+ CD25+ regulatory T cells. J Cell Mol Med. 2020;24(2):2027–2039. doi:10.1111/jcmm.14902
- Luo D, Chen Y, Zhou N, Li T, Wang H. Blockade of Th17 response by IL-38 in primary Sjogren’s syndrome. Mol Immunol. 2020;127:107–111. doi:10.1016/j.molimm.2020.09.006
- Wang X, Yang S, Ke X, Hong S. Anti-inflammatory Mechanisms of IL-38 in Chinese patients with allergic rhinitis. Iran J Immunol. 2023;20(1):92–103. doi:10.22034/iji.2023.94361.2305
- Zhang M, Zhou JX, Huang CQ, et al. IL-38 alleviates airway remodeling in chronic asthma via blocking the profibrotic effect of IL-36gamma. Clin Exp Immunol. 2023;214(3):260–274. doi:10.1093/cei/uxad099
- Li H, Zhu L, Wang R, et al. Therapeutic effect of IL-38 on experimental autoimmune uveitis: reprogrammed immune cell landscape and reduced Th17 cell pathogenicity. Invest Ophthalmol Vis Sci. 2021;62(15):31. doi:10.1167/iovs.62.15.31
- Huard A, Wilmes C, Kiprina A, et al. Cell Intrinsic IL-38 Affects B cell differentiation and antibody production. Int J Mol Sci. 2023;24(6):5676. doi:10.3390/ijms24065676
- Li T, Chubinskaya S, Esposito A, et al. TGF-beta type 2 receptor-mediated modulation of the IL-36 family can be therapeutically targeted in osteoarthritis. Sci Transl Med. 2019;11(491):eaan2585. doi:10.1126/scitranslmed.aan2585
- Yi YH, Chen G, Gong S, et al. Injectable temperature-sensitive hydrogel loaded with IL-36Ra for the relief of osteoarthritis. ACS Biomater Sci Eng. 2023;9(3):1672–1681. doi:10.1021/acsbiomaterials.2c01144
- Zhou Y, Li J, Xu F, Ji E, Wang C, Pan Z. Long noncoding RNA H19 alleviates inflammation in osteoarthritis through interactions between TP53, IL-38, and IL-36 receptor. Bone Joint Res. 2022;11(8):594–607. doi:10.1302/2046-3758.118.BJR-2021-0188.R1
- Rai V, Dilisio MF, Samadi F, Agrawal DK. Counteractive Effects of IL-33 and IL-37 on Inflammation in Osteoarthritis. Int J Environ Res Public Health. 2022;19(9):5690. doi:10.3390/ijerph19095690
- Ding L, Hong X, Sun B, et al. IL-37 is associated with osteoarthritis disease activity and suppresses proinflammatory cytokines production in synovial cells. Sci Rep. 2017;7(1):11601. doi:10.1038/s41598-017-11397-5
- Duman BA, Duman S, Çamurcu Y, Gem M, Erdinç L. Evaluation of Serum Interleukin-38 levels in different radiographic grades of idiopathic knee osteoarthritis. J Interferon Cytokine Res. 2021;41(11):425–430. doi:10.1089/jir.2020.0109
- Jiang L, Zhou X, Huang C, et al. The elevated expression of IL-38 serves as an anti-inflammatory factor in osteoarthritis and its protective effect in osteoarthritic chondrocytes. Int Immunopharmacol. 2021;94:107489. doi:10.1016/j.intimp.2021.107489
- Abassifard M, Khorramdelazad H, Rezaee S, Jafarzadeh A. Higher circulating concentration of interleukin-38 in patients with knee osteoarthritis: its association with disease severity. Iran J Allergy Asthma Immunol. 2021;20(1):114–119. doi:10.18502/ijaai.v20i1.5418
- Zheng W, Hu X, Zou M, et al. Plasma IL-36alpha and IL-36gamma as potential biomarkers in interstitial lung disease associated with rheumatoid arthritis: a pilot study in the Chinese population. Inflammation. 2023;46(1):285–296. doi:10.1007/s10753-022-01733-x
- Mai SZ, Li CJ, Xie XY, et al. Increased serum IL-36alpha and IL-36gamma levels in patients with systemic lupus erythematosus: association with disease activity and arthritis. Int Immunopharmacol. 2018;58:103–108. doi:10.1016/j.intimp.2018.03.011
- Boutet MA, Bart G, Penhoat M, et al. Distinct expression of interleukin (IL)-36alpha, beta and gamma, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn’s disease. Clin Exp Immunol. 2016;184(2):159–173. doi:10.1111/cei.12761
- Frey S, Derer A, Messbacher ME, et al. The novel cytokine interleukin-36alpha is expressed in psoriatic and rheumatoid arthritis synovium. Ann Rheum Dis. 2013;72(9):1569–1574. doi:10.1136/annrheumdis-2012-202264
- Meng Y, Cai XL, Cong S, et al. Role of platelet/lymphocyte, neutrophil/lymphocyte, and interleukin-37/Interleukin-17 ratios in the occurrence and treatment of rheumatoid arthritis. Immunol Invest. 2024;53:464–474. doi:10.1080/08820139.2023.2299687
- Ragab D, Mobasher S, Shabaan E. Elevated levels of IL-37 correlate with T cell activation status in rheumatoid arthritis patients. Cytokine. 2019;113:305–310. doi:10.1016/j.cyto.2018.07.027
- Xia L, Shen H, Lu J. Elevated serum and synovial fluid levels of interleukin-37 in patients with rheumatoid arthritis: attenuated the production of inflammatory cytokines. Cytokine. 2015;76(2):553–557. doi:10.1016/j.cyto.2015.06.005
- Zhu J, Xie C, Qiu H, Shi L. Correlation between level of interleukin-37 and rheumatoid arthritis progression. Int J Gen Med. 2021;14:1905–1910. doi:10.2147/IJGM.S309436
- Song L, Wang Y, Sui Y, et al. High Interleukin-37 (IL-37) expression and increased mucin-domain containing-3 (TIM-3) on peripheral T cells in patients with rheumatoid arthritis. Med Sci Monit. 2018;24:5660–5667. doi:10.12659/MSM.909254
- Yang L, Zhang J, Tao J, Lu T. Elevated serum levels of Interleukin-37 are associated with inflammatory cytokines and disease activity in rheumatoid arthritis. APMIS. 2015;123(12):1025–1031. doi:10.1111/apm.12467
- Al-Tae FMD, Al-Harbi AA, Turki KW, Sood M. Interleukin −37 in rheumatoid arthritis: correlation with clinical severity and genetic polymorphisms in Mosul city, Iraq. Egypt J Immunol. 2023;30(2):162–173. doi:10.55133/eji.300215
- Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan YX, Jiang YF. Plasma levels of IL-37 and correlation with TNF-alpha, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS One. 2014;9(5):e95346. doi:10.1371/journal.pone.0095346
- Yuan ZC, Wang JM, Huang AF, Su LC, Li SJ, Xu WD. Elevated expression of interleukin-37 in patients with rheumatoid arthritis. Int J Rheum Dis. 2019;22(6):1123–1129. doi:10.1111/1756-185X.13539
- Xia T, Zheng XF, Qian BH, et al. Plasma Interleukin-37 is elevated in patients with rheumatoid arthritis: its correlation with disease activity and Th1/Th2/Th17-related cytokines. Dis Markers. 2015;2015:795043. doi:10.1155/2015/795043
- Wang L, Wang Y, Xia L, Shen H, Lu J. Elevated frequency of IL-37- and IL-18Ralpha-positive T cells in the peripheral blood of rheumatoid arthritis patients. Cytokine. 2018;110:291–297. doi:10.1016/j.cyto.2018.02.015
- Liang S, Chen L, Liang R, et al. Emerging role of interleukin-38 (IL-38) in the development of rheumatoid arthritis. Rheumatol Ther. 2024;11(2):349–362. doi:10.1007/s40744-024-00640-x
- Xu WD, Su LC, He CS, Huang AF. Plasma interleukin-38 in patients with rheumatoid arthritis. Int Immunopharmacol. 2018;65:1–7. doi:10.1016/j.intimp.2018.09.028
- Takenaka SI, Kaieda S, Kawayama T, et al. IL-38: a new factor in rheumatoid arthritis. Biochem Biophys Rep. 2015;4:386–391. doi:10.1016/j.bbrep.2015.10.015
- Luo P, Feng C, Jiang C, et al. IL-37b alleviates inflammation in the temporomandibular joint cartilage via IL-1R8 pathway. Cell Prolif. 2019;52(6):e12692. doi:10.1111/cpr.12692
- Luo P, Peng S, Yan Y, Ji P, Xu J. IL-37 inhibits M1-like macrophage activation to ameliorate temporomandibular joint inflammation through the NLRP3 pathway. Rheumatology. 2020;59(10):3070–3080. doi:10.1093/rheumatology/keaa192
- van Geffen EW, van Caam APM, Schreurs W, et al. IL-37 diminishes proteoglycan loss in human OA cartilage: donor-specific link between IL-37 and MMP-3. Osteoarthritis Cartilage. 2019;27(1):148–157. doi:10.1016/j.joca.2018.08.016
- Ye C, Zhang W, Hang K, et al. Extracellular IL-37 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells via activation of the PI3K/AKT signaling pathway. Cell Death Dis. 2019;10(10):753. doi:10.1038/s41419-019-1904-7
- Luo P, Zhao T, He H. IL-38-mediated NLRP3/caspase-1 inhibition is a disease-modifying treatment for TMJ inflammation. Ann N Y Acad Sci. 2022;1508(1):92–104. doi:10.1111/nyas.14704
- Hao Z, Liu Y. IL-38 and IL-36 target autophagy for regulating synoviocyte proliferation, migration, and invasion in rheumatoid arthritis. Dis Markers. 2021;2021:7933453. doi:10.1155/2021/7933453
- Lamacchia C, Palmer G, Rodriguez E, et al. The severity of experimental arthritis is independent of IL-36 receptor signaling. Arthritis Res Ther. 2013;15(2):R38. doi:10.1186/ar4192
- Derer A, Groetsch B, Harre U, et al. Blockade of IL-36 receptor signaling does not prevent from TNF-induced arthritis. PLoS One. 2014;9(8):e101954. doi:10.1371/journal.pone.0101954
- El-Sayed EH, Saleh MH, Al-Shahaly MH, Toraih EA, Fathy A. IL-37 gene variant (rs3811047): a marker of disease activity in rheumatoid arthritis: a pilot study. Autoimmunity. 2018;51(8):378–385. doi:10.1080/08916934.2018.1551373
- Zhang XY, Zuo Y, Li C, et al. IL1F7 gene polymorphism is not associated with rheumatoid arthritis susceptibility in the northern Chinese han population: a case-control study. Chin Med J (Engl). 2018;131(2):171–179. doi:10.4103/0366-6999.222340
- Ren C, Chen J, Che Q, et al. IL-37 alleviates TNF-alpha-induced pyroptosis of rheumatoid arthritis fibroblast-like synoviocytes by inhibiting the NF-kappaB/GSDMD signaling pathway. Immunobiology. 2023;228(3):152382. doi:10.1016/j.imbio.2023.152382
- Wang Y, Zhang G, Huang W. MiR-300 promotes the proliferation, migration and invasion of fibroblast-like synoviocytes in rheumatoid arthritis by targeting IL-37. Autoimmunity. 2022;55(6):371–377. doi:10.1080/08916934.2022.2081842
- Ye L, Jiang B, Deng J, et al. IL-37 alleviates rheumatoid arthritis by suppressing IL-17 and IL-17-triggering cytokine production and limiting Th17 cell proliferation. J Immunol. 2015;194(11):5110–5119. doi:10.4049/jimmunol.1401810
- Cavalli G, Koenders M, Kalabokis V, et al. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatology. 2016;55(12):2220–2229. doi:10.1093/rheumatology/kew325
- Ding Y, Shao J, Liu C, et al. Whether full-length IL −38 acts as a promoter or inhibitor in activating rheumatoid arthritis fibroblast-like synoviocytes depends on IL −1β. Int J Rheum Dis. 2024;27(1):e15020. doi:10.1111/1756-185X.15020
- Pei B, Chen K, Zhou S, Min D, Xiao W. IL-38 restrains inflammatory response of collagen-induced arthritis in rats via SIRT1/HIF-1alpha signaling pathway. Biosci Rep. 2020;40(5):BSR20182431. doi:10.1042/BSR20182431
- Boutet MA, Najm A, Bart G, et al. IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro. Ann Rheum Dis. 2017;76(7):1304–1312. doi:10.1136/annrheumdis-2016-210630
- Riaz MF, Garg G, Umeano L, et al. Comparison of low-dose interleukin 2 therapy in conjunction with standard therapy in patients with systemic lupus erythematosus vs rheumatoid arthritis: a systematic review. Cureus. 2024;16(3):e56704. doi:10.7759/cureus.56704
- Byravan S, Samarasinghe H, Yuan JSJ, Tahir SH, Moorthy A, Tahir H. From bench to bedside- is there a role of IL-17 drugs in rheumatoid arthritis? Expert Opin Investig Drugs. 2024. doi:10.1080/13543784.2024.2351505
- Kunwar S, Dahal K, Sharma S. Anti-IL-17 therapy in treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatol Int. 2016;36(8):1065–1075. doi:10.1007/s00296-016-3480-9
- Bliddal H, Beier J, Hartkopp A, Conaghan PG, Henriksen M. Effectiveness and safety of polyacrylamide hydrogel injection for knee osteoarthritis: results from a 12-month follow up of an open-label study. J Orthop Surg Res. 2024;19(1):274. doi:10.1186/s13018-024-04756-2
- Gaissmaier C, Angele P, Spiro RC, Köhler A, Kirner A, Niemeyer P. Hydrogel-based matrix-associated autologous chondrocyte implantation shows greater substantial clinical benefit at 24 months follow-up than microfracture: a propensity score matched-pair analysis. Cartilage. 2024;19476035241235928. doi:10.1177/19476035241235928
- Russu OM, Pop TS, Feier AM, et al. Treatment efficacy with a novel hyaluronic acid-based hydrogel for osteoarthritis of the knee. J Pers Med. 2021;11(4):303. doi:10.3390/jpm11040303
- Yi J, Liu Y, Xie H, et al. Hydrogels for the treatment of rheumatoid arthritis. Front Bioeng Biotechnol. 2022;10:1014543. doi:10.3389/fbioe.2022.1014543
