Abstract
The bacterial pathogen Helicobacter pylori commonly colonizes the human gastric mucosa during early childhood and persists throughout life. The organism has evolved multiple mechanisms for evading clearance by the immune system and, despite inducing inflammation in the stomach, the majority of infections are asymptomatic. H. pylori is the leading cause of peptic ulcer disease and gastric cancer. However, disease outcomes are related to the pattern and severity of chronic inflammation in the gastric mucosa, which in turn is influenced by both bacterial and host factors. Despite over 2 decades of intensive research, there remains an incomplete understanding of the circumstances leading to disease development, due to the fascinating complexity of the host–pathogen interactions. There is accumulating data concerning the virulence factors associated with increased risk of disease, and the majority of these have pro-inflammatory activities. Despite this, only a small proportion of those infected with virulent strains develop disease. Several H. pylori virulence factors have multiple effects on different cell types, including the induction of pro- and anti-inflammatory, immune stimulatory, and immune modulatory responses. The expression of multiple virulence factors is also often linked, making it difficult to assess the meaning of their effects in isolation. Overall, H. pylori is thought to usually modulate inflammation and limit acute damage to the mucosa, enabling the bacteria to persist. If this delicate balance is disturbed, disease may then develop.
Introduction
Barry Marshall and Robin Warren were the first to isolate a spiral bacterium, now known as Helicobacter pylori, from inflamed mucosal tissue of the human stomach.Citation1 In most cases, the infection is asymptomatic. The severity and type of disease depend on the characteristics of the colonizing strain and how it interacts with the host to cause chronic inflammation. Many of the main H. pylori virulence factors have multiple effects on different cell types and may have both pro- and anti-inflammatory activities. It is therefore necessary to assess the relative importance and net effects of these factors in order to understand the circumstances leading to disease development.
H. pylori infection
H. pylori has coevolved with humans over the last 60,000 years.Citation2 It typically first colonizes the gastric mucosa during early childhood and persists lifelong in the absence of effective eradication treatment.Citation3 It is estimated that approximately 50% of the world’s population is colonized, although the prevalence differs between countries. Developing countries have a much higher infection rate than developed countries, and this is thought to be due to differences in living conditions and the use of antibiotics, especially in childhood.Citation4 Globally, H. pylori prevalence is declining. In the US, approximately 10% of individuals under the age of 20 are infected compared to 40% over 60 years of age.Citation5 This higher rate of H. pylori infection seen with increasing age is not due to acquisition of the infection at a later age, but a birth cohort effect.
H. pylori is found almost exclusively in humans. Other Helicobacter species are occasionally found in humans and these are thought to be acquired from domestic pets. The exact route of infectious transmission is not clear, but person-to-person transmission is likely to be a combination of fecal–oral and oral–oral routes. H. pylori strains are usually isolated from gastric biopsy tissue, but it is also possible for the bacterium to be isolated from saliva, gastric reflux fluid, and vomitus.Citation6
Consequences of H. pylori infection
Acute infection
Acute infectious symptoms (such as nausea, halitosis, dyspepsia, and malaise) are experienced by most infected adults but the symptoms are variable. These tend to resolve within 2 weeks. Supporting evidence for the above is mainly from cases of deliberate ingestion. When examined histologically, acute infection is accompanied by severe gastritis, characterized by infiltration of neutrophils and inflammatory cells with marked persistent lymphocyte penetration. A reduction in stomach acid secretion also occurs simultaneously.Citation7 It is unknown whether children suffer similar symptoms or whether histological features are concordant.Citation8
Chronic infection and disease outcome
Chronic H. pylori infection leads to local inflammation of the gastric mucosa (gastritis). Disease risk increases with the level of inflammation, but the pattern of inflammation determines the disease outcome. Host genetic factors, bacterial virulence, environmental factors, and age of infection all influence the distribution of resulting gastritis.Citation8 These complex and only partially understood interactions are thought to explain why only 15% of infected individuals develop disease in their lifetime.Citation9
The most common and serious complications of H. pylori infection include peptic ulcer disease, distal gastric adenocarcinoma, and primary gastric mucosa associated lymphoid tissue (MALT) lymphoma. Other conditions associated with H. pylori infection include dyspepsia, atrophic gastritis, iron deficiency anemia, and idiopathic thrombocytopenia purpura. In contrast, epidemiological evidence also suggests a protective association between H. pylori infection and disorders such as gastroesophageal reflux disease (GERD), esophageal adenocarcinoma, inflammatory bowel disease, multiple sclerosis, and asthma.Citation10,Citation11
Peptic ulceration
Peptic ulcers are breaks in the lining of the duodenal or gastric mucosa, most commonly caused by H. pylori and nonsteroidal anti-inflammatory drugs. Peptic ulcer disease is associated with significant mortality and complications include hemorrhage and perforation. H. pylori eradication heals existing ulcers and prevents their recurrence.Citation12
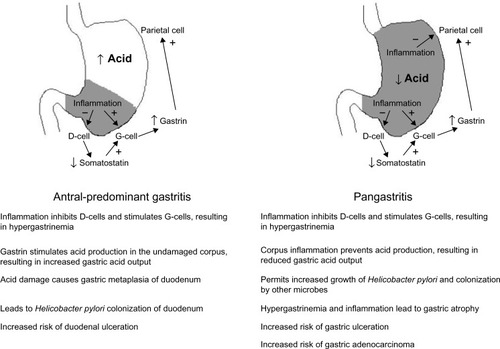
H. pylori is the causative agent in over 75% of duodenal ulcer cases. Antral-predominant inflammation leads to increased gastric acid output (). Gastric metaplasia of the duodenal epithelium then permits H. pylori to colonize and cause inflammation, which may lead to duodenal ulceration. H. pylori is also the leading cause of gastric ulcers, which develop in patients with pangastritis. Here the acid output is normal or reduced, thus preventing the development of duodenal ulcers, but gastric ulcers may develop. Premalignant lesions and gastric adenocarcinoma may also arise.Citation2,Citation13,Citation14
Gastric adenocarcinoma
Gastric cancer is ranked the fifth most common malignancy worldwide with an estimated 100,000 new cases per year.Citation15 Most cases are found in Asia, with over two-thirds occurring in the People’s Republic of China.Citation16 Gastric cancer is the third most common cause of cancer-related deaths, since initial diagnosis is usually at a late stage.Citation17,Citation18 It can be divided into two subtypes depending on the location: cardia (arising from the gastroesophageal junction) and noncardia (arising from the distal stomach). Cardia gastric cancers share risk factors with esophageal adenocarcinoma, Barrett’s esophagus, obesity, and GERD.Citation15 Noncardia gastric cancer is strongly associated with H. pylori, and it is thought that up to 89% may be attributed to the infection. Thus H. pylori has been classified as a human carcinogen.Citation19 The lifetime risk of an H. pylori-infected individual developing gastric cancer is 1%–2%.Citation14,Citation20
There are two histological types of gastric adenocarcinoma: intestinal and diffuse. The intestinal type develops gradually, following a stepwise progression driven by inflammation. H. pylori infection of the normal gastric mucosa leads to a state of chronic gastritis, which later leads to atrophic gastritis (characterized by gland loss and infiltration of inflammatory cells), intestinal metaplasia (where gastric epithelial cells are replaced with those of an intestinal type), dysplasia (neoplasia confined to epithelial cells), and finally adenocarcinoma.Citation21 The diffuse type usually affects younger patients and is not associated with intestinal metaplasia. Although thought to be triggered by H. pylori infection, the exact mechanism is not known.Citation17 H. pylori eradication has been shown to reduce the incidence of atrophic gastritis but does not result in a reduction in gastric cancer incidence unless achieved before the appearance of premalignant changes.Citation22
MALT lymphoma
H. pylori colonization is strongly linked to MALT lymphoma.Citation23 Due to the rarity of this condition, the exact number of individuals coinfected with H. pylori is not known but the condition occurs in less than 1% of those who are colonized. Low-grade B-cell MALT lymphomas normally regress following H. pylori eradication treatment.Citation24
Host response to H. pylori and its association with disease risk
H. pylori elicits a strong immune response, stimulating the expression of cytokines and chemokines from gastric epithelial cells. These factors attract neutrophils, macrophages, dendritic cells (DCs), natural killer (NK) cells, and lymphocytes,Citation8,Citation25 and induce the release of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Gastric carcinogenesis is associated with ROS/RNS-mediated DNA damage, silencing of tumor suppressor genes via DNA methylation, histone epigenetic modification, and epithelial–mesenchymal transition.Citation26 The level and nature of the immune response varies and this affects the risk of disease development.Citation27
Interactions of H. pylori with gastric epithelium
The surface of the gastric mucosa is covered by protective mucus consisting of a cell-associated layer (predominantly MUC1) and secreted mucin (mainly MUC5AC).Citation28 This layer has a profound impact on H. pylori adhesion to the gastric mucosa. H. pylori interacts with mucin fucosylated Lewisb blood group antigen moieties via the BabA adhesin.Citation29 During gastritis, there is an increase in sialylated mucin structures such as sialyl-Lewisx and sialyl-Lewisa, and these bind to the adhesin SabA.Citation30 Recently, the LabA adhesin was identified as binding a motif on MUC5AC.Citation31 The mucus layer is also important for H. pylori motility; the organism reduces its viscosity in order to move through it.Citation32 Mucins also have natural antibiotic activity against the bacterium,Citation33 and H. pylori binding to MUC1 induces multiple effects on host cells including the modulation of inflammation.Citation34
Innate immunity and inflammation
Pattern recognition receptors (PRRs) expressed by gastric epithelial cells interact with H. pylori and activate inflammatory gene expression. These molecules, which include the toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), recognize pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), flagellins, and cell wall peptides.Citation35 Some H. pylori PAMPs are modified to weakly activate PRRs, since its tetra-acetylated LPS is poorly recognized by TLR4, and the FlaA flagellin binds much less efficiently to TLR5.Citation36 Unlike other bacteria, TLR2 appears to be the main receptor for H. pylori LPS, but TLR2 is also activated by other components ().Citation37 Polymorphisms in TLR1, TLR2, TLR4, TLR5, and TLR9 genes have been associated with more severe gastritis and increased risk of premalignant pathology.Citation38,Citation39
Table 1 Major virulence factors and their effects on inflammation and the immune response
Interaction of the cytotoxin-associated gene pathogenicity island (cagPAI) encoded type IV secretion system (T4SS) with gastric epithelial cells results in the transfer of soluble peptidoglycan components into the cytoplasm, NOD1 activation, and pro-inflammatory gene expression.Citation40 The largest NLR subfamily includes the NLRPs, which are the scaffolding proteins of inflammasomes. NLRPs interact with adaptor proteins leading to the activation of caspase-1, which controls the maturation of inflammatory cytokines such as IL-1β and IL-18.Citation41 There is increased expression of these factors and other NLRP3-related molecules in infected gastric tissue.Citation39,Citation42
Autophagy, the pathway for breakdown and removal of damaged cellular components, is an important homeostatic mechanism which regulates inflammatory signaling.Citation43 Dysregulation of autophagy has been reported to result in increased production of ROS and DNA damage. This leads to accumulations of damaged organelles, changes in cell metabolism, and carcinogenesis.Citation44 H. pylori-mediated induction of autophagy has been reported;Citation45,Citation46 however, more virulent H. pylori isolates rapidly downregulate autophagy in gastric epithelial and monocytic cells lines.Citation47 Characterization of the H. pylori B128 7.13 strain, which causes gastric cancer in Mongolian gerbils, revealed a mutation in a peptidoglycan deacetylase gene (pgdA). This led to reduced autophagy in vitro and cancer development in animals.Citation48
Secreted antimicrobial peptides are produced in response to H. pylori. Elevated levels of human beta defensin 2 (hBD2), hBD3, hBD4, adrenomedullin, angiogenin, alpha defensins 1, 2, and 3, and the human cationic antimicrobial peptide 18 (LL-37) are present in the gastric mucosa of H. pylori-infected patients and/or infected human gastric epithelial cells in vitro.Citation49–Citation54
As a consequence of H. pylori interactions with the epithelium, pro-inflammatory chemokines and cytokines, including IL-8, IL-1β, tumor necrosis factor alpha (TNFα), IL-6, IL-12, CCL2-5, CCL20, and CXCL1-3, are upregulated in the infected gastric mucosa.Citation55,Citation56 Gene polymorphisms resulting in increased expression of pro-inflammatory cytokines (IL-6, IL-8, TNFα, IL-1β), or reduced expression of anti- inflammatory cytokines (IL-10), are associated with higher risk of disease.Citation27,Citation57,Citation58 The presence of chemokines leads to the recruitment of immune cells, including neutrophils, macrophages, DCs, NK cells, and lymphocytes.Citation2 Neutrophils contribute to gastritis by secreting inflammatory cytokines and releasing tissue damaging factors from neutrophilic granules. They also phagocytose bacteria, and within the phagolysosomes the bacteria are exposed to bactericidal factors, including myeloperoxidase and matrix metalloproteinases which degrade cell walls and proteins, and ROS and RNS, which induce DNA damage. H. pylori prevents the oxidative burst and can survive intracellularly within neutrophils.Citation59 Helicobacter-infected neutrophil-depleted mice appear to be colonized at the same densities as normal mice.Citation60 These data imply that neutrophils may play a lesser role in protective immunity, but contribute to mucosal damage.
Macrophage-depleted mice have a significantly reduced H. pylori gastritis severity.Citation61 Both M1 and M2 macrophages are present in the infected gastric mucosa.Citation62 M1 macrophages secrete pro-inflammatory cytokines and nitric oxide and have potent bactericidal activity compared with M2 macrophages, which promote cell proliferation and tissue repair.Citation63 H. pylori is able to survive phagocytosis by macrophages, since it induces the fusing together of phagosomes to form megasomes without lysosomal fusion.Citation64 The megasomes provide a protected intracellular niche and may even contribute to the persistence of infection.Citation65 H. pylori is also able to neutralize the released ROS via catalase activity, and arginase production by the bacteria inhibits nitric oxide production.Citation66,Citation67 Chronic exposure to ROS and RNS, however, results in host cell DNA damage and favors cancer development.
Mast cells are also present at higher frequencies in the H. pylori-infected human gastric mucosa.Citation68 The role of these cells has not been widely studied, but they may be involved in tissue repair, inflammation, and vaccine-mediated clearance of the infection.Citation69
DCs in H. pylori-infected gastric tissue tend to be of a myeloid type (mDCs) and express DC-SIGN and high levels of HLA-DR, but are semi-mature and tolerogenic.Citation70–Citation75 Together with the DC response, macrophage-derived cytokines also have an important influence on the development and balance of the adaptive immune response.Citation76 It has recently been shown that both human gastric epithelial cells and gastric mucosal DCs produce retinoic acid (RA), an important factor that regulates inflammation. When infected with H. pylori, however, mucosal RA production is impaired, leading to increased inflammation and possibly resulting in incresed risk of peptic ulceration and gastric carcinogenesis.Citation77
Despite recent interest in invariant lymphoid and NK cell populations there is very little data on these in the context of H. pylori infection. NKT cells are more abundant in the infected gastric mucosa, and a larger NK cell population was detected in the peripheral blood of infected donors.Citation78,Citation79 How these cell types contribute to disease is not understood; however, NK cell-derived perforin and granzymes may cause damage to host cells. NK cells respond to incubation with H. pylori or its secreted products by secreting inflammatory cytokines such as interferon-gamma (IFNγ) and TNFα.Citation80
Adaptive immunity
Strong IgG and IgA antibody responses are present in H. pylori-infected individuals and these may trigger autoimmunity.Citation66 Molecular mimicry by H. pylori induces antibodies that react with host antigens in the gastric mucosa, such as the parietal cell H+, K+-ATPase.Citation81 Such autoreactive antibodies are frequently present in the serum of infected patients, and these may increase local inflammation and damage in the stomach or elsewhere.Citation82
H. pylori infection induces a vigorous T-cell response, which includes both CD4+ and CD8+ cells. The gastric mucosa of infected humans and mice contains increased numbers of CD8+ cells and these contribute to inflammation and disease.Citation83 More is known about the CD4+ T-helper (Th) response. The main Th subsets induced by H. pylori infection are pro-inflammatory Th17 and Th1 and anti-inflammatory regulatory T-cell (Treg) populations; however, Th2 and Th22 responses have also been reported.Citation84–Citation88 Th-derived cytokines orchestrate the host response, having an impact on H. pylori-induced inflammation and immunity, as well as playing an important role in determining H. pylori-associated disease risk.
Th1 cells secrete cytokines IFNγ and TNFα, which stimulate macrophages to secrete further pro-inflammatory factors and have more bactericidal activity.Citation89 Th17 cells secrete IL-17A, IL-17F, IL-21, and IL-22, and stimulate the expression of antimicrobial peptides, ROS, RNS, and chemokines. This leads to increased inflammation and neutrophil recruitment.Citation90 H. pylori-induced expression of B-cell activating factor of TNF family (BAFF) by macrophages is important for the differentiation of Th17 cells.Citation76 In H. pylori-infected mice, a Th17 response is observed in addition to the Th1 response, leading to more severe gastritis.Citation91
In the infected human and mouse gastric mucosa, the severity of gastritis correlates with the number of Th1 and Th17 cells.Citation92–Citation94 Although a strong Th1 response may contribute to carcinogenesis, there is evidence that a high Th1 response leads to a better prognosis for gastric cancer patients due to stronger antitumor immunity.Citation95 On the other hand, high-level Th17 and Th22 responses are associated with gastric cancer progression and poor survival, possibly due to the role of their cytokines in angiogenesis and tumor invasiveness.Citation96 Gastric Th1 cells from the antrum of patients with peptic ulcer disease provide help for B-cell antibody isotype switching, induce epithelial cells to express higher levels of MHC class II, and also have H. pylori-specific cytolytic activity.Citation94 They are proposed to contribute to disease via cytotoxicity against antigen-presenting epithelial cells, and may also promote autoimmune reactions such as in autoimmune gastritis and gastric MALT lymphoma.Citation81,Citation94 T-cell clones from patients with MALT lymphoma, however, are commonly Th0 rather than Th1 types. These have a markedly reduced cytotoxic activity against B-cells and an impaired ability to induce apoptosis in T-cells. This may explain the unchecked B-cell expansion in MALT lymphoma.Citation97
H. pylori has multiple mechanisms for directing the immune system away from a pro-inflammatory T-cell response and toward a suppressive Treg response.Citation98 Increased numbers of Tregs are observed in the gastric mucosa and peripheral blood of H. pylori-infected patients, and peptic ulceration is more frequently found in those with reduced Treg numbers in their gastric mucosa.Citation56,Citation84,Citation92,Citation99 Tregs may act by secreting cytokines such as IL-10 and transforming growth factor beta to modulate inflammation, or they may act via contact-mediated mechanisms.Citation100 H. pylori influences DCs to promote the differentiation of naïve T-cells into Tregs. Such responses are reported to protect against extra-gastric immune and inflammatory conditions including asthma and inflammatory bowel disease.Citation74,Citation101
In addition to Treg induction, H. pylori utilizes many other mechanisms to modulate the immune and inflammatory response. Several virulence factors have anti- as well as pro-inflammatory functions (), and expression of B7-H1 is upregulated in gastric epithelial cells during H. pylori infection. Interaction with this molecule suppresses T-cell activity.Citation102
Virulence factors and inflammation
H. pylori produces numerous virulence factors, many of which are highly polymorphic, phase variable, genetically linked, and/or have diverse and sometimes opposing functions. This diversity, together with the complexity of the host immune response, makes it difficult to define clearly the relative roles of individual virulence factors in H. pylori-mediated inflammation and disease. Pro- and anti-inflammatory influences of some of the best-studied H. pylori virulence factors are briefly summarized in this section.
The cag pathogenicity island and CagA
The cagPAI is a 40 kb horizontally transmitted segment of DNA. It encodes a T4SS, with CagL at the tip of the needle-like structure which binds to α5β1 integrin on host cells.Citation103 CagA, an immunodominant 120–145 kDa protein, is injected into cells through the T4SS together with peptidoglycan peptides. This process activates NF-κB, triggering the secretion of pro-inflammatory cytokines and chemokines, most notably IL-8. Once inside the host cell, CagA is rapidly tyrosine phosphorylated at its EPIYA (Glu-Pro-Ile-Tyr-Ala) motifs by Src kinases and then interacts with the SHP-2 cellular phosphatase. This ultimately leads to cytoskeletal changes via actin rearrangement.Citation104 Unphosphorylated CagA also interacts with numerous targets inside the host cell including the tight junction protein ZO-1 (causing tight junction disruption) and E-cadherin (disrupting E-cadherin/β-catenin complexes to promote β-catenin mediated upregulation of genes with oncogenic potential).Citation105,Citation106 Taken together, cagPAI activity drives a scattering/elongation, or “hummingbird”, phenotype and pro-inflammatory responses in gastric epithelial cells. However, cagPAI-mediated NF-κB activation also downregulates the expression of the antimicrobial and pro-inflammatory defensin hBD1, and the activation of SHP-2 by CagA prevents EGFR-mediated expression of hBD-3.Citation53,Citation107 Downregulation of these β-defensins may help promote the persistence of CagA-positive H. pylori strains.
The cagPAI may be present fully, partially, or not at all. Strains with a functional cag T4SS are strongly associated with increased gastric cancer risk. The cagA gene sequence is itself polymorphic. EPIYA motifs may be categorized as EPIYA-A, B, C, or D depending on their flanking sequences, with EPIYA-A, B, and C found in Western CagA types and EPIYA-A, B, and D found in East Asian CagA. A larger number of EPIYA-C motifs or the presence of an EPIYA-D increases interactions with SHP-2, and is associated with a higher risk of intestinal metaplasia and gastric cancer.Citation108 Strains lacking CagA may induce inflammation via other cagPAI-dependent mechanisms. If the T4SS is functional, peptidoglycan peptides enter the cell and activate NOD1-mediated signaling.Citation40 Additionally, interaction of CagL with the α5β1 integrin is sufficient to activate NF-κB and induce IL-8 expression.Citation109
Vacuolating cytotoxin (VacA)
Virtually all H. pylori strains possess the vacA gene but it is highly polymorphic, with two alternative allelic variants for the signal (s1/s2), intermediate (i1/i2), and mid- (m1/m2) regions. The mid-region plays a role in host cell binding, and m1 forms are able to bind a wider range of cell types than m2. s2 and i2 VacA have reduced activity compared to the s1 and i1 variants.Citation110,Citation111 VacA is a pore-forming toxin, originally named for its ability to induce vacuolation in gastric epithelial cells in vitro. A myriad of other functions have also been attributed to it, including the induction of epithelial cell apoptosis, autophagy, and inhibition of T-cell activation ().Citation46,Citation112,Citation113
The vacA s1 and i1 alleles are associated with increased risk of peptic ulceration, atrophy, and gastric adenocarcinoma, but genetic linkage between these alleles and the presence of cagA makes it difficult to determine with certainty the contribution of each individual factor.Citation111 There is also functional linkage between VacA and CagA, for example, VacA induces apoptosis in gastric epithelial cells, but CagA blocks this activity and can also prevent VacA gaining access into host cells.Citation114,Citation115 This may protect the host cell to which the bacterium has adhered, while allowing continued VacA-mediated disruption of more distant cells. Conversely, VacA inhibits the induction of the hummingbird phenotype by CagA.Citation116 Recently, VacA and another secreted H. pylori protein, γ-glutamyl transferase have been shown to tolerize DCs, promoting Treg responses and protecting against asthma in a mouse model.Citation117,Citation118 Since both s1i1m1 and s2i2m2 VacA can tolerize DCs, this anti-inflammatory function may be one reason for the otherwise unexplained maintenance of apparently nonfunctional type 2 toxin variants in the H. pylori genome.
DupA and tfs4
H. pylori genomes contain regions of low GC content and high diversity, known as “plasticity zones”. The number and contents of PZs vary between strains, and several PZ-specific genes are associated with disease. Of these, one of the best studied is the duodenal ulcer-promoting gene, dupA. The tfs4 gene cluster comprises dupA and other vir homologues which are thought to encode a type IV secretion system.Citation119
Although dupA was initially identified as a duodenal ulcer-promoting virulence factor, numerous subsequent conflicting studies have left the role of dupA in disease unclear. This is likely due to the requirement for other components of the tfs4 to produce a functional type IV secretion system, making dupA alone an imperfect marker.Citation120,Citation121 The presence of dupA in clinical H. pylori isolates is associated with increased IL-8 levels in the antrum of infected individuals.Citation122,Citation123
H. pylori neutrophil-activating protein (HP-NAP)
HP-NAP is a highly conserved dodecameric 150 kDa protein, named for its ability to stimulate endothelial adhesion and production of oxygen radicals by neutrophils. The protein is also a neutrophil chemottractant and stimulates these cells to produce pro-inflammatory cytokines and chemokines. Since neutrophil infiltration is a dominant characteristic of H. pylori gastritis, HP-NAP may play a central role in H. pylori-associated disease. HP-NAP may also associate with the outer membrane of intact bacteria and play a role in binding to host mucin carbohydrates.Citation124,Citation125
Adhesins
Adherent bacteria might be expected to induce stronger inflammatory responses than nonadherent bacteria. H. pylori possesses several major adhesins including the blood group antigen binding adhesin (BabA), sialic acid binding adhesin (SabA), and OipA.
BabA is expressed by a subset of H. pylori strains, and it binds to difucosylated Leb blood group antigens on host epithelial cells.Citation29 BabA may facilitate close association with the epithelium for delivery of other virulence factors, and indeed babA2-positive strains are associated with increased gastric mucosal granulocyte infiltration and IL-8 expression.Citation126
sabA is a phase-variable gene that may be switched “on” or “off”. SabA allows H. pylori to adhere to sialylated Lewis antigens, which are present during gastritis.Citation30 BabA plays the major role in bacterial adhesion soon after colonization, and SabA becomes the predominant adhesin once chronic inflammation is established. Colonization with SabA-producing strains is associated with increased risk of gastric cancer, atrophy, and intestinal metaplasia; however, there is a negative association between SabA expression and neutrophil infiltration.Citation127
OipA is another phase-variable adhesin, and it has several other putative functions including the induction of actin stress fiber formation and IL-8 production by epithelial cells. OipA shares some activities and host cell signaling pathways with the cagPAI, and IL-8 expression may be induced synergistically. There is also OipA-specific signaling, however. While “on” OipA is associated with increased risk of duodenal ulcer and gastric cancer, defining the relative roles of cagPAI and oipA is not straightforward because oipA “on” strains are also likely to be cagPAI-positive.Citation128,Citation129
Conclusion
H. pylori infection strongly stimulates gastric mucosal inflammation and both the innate and acquired immune response. The usual consequence of H. pylori infection is chronic asymptomatic gastritis, probably because the bacteria have adapted to evade and suppress the immune response. The inflammatory response is important in the development of gastric adenocarcinoma; however, there is growing evidence that other aspects of the local and systemic response are also central to disease pathogenesis. It may ultimately be possible to develop prognostic tests based on these parameters, along with bacterial virulence types, to predict who is at risk of developing gastric cancer. However, since many of the major virulence factors have both pro- and anti-inflammatory activities, further research is necessary to gain a complete understanding of the circumstances leading to disease occurrence.
Acknowledgments
KR’s research is supported by the National Institute for Health Research (NIHR), through the Biomedical Research Unit in Gastrointestinal and Liver Diseases at Nottingham University Hospitals NHS Trust and the University of Nottingham. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosure
The authors report no conflicts of interest in this work.
References
- MarshallBJWarrenJRUnidentified curved bacilli in the stomach of patients with gastritis and peptic ulcerationLancet198418390131113156145023
- AthertonJCBlaserMJCoadaptation of Helicobacter pylori and humans: ancient history, modern implicationsJ Clin Invest200911992475248719729845
- BlaserMJAthertonJCHelicobacter pylori persistence: biology and diseaseJ Clin Invest2004113332133314755326
- PeleteiroBBastosAFerroALunetNPrevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverageDig Dis Sci20145981698170924563236
- GradYHLipsitchMAielloAESecular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparitiesAm J Epidemiol20121751545922085628
- GoodmanKJCorreaPTransmission of Helicobacter pylori among siblingsLancet2000355920135836210665555
- MorrisANicholsonGIngestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pHAm J Gastroenterol19878231921993826027
- RobinsonKArgentRHAthertonJCThe inflammatory and immune response to Helicobacter pylori infectionBest Pract Res Clin Gastroenterol200721223725917382275
- AthertonJCThe pathogenesis of Helicobacter pylori-induced gastroduodenal diseasesAnnu Rev Pathol20061639618039108
- RobinsonKHelicobacter pylori-mediated protection against extra-gastric immune and inflammatory disorders: the evidence and controversiesDiseases2015323455
- ArnoldICHitzlerIMullerAThe immunomodulatory properties of Helicobacter pylori confer protection against allergic and chronic inflammatory disordersFront Cell Infect Microbiol201221022919602
- MalfertheinerPChanFKMcCollKEPeptic ulcer diseaseLancet200937496991449146119683340
- MalfertheinerPThe intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancerDig Dis201129545946422095010
- KustersJGvan VlietAHKuipersEJPathogenesis of Helicobacter pylori infectionClin Microbiol Rev200619344949016847081
- ColquhounAArnoldMFerlayJGoodmanKJFormanDSoerjomataramIGlobal patterns of cardia and non-cardia gastric cancer incidence in 2012Gut2015 Epub201536
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- PeekRMJrBlaserMJHelicobacter pylori and gastrointestinal tract adenocarcinomasNat Rev Cancer200221283711902583
- HerreroRParkJYFormanDThe fight against gastric cancer – the IARC Working Group reportBest Pract Res Clin Gastroenterol20142861107111425439075
- PlummerMFranceschiSVignatJFormanDde MartelCGlobal burden of gastric cancer attributable to Helicobacter pyloriInt J Cancer2015136248749024889903
- KuipersEJReview article: exploring the link between Helicobacter pylori and gastric cancerAliment Pharmacol Ther199913Suppl 131110209681
- CorreaPHelicobacter pylori and gastric carcinogenesisAm J Surg Pathol199519Suppl 1S37S437762738
- MalfertheinerPFryLCMonkemullerKCan gastric cancer be prevented by Helicobacter pylori eradication?Best Pract Res Clin Gastroenterol200620470971916997155
- WotherspoonACOrtiz-HidalgoCFalzonMRIsaacsonPGHelicobacter pylori-associated gastritis and primary B-cell gastric lymphomaLancet19913388776117511761682595
- ParsonnetJIsaacsonPGBacterial infection and MALT lymphomaN Engl J Med2004350321321514724298
- SundquistMQuiding-JarbrinkMHelicobacter pylori and its effect on innate and adaptive immunity: new insights and vaccination strategiesExpert Rev Gastroenterol Hepatol20104673374421108593
- NaHKWooJHHelicobacter pylori induces hypermethylation of CpG islands through upregulation of DNA methyltransferase: possible involvement of reactive oxygen/nitrogen speciesJ Cancer Prev201419425926425574460
- MacarthurMHoldGLEl-OmarEMInflammation and cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancyAm J Physiol Gastrointest Liver Physiol20042864G515G52015010360
- DunneCDolanBClyneMFactors that mediate colonization of the human stomach by Helicobacter pyloriWorld J Gastroenterol201420195610562424914320
- IlverDArnqvistAOgrenJHelicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retaggingScience199827953493733779430586
- MahdaviJSondenBHurtigMHelicobacter pylori SabA adhesin in persistent infection and chronic inflammationScience2002297558157357812142529
- RossezYGossetPBonecaIGThe lacdiNAc-specific adhesin LabA mediates adhesion of Helicobacter pylori to human gastric mucosaJ Infect Dis201421081286129524755437
- BansilRCelliJPHardcastleJMTurnerBSThe influence of mucus microstructure and rheology in Helicobacter pylori infectionFront Immunol2013431024133493
- FukudaMKawakuboMItoYKobayashiMLeeHNakayamaJAssay of human gastric mucin as a natural antibiotic against Helicobacter pyloriMethods Enzymol200641516417917116474
- ParkYSGuangWBlanchardTGKimKCLillehojEPSuppression of IL-8 production in gastric epithelial cells by MUC1 mucin and peroxisome proliferator-associated receptorgammaAm J Physiol Gastrointest Liver Physiol20123036G765G77422766852
- SmithSMRole of Toll-like receptors in Helicobacter pylori infection and immunityWorld J Gastrointest Pathophysiol20145313314625133016
- SalamaNRHartungMLMullerALife in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pyloriNat Rev Microbiol201311638539923652324
- SmithSMMoranAPDugganSPTribbles 3: a novel regulator of TLR2-mediated signaling in response to Helicobacter pylori lipopolysaccharideJ Immunol201118642462247121220698
- El-OmarEMNgMTHoldGLPolymorphisms in Toll-like receptor genes and risk of cancerOncogene200827224425218176606
- Castano-RodriguezNKaakoushNOMitchellHMPattern-recognition receptors and gastric cancerFront Immunol2014533625101079
- VialaJChaputCBonecaIGNod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity islandNat Immunol20045111166117415489856
- VanajaSKRathinamVAFitzgeraldKAMechanisms of inflammasome activation: recent advances and novel insightsTrends Cell Biol201525530831525639489
- JiangJLiuSLuoJThe expressions of NLRP3 inflammasome and its downstream molecules in the mouse model of Helicobacter pylori infectionXi Bao Yu Fen Zi Mian Yi Xue Za Zhi2013298785788 Chinese23948399
- JovenJGuirroMMarine-CasadoRRodriguez-GallegoEMenendezJAAutophagy is an inflammation-related defensive mechanism against diseaseAdv Exp Med Biol2014824435925038993
- FilomeniGDe ZioDCecconiFOxidative stress and autophagy: the clash between damage and metabolic needsCell Death Differ201522337738825257172
- RajuDHusseySAngMVacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humansGastroenterology201214251160117122333951
- TerebiznikMRRajuDVazquezCLEffect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cellsAutophagy20095337037919164948
- Castano-RodriguezNKaakoushNOGohKLFockKMMitchellHMAutophagy in Helicobacter pyloriinfection and related gastric cancerHelicobacter Epub201529
- SuarezGRomero-GalloJPiazueloMBModification of Helicobacter pylori peptidoglycan enhances NOD1 activation and promotes cancer of the stomachCancer Res20157581749175925732381
- IsomotoHMukaeHIshimotoHElevated concentrations of alpha-defensins in gastric juice of patients with Helicobacter pylori infectionAm J Gastroenterol200499101916192315447750
- AllakerRPKapasSAdrenomedullin and mucosal defence: interaction between host and microorganismRegul Pept20031121–314715212667636
- HaseKMurakamiMIimuraMExpression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pyloriGastroenterology200312561613162514724813
- BoughanPKArgentRHBody-MalapelMNucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: critical regulators of beta-defensins during Helicobacter pylori infectionJ Biol Chem200628117116371164816513653
- PatelSRSmithKLetleyDPHelicobacter pylori downregulates expression of human beta-defensin 1 in the gastric mucosa in a type IV secretion-dependent fashionCell Microbiol201315122080209223870035
- OtteJMNeumannHMBrandSSchraderHSchmidtWESchmitzFExpression of beta-defensin 4 is increased in human gastritisEur J Clin Invest200939212613819200166
- PeekRMJrMillerGGThamKTHeightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strainsLab Invest19957367607708558837
- CookKWLetleyDPIngramRJCCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosaGut201463101550155924436142
- RamisIBViannaJSGoncalvesCVvon GrollADellagostinOAda SilvaPEPolymorphisms of the IL-6, IL-8 and IL-10 genes and the risk of gastric pathology in patients infected with Helicobacter pyloriJ Microbiol Immunol Infect Epub2015324
- El-OmarEMRabkinCSGammonMDIncreased risk of non-cardia gastric cancer associated with pro-inflammatory cytokine gene polymorphismsGastroenterology200312451193120112730860
- AllenLABeecherBRLynchJTRohnerOVWittineLMHelicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide releaseJ Immunol200517463658366715749904
- IsmailHFFickPZhangJLynchRGBergDJDepletion of neutrophils in IL-10(-/-) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to HelicobacterJ Immunol200317073782378912646644
- KaparakisMWalduckAKPriceJDMacrophages are mediators of gastritis in acute Helicobacter pylori infection in C57BL/6 miceInfect Immun20087652235223918332213
- Quiding-JarbrinkMRaghavanSSundquistMEnhanced M1 macrophage polarization in human Helicobacter pylori-associated atrophic gastritis and in vaccinated micePLoS One2010511e1501821124899
- ItalianiPBoraschiDFrom monocytes to M1/M2 macrophages: phenotypical vs functional differentiationFront Immunol2014551425368618
- SchwartzJTAllenLARole of urease in megasome formation and Helicobacter pylori survival in macrophagesJ Leukoc Biol20067961214122516543403
- AllenLAThe role of the neutrophil and phagocytosis in infection caused by Helicobacter pyloriCurr Opin Infect Dis200114327327711964843
- WilsonKTCrabtreeJEImmunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studiesGastroenterology2007133128830817631150
- GobertAPMcGeeDJAkhtarMHelicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survivalProc Natl Acad Sci U S A20019824138441384911717441
- BambaNNakajimaSAndohAStem cell factor expressed in human gastric mucosa in relation to mast cell increase in Helicobacter pylori-infected gastritisDig Dis Sci200247227428211855541
- VelinDBachmannDBouzoureneHMichettiPMast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse modelGastroenterology2005129114215516012944
- DrakesMLCzinnSJBlanchardTGRegulation of murine dendritic cell immune responses by Helicobacter felis antigenInfect Immun20067484624463316861650
- AlgoodHMGallo-RomeroJWilsonKTPeekRMJrCoverTLHost response to Helicobacter pylori infection before initiation of the adaptive immune responseFEMS Immunol Med Microbiol200751357758617919297
- BimczokDClementsRHWaitesKBHuman primary gastric dendritic cells induce a Th1 response to H pyloriMucosal Immunol20103326026920237463
- KhamriWWalkerMMClarkPHelicobacter pylori stimulates dendritic cells to induce interleukin-17 expression from CD4+ T lymphocytesInfect Immun201078284585319917709
- OertliMSundquistMHitzlerIDC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protectionJ Clin Invest201212231082109622307326
- RizzutiDAngMSokollikCHelicobacter pylori inhibits dendritic cell maturation via interleukin-10-mediated activation of the signal transducer and activator of transcription 3 pathwayJ Innate Immun20157219921125412627
- MunariFFassanMCapitaniNCytokine BAFF released by Helicobacter pylori-infected macrophages triggers the Th17 response in human chronic gastritisJ Immunol2014193115584559425339679
- BimczokDKaoJYZhangMHuman gastric epithelial cells contribute to gastric immune regulation by providing retinoic acid to dendritic cellsMucosal Immunol Epub2014924
- O’KeeffeJGatelyCMO’DonoghueYZulquernainSAStevensFMMoranAPNatural killer cell receptor T-lymphocytes in normal and Helicobacter pylori-infected human gastric mucosaHelicobacter200813650050519166415
- RudnickaKMatusiakAMiszczykERudnickaWTenderendaMChmielaMImmunophenotype of peripheral blood natural killer cells and IL-10 serum levels in relation to Helicobacter pylori statusAPMIS2013121980681323758061
- YunCHLundgrenAAzemJNatural killer cells and Helicobacter pylori infection: bacterial antigens and interleukin-12 act synergistically to induce gamma interferon productionInfect Immun20057331482149015731046
- D’EliosMMAppelmelkBJAmedeiABergmanMPDel PreteGGastric autoimmunity: the role of Helicobacter pylori and molecular mimicryTrends Mol Med200410731632315242679
- SmykDSKoutsoumpasALMytilinaiouMGRigopoulouEISakkasLIBogdanosDPHelicobacter pylori and autoimmune disease: cause or bystanderWorld J Gastroenterol201420361362924574735
- Figueiredo SoaresTAguiar RochaGCamargos RochaAMDifferences in peripheral blood lymphocyte phenotypes between Helicobacter pylori-positive children and adults with duodenal ulcerClin Microbiol Infect200713111083108817727687
- RobinsonKKenefeckRPidgeonELHelicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responsesGut200857101375138518467372
- Serelli-LeeVLingKLHoCPersistent Helicobacter pylori specific Th17 responses in patients with past Hpylori infection are associated with elevated gastric mucosal IL-1betaPLoS One201276e3919922761739
- EnarssonKLundgrenAKindlundBFunction and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinomaClin Immunol2006121335836816934529
- ZhuangYChengPLiuXFA pro-inflammatory role for Th22 cells in Helicobacter pylori-associated gastritisGut Epub2014818
- BuzelliJNChalinorHVPavlicDIIL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infectionCell Mol Gastroenterol Hepatol201511203221
- PopovaAKzhyshkowskaJNurgazievaDGoerdtSGratchevAPro- and anti-inflammatory control of M-CSF-mediated macrophage differentiationImmunobiology20112161–216417220619482
- WilkeCMBishopKFoxDZouWDeciphering the role of Th17 cells in human diseaseTrends Immunol2011321260361121958759
- ShiYLiuXFZhuangYHelicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in miceJ Immunol201018495121512920351183
- SerranoCWrightSWBimczokDDownregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected childrenMucosal Immunol20136595095923299619
- HitzlerIKohlerEEnglerDBYazganASMullerAThe role of Th cell subsets in the control of Helicobacter infections and in T cell-driven gastric immunopathologyFront Immunol2012314222675328
- D’EliosMMManghettiMDe CarliMT helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer diseaseJ Immunol199715829629678993017
- ChangWJDuYZhaoXMaLYCaoGWInflammation-related factors predicting prognosis of gastric cancerWorld J Gastroenterol201420164586459624782611
- LiuTPengLYuPIncreased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancerJ Clin Immunol20123261332133922760549
- D’EliosMMAmedeiAManghettiMImpaired T-cell regulation of B-cell growth in Helicobacter pylori-related gastric low-grade MALT lymphomaGastroenterology199911751105111210535873
- KaoJYZhangMMillerMJHelicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in miceGastroenterology201013831046105419931266
- RadRBrennerLBauerSCD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivoGastroenterology2006131252553716890606
- AiTLSolomonBDHsiehCST-cell selection and intestinal homeostasisImmunol Rev20142591607424712459
- LutherJOwyangSYTakeuchiTHelicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitisGut201160111479148621471567
- DasSSuarezGBeswickEJSierraJCGrahamDYReyesVEExpression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infectionJ Immunol200617653000300916493058
- KwokTZablerDUrmanSHelicobacter exploits integrin for type IV secretion and kinase activationNature2007449716486286617943123
- TegtmeyerNWesslerSBackertSRole of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesisFEBS J201127881190120221352489
- AmievaMRVogelmannRCovacciATompkinsLSNelsonWJFalkowSDisruption of the epithelial apical-junctional complex by Helicobacter pylori CagAScience200330056241430143412775840
- FrancoATIsraelDAWashingtonMKActivation of beta-catenin by carcinogenic Helicobacter pyloriProc Natl Acad Sci U S A200510230106461065116027366
- BauerBPangEHollandCKesslerMBartfeldSMeyerTFThe Helicobacter pylori virulence effector CagA abrogates human beta-defensin 3 expression via inactivation of EGFR signalingCell Host Microbe201211657658622704618
- PeekRMJrFiskeCWilsonKTRole of innate immunity in Helicobacter pylori-induced gastric malignancyPhysiol Rev201090383185820664074
- GorrellRJGuanJXinYA novel NOD1- and CagA-independent pathway of interleukin-8 induction mediated by the Helicobacter pylori type IV secretion systemCell Microbiol201315455457023107019
- AthertonJCCaoPPeekRMJrTummuruMKBlaserMJCoverTLMosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulcerationJ Biol Chem19952703017771177777629077
- RheadJLLetleyDPMohammadiMA new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancerGastroenterology2007133392693617854597
- KimIJBlankeSRRemodeling the host environment: modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA)Front Cell Infect Microbiol201223722919629
- GebertBFischerWWeissEHoffmannRHaasRHelicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activationScience200330156361099110212934009
- OldaniACormontMHofmanVHelicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cellsPLoS Pathog2009510e100060319798427
- AkadaJKAokiHTorigoeYHelicobacter pylori CagA inhibits endocytosis of cytotoxin VacA in host cellsDis Model Mech201039–1060561720682750
- ArgentRHThomasRJLetleyDPRittigMGHardieKRAthertonJCFunctional association between the Helicobacter pylori virulence factors VacA and CagAJ Med Microbiol200857Pt 214515018201978
- OertliMNobenMEnglerDBHelicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune toleranceProc Natl Acad Sci U S A201311083047305223382221
- EnglerDBReuterSvan WijckYEffective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10Proc Natl Acad Sci U S A201411132118101181525074917
- FischerWWindhagerLRohrerSStrain-specific genes of Helicobacter pylori: genome evolution driven by a novel type IV secretion system and genomic island transferNucleic Acids Res201038186089610120478826
- JungSWSugimotoMShiotaSGrahamDYYamaokaYThe intact dupA cluster is a more reliable Helicobacter pylori virulence marker than dupA aloneInfect Immun201280138138722038914
- ShiotaSMatsunariOWatadaMHanadaKYamaokaYSystematic review and meta-analysis: the relationship between the Helicobacter pylori dupA gene and clinical outcomesGut Pathog2010211321040520
- LuHHsuPIGrahamDYYamaokaYDuodenal ulcer promoting gene of Helicobacter pyloriGastroenterology2005128483384815825067
- HusseinNRArgentRHMarxCKPatelSRRobinsonKAthertonJCHelicobacter pylori dupA is polymorphic, and its active form induces pro-inflammatory cytokine secretion by mononuclear cellsJ Infect Dis2010202226126920533870
- de BernardMD’EliosMMThe immune modulating activity of the Helicobacter pylori HP-NAP: friend or foe?Toxicon20105671186119219818802
- SatinBDel GiudiceGDella BiancaVThe neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factorJ Exp Med200019191467147610790422
- RadRGerhardMLangRThe Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a nonspecific immune responseJ Immunol200216863033304111884476
- YamaokaYOjoOFujimotoSHelicobacter pylori outer membrane proteins and gastroduodenal diseaseGut200655677578116322107
- YamaokaYGrahamDYHelicobacter pylori virulence and cancer pathogenesisFuture Oncol20141081487150025052757
- YamaokaYKikuchiSel-ZimaityHMGutierrezOOsatoMSGrahamDYImportance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 productionGastroenterology2002123241442412145793