Abstract
Previous studies have reported the existence of three promoters for the human type I interleukin-1 receptor (hIL-1R1) gene. These promoters were discovered by identifying discrete transcription start sites (TSS) from limited human cell lines. In this study, we examined the TSSs of hIL-1R1 mRNA from 24 different tissues and identified several novel TSSs in hIL-1R1 that suggest the existence of seven hIL-1R1 promoters: three of them are the same as those reported previously and four are putative novel promoters. Using a promoter-reporter assay, we show here that these promoters can drive the transcription of the reporter gene. In addition, these promoters exhibit cell type specific expression patterns and they can be regulated by enhancer elements in a cell type specific manner. Only one of the promoters was found to be sensitive to the stimulation by glucocorticoids. Similar to our recent report on murine IL-1R1, two of the hIL-1R1 promoters appear to be the dominant promoters, one of which was published previously and the other is identified in the present study. We also found an internal promoter that drives the expression of IL-1R1 after the conventional translation start codon, suggesting that a truncated hIL-1R1 may be expressed by this promoter. These results provide additional information regarding the transcription of hIL-1R1.
Introduction
Interleukin-1 (IL-1) is a pleiotropic factor that is active in multiple systems. In the immune system, IL-1 can act on neutrophils,Citation1 macrophages,Citation2 and natural killer cellsCitation3 to initiate innate immune responses, and on dendritic cellsCitation4 and T cellsCitation5 to modulate adaptive immune responses. In the central nervous system, IL-1 can act on endothelial cells,Citation6 astrocytes,Citation7 microglia,Citation8 and neuronsCitation9 to modulate neuroinflammation, neurogenesis, learning and memory, pain,Citation10 and sickness behavior.Citation11 In the neuroendocrine system, IL-1 can act on secretory cells of the pituitary to influence the production and release of stress hormones.Citation12 In the liver, IL-1 can act on hepatic cells to induce the production of acute phase proteins.Citation13
In the context of this vast array of the distinct IL-1 activities, a surprising aspect of the IL-1 biology is that IL-1 exerts its function mostly through a single receptor, the type I IL-1 receptor (IL-1R1).Citation14 We showed in a recent study, that three different promoters control murine IL-1R1 (mIL-1R1) expression in a tissue- and cell type-specific manner, Citation15 suggesting that the regulation of IL-1R1 by multiple promoters is an important mechanism for controlling the diverse functions of IL-1R1. Screening transcription start sites of mIL-1R1 from a large panel of different tissue samples was necessary for the discovery of these mIL-1R1 promoters because some promoters are not active in all tissues. Previous studies have reported the existence of three human IL-1R1 (hIL-1R1) promoters in several cell lines.Citation16 It is not clear whether other hIL-1R1 promoters exist in other cell types. In this study, we screened 24 different human tissues to identify additional hIL-1R1 promoters that might regulate hIL-1R1 transcription.
Material and methods
Reagents and cell lines
Luciferase reporter vector pGL4.10 was obtained from Promega (Madison, WI, USA). PolyJet™ in vitro DNA transfection reagent was purchased from SignaGen (Ijamsville, MD, USA). Steady-Lite™ HTS for luciferase assay was purchased from PerkinElmer Life Sciences (Waltham, MA, USA). CCD-18co (human fibroblast), SH-SY5Y (human neuronal cell), H1299 (human lung cell), 293 (human kidney), HeLa (human cervix adenocarcinoma), RAW 264.7 (murine macrophage), Neuro- 2a (murine neuroblast cell) and SVEC4-10 (murine peripheral endothelial cell) were purchased from ATCC (Manassas, VA, USA). These cell lines were maintained according to the instructions of the ATCC protocols. The pSG5-hGR (a plasmid that expresses human glucocorticoid receptor) was generously provided by Keith Yamamoto (University of California, San Francisco CA, USA) and the pGRE-Luc (glucocorticoid receptor (GR) reporter construct) was provided by Jeanette Marketon (Ohio State University OH, USA).
5′-rapid amplification of cdna ends
The Human Sure-RACE panel HRAA-101 (OriGene, Rockville, MD, USA) containing PCR-ready human cDNAs (generated from 24 different tissues) was used. The 5′ end of mRNA in this kit has been modified to contain 5′ adaptor sequences (ADP) to facilitate polymerase chain reaction (PCR) amplification of the 5′ ends of mRNAs. Two gene-specific primers (GSP) for hIL-1R1 were designed from the published hIL-1R1 mRNA sequence in the GenBank (accession number: NM_0008770). The sequence for GSP1 is 5′-TGA TGAATCCTGGAGGCTTGTTC, and the sequence for GSP2 is 5′-GGACAGGGACGAACATCAATTTC. These primers are located in exon 4 of the published hIL-1R1 mRNA sequence, downstream of the start codon in exon 3. Nested PCR was performed with the pair of outer anchor primers, ADP1 and GSP1, followed by the pair of inner anchor primers, ADP2 and GSP2. A graphic depiction of the RACE design is shown in . The PCR products were separated by electrophoresis using a Bioanalyzer (Agilent, Santa Clara, CA, USA). For the RACE-PCR amplicons with a single major band, the products were directly cloned into the pCRII-TOPO vector by TOPO TA cloning® (Invitrogen, Carlsbad, CA, USA). For the RACE-PCR amplicons containing multiple bands, all of the visible bands were resolved by 1.0% agarose gel electrophoresis and purified. The isolated bands were subsequently cloned into the PCR II-TOPO vector. The cloned cDNAs were sequenced by an automatic sequencer (Plant-Microbe Genomics Facility at Ohio State University). The sequence data were aligned to the human genomic database of the National Center for Biotechnology Information.
Figure 1 A) diagrammatic illustration of the 5′-RACE design used in the present study. Nested PCR was performed first with ADP 1 and GSP1, followed with ADP 2 and GSP2. The arrows denote the positions of PCR primers in the context of hIL-1R1 genomic DNA structure. B) electrophoresis results of 5′-RACE PCR from 24 human tissues. Molecular weight markers (in base pairs) are shown in the far left lane. Lane 1. Brain; lane 2. Heart; lane 3. Kidney; lane 4. Spleen; lane 5. Liver; lane 6. Colon; lane 7. Lung; lane 8. Small intestine; lane 9. Muscle; lane 10. Stomach; lane 11. Testis; lane 12. Placenta; lane 13. Pituitary; lane 14. Thyroid gland; lane 15. Adrenal gland; lane 16. Pancreas; lane 17. Ovary; lane 18. Uterus; lane 19. Prostate; lane 20. PBL (leukocyte); lane 21. Fetal brain; lane 22. Fetal liver; lane 23. Fat (adipose); lane 24. Mammary gland. C) annotation of TSSs found in the present study in the context of the known genomic DNA structure of human IL-1R1. P1D, P1A, P1E, P1B, P1C, P6 and P7 denote the position of the putative promoters of hIL-1R1 deduced from the TSSs. EX1D, EX1A, EX1E, EX1B and EX1C denote the five separate alternative exons 1 found in this study.
Abbreviations: ADP, adaptor primer; GSP, gene-specific primer.
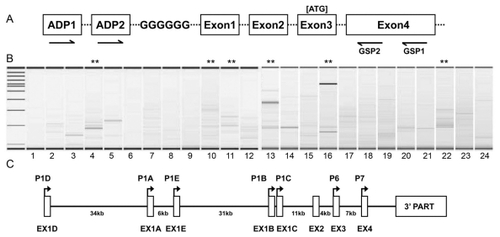
Sequence data from the RACE assay revealed seven clusters of Transcriptional Start Sites (TSSs). Five TSS clusters were located upstream of exon 2 and two clusters were located immediately upstream of exon 3 and exon 4. These TSS sites are annotated in .
Promoter-reporter constructs
To determine promoter activities associated with these TSSs, 1.2 kb to 2.2 kb of genomic sequences were PCR-amplified. Each PCR product contains at least 1 kb upstream sequence of each TSS cluster and at least 100 bp downstream sequence. The sequences corresponding to the previously published three hIL-1R1 promoters are designated as P1A, P1B, and P1C (they are associated with three alternative first exons). The sequences associated with the two newly discovered alternative exons 1 are designated as P1D and P1E. The sequences associated with TSSs immediately upstream of exon 3 and exon 4 are designated as P6 and P7, respectively. The following primer pairs were used: 5′-CCACTAGTCATCTCTCAGTG /5′-AGTCACAAACTACAGGCCCAAGGAAG (P1D); 5′-GCTCACTTCTGCTATCTCCGTTATCATC/ 5′-TCTTACCGTCTCCGTTCTTCATCCTTG (P1E); 5′-CTGGCCTGGACAACTGAGTGCTG/5′-CTCACCT TGGCCTCCCTTCCAC (P1A); 5′-TCTAGCAGGA CACTGGGTAGGAGTG/5′-GAGTTAGTTGAGACAAC TCACCTAG (P1B); 5′-CAAAGTGAGCTGGTGGGCAT AAGTG/5′-CACACTCTGACAACCAAGTGCAACTTAG (P1C); 5′-CACAGAAAGAGCTCATAGACCAGACTG/ 5′-GTATGAGAAATGACTCTGTTAGGGAAGGTAC (P6); 5′-TTCATGGGATATGGAATTCTAGGTAGAC/ 5′-TGGGTTAAGAGGACAGGGACGAAC (P7).
Each resulting amplicon (P1A, P1B, P1C, P1D, P1E, P6 and P7) was sequenced and cloned separately into the pGL4.10 vector between the restriction sites KpnI/XhoI, immediately before the luciferase reporter sequence. To study the influence of potential enhancers on the activities of these putative promoters, an SV40 enhancer was added to each promoter-reporter construct downstream of the luciferase reporter sequence to generate the promoter-reporter-enhancer constructs.
Promoter-reporter assay
Promoter-reporter constructs were transfected into CCD-18co, SH-SY5Y, H1299, 293, HeLa, RAW 264.7, Neuro-2a and SVEC4-10 cells using the PolyJet reagent. Briefly, in a 24-well culture plate, the promoter construct (0.1 μg) was added in the PolyJet reagent according to the manufacturer’s instructions. The cells were always grown to be 90% confluent at the time of transfection. Transfected cells were incubated at 37 °C in a CO2 incubator for 24 hours. Luciferase activity was then measured using the Steady-Lite HTS assay system and a VICTOR3 Multi-Label Reader (both from PerkinElmer Life Sciences).
In silico analysis of the hil-1R1 promoters
The core promoter sequences (sequences encompassing the TSSs containing 500 bp upstream and 100 bp downstream sequence) of the seven hIL1-R1 promoters were subjected to a web-based transcription factor binding site analysis (Patch 1.0 using TRANSFAC 6.0 public sites).
Dexamethasone treatment
DNA transfection was carried out in HeLa cells using PolyJet transfection reagent as described. Empty vector, pGRE-Luc and the seven promoter-reporter constructs were cotransfected with pSG5-hGR. Twelve hours after transfection, media was changed and 1 μM dexamethasone (Dex) was added. Fourteen hours after the Dex treatment, luciferase activity was measured.
Statistical analysis
The data are presented as the means ± standard error of mean (SEM). Variations in mRNA levels were evaluated by one-way analysis of variance followed by post-hoc analysis (Tukey test). A P-value of < 0.05 was considered significant in all statistical comparisons.
Results
shows results of electrophoresis of 5′-RACE PCR products from various human tissues listed in the legend. Multiple band patterns were obvious when PCR products from different tissues were compared. In some tissues, for example; heart (lane 2), spleen (lane 4), stomach (lane 10), testis (lane 11), pituitary (lane 13), pancreas (lane 16), and fetal liver (lane 22) and mammary gland (lane 24), multiple bands (indicated by asterisks) were generated by the RACE-PCR. These bands were isolated, cloned into the TOPO pCRII vector, and sequenced. In other tissue, for example, liver (lane 5), only one major band was generated. The PCR products from these tissues were directly cloned into TOPO vector and sequenced.
The sequences generated from the RACE clones were aligned to the genomic DNA sequence of human IL-1R1 by BLAST. The results are summarized in . Only the unique sequences are listed. Many TSSs were identified in different tissues. Seven major groups of TSS in IL-1R1 were found. Five groups are upstream of exon 2; the resulting 5 alternative first exons are all followed by the published exon 2 and the rest of the IL-1R1 mRNA sequence. The other two groups are downstream of exon 2. The first group of TSSs spans 147 bp, aligning to nucleotide positions 7360679- 7360825 of the reference contiguous sequence NT_022171.15. This group of TSSs is close to a published mRNA start site (accession number AK314433.1). The second group of TSSs was found clustered close to the nucleotide 7394858 of the NT_022171.15 sequence, aligning to a published mRNA start site (CR858295.1). The third kind of TSS aligns to 7401174 of the NT_022171.15 sequence, and the corresponding transcript is not recorded in the current database. The fourth and the fifth TSSs were found in proximity to each other, aligning to 7432595 and 7433270 of the NT_022171.15 sequence, respectively. These two TSSs are close to the start sites of a published EST (DA333942.1) and a cDNA (CR595183.1). The sixth group of TSSs was found immediately upstream of exon 3, and the corresponding transcript is not recorded in current database. The seventh group of TSSs was found immediately upstream of exon 4, and it is close to the start site of a published cDNA (BP233286.1).
Table 1 Sequence analysis of human IL-1R1 TSS. The sequences are aligned to the latest version of NT _022171.15
These seven TSS groups suggest the existence of seven promoters, including five promoters which yield five alternative exons 1 and two internal promoters. The second, the fourth and the fifth TSS groups correspond to the three promoters published in previous reports (resulting in three different exon1s: EX1A, EX1B and EX1C).Citation16,Citation17 We designated these seven putative promoters as P1D, P1A, P1E, P1B, P1C, P6 and P7 of the hIL-1R1, in the 5′ to 3′ order. The positions of the hIL-1R1 promoters are annotated in in the context of known genomic structure of the hIL-1R1 gene.
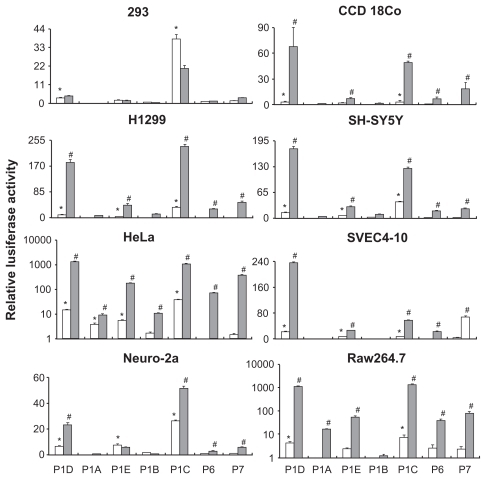
shows the results of the promoter-reporter assay. We define a detectible promoter activity as an observed luciferase activity in cells transfected with a promoter-reporter construct that is at least 3 times higher than that seen in cells transfected with the empty vector. In 293 cells, only P1D and P1C showed promoter activity. In the presence of the SV40 enhancer, P1C activity was significantly reduced and the activities of other promoters remain unchanged. In CCD-18Co cells, again, only P1D and P1C were active without the SV40 enhancer. The presence of the SV40 enhancer significantly increased the activity of these promoters and caused P1E and P7 to become active. In H1299, and SH-SY5Y cells, P1D, P1E, and P1C were active without the SV 40 enhancer, and P6 and P7 promoters became active in the presence of the SV 40 enhancer. In HeLa cells, P1D, P1E, P1A and P1C were active without SV40 enhancer, and all 7 promoters became active in the presence of the SV40 enhancer. In the three mouse cell lines, similar promoter activity patterns emerged. In the SVEC-10 and Neuro-2a cells, P1D, P1E and P1C were active without the SV40 enhancer and P1D, P1E, P1C, P6 and P7 were active in presence of the SV40 enhancer. In the RAW cells, P1D and P1C were active without SV40 enhancer and all the promoters except P1B were active in the presence of the SV40 enhancer. Except for the 293 cells, the SV40 enhancer was able to increase the promoter activity in all the human cells lines. In the three murine cell lines, the presence of SV40 enhancer failed to increase P1A, P1E, and P1B activity in Neuro-2a cells, failed to increase P1A and P1B activity in SVEC4-10 cells, and failed to increase P1B activity in RAW cells.
Figure 2 Results of promoter-reporter assay carried out in 8 different cell lines are shown. Relative luciferase activity was calculated as the ratio of the luciferase activity in cells transfected with a promoter-reporter construct over that in cells transfected with a promoter-less vector control. Means and standard errors of the mean calculated from three separate experiments are presented. The open bars show promoter activities without the SV40 enhancer; relative luciferase activity over 3 was defined as an active promoter (indicated by *). Filled bars show promoter activities in the presence of the SV40 enhancer; significant increase of promoter activity by the SV40 enhancer is indicated by # (#: P < 0.05, promoter + enhancer versus. promoter).
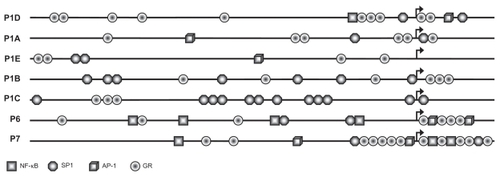
shows the results of the in-silico analysis of the promote sequences. None of the seven promoters contains any TATA box or CAAT box. The core promoter regions containing 500 bp upstream and 100 bp downstream sequences are shown; NF-κB, SP1, AP-1 and GR are annotated. P1D contains NF-κB, SP1 and AP-1 sites which are close to P1D’s TSS cluster, whereas transcription factor binding sites in P1A and P1E are relatively dispersed. Notably, a dense array of SP1 sites was found in the proximity of P1C TSS. In addition, numerous GR-binding sites were found in all the promoters.
Figure 3 Annotation of the hIL-1R1 promoter sequences by in silico analysis. Arrows indicate the positions of the TSSs. The core promoter regions containing 500 bp upstream and 100 bp downstream sequence are shown. SP1, NF-κB, AP 1, and GR sites are shown. All indicated sites contain the transcription factor binding sites with 100% sequence homology to the corresponding consensus sequences.
Abbreviations: TSS, transcription start sites; GR, glucocorticoid receptor.
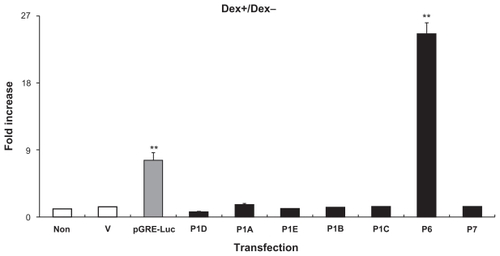
shows the influence of GR activation on the transcription activity of the seven hIL-1R1 promoters in HeLa cells. Data show ratios of luciferase activity of Dex treated cells (Dex+) over those in untreated (Dex−) cells. The first three bars represent control experiments: background luciferase activity is shown in untransfected cells (non); cells transfected with hGR and a vector containing luciferase cDNA without a promoter (V) did not respond to the Dex treatment; cells transfected with hGR and the GR reporter construct, pGRE-luc (pGRE-luc), showed a 9 fold increase of luciferase activity when they were treated with Dex. The rest of the bars represent results from cells transfected with hGR together with a vector containing one of the hIL-1R1 promoter-reporter constructs. Dex treatment did not change promoter activity in 6 out of the 7 hIL-1R1 promoters. However in P6-transfected cells, Dex treatment increased the promoter activity by over 20-fold.
Figure 4 Influence of glucocorticoid on the hIL-1R1 promoter activity in HeLa cells. Fold increases (luciferase activity in Dex-treated cells over that in Dex-untreated cells) are shown. Means and standard errors of the mean calculated from 3 separate experiments are presented.
Notes: Non: cells without transfection as negative control. V: cells transfected with hGR and the luciferase cDNA without a promoter as an additional negative control. pGRE-luc: cells transfected with hGR together with the GR reporter construct as a positive control for the assay.
Abbreviations: GR, glucocorticoid receptor; Dex, dexamethasone.
Discussion
The results of this study revealed seven clusters of TSSs, each associated with a unique promoter in the human IL-1R1 gene. Using RNA generated from 24 different tissues and the sensitive 5′-RACE method, we found many novel TSS sites, including two TSS clusters (associated with P1E and P6) that are not in the current database which includes randomly sequenced mRNAs.
The 5′-RACE may produce artificial putative TSSs by identifying 5′ truncated RNA. However, all of the TSSs associated with the novel P1D, P1E, P6, and P7 promoters were found in multiple tissues and multiple clones, suggesting that the results were unlikely to be the consequence of faulty RACE PCR amplification of randomly truncated RNAs. In addition, TSSs associated with the P1A, P1B, and P1C found in this study are the same as those found in the previous studies using the primer extension assay,Citation16 affirming the accuracy of the method used in the present study. Inspection of the core promoter sequences showed no TATA box consensus sequence in any of the hIL-1R1 promoters. This is consistent with previous reports that IL-1R1 uses TATA-less promoters.Citation15,Citation16 Other studies have demonstrated that TATA-less promoters often start transcription from several TSSs downstream of the promoter.Citation18 This may account for the fact that P1D, P1A, P6 and P7 are associated with multiple closely positioned TSSs found in the present study ().
Experimental verification of promoter activity for P1A, P1B, and P1C has been reported previously.Citation16 using a promoter-reporter assay. We show in the present study that all 7 promoters can drive the luciferase reporter expression in the promoter-reporter assay. In addition, the promoter activity can be modulated by the SV40 enhancer, a viral enhancer sequence known to generally increase promoter activity. Interestingly, the promoter activity of these hIL-1R1 promoters showed dramatic cell type-specific effects. For example, although SV40 enhancer increased the activity of these promoters in most human cell lines tested, it did not do so in the 293 cells. In addition, 4 promoters, P1D, P1E, P1A and P1C, were active in the HeLa cells without SV40 enhancer, but only two promoters, P1D and P1C, were active in 293 cells. This is similar to the mIL-1R1 promoters we reported recently that different promoters can be active in different cell types.Citation15
The two dominant hIL-1R1 promoters are P1D and P1C: they are active in all the cell lines tested including the 3 murine cell lines. In the mouse, we reported the existence of three mIL-1R1 promoters, P1, P2, and P3, with P1 and P2 being the dominant promoters and P3 being expressed only in limited cell types of certain tissues. It is possible that P1D and P1C in the hIL-1R1 correspond to P1 and P2 in mIL-1R1 in evolutionarily conserved functions. Surprisingly, no significant sequence homology between these promoters can be identified by BLAST (basic local alignment search tool analysis: data not shown), suggesting that the functional conservation of these promoters may have to be explained at the level of sequence framework, but not simple sequence homology. The P1C promoter was also the earliest reported hIL-1R1 promoter,Citation19 probably because it is the most active hIL-1R1 promoter in most tissues. This is consistent with our result of the in silico analysis that P1C contained many more SP1 sites than the other hIL-1R1 promoters; SP1 sites are critical for the activity of TATA-less promoters.Citation20 It is interesting to note that the mouse P1 is 41 kb upstream of P2 and the human P1D is about 71 kb upstream of P1C. Thus, transcribing IL-1R1 via P1 in mouse and via P1D in human might cost significantly more energy, due to the need to transcribe much longer intron1, than transcribing IL-1R1 via P2 in the mouse and via P1C in human. The purpose of using these less efficient promoters remains to be elucidated. We have shown previously that the mouse P1 contributes to cell type- or tissue-specific regulation of mIL-1R1. This could also be one of the reasons for the existence of the multiple promoters in the hIL-1R1 gene.
The other five promoters, P1E, P1A, P1B, P6, and P7, may be considered as minor promoters because they are much less active compared to P1D and P1C. The physiological significance of these promoters, however, may not be minor. For example, a PstI polymorphism near P1B was found to have significant association with insulin-dependent diabetes mellitus.Citation21 and a polymorphism within exon 1B has been associated with protective effects against endometriosis development.Citation22 It is conceivable that polymorphisms in the new promoter sequences discovered in the present study can be correlated to other disease conditions.
The existence of P7 was surprising because IL-1R1 mRNA expressed via this promoter would be missing the traditional IL-1R1 translation start codon in exon 3. In fact, the sequence of the P7-derived IL-1R1 mRNA predicts the translation of a hIL-1R1 from a downstream start codon in exon 5 that potentially results in the production of a truncated IL-1R1 molecule, lacking a segment of the N-terminus amino acid sequence of the IL-1R1. Whether this truncated IL-1R1 represents a novel IL-1 receptor remains to be determined.
The results of the in silico analysis of the hIL-1R1 promoters revealed multiple GR binding sites in all the promoters. We therefore tested whether these promoters are susceptible to glucocorticoid regulation. Surprisingly, only P6 responded to the glucocorticoid agonist Dex in HeLa cells. Therefore, GR regulates hIL-1R1 expression in a promoter-specific manner in HeLa cells. The effects of glucocorticoids in other cell types remain to be determined.
In summary, the present study reveals the existence of 7 hIL-1R1 promoters. The activities of these promoters are cell type-specific and are susceptible to regulations by enhancers and transcription factors in a promoter-specific manner.
Disclosures
The authors report no conflicts of interest in this work.
References
- ForsythKDLevinskyRJFibronectin degradation; an in-vitro model of neutrophil mediated endothelial cell damageJ Pathol19901613133192213372
- HanazawaSAmanoSHanaizumiCInductive effect of human recombinant IL-1 on differentiation of a macrophage-like tumor cell lineAdv Dent Res198823723753271032
- HermanJDinarelloCAKewMCRabsonARThe role of interleukin-1 (IL-1) in tumor-NK cell interactions: correction of defective NK cell activity in cancer patients by treating target cells with IL-1J Immunol1985135288228862993420
- JonuleitHKnopJEnkAHCytokines and their effects on maturation, differentiation and migration of dendritic cellsArch Dermatol Res1996289189017128
- IgarashiKMitsuyamaMMuramoriKTsukadaHNomotoKInterleukin-1-induced promotion of T-cell differentiation in mice immunized with killed Listeria monocytogenesInfect Immun199058397339792123829
- ChingSZhangHBelevychNEndothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administrationJ Neurosci200727104761048617898219
- JohnGRLeeSCSongXRivieccioMBrosnanCFIL-1-regulated responses in astrocytes: relevance to injury and recoveryGlia20054916117615472994
- KawanokuchiJShimizuKNittaAProduction and functions of IL-17 in microgliaJ Neuroimmunol2008194546118164424
- WangXFuSWangYInterleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathwayMol Cell Neurosci20073634335417822921
- ZhangRXLiALiuBIL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in ratsPain200813523223917689191
- QuanNBanksWABrain-immune communication pathwaysBrain Behav Immun20072172773517604598
- ArimuraAACTH regulation and IL-1Science198823913132830676
- BevanSRaynesJGIL-1 receptor antagonist regulation of acute phase protein synthesis in human hepatoma cellsJ Immunol1991147257425781717568
- SimsJEGiriJGDowerSKThe two interleukin-1 receptors play different roles in IL-1 actionsClin Immunol Immunopathol1994729148020198
- ChenQZhangHLiQThree promoters regulate tissue- and cell type-specific expression of murine interleukin-1 receptor type IJ Biol Chem20092848703871319196714
- YeKVannierEClarkBDSimsJEDinarelloCAThree distinct promoters direct transcription of different 5′ untranslated regions of the human interleukin 1 type I receptor: a possible mechanism for control of translationCytokine199684214298818538
- SimsJEPainterSLGowIRGenomic organization of the type I and type II IL-1 receptorsCytokine199574834908580363
- Juven-GershonTHsuJYTheisenJWKadonagaJTThe RNA polymerase II core promoter – the gateway to transcriptionCurr Opin Cell Biol20082025325918436437
- YeKDinarelloCAClarkBDIdentification of the promoter region of human interleukin 1 type I receptor gene: multiple initiation sites, high G + C content, and constitutive expressionProc Natl Acad Sci U S A199390229522998460136
- BlockKLShouYThortonMPonczMThe regulated expression of a TATA-less, platelet-specific gene, alphaIIbStem Cells199614Suppl 1384711012201
- BergholdtRKarlsenAEJohannesenJCharacterization of polymorphisms of an interleukin-1 receptor type 1 gene (IL1RI) promotor region (P2) and their relation to insulin-dependent diabetes mellitus (IDDM). The Danish Study Group of Diabetes in ChildhoodCytokine199577277338580383
- D’AmoraPSatoHGiraoMJSilvaIDSchorEPolymorphisms in exons 1B and 1C of the type I interleukin-1 receptor gene in patients with endometriosisAm J Reprod Immunol20065617818416911713