Abstract
Background
Mesenchymal stem cells (MSCs) have been proposed as autologous therapy for inflammatory diseases in neonates. MSCs from umbilical cord Wharton’s jelly (WJ-MSCs) are accessible, with high proliferative capacity. The effects of WJ-MSCs on neutrophil activity in neonates are not known. We compared the effects of WJ-MSCs on apoptosis and the expression of inflammatory, oxidant, and antioxidant mediators in adult and neonatal neutrophils.
Methods
WJ-MSCs were isolated, and their purity and function were confirmed by flow cytometry. Neutrophils were isolated from cord and adult blood by density centrifugation. The effects of neutrophil/WJ-MSC co-culture on apoptosis and gene and protein expression were measured.
Results
WJ-MSCs suppressed neutrophil apoptosis in a dose-dependent manner. WJ-MSCs decreased gene expression of NADPH oxidase-1 in both adult and neonatal neutrophils, but decreased heme oxygenase-1 and vascular endothelial growth factor and increased catalase and cyclooxygenase-2 in the presence of lipopolysaccharide only in adult cells. Similarly, generation of interleukin-8 was suppressed in adult but not neonatal neutrophils. Thus, WJ-MSCs dampened oxidative, vascular, and inflammatory activity by adult neutrophils, but neonatal neutrophils were less responsive. Conversely, Toll-like receptor-4, and cyclooxygenase-2 were upregulated in WJ-MSCs only in the presence of adult neutrophils, suggesting an inflammatory MSC phenotype that is not induced by neonatal neutrophils.
Conclusion
Whereas WJ-MSCs altered gene expression in adult neutrophils in ways suggesting anti-inflammatory and antioxidant effects, these responses were attenuated in neonatal cells. In contrast, inflammatory gene expression in WJ-MSCs was increased in the presence of adult but not neonatal neutrophils. These effects should be considered in clinical trial design before WJ-MSC-based therapy is used in infants.
Introduction
Mesenchymal stem cells (MSCs) are multipotent progenitor cells that mediate immune tolerance in recipient hosts. These cells are commonly isolated from bone, blood, and adipose tissues, as well as from umbilical cord Wharton’s jelly. They home to damaged tissues and contribute to their repair by secretion of cytokines, chemokines, and extracellular matrix proteins.Citation1 Bone marrow-derived (BM)-MSCs, the most commonly studied MSCs, are also highly immunosuppressive both in vitro and in vivo in animal models.Citation2,Citation3 BM-MSCs modulate immune responses by dendritic cells, lymphocytes, neutrophils, and monocytes, in part through the release of cytokines and lipid mediators.Citation4 These cells also inhibit apoptosis and reduce the formyl-methionyl-leucyl-phenylalanine-induced respiratory burst in neutrophils by constitutive release of interleukin (IL)-6.Citation5 Some evidence suggests that immunosuppression by BM-MSCs is dependent on their expression of the adhesion molecules, ie, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1, indicating that cell-to-cell contact is required.Citation3 However, the mechanisms of immunosuppression by MSCs are not known.
MSCs may have therapeutic utility in neonates, who are susceptible to inflammatory diseases, such as bronchopulmonary dysplasia. However, the invasiveness of bone marrow aspiration and the age-dependent degradation in quantity and quality of BM-MSCs limit their clinical potential. Human umbilical cord Wharton’s jelly (WJ) provides an alternative source of MSCs that exhibit high proliferative capacity and multidifferentiation potential.Citation6 These cells are abundant and accessible, and when compared with MSCs from adult bone marrow or adipose tissue, WJ-MSCs are more robustly proliferative and immunosuppressive.Citation7 Of note, these cells can be expanded from the umbilical cord at the time of birth, raising the possibility of autologous treatment of newborns.
Neutrophils are nonproliferative circulating phagocytes that produce reactive oxygen intermediates and inflammatory cytokines, and also promote vascular remodeling by secretion of angiogenic growth factors.Citation8 However, prolonged activation of these cells following hypoxic or infectious insults has been implicated in chronic inflammatory diseases associated with alterations in tissue architecture, such as bronchopulmonary dysplasia.Citation9 Current therapies to ameliorate neutrophil cytotoxicity have not been completely effective in treating or preventing these conditions. WJ-MSCs have been proposed as cell-based prophylaxis or treatment for neonatal inflammatory diseases.Citation10 However, the effects of WJ-MSCs on neonatal neutrophil activity are not known.
In the current studies, we compared the dose-dependent effects of WJ-MSCs on apoptosis and inflammatory function in neonatal and adult neutrophils. We hypothesized that WJ-MSCs would downregulate expression of oxidant and inflammatory mediators, and increase antioxidant expression in neutrophils. Experiments were performed in the presence or absence of IL-6 or ICAM-1 (CD54) blocking antibodies to investigate the possibility that these effects are mediated by soluble mediators or cell contact, respectively.Citation3,Citation11
Materials and methods
Reagents
Dulbecco’s Modified Eagle’s Medium, phosphate- buffered saline, and bacterial lipopolysaccharide (serotype 0128:B12) were purchased from Sigma Chemical Company (St Louis, MO, USA). Dextran was from ThermoFisher (Fairlawn, NJ, USA). Ficoll-Paque was from GE Healthcare (Piscataway, NJ, USA), and heat inactivated fetal bovine serum was from Biosera US (Kansas City, MO, USA). Annexin V-APC, 7-actinomycin D (7-AAD) and cytometric bead array flex sets were from BD Biosciences (San Jose, CA, USA). RNeasy RNA purification kits were purchased from Qiagen (Chatsworth, CA, USA). Primers for real time polymerase chain reaction were obtained from Integrated DNA Technologies, Inc. (Coralville, IA, USA) and Power SYBR green master mix from Applied Biosystems (Foster City, CA, USA). Anti-human IL-6 and anti-human CD54 (ICAM-1) were from eBioscience (San Diego, CA, USA).
Subjects
These studies were approved by the institutional review board of Robert Wood Johnson Medical School. Umbilical cord blood samples were collected at the time of delivery of healthy term newborns (≥37 weeks gestation) by elective cesarean section prior to labor, with tissue-specific informed consent. Subjects with clinical evidence of chorioamnionitis, funisitis, or other perinatal infections were excluded. For comparison, neutrophils were isolated from peripheral venous blood of healthy adult volunteers.
WJ-MSC isolation
WJ-MSCs from nine infants were isolated by peeling the outer membrane of the umbilical cord and dissecting out the arteries and vein, as previously described.Citation12 WJ was cut into 1–3 mm pieces and placed in 10 cm tissue culture dishes. After drying (15 minutes), Dulbecco’s Modified Eagle’s Medium + 10% fetal bovine serum was added. Half of the medium was changed on day 5, and all media were changed every 3 days subsequently. Adherent cells reached confluence after 10–12 days. Cells from each subject were treated as a new cell line and tested for purity and expression of characteristic MSC markers by flow cytometry. Similar to previous reports,Citation1–Citation3 all nine lines expressed CD105, CD73, and CD90, but not CD45, CD34, or CD31. All experiments were performed no later than passage five, to maintain consistency.
Neutrophil isolation and co-culture
Polymorphonuclear neutrophils (PMN) were isolated by dextran sedimentation, Ficoll gradient centrifugation, and hypotonic lysis. Cells were resuspended in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum (1×106 cells/mL). Neutrophil suspensions were incubated with adherent WJ-MSCs in 24-well plates at 37°C, at WJ-MSC to neutrophil ratios of 1:20–1:640 for apoptosis studies and 1:20 for analysis of gene and protein expression. Cell viability (defined by absence of uptake of 7-AAD fluorescence dye) was >97% in all experiments.
Measurement of neutrophil apoptosis
After 24 hours in co-culture with WJ-MSCs, neutrophils were washed out of the wells, pelleted, and resuspended in phosphate-buffered saline at 1×107 cells/mL. Triplicate aliquots of cells (100 mL) were then incubated with Annexin V-APC (1:20) and 7-AAD (1:10; 15 minutes, room temperature). The remaining cells were lysed for measurement of gene expression, as described below. Cells were analyzed by flow cytometry on a FACSArray Bioanalyzer (BD Biosciences). Neutrophils were identified based on forward scatter and side scatter characteristics, and all analyses were performed on this population. Viable neutrophils were defined as those not taking up either Annexin V or 7-AAD. Necrotic cells were defined as those taking up only 7-AAD, and apoptotic cells as those binding Annexin V. Quadrant boundaries were determined using unstained, as well as positive control cells stained with either 7-AAD or Annexin V-APC, and statistics were performed based on the relative cell numbers in these quadrants.
Gene expression
PMN were incubated with WJ-MSCs (WJ-MSC + PMN, 1:20) or medium control (PMN) for 24 hours, in the presence or absence of lipopolysaccharide (1 μg/mL) and antibodies to ICAM-1 or IL-6 (1 μg/mL). Neutrophils and WJ-MSCs were separated after co-culture by adherence. Nonadherent neutrophils were harvested from the co-culture supernatant, and their purity was confirmed by morphology. Viable WJ-MSCs were identified as adherent to the culture plate after two washes with phosphate-buffered saline. Absence of residual neutrophils and WJ-MSC morphology were confirmed by microscopy. Total RNA was extracted from neutrophils or WJ-MSCs, complementary DNA generated, and gene expression quantified by real-time polymerase chain reaction using RT2 qPCR master mix (Qiagen) and amplified using a Stratagene MX300p instrument, with β-actin as standard. Full-length coding sequences were obtained from GenBank™. Primers were designed using Primer Express software. Forward and reverse primers used were: NOX-1, 5′-CCTTGCACCGGTCATTCTTT-3′ and 5′-CGGTAAAACCGGAGGATCCT-3′; COX-2, 5′-GCCTGATGATTGCCCGACT-3′ and 5′-GCTGGCCCTCGCTTATGATCT-3′; HO-1, 5′-GCTCAAAAAGATTGCCCAGA-3′ and 5′-GCGGTAGAGCTGCTTGAACT-3′; catalase, 5′-CGGAGATTCAACACTGCCAA-3′ and 5′-GAATGCCCGCACCTGAGTAA-3′; VEGF, 5′-GGCGTCGCACTGAAACTTTT-3′ and 5′-TCCGAAGCGAGAACAGCC-3′; TLR4, 5′-CCAGAGCCGCTGGTGTATCT-3′ and 5′-AACTGCCAGGTCTGAGCAATCT-3′; β-actin, 5′-AAAGACCTGTACGCCAACAC-3′ and 5′-GTCATACTCCTGCTTGCTGAT-3′.
Protein expression
Supernatants from 24-hour cultures of neutrophils and/or WJ-MSCs were incubated with premixed flex set beads coated with antibodies to IL-8, macrophage inflammatory protein (MIP)-1β, and ICAM-1 for 1 hour in 96-well filtration plates. Premixed phycoerythrin-labeled detection reagent was then added to the wells, and the plates incubated at room temperature in the dark for 2 hours. Bead/protein complexes were then washed and analyzed for fluorescence intensity using a BD FACSArray Bioanalyzer. Data were analyzed using BD FCAP software (version 2.0) with five parameter curve fitting. Measurements from co-cultures (observed) were compared with the sum of the measurements from cultures of neutrophils and WJ-MSCs alone (expected) and data are presented as the mean ± 95% confidence interval.
Statistical analysis
Data were analyzed using Statistica version 5.5 (StatSoft, Inc., Tulsa, OK, USA). The effects of treatments were compared pairwise by Student’s t-test.
Results
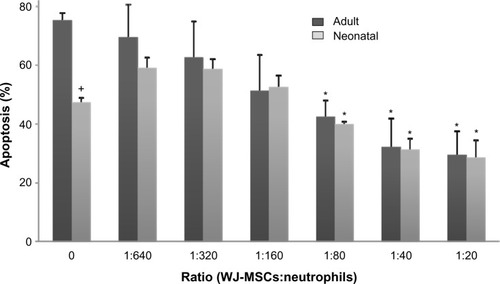
WJ-MSCs suppressed neutrophil apoptosis in a dose-dependent manner (). WJ-MSC to neutrophil ratios greater than 1:80 resulted in significant decreases in neutrophil apoptosis over 24 hours, in both adult and neonatal cells, with optimal efficacy at a WJ-MSC to neutrophil ratio greater than 1:40. In order to have enough cells for gene expression and protein assays, a ratio of 1:20 was chosen for all experiments. As previously reported,Citation13 apoptosis was significantly reduced in neonatal neutrophils when compared with adult cells, but this difference was not sustained in the presence of WJ-MSCs. Incubation with IL-6 or ICAM-1 blocking antibodies did not affect apoptosis (not shown).
Figure 1 Effects of WJ-MSCs on neutrophil apoptosis.
Notes: Adult and neonatal neutrophils were incubated with WJ-MSCs at varying ratios for 24 hours and then analyzed for apoptosis by flow cytometry using BD FACSArray quadrant and two-dimensional histogram statistics based on relative fluorescence. Each bar represents the mean ± standard error (n=3–8). +Significantly different (P<0.05) from adult; *significantly different (P<0.05) from untreated control (0).
Abbreviation: MSCs, mesenchymal stem cells; WJ-MSCs, MSCs derived from Wharton’s jelly.
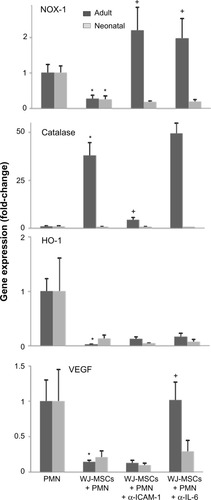
NADPH oxidase-1 (NOX-1) is a membrane-bound enzyme that is important in generating the superoxide anion that drives respiratory burst activity in neutrophils. We found that WJ-MSCs suppressed NOX-1 expression in both adult and neonatal neutrophils (). WJ-MSCs also markedly induced expression of the antioxidant enzyme catalase and suppressed expression of heme oxygenase-1 (HO-1) and vascular endothelial growth factor (VEGF), but these changes were significant only in adult cells. Effects of WJ-MSCs on neutrophil expression of the NOX-1 gene were decreased by antibodies to IL-6 or ICAM-1, catalase gene by anti-ICAM-1 only, VEGF by anti-IL-6 only, and HO-1 by neither antibody.
Figure 2 Expression of VEGF, catalase, HO-1, and NOX-1 in neutrophils exposed to WJ-MSCs.
Notes: Adult and neonatal neutrophils were incubated with WJ-MSCs (WJ-MSCs + PMN, 1:20) or medium control (PMN) for 4 hours, in the presence or absence of neutralizing antibodies to ICAM-1 or IL-6 (1 μg/mL). Total RNA was extracted, complementary DNA generated, and gene expression quantified by real-time polymerase chain reaction. Results were normalized to β-actin expression. Each bar represents the mean ± standard error (n=5–7). *Significantly different (P<0.05) from control; +significantly different (P<0.05) from MSCs + PMN.
Abbreviations: NOX-1, NADPH oxidase-1; HO-1, heme oxygenase-1; VEGF, vascular endothelial growth factor; MSCs, mesenchymal stem cells; WJ-MSCs, MSCs derived from Wharton’s jelly; PMN, polymorphonuclear neutrophils; IL-6, interleukin-6; ICAM-1, intercellular adhesion molecule-1.
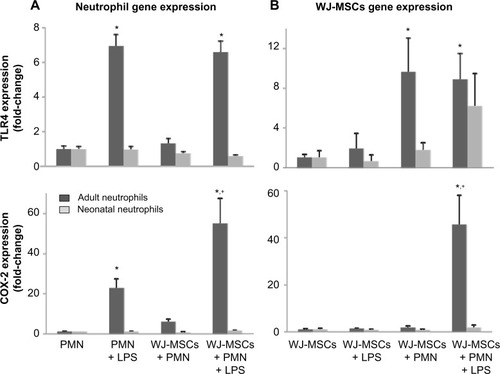
Lipopolysaccharide induced Toll-like receptor-4 (TLR4) expression in adult but not neonatal neutrophils (). These responses were not affected by co-culture with WJ-MSCs. Some previous reports have suggested that WJ-MSCs may be distinguished from other MSCs by their hyporesponsiveness to lipopolysaccharide associated with low expression of TLR4.Citation14 Consistent with this, we found that lipopolysaccharide did not induce TLR4 in WJ-MSCs (). In contrast, co-culture with adult neutrophils markedly increased TLR4 expression in WJ-MSCs. Cyclooxygenase-2 (COX-2), which catalyzes the production of inflammatory eicosanoids in neutrophils, is regulated in part by TLR4.Citation15 We found that lipopolysaccharide markedly induced COX-2 expression (20-fold) in adult neutrophils (). COX-2 expression in adult neutrophils further increased (60-fold) in the presence of both lipopolysaccharide and WJ-MSCs. As for TLR4, neonatal neutrophils were not affected by lipopolysaccharide and/or WJ-MSCs. While WJ-MSCs did not express COX-2 in response to lipopolysaccharide or to co-culture with neutrophils, the combination of lipopolysaccharide and adult neutrophils markedly induced generation of COX-2 (40-fold; ).
Figure 3 Expression of TLR-4 and COX-2 in neutrophils and WJ-MSCs.
Notes: (A) Adult and neonatal neutrophils were incubated in medium control (PMN), LPS (1 μg/mL) and/or WJ-MSCs (WJ-MSCs to neutrophils, 1:20) (B) WJ-MSCs were incubated in medium control (MSCs), with LPS (1 μg/mL), and/or adult or neonatal neutrophils (WJ-MSCs to neutrophils, 1:20). Total RNA was extracted from neutrophils (A) or WJ-MSCs (B), complementary DNA generated, and gene expression quantified by real-time polymerase chain reaction. Results were normalized to β-actin expression. Each bar represents the mean ± standard error (n=5–7). *Significantly different (P<0.05) from PMN control; +significantly different (P<0.05) from PMN + LPS (A) or MSCs + LPS (B).
Abbreviations: COX-2, cyclooxygenase-2; MSCs, mesenchymal stem cells; WJ-MSCs, MSCs derived from Wharton’s jelly; PMN, polymorphonuclear neutrophils; LPS, lipopolysaccharide; TLR4, Toll-like receptor-4.
Both neutrophils and WJ-MSCs constitutively produced IL-8 (). Consistent with previous reports, production of IL-8 was markedly decreased in neonatal neutrophils when compared with adult neutrophils. The combined generation of IL-8 by neutrophils and WJ-MSCs in co-culture was significantly decreased in adult but not neonatal cells. In contrast, expression of MIP-1β, another chemokine associated with neutrophil recruitment, by neutrophils and WJ-MSCs was not affected by co-culture of these cells. Expression of ICAM-1 by neutrophils and WJ-MSCs was also not significantly affected by co-culture.
Table 1 Production of inflammatory mediators by neutrophils and WJ-MSCs
Discussion
Our finding that WJ-MSCs suppress neutrophil apoptosis in a dose-dependent manner indicates that the physiologic effects of WJ-MSCs are similar in some respects to those demonstrated by BM-MSCs. Previous reports have shown that BM-MSCs protect adult neutrophils from apoptosis by triggering STAT-3 signalingCitation5 or by decreasing expression of Bax, a proapoptotic member of the Bcl-2 family.Citation16 BM-MSCs secrete mediators (VEGF, transforming growth factor-β) that promote fibroblast activation, angiogenesis, and alteration of tissue architecture, ie, processes that may be facilitated by neutrophils.Citation17 Thus, prolonged viability of neutrophils in the presence of MSCs may facilitate MSC-mediated tissue repair. Our findings also suggest that the immunomodulatory effects of WJ-MSCs do not occur by facilitating neutrophil clearance, but most likely by decreasing their activity.
To investigate this, we next quantified the effects of WJ-MSCs on the expression of pro-oxidant and antioxidant enzymes in neutrophils. Neutrophils exert their bactericidal and inflammatory activity, in part, by the release of reactive oxygen intermediates, but prolonged neutrophil activity can cause cytotoxicity.Citation18 NOX-1 is expressed in these cells, mediating the transfer of electrons to molecular oxygen to produce the superoxide anion. We found that WJ-MSCs suppressed NOX-1 activity in both adult and neonatal neutrophils, which may serve to modulate oxidative activity and ameliorate potential tissue injury. WJ-MSCs also induced expression of the antioxidant catalase, but only in adult neutrophils. Catalase protects cells from reactive oxygen intermediates by catalyzing the decomposition of hydrogen peroxide to water and oxygen, and its basal expression is decreased in neonatal neutrophils. Our observation that catalase is further upregulated in adult cells is consistent with previous reports that MSCs ameliorate hepatic injury by upregulating catalase expression in the liver.Citation19 Upregulation of catalase may protect adults from neutrophil-mediated cytotoxicity in the presence of WJ-MSCs, but neonates appear to be resistant to this antioxidant effect.
WJ-MSCs also significantly decreased expression of the anti-inflammatory enzyme HO-1 in adult neutrophils but not in neonatal cells. HO-1 exerts anti-inflammatory effects by upregulating IL-10 and IL-1R antagonist expression.Citation20 This results in decreased neutrophil rolling and adhesion, as well as transmigration of these cells to inflammatory sites. Thus, the relative preservation of HO-1 activity in neonatal neutrophils exposed to WJ-MSCs may be a marker of preserved immune responses. Similarly, WJ-MSCs suppressed VEGF gene expression in adult, but not neonatal cells. VEGF is important in physiologic angiogenesis that drives tissue differentiation and growth.Citation8 Taken together, the hyporesponsiveness of neonatal neutrophils suggests that interactions between WJ-MSCs and neutrophils in neonates may be characterized by sustained immune activity and ongoing tissue modeling and repair, whereas those in adult cells are predominantly characterized by suppression of oxidant activity and immunosuppression.
In contrast with earlier reports indicating that ICAM-1 is important in mediating the immunosuppressive effects of BM-MSCs,Citation3 antibodies to ICAM-1 did not alter the effects of WJ-MSCs on apoptosis or expression of antioxidant or inflammatory mediators in neonatal neutrophils. Thus, ICAM-mediated cell contact is not likely an obligatory pathway for these effects. It is possible that this represents a mechanistic distinction between BM-MSCs and WJ-MSCs. Alternatively, our findings are consistent with previous studies reporting that the biologic effects of MSCs are mediated by soluble mediators, rather than direct contact.Citation21,Citation22 However, blocking antibodies to IL-6 also had no effect on WJ-MSC-mediated changes in neonatal neutrophil apoptosis or secretory activity. It is possible that MSCs exert paracrine effects via membrane-bound exosomes containing multiple soluble mediators affecting inflammatory and oxidative activity.Citation23
TLR4 expression in neutrophils was upregulated by lipopolysaccharide in adult neutrophils, and this was not affected by WJ-MSCs. Previous studies have suggested that WJ-MSCs are distinct from BM-MSCs in that they do not express TLR4 and are nonresponsive to lipopolysaccharide.Citation24 However, co-culture with adult neutrophils markedly upregulated expression of TLR4 in WJ-MSCs. This may represent a biologically relevant trigger for inflammation in adults, since TLR4-primed MSCs can differentiate into a phenotype (“MSC1”) characterized by secretion of proinflammatory mediators.Citation25 This pathway appears to be developmentally impaired in neonatal neutrophils, which do not upregulate TLR4 expression and do not induce it in WJ-MSCs. COX-2 expression is regulated in part by TLR4.Citation15 BM-MSCs bind lipopolysaccharide via surface TLR4 receptors, initiating a signaling cascade involving sequential activation of NF-κB and upregulation of COX-2 production.Citation26 We found that gene expression of COX-2 by WJ-MSCs is markedly upregulated in the presence of adult neutrophils and lipopolysaccharide, but not of either of those alone. Similarly, COX-2 is induced in adult neutrophils by lipopolysaccharide, and significantly further increased by WJ-MSCs. Our findings suggest that WJ-MSCs and neutrophils generate prostaglandins or other eicosanoids under inflammatory conditions, but that these signaling pathways are developmentally impaired in neonates.
We also found that WJ-MSCs and adult neutrophils in co-culture generate significantly less IL-8 than they do alone. IL-8 is a chemokine that activates neutrophil calcium mobilization and chemotaxis,Citation27,Citation28 so this may represent a mechanism for immunomodulation by WJ-MSCs. In contrast, WJ-MSCs did not affect the generation of ICAM-1 or the chemokine MIP-1β in either adult or neonatal neutrophils. ICAM-1 mediates transendothelial migration of neutrophils, and MIP-1β is secreted in large quantities by lipopolysaccharide-activated neutrophils.Citation29 Our finding that WJ-MSCs do not affect expression of IL-8, ICAM-1, or MIP-1β in neonatal neutrophils suggests that WJ-MSCs may not suppress neutrophilic inflammation in this population as anticipated.
Conclusion
Hospitalized premature neonates are susceptible to diseases characterized by excessive inflammation, including bronchopulmonary dysplasia, and MSC-based therapies are thought to have potential in preventing or ameliorating these conditions. Animal studies of such therapies have shown efficacy in conditions including sepsisCitation30 and acute lung injury,Citation31 and initial trials in human neonatesCitation32 and adultsCitation33 have indicated feasibility and possible efficacy in bronchopulmonary dysplasia and chronic lung disease, respectively. In the current studies, WJ-MSCs suppressed apoptosis and dampened oxidative, vascular, and inflammatory activity by adult neutrophils, but neonatal neutrophils were less responsive. Conversely, TLR4 and COX-2 were upregulated in WJ-MSCs only when they were co-cultured with adult neutrophils, suggesting an inflammatory MSC phenotype that is not induced by neonatal neutrophils. Moreover, WJ-MSCs suppressed apoptosis of both adult and neonatal cells. In summary, WJ-MSCs may alter neutrophilic inflammation in adults and neonates via pathways that are distinct. These effects should be carefully considered and tested in clinical trials before WJ-MSC-based-therapy is promoted for prophylaxis or treatment in infants.
Acknowledgments
This research was supported by grants from the Gerber Foundation and the National Institutes of Health (R21ES021170, P30ES005022).
Disclosure
The authors report no conflicts of interest in this work.
References
- ShiYHuGSuJMesenchymal stem cells: a new strategy for immunosuppression and tissue repairCell Res20102051051820368733
- RenGSuJZhangLSpecies variation in the mechanisms of mesenchymal stem cell-mediated immunosuppressionStem Cells2009271954196219544427
- RenGZhaoXZhangLInflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppressionJ Immunol20101842321232820130212
- MatthayMAThompsonBTReadEJTherapeutic potential of mesenchymal stem cells for severe acute lung injuryChest201013896597220923800
- RaffaghelloLBianchiGBertolottoMHuman mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow nicheStem Cells20082615116217932421
- ZhouCYangBTianYImmunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytesCell Immunol2011272333822004796
- KimDWStaplesMShinozukaKPantchevaPKangSDBorlonganCVWharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applicationsInt J Mol Sci201314116921171223727936
- GaudryMBregerieOAndrieuVEl BennaJPocidaloMAHakimJIntracellular pool of vascular endothelial growth factor in human neutrophilsBlood199790415341619354686
- ChessPRD’AngioPryhuberGSManiscalcoWMPathogenesis of bronchopulmonary dysplasiaSemin Perinatol20063017117816860156
- BorghesiACovaCGazzoloDStronatiMStem cell therapy for neonatal diseases associated with preterm birthJ Clin Neonatol201321724027735
- GroppoRRichterJDCPEB control of NF-kappaB nuclear localization and interleukin-6 production mediates cellular senescenceMol Cell Biol2011312707271421536657
- BongsoAFongCYThe therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cordStem Cell Rev2013922624023233233
- HannaNVasquezPPhamPMechanisms underlying reduced apoptosis in neonatal neutrophilsPediatr Res200557566215557111
- RaicevicGNajarMStamatopoulosBThe source of human mesenchymal stromal cells influences their TLR profile as well as their functional propertiesCell Immunol201127020721621700275
- KirkbyNSZaissAKWrightWRDifferential COX-2 induction by viral and bacterial PAMPs: consequences for cytokine and interferon responses and implications for anti-viral COX-2 directed therapiesBiochem Biophys Res Commun201343824925623850620
- OttonelloLFrumentoGArduinoNDifferential regulation of spontaneous and immune complex-induced neutrophil apoptosis by proinflammatory cytokines. Role of oxidants, Bax and caspase-3J Leukoc Biol20027212513212101271
- HayashiYTsujiSTsujiiMTopical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in ratsJ Pharmacol Exp Ther200832652353118448866
- HamptonMBKettleAJWinterbournCCInside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killingBlood199892300730179787133
- BurraPArcidiaconoDBizzaroDSystemic administration of a novel human umbilical cord mesenchymal stem cells population accelerates the resolution of acute liver injuryBMC Gastroenterol2012128822788801
- PiantadosiCAWithersCMBartzRRHeme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expressionJ Biol Chem2011286163741638521454555
- ChenLTredgetEEWuPYWuYParacrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healingPLoS One20083e188618382669
- GnecchiMZhangZNiADzauVJParacrine mechanisms in adult stem cell signaling and therapyCirc Res20081031204121919028920
- LeeCMitsialisSAAslamMExosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertensionCirculation20121262601261123114789
- TomchuckSLZwezdarykKJCoffeltSBWatermanRSDankaESScandurroABToll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responsesStem Cells2008269910717916800
- WatermanRSTomchuckSLHenkleSLBetancourtAMA new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotypePLoS One20105e1008820436665
- TyndallAPistoiaVMesenchymal stem cells combat sepsisNat Med200915182019129775
- BaggioliniMDewaldBMoserBInterleukin-8 and related chemotactic cytokines – CXC and CC chemokinesAdv Immunol199455971798304236
- ZeilhoferHUSchorrWRole of interleukin-8 in neutrophil signalingCurr Opin Hematol2000717818210786656
- IssekutzACRowterDSpringerTARole of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migrationJ Leukoc Biol1999651171269886254
- HallSRTsoyiKIthBMesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophilsStem Cells20133139740723132816
- ShalabySMEl-ShalASAbd-AllahSHMesenchymal stromal cell injection protects against oxidative stress in Escherichia coli-induced acute lung injury in miceCytotherapy20141676477524525173
- ChangYSAhnSYYooHSMesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trialJ Pediatr201416496697224508444
- AntunesMALaffeyJGPelosiPRoccoPRMesenchymal stem cell trials for pulmonary diseasesJ Cell Biochem20141151023103224515922
