Abstract
Purpose
To investigate the ability of a commercial extract from the medicinal plant Artemisia annua to modulate production of the cytokine, tumor necrosis factor-alpha (TNF-α), and the cyclooxygenase (COX) inflammatory marker, prostaglandin E2 (PGE2) in activated neutrophils.
Methods
Neutrophils were harvested from rat whole blood and cultured in the presence of plant extract or control samples. Neutrophils, except unactivated control cells, were activated with 10 μg/mL lipopolysaccharide (LPS). The cells were cultured with a range of different concentrations of the A. annua extracts (400–1 μg/mL) and artemisinin (200 and 100 μg/mL) and the supernatants were then tested by enzyme-linked immunosorbent assay (ELISA) for the concentrations of TNF-α and PGE2. Each sample was assayed in triplicate. Positive controls with an inhibitor were assayed in triplicate: chloroquine 2.58 and 5.16 μg/mL for TNF-α, and ibuprofen 400 μg/mL for PGE2. An unsupplemented group was also assessed in triplicate as a baseline control.
Results
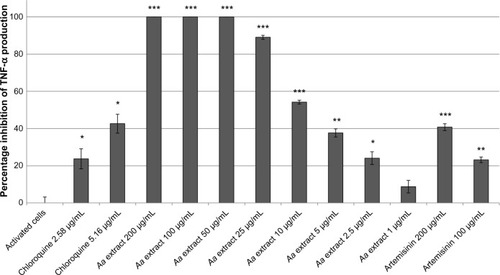
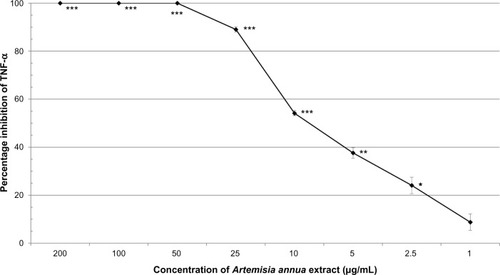
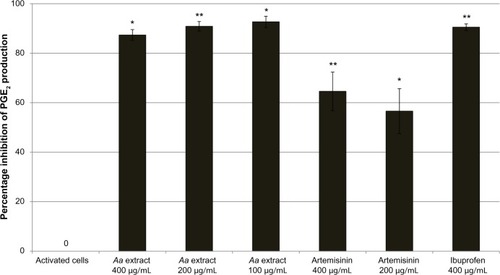
Neutrophils were stimulated to an inflammatory state by the addition of LPS. A. annua extract significantly inhibited TNF-α production by activated neutrophils in a dose-dependent manner. There was complete inhibition by the A. annua extract at 200, 100, and 50 μg/mL (all P≤0.0003). At A. annua extract concentrations of 25, 10, and 5 μg/mL, TNF-α production was inhibited by 89% (P<0.0001), 54% (P=0.0002), and 38% (P=0.0014), respectively. A. annua 1 μg/mL did not significantly inhibit TNF-α production (8.8%; P>0.05). Concentrations of 400, 200, and 100 μg/mL A. annua extract significantly inhibited PGE2 production by 87% (P=0.0128), 91% (P=0.0017), and 93% (P=0.0114), respectively.
Conclusion
An extract of A. annua was shown to be a potent inhibitor of TNF-α and a strong inhibitor of PGE2 production in activated neutrophils at the concentrations tested. Further studies are warranted with this promising plant extract.
Introduction
Much recent attention has been given to traditional medicines and natural products with potential and promising anti-inflammatory properties.Citation1–Citation4 However, much of the evidence is minimal or anecdotal, and it is clear that more research is needed in this area.Citation2
The medicinal plant Artemisia annua L. (Asteraceae) is native to the People’s Republic of China but has been introduced and grows wild throughout Asia, North America, and Europe, and is now broadly cultivated for medicinal purposes.Citation5 A. annua has been used as a medicinal herb for more than 2,000 years.Citation6 Traditional uses of the plant include as an antimalarial, a food additive, an anti-inflammatory, and to treat hemorrhoids, lice, and boils.Citation2 Texts on Chinese herbal medicines, written as early as 200 AD, also reported that it relieved joint pain.Citation5
In the 1970s, researchers in the People’s Republic of China identified one of the main components of A. annua; a sesquiterpene lactone, artemisinin.Citation5,Citation6 Artemisinin-based therapy is one of the most effective agents for the prevention and treatment of malaria and has been used successfully to treat millions of people worldwide.Citation7–Citation11 The mechanism of action against malaria is still debated, even though a number of potential targets have been proposed, such as alkylation of heme or proteins, inhibition of a parasite gene, or damage to the parasite’s membrane.Citation12 The compounds in A. annua appear to have other bioactive properties and may have broader anti-disease applications beyond the treatment of malaria.Citation13
Artemisinin appears to have anti-inflammatory properties, probably due to the inhibition of inflammatory factors and mediators such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1β, and nitric oxide.Citation14,Citation15 Other antimalarial drugs, especially quinine derivatives, are standard therapies for the treatment of rheumatoid arthritis and systemic lupus erythematosus, where they appear to have both disease-modifying and anti-inflammatory effects.Citation16
The aim of this study was to investigate the ability of an extract of A. annua to modulate production of the cytokine TNF-α in activated neutrophils. Preliminary investigations were also conducted on the ability of A. annua to modulate production of the cyclooxygenase (COX) inflammatory marker, prostaglandin E2 (PGE2) in activated neutrophils. These commonly studied markers were chosen as they are known to be produced by neutrophils from many species and can also be easily studied and quantified using well-documented in vitro assays.
Materials and methods
Plant material
A commercial supercritical carbon dioxide extract of A. annua was used in the assays. The extract is used in Arthrem™ capsules (Promisia Integrative Ltd, Wellington, New Zealand), a dietary supplement for joint support. Commercially available artemisinin capsules (Super Artemisinin; NutriCology, Alameda, CA, USA) were also tested.
Cell culture
Rat blood was taken by cardiac puncture of animals under an animal ethics protocol approved by the Animal Ethics Committee, University of Otago, Wellington, New Zealand. Blood was collected in an anticoagulant (ethylenediaminetetraacetic acid) tube, inverted several times, and kept at 18°C–22°C. Polymorphprep™ (Axis-Shield, Oslo, Norway) 2.5 mL was added to each centrifuge tube, overlayered with 7.0 mL whole blood, and centrifuged at 500 g for 30 minutes at 20°C. After centrifugation, the polymorphonuclear fraction was suspended with Hank’s Balanced Salt Solution (HBSS) and centrifuged at 125 g for 5 minutes at 4°C. The supernatant was discarded and the cell pellet was resuspended, washed with HBSS, and centrifuged at 125 g again. The supernatant was discarded and the cell pellet was resuspended in RPMI-1640 medium (Gibco, Auckland, New Zealand). The cell number was counted and the concentration adjusted to 5.0×106 cells/mL with RPMI-1640 medium. The cell suspensions containing isolated neutrophils were kept on ice for up to 10 minutes until used in the assays.
Experimental assays
In the TNF-α assay, A. annua extract was tested at a range of concentrations from 200 μg/mL to 1 μg/mL. The concentrations of A. annua for this dose–response study were selected because preliminary tests (not shown here) indicated that A. annua 400 μg/mL completely inhibited TNF-α production by activated neutrophils. Artemisinin was tested at 200 μg/mL and 100 μg/mL. The positive control, chloroquine, was tested at 5 μM (2.58 μg/mL) and 10 μM (5.16 μg/mL). The investigation into PGE2 production was preliminary, with only three concentrations of A. annua tested: 400 μg/mL, 200 μg/mL, and 100 μg/mL. The concentrations selected for this analysis were arbitrary as, to our knowledge, there have been no previous reports of A. annua modulating the production of PGE2 in activated neutrophils. Artemisinin was tested at 400 μg/mL and 200 μg/mL. The positive control, ibuprofen, which is a COX-2 inhibitor, was tested at 400 μg/mL.
Plant extracts and positive controls were dissolved in 100% ethanol. For each sample of plant extract or positive control, 3 μL was added to a 96-well plate. The ethanol was allowed to dry and 20 μL of HBSS was then added to the test wells. A total of 160 μL of the cell suspension was added to each test well. The plate was incubated in a humidified incubator at 37°C in 95% air and 5% carbon dioxide for 20 minutes. Twenty microliters of lipopolysaccharide (LPS; Sigma-Aldrich Co, St Louis, MO, USA) at 100 μg/mL was added to each well (except the unactivated control cells). The plate was incubated at 37°C in 95% air and 5% carbon dioxide. After 24 hours, the plate was centrifuged at 44 g for 5 minutes. A 50 μL aliquot from each well was transferred to new 96-well plates for either TNF-α or PGE2 determination and stored at −20°C until used. Each sample was assayed in triplicate. As a positive control, triplicate wells with an inhibitor were assayed. As a baseline control, an unsupplemented group was also assessed in triplicate.
Enzyme-linked immunosorbent assay
The enzyme-linked immunosorbent assays (ELISAs) for TNF-α and PGE2 were performed according to the instruction manual provided by the kit manufacturer (R&D Systems, Inc., Minneapolis, MN, USA) and the absorbance read at 450 nm using a VersaMax™ 96-well plate reader.
Statistical analysis
The percentage standard error of the mean (SEM) for each sample was assessed and extreme outliers were removed if the SEM% was greater than 15%. Preliminary statistical significance was assessed with an independent Student’s t-test at α≤0.05 (with and without outliers).
Results
In both assays, the addition of the LPS to the neutrophil cells stimulated them to an inflammatory state.
Inhibition of TNF-α production
For the control cells, the concentration of TNF-α increased 11.89-fold when LPS was included. The positive control, chloroquine, resulted in 23.7% and 42.6% reductions in TNF-α production at 5 μM and 10 μM, respectively.
A. annua extract significantly inhibited TNF-α production by activated neutrophils in a dose-dependent manner (). There was complete inhibition by the extract at 200, 100, and 50 μg/mL (all P≤0.0003). At 25, 10, and 5 μg/mL, A. annua extract inhibited TNF-α production by 89% (P<0.0001), 54% (P=0.0002), and 38% (P=0.0014), respectively. At an A. annua concentration of 1 μg/mL, TNF-α production was not significantly inhibited (8.8%; P>0.05). shows a dose–response curve of the percentage inhibition of TNF-α production. Artemisinin at 200 μg/mL and 100 μg/mL inhibited TNF-α production by 40.7% and 23.2%, respectively. This is less than that seen with the same concentration of the whole A. annua plant extract.
Inhibition of PGE2
In control cells, the concentration of PGE2 increased 4.95-fold compared to unactivated cells. Ibuprofen at 400 μg/mL was a very potent inhibitor of COX-2 activity, with a 91% reduction in PGE2 production. A. annua extract significantly inhibited PGE2 production by activated neutrophils. At concentrations of 400, 200, and 100 μg/mL, A. annua extract significantly inhibited PGE2 production by 87% (P=0.0128), 91% (P=0.0017), and 93% (P=0.0114), respectively. shows the effects of the samples on PGE2 inhibition by activated neutrophils. As in the TNF-α assay, artemisinin significantly inhibited production of PGE2, but was not as potent as the whole A. annua extract at the same concentration; artemisinin 400 and 200 μg/mL inhibited PGE2 production by 65% (P=0.0063) and 57% (P=0.0101), respectively.
Discussion
In this study, the A. annua extract was shown to be a potent inhibitor of TNF-α by activated neutrophils with a clear dose–response effect. There was complete inhibition of TNF-α production at concentrations of 50 μg/mL and above. The extract showed statistically significant inhibition of TNF-α production at all concentrations down to 2.5 μg/mL (24% inhibition).
Artemisinin, a well-established bioactive derived from A. annua, also inhibited the production of TNF-α by activated neutrophil cells in this study. However, the artemisinin was not as potent as the whole extract of the plant. The inhibitory effects of 200 μg/mL and 100 μg/mL artemisinin were 40.7% and 23.2%, respectively, while the equivalent concentrations in the whole plant extract were both 100% inhibitory. These results suggest that artemisinin is a strong inhibitor of TNF-α production but that it is not the only antagonist present in the plant extract. It appears likely therefore, that other components of the A. annua extract also contribute to its anti-inflammatory bioactivity.
Similar results were seen in the PGE2 assay, with a significant inhibitory effect displayed for all concentrations of the extract tested. Again, the inhibitory effects of the compound artemisinin were not as potent as the effect of the whole plant extract. This suggests again that there are other bioactive components in the A. annua extract, as well as artemisinin, that inhibit the COX-2 activity. This was a preliminary investigation of activity at a small number of concentrations of the plant extract. Inhibition of PGE2 was similar for all concentrations of A. annua extract tested. This implies that 400, 200, and 100 μg/mL A. annua extract produced a maximal level of inhibition. Further investigations on dose–response below 100 μg/mL would be needed to find out the potency of A. annua extract at inhibiting PGE2 production.
These results corroborate previous reports suggesting that artemisinin is not the only bioactive compound in A. annua.Citation17,Citation18 A review on traditional A. annua use in malaria suggests that the activity of A. annua extracts cannot be accounted for by their artemisinin content alone.Citation17 Another study suggests that artemisinin may act synergistically with flavonoids and polyphenols also present in A. annua.Citation18 It is not known whether either of these classes of compounds are present in the extract tested in this study. Interestingly, in humans, it appears that the bioavailability of artemisinin is enhanced when the entire plant extract is consumed, compared with consumption of pure artemisinin.Citation19 It is possible that, of the many types of phytochemicals isolated from A. annua (sesquiterpenoids, monoterpenes, triterpenoids, flavonoids, coumarins, phenolics, and lipids), several may be responsible for the overall activity and properties of crude plant A. annua extracts compared to that of pure artemisinin.Citation5,Citation20
While artemisinin may not be responsible for all of the bioactivity in this A. annua extract, it is likely that it is one of the most important compounds in the extract. Dihydroartemisinin, a semi-synthetic analog of artemisinin, has been reported to significantly inhibit LPS-induced release of TNF-α, IL-6, and nitric oxide from mouse mononuclear macrophages.Citation14 Pure artemisinin has been reported to have an anti-inflammatory effect on phorbol myristate acetate–induced THP-1 monocytes.Citation15
The extract of A. annua used in this study seems to have potent bioactivity. This could partly be due to the physical properties of artemisinin, which is poorly water soluble and is heat labile.Citation21 The commercial extract used in this study was produced by supercritical extraction of the plant material with carbon dioxide. This type of extraction allows the processing of plant material at low temperatures, limiting thermal degradation, and avoids the use of toxic solvents such as hexane or methane.Citation22,Citation23
Studies have previously tested extracts of A. annua in vitro, with results reporting a variety of bioactive properties, including protection against oxidative stress,Citation24 and antioxidant,Citation25 anthelminthic,Citation26 and anti-pestCitation27 properties. However, to our knowledge, this is the first report of in vitro anti-inflammatory properties in this interesting plant.
This study has some limitations. While a dose–response effect was established for TNF-α inhibition in activated neutrophils, the number of concentrations of A. annua tested should be increased to establish a dose–response for PGE2. Similarly, artemisinin was only tested at two concentrations in each assay in this study; further studies would be needed to establish a dose–response for artemisinin. This study was conducted only in activated neutrophils; it would be interesting to establish whether the A. annua extract shows similar activity against the production of other pro-inflammatory cytokines in activated macrophages. Toxicity of the plant extract to activated neutrophils was not assessed; further studies should assess any effect of the medicinal plant on cell survival.
Conclusion
In this study in activated neutrophils, an extract of A. annua was shown to be a potent inhibitor of TNF-α and a strong inhibitor of PGE2 production at the concentrations tested. Further studies are needed with this promising plant extract to ascertain whether these in vitro anti-inflammatory effects may translate into in vivo or clinical benefits.
Author contributions
Sheena Hunt designed the study and drafted the manuscript. Mayumi Yoshida and Catherine EJ Davis conducted the experiments and analyzed the data. Nicholas S Greenhill supervised the study and analyzed the data. Paul F Davis designed the study, analyzed the data, and helped draft the manuscript. All authors revised the manuscript for important intellectual content and read and approved the final manuscript.
Acknowledgments
The authors gratefully acknowledge the financial support of a Callaghan Innovation R&D Project Grant.
Disclosure
This study was funded by Promisia Integrative Ltd. Sheena Hunt is an employee of Promisia Integrative Ltd. The other authors report no conflicts of interest in this work.
References
- GautamRJachakSMRecent developments in anti-inflammatory natural productsMed Res Rev200929576782019378317
- FürstRZündorfIPlant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progressMediators Inflamm2014201414683224987194
- GuRWangYLongBProspecting for bioactive constituents from traditional medicinal plants through ethnobotanical approachesBiol Pharm Bull201437690391524882403
- SalimEKumolosasiEJantanIInhibitory effect of selected medicinal plants on the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated human peripheral blood mononuclear cellsJ Nat Med201468364765324799081
- WillcoxMBodekerGBourdyGArtemisia annua as a traditional herbal antimalarialWilcoxMLBodekerGRasoanaivoPAddae-KyeremeJTraditional Medicinal Plants and MalariaBoca Raton, ILCRC Press2004
- GrazioseRLilaMARaskinIMerging traditional Chinese medicine with modern drug discovery technologies to find novel drugs and functional foodsCurr Drug Discov Technol20107121220156139
- BrownGDThe biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao)Molecules201015117603769821030913
- JelinekTArtemisinin based combination therapy in travel medicineTravel Med Infect Dis2013111232823465532
- Olupot-OlupotPMaitlandKManagement of severe malaria: results from recent trialsAdv Exp Med Biol201376424125023654072
- RathoreDMcCutchanTFSullivanMKumarSAntimalarial drugs: current status and new developmentsExpert Opin Investig Drugs2005147871883
- World Health OrganizationWHO informal consultation with manufacturers of artemisinin-based pharmaceutical products in use for the treatment of malariaGeneva, SwitzerlandWorld Health Organization2007 Available from: http://www.who.int/malaria/publications/mtgmanufacturersartemisininderivatives.pdfAccessed August 29, 2014
- O’NeillPMBartonVEWardSAThe molecular mechanism of action of artemisinin – the debate continuesMolecules20101531705172120336009
- KrishnaSBustamanteLHaynesRKStainesHMArtemisinins: their growing importance in medicineTrends Pharmacol Sci2008291052052718752857
- YuWYKanWJYuPXLiMMSongJSZhaoFAnti-inflammatory effect and mechanism of artemisinin and dihydroartemisininZhongguo Zhong Yao Za Zhi2012371726182621 Chinese23236763
- WangYHuangZWangLThe anti-malarial artemisinin inhibits pro-inflammatory cytokines via the NF-κB canonical signaling pathway in PMA-induced THP-1 monocytesInt J Mol Med201127223324121165548
- BellamyNBrooksPMCurrent practice in antimalarial drug prescribing in rheumatoid arthritisJ Rheumatol19861335515553735276
- RasoanaivoPWrightCWWillcoxMLGilbertBWhole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactionsMalar J201110Suppl 1S421411015
- FerreiraJFLuthriaDLSasakiTHeyerickAFlavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancerMolecules20101553135317020657468
- RäthKTaxisKWalzGGleiterCHLiSMHeideLPharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L. (annual wormwood)Am J Trop Med Hyg200470212813214993622
- BhakuniRSJainDCSharmaRPPhytochemistry of Artemisia annuaand the development of artemisinin-derived antimalarial agentsWrightCWArtemisiaLondon, UKTaylor and Francis2002
- CuiLSuXZDiscovery, mechanisms of action and combination therapy of artemisininExpert Rev Anti Infect Ther200978999101319803708
- CapuzzoAMaffeiMEOcchipintiASupercritical fluid extraction of plant flavors and fragrancesMolecules20131867194723823783457
- CoelhoJPCristinoAFMatosPGExtraction of volatile oil from aromatic plants with supercritical carbon dioxide: experiments and modelingMolecules2012179105501057322951395
- KimMHSeoJYLiuKHKimJSProtective effect of Artemisia annua L. extract against galactose-induced oxidative stress in micePLoS One201497e10148624988450
- IqbalSYounasUChanKWZia-Ul-HaqMIsmailMChemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solventsMolecules20121756020603222614857
- CalaACFerreiraJFChagasACAnthelmintic activity of Artemisia annua L. extracts in vitro and the effect of an aqueous extract and artemisinin in sheep naturally infected with gastrointestinal nematodesParasitol Res201411362345235324802864
- ChagasACGeorgettiCSCarvalhoCOIn vitro activity of Artemisia annua L (Asteraceae) extracts against Rhipicephalus (Boophilus) microplusRev Bras Parasitol Vet2011201313521439229