Abstract
Background
In patients polysensitized to pollen allergens, the priming effect, by which the sensitivity of the nasal mucosa to an allergen is increased by the previous exposure to another allergen, is a known phenomenon. This study was aimed at evaluating the degree of nasal inflammation, assessed by nasal cytology, in children with allergic rhinitis (AR) from ragweed pollen according to being monosensitized or polysensitized.
Methods
The study included 47 children. Of them, 24 suffered from AR caused by sensitization to grass pollen and ragweed pollen (group A) and 23 were sensitized only to ragweed pollen (group B). In all patients, the severity of AR was assessed according to the Allergic Rhinitis and Its Impact on Asthma guidelines, and comorbidities were also evaluated.
Results
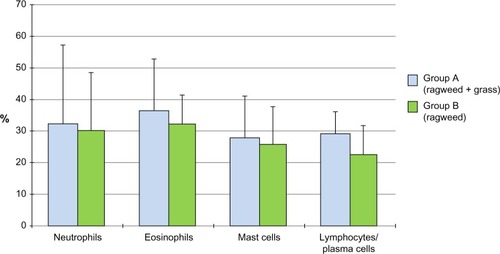
In group A, 16.7% of children had a mild intermittent AR, 4.2% a moderate-to-severe intermittent, 33.3% a mild persistent, and 45.8% a moderate-to-severe persistent; in group B, 26.1% of children had a mild intermittent AR, 0% a moderate-to-severe intermittent, 52.2% a mild persistent, and 21.7% a moderate-to-severe persistent. No significant difference was detected in the number of the considered comorbidities between the two groups. The cell counts of neutrophils, eosinophils, lymphocytes/plasma cells, and mast cells were high but not significantly different in the two groups.
Conclusion
These findings show that the degree of nasal inflammation found in children with ragweed-induced AR is not influenced by additional allergy to grass pollen and confirm the previously reported absence of priming effect in ragweed allergy.
Introduction
Ragweed pollen is a major cause of respiratory allergy. In the pioneering age of allergy in the 19th century, when Blackley discovered that grass pollen was the cause of hay fever, Wyman demonstrated that the so-called autumnal catarrh (hay fever) was due to ragweed pollen.Citation1 Ragweed has optimal characteristics to cause allergy because its pollen is able to induce respiratory allergy starting from concentrations as low as five to ten pollen grains per cubic meter of air and because each plant (as demonstrated for Ambrosia artemisiifolia) can produce up to 60,000 seeds, which can germinate even after lying up to 40 years in the ground.Citation2 For a long time, ragweed allergy was apparently confined to northern America,Citation3 but in the last few decades, a worldwide diffusion of this plant occurred. In Europe, the four species of ragweed, A. artemisiifolia (short ragweed), Ambrosia trifida (giant ragweed), Ambrosia coronopifolia (perennial ragweed), and Ambrosia tenuifolia (slimleaf bur ragweed), were accidentally introduced to Europe, where previously only Ambrosia maritima was known.Citation4 Ragweed allergy was first detected in the Lyon region in France,Citation5 followed by northern Italy, Austria, and Hungary,Citation6 and more recently, Switzerland.Citation7 In Italy, a spreading of this allergy also to central regions was found, mainly caused by long distance transport of pollen.Citation8 These aspects make ragweed allergy an individual and social burden in Italy, which needs effective treatments. In this study, we aimed to evaluate the degree of nasal inflammation, assessed by nasal cytology, in children with allergic rhinitis (AR) from ragweed pollen according to being monosensitized or polysensitized, ie, sensitized also to grass pollen.
Methods
Patients
The population of this study was 47 children. Their demographic data and kind of sensitization are reported in . Oral informed consent was obtained by both parents before entering the study, and the study was conducted in accordance with good clinical practice guidelines. Allergic sensitization was evaluated by skin prick tests performed by allergen extracts from Stallergenes (Antony, France) using a complete panel of inhalant allergens, which included for grasses Dactylis glomerata, Phleum pratense, Anthoxanthum odoratum, Poa pratensis, and Lolium perenne. To be included in the study, the patients must not have any other positive tests in addition to those for grass and ragweed pollen. The symptomatic periods in the area where patients live are from April to June for grass pollen and from late August to early October for ragweed pollen. All patients were allowed to use antihistamines as needed to treat their symptoms, while corticosteroids were not permitted to avoid interference with nasal cytology. The severity of AR was assessed according to the Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines,Citation9 and comorbidities were also evaluated. The local ethical committee, Luigi Sacco Hospital, Milan, Italy, after being informed about the procedures to be carried out gave the authorization to perform the study. Parents or legal guardians gave their written informed consent allowing the inclusion of children in the study.
Table 1 Demographic data and kind of sensitization in the two groups of patients
Nasal cytology
In all study subjects, nasal cytology was performed by anterior rhinoscopy using a nasal speculum and adequate lighting. The cytological sampling consists of collecting the nasal mucosa surface cells by scraping from the middle portion of the inferior turbinate using a Rhino-probe™ (Arlington Scientific, Springville, UT, USA). When the sampling is obtained, the material is placed on a glass slide, fixed by air drying, and stained using the May-Grünwald-Giemsa method, which allows the detection of all the cellular components of the nasal mucosa, including those cells that are associated with the immune inflammation process (such as neutrophils, eosinophils, lymphocytes/plasma cells, and mast cells). The slide is then observed through a light microscope supplied with an object-glass, able to magnify up to ×1,000. For the rhinocytogram analysis, at least 50 microscopic fields have to be read in order to detect all the cells present in the sample.Citation10 The sampling was performed in all subjects in mid-May and mid-September, which are periods corresponding to the peak of symptoms from grass pollen and ragweed pollen, respectively, in Italy. In the same time periods, the total nasal symptom score as reported by Ford et alCitation11 was calculated for each patient by the sum of four individual scores, nasal congestion, nasal itching, rhinorrhea, and sneezing, in which each symptom was scored on a scale of 0–3.
Statistical analysis
The comparison between the two groups of patients for the variables of the continuous type was performed by means of Student’s t-test by calculating the 95% confidence interval. In the case of nonhomogeneity of the variances of the two groups, the nonparametric Mann–Whitney U test was used. The analysis of discrete or nominal parameters was performed using the Fisher’s exact test or the chi-square test. A P-value of at least <0.05 was considered significant.
Results
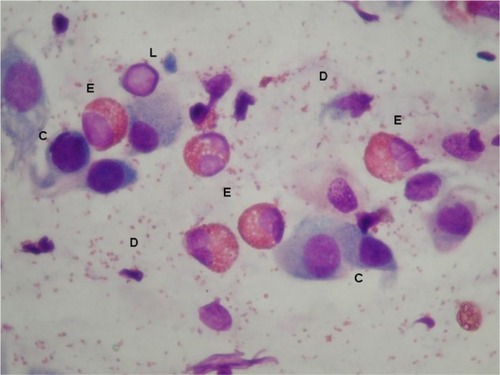
Twenty-four children were allergic to grass pollen and ragweed pollen and formed group A, while 23 were allergic only to ragweed pollen and formed group B (). The distribution of the clinical forms according to ARIA classification was as follows: in group A, four (16.7%) children had a mild intermittent AR, one (4.2%) a moderate-to-severe intermittent, eight (33.3%) a mild persistent, and eleven (45.8%) a moderate-to-severe persistent; in group B, six (26.1%) children had a mild intermittent AR, zero (0%) a moderate-to-severe intermittent, 12 (52.2%) a mild persistent, and five (21.7%) a moderate-to-severe persistent. None of these differences were statistically significant. Asthma was present in 25% of subjects in group A and in 30.4% of subjects in group B. The number of the considered comorbidities in the two groups is reported in . No significant difference was detected. The mean total nasal symptom score was 8.5±4.7 in patients allergic only to ragweed and 8.0±5.2 in patients allergic to both grass and ragweed pollen. The cell counts of neutrophils, eosinophils, lymphocytes/plasma cells, and mast cells, as assessed by nasal cytology, were high but not significantly different in subjects of group A and group B (). shows a typical rhinocytogram obtained in patients with ragweed pollen allergy.
Table 2 Comorbidities in group A and group B
Discussion
Pollen allergy is a very common form of immunoglobulin E (IgE)-mediated hypersensitivity. The process of sensitization takes place when during seasonal exposure the antigenic proteins contained in pollen grains come into contact with antigen-presenting cells, which present them to T and B lymphocytes, with subsequent production of specific antibodies. In atopic subjects, the prevalence of a Th2 cytokine pattern results in an IgE response.Citation12 Then, the Fc portion of IgE antibodies binds to the high-affinity receptors on the surface of mast cells, and when subsequent exposure to the same allergens occurs, the cross-linking of two or more IgE molecules elicits degranulation of mast cells with release of mediators and development of clinical symptoms. This corresponds to the early phase of the allergic reaction, but the ensuing recruitment of inflammatory cells results in a late-phase reaction that sustains the ongoing symptomatology. A phenomenon occurring in the initial phases of pollen allergy is the priming effect, by which the sensitivity of the nasal mucosa makes possible that a much reduced dose of allergen can elicit a full response.Citation13 In patients allergic to grass pollen undergoing a nasal challenge with orchard (D. glomerata) before the pollen season, Bousquet et al showed that subjects polysensitized also to other pollens, such as cypress, olive, and Parietaria, had symptoms occurring earlier than subjects monosensitized to grass pollen, this confirming the priming effect.Citation14 Of interest, in the same years, it was reported that a priming effect was apparently absent in ragweed-allergic patients, as showed by similar symptom scores in early and late seasonal periods of ragweed pollination, but the patients were allergic only to ragweed.Citation15 In this study, we compared the degree of inflammation, as evaluated by nasal cytology, in two groups of children monosensitized to ragweed pollen or sensitized to grass pollen and ragweed pollen, that have different pollination periods, with no other sensitization to inhalant allergens. Nasal cytology is a useful tool to evaluate AR, and a study demonstrated that the ARIA classification of AR severity is associated with different patterns of inflammatory cells, because patients with moderate-to-severe AR have an increased number of mast cells/lymphocytes, while the intermittent or persistent nature of the disease does not influence the cytological pattern.Citation16 We chose to limit the study to children to avoid influence on nasal inflammation by factors other than allergy, particularly cigarette smoking. Concerning the main objective of the study, we found an abundant presence of inflammatory cells, but no significant difference was detected in their number, including neutrophils, eosinophils, mast cells, and lymphocytes/plasma cells, comparing children allergic only to ragweed and allergic to grass pollen and ragweed. The high cell counts in both groups of children may have made it difficult to achieve a statistical significance, but still show that ragweed allergy alone elicits a grade of inflammation similar to double allergy to ragweed and grass pollen. These findings do not disprove the existence of the priming effect in ragweed allergy, but they may suggest that it is not as robust as previously found for other pollens. On the other hand, ragweed pollen contains nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that induces reactive oxygen species in mucosal cells independent of adaptive immunity, facilitating antigen-induced allergic inflammation.Citation17 In the biology of plants, intrinsic pollen NADPH oxidases are required to generate reactive oxygen species and induce pollen-tube growth.Citation18 In addition, the climatic changes associated with the so-called global warming may cooperate, having being reported that ragweed plants grown in CO2-enriched environments produced more allergenic pollens.Citation19 Such factors are likely to result in a higher inflammation induced by ragweed pollen, as confirmed by our data.
Conclusion
The findings from this study suggest that the nasal inflammation in children with ragweed-induced AR is not influenced by additional allergy to grass pollen. This confirm the previously reported absence of priming effect in ragweed allergy. Therefore, ragweed pollen is able to elicit by itself a strong inflammatory response in allergic patients. This suggests to manage this allergy by treatments not limited to symptomatic action but instead addressing the cause of inflammation, such as allergen-specific immunotherapy.
Disclosure
F Frati and S Buttafava are employees of Stallergenes Italia Srl. C Incorvaia is a scientific consultant for Stallergenes Italia Srl. The other authors have no conflicts of interest that are directly relevant to the content of the study.
References
- KayABLandmarks in allergy during the 19th centuryChem Immunol Allergy2014100212624925381
- BassetICromptonCWThe biology of canadian weeds. 11. Ambrosia artemisiifolia L. and A. psilostachya DCCan J Plant Sci197555463476
- GirshLSRagweed pollen distribution in the USA: utilization of graphic mapsAnn Allergy19824923287091785
- TutinTFlora Europaea1–5CambridgeCambridge University Press1964–1980
- TouraineRCornillonJde PoumeyrolBAllergy to Ambrosia pollen in the Lyon regionRev Fr Allergol1965582945828014
- D’AmatoGSpieksmaFTLiccardiGPollen-related allergy in EuropeAllergy1998535675789689338
- TaramarcazPLambeletBClotBKeimerCHauserCRagweed (Ambrosia) progression and its health risk: will Switzerland resist this invasion?Swiss Med Wkly200513553854816333764
- CecchiLMorabitoMDomeneghettiPMCrisciAOnorariMOrlandiniSLong distance transport of ragweed pollen as a potential cause of allergy in central ItalyAnn Allergy Asthma Immunol200696868916440538
- BousquetJvan CauwenbergePKhaltaevNARIA Workshop GroupWorld Health OrganizationAllergic rhinitis and its impact on asthmaJ Allergy Clin Immunol2001108suppl 5147334
- GelardiMIncorvaiaCFiorellaMLItalian Academy of Nasal CytologyThe clinical stage of allergic rhinitis is correlated to inflammation as detected by nasal cytologyInflamm Allergy Drug Targets20111047247621999180
- FordLBMatzJHankinsonTPrillamanBGeorgesGA comparison of fluticasone propionate nasal spray and cetirizine in ragweed fall seasonal allergic rhinitisAllergy Asthma Proc20153631331926108088
- ParikhAScaddingGKSeasonal allergyBMJ1997314129212959158456
- BousquetJVignolaAMCampbellAMMichelFBPathophysiology of allergic rhinitisInt Arch Allergy Immunol19961102072188688666
- BousquetJHejjaouiABeckerWMMichelFBClinical and immunologic reactivity of patients allergic to grass pollens and to multiple pollen species. I. Clinical and immunologic characteristicsJ Allergy Clin Immunol1991877377462005328
- GrammerLWigginsCShaughnessyMAChmielJAbsence of nasal priming as measured by rhinitis symptoms scores of ragweed allergic patients during seasonal exposure to ragweed pollenAllergy Proc1990112432462258045
- GelardiMIncorvaiaCPassalacquaGQuarantaNFratiFThe classification of allergic rhinitis and its cytological correlateAllergy2011661624162522029868
- BoldoghIBacsiAChoudhuryBROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammationJ Clin Invest20051152169217916075057
- DharajiyaNBoldoghICardenasVSurSRole of pollen NAD(P)H oxidase in allergic inflammationCurr Opin Allergy Clin Immunol20088576218188019
- WaynePFosterSConnollyJBazzazFEpsteinPProduction of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheresAnn Allergy Asthma Immunol20028827928211926621