Abstract
Purpose
Reporting guidelines (eg, Consolidated Standards of Reporting Trials [CONSORT] statement) are intended to improve reporting standards and enhance the transparency and reproducibility of research findings. Despite accessibility of such guidelines, researchers are not required to adhere to them. Our goal was to determine the current status of reporting quality in the medical literature and examine whether adherence of reporting guidelines has improved since the inception of reporting guidelines.
Materials and methods
Eight reporting guidelines, such as CONSORT, Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), STrengthening the Reporting of OBservational studies in Epidemiology (STROBE), Quality of Reporting of Meta-analysis (QUOROM), STAndards for Reporting of Diagnostic accuracy (STARD), Animal Research: Reporting In Vivo Experiments (ARRIVE), Consolidated Health Economic Evaluation Reporting Standards (CHEERS), and Meta-analysis of Observational Studies in Epidemiology (MOOSE) were examined. Our inclusion criteria included reviews published between January 1996 to September 2016 which investigated the adherence to reporting guidelines in the literature that addressed clinical trials, systematic reviews, observational studies, meta-analysis, diagnostic accuracy, economic evaluations, and preclinical animal studies that were in English. All reviews were found on Web of Science, Excerpta Medical Database (EMBASE), MEDLINE, and Cumulative Index to Nursing and Allied Health Literature (CINAHL).
Results
Among the general searching of 26,819 studies by using the designed searching method, 124 studies were included post screening. We found that 87.9% of the included studies reported suboptimal adherence to reporting guidelines. Factors associated with poor adherence included non-pharmacological interventions, year of publication, and trials concluding with significant results. Improved adherence was associated with better study designs such as allocation concealment, random sequence, large sample sizes, adequately powered studies, multiple authorships, and being published in journals endorsing guidelines.
Conclusion
We conclude that the level of adherence to reporting guidelines remains suboptimal. Endorsement of reporting guidelines by journals is important and recommended.
Keywords:
Introduction
Medical science is an evolving and dynamic field of research that impacts health care, disease outcomes, and health care systems in general. The evidence generated from millions of medical publications is meant to inform these dynamic changes and therefore has to be presented in a clear, consistent, and transparent fashion. There are more than 26 million citations for biomedical literature in the PubMedCitation1 database alone. To understand and evaluate the evidence presented in these citations, a harmonized method of reporting the research findings is needed to ensure clarity, consistency, and the uptake and dissemination of knowledge.Citation2 Tremendous efforts have been made to provide guidelines for different types of research designs to assist in the process of transparent and clear reporting, eg, Enhancing the QUAlity and Transparency Of health Research (EQUATOR) Network website.Citation3 However, despite the wide availability of such guidelines since the inception of the Consolidated Standards of Reporting Trials (CONSORTCitation4) statement in 1996, the uptake remains suboptimal in the face of the exponential volume of medical literature leaving the readers confused. For example, some studies show positive harmful results from eating red meat on the risk of having colorectal cancer,Citation5 while others are showing inconsistent effect marked by substantial methodological differences, type of red meat investigated, and the population selection limitations.Citation6 Therefore, the reader is unable to decide whether red meat has an effect on bowel cancer risk. Poor reporting without using well-designed guidelines in primary studies may lead to a bias in the treatment effects found in systematic reviews. In addition, poorly conducted systematic reviews may not be able to detect the bias effect that the studies included. In a previous study, we conducted a scoping review and examined the level of adherence to six reporting guidelines and found the level of adherence to be suboptimal in 86% of the included studies.Citation7
The aim of this review was to conduct a systematic review of reviews to update the state of adherence to guidelines since 2012 and to identify factors associated with improved adherence. Our hypothesis was that the reporting standards have improved since our last examination in 2012 given that a longer period has passed after guideline statements were first introduced for researchers and more journals started to endorse the guidelines. Our search was looking at reviews published between January 1, 1996, and September 30, 2016.
Materials and methods
This systematic review was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.Citation8 A protocol for a series of three reviews including the current systematic review has been peer reviewed and published elsewhere.Citation9
Study inclusion and exclusion criteria
Systematic reviews which investigated the adherence to commonly used reporting guidelines in medical literature that addressed clinical trials, systematic reviews, observational studies, meta-analysis, diagnostic accuracy, economic evaluations, and preclinical animal studies that have been reported in English were selected. Eight guidelines included in this review were as follows: CONSORT,Citation4 PRISMA,Citation8 STrengthening the Reporting of OBservational studies in Epidemiology (STROBE),Citation10 Quality of Reporting of Meta-analysis (QUO-ROM),Citation11 STAndards for Reporting of Diagnostic accuracy (STARD),Citation12 Animal Research: Reporting In Vivo Experiments (ARRIVE),Citation13 Consolidated Health Economic Evaluation Reporting Standards (CHEERS),Citation14 and Meta-analysis of Observational Studies in Epidemiology (MOOSE).Citation15
The exclusion criteria included studies that 1) were not systematic reviews; 2) did not explore adherence to the aforementioned reporting guidelines; 3) did not provide data on guideline adherence; 4) were subsets of the included studies; 5) published abstracts, letters, editorials, or commentaries; and 6) reviews in languages other than English for feasibility and resource purposes.
Search strategy
The search strategy was based on the previously published reviewCitation7 and was updated for this systematic review. We searched four databases (Excerpta Medical Database [EMBASE], MEDLINE, Cumulative Index to Nursing, and Allied Health Literature [CINAHL], and Web of Science) from 1996 (CONSORT inception – first created guideline among all eight included guidelines) to September 30, 2016.
We used the following search terms for each of the four databases: (Systematic reviews OR reviews OR quality of reporting OR completeness of reporting) AND (CONSORT OR STROBE OR QUOROM OR PRISMA OR MOOSE OR STARD OR ARRIVE OR CHEERS) OR adherence. Detailed search terms have been reported in the published protocol.Citation9 All stages of search, inclusion, exclusion, and data abstraction were performed independently in duplicate, and agreement was reached through team discussion and consensus.
Outcome measures
The primary outcome was the level of adherence to reporting guidelines and their checklists as reported in the systematic reviews. The secondary outcome included the factors that were associated with improved adherence to guidelines.
Data extraction
A specific data abstraction form was designed to include the following data: 1) general characteristics of the included studies (first author, publication year, country, journal, study field, search time frame, data sources, numbers of included primary studies, and study design), 2) main findings from the included studies, 3) authors’ summaries and conclusions, and 4) factors reported to be related to improved guideline reporting adherence. Each assessment of the systematic reviews was conducted in duplicate. Calibration was performed on the data extraction form. If the pair of evaluators was unable to come to a conclusion, a third-party reviewer would have settled the dispute.
Quality evaluation
We used the modified Assessing the Methodological Quality of Systematic Reviews/Overview of Quality Assessment Questionnaire (Assessment of Multiple Systematic Reviews [AMSTAR]/Overview Quality Assessment Questionnaire [OQAQ]), a 10-item scale,Citation7 to assess the quality of the systematic reviews included in this review. We assigned a number out of a maximum of 20 points for each included study. The higher the number assigned, the better the quality of the systematic review.
Data synthesis
We provided a qualitative summary and characteristics of the included studies. We summarized the factors associated with adherence based on the included study results; no quantitative analysis was possible in this review. We also reported the percentage of studies in which the level of adherence to reporting each guideline was suboptimal. This was calculated by dividing the number of studies with this finding by the total number of studies evaluating the guideline.
Results
Our search resulted in a total of 9,123 publications, of which 124 systematic reviews that included 26,819 primary studies were included in this systematic review of reviews. shows the PRISMA flowchart for the included studies.
Figure 1 PRISMA flow diagram.
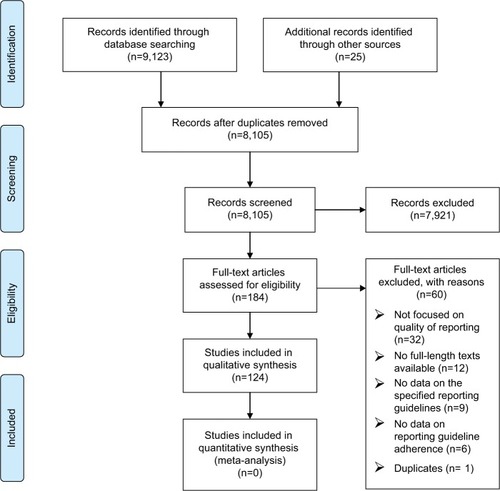
The characteristics of the included studies are described in . The majority of the studies (65% of the total 124 included studies) investigated the adherence to the CONSORT statement as expected since it is the first and oldest guideline. The second most commonly investigated guideline is the PRISMA with 19 studies (15%; ).
Table 1 Characteristics of the included studies
The majority of studies used the guideline checklist to evaluate the level of adherence and generated a mean score as summarized in Table S1. Table S1 summarizes the studies’ findings by guideline with authors’ conclusions for each study. Most studies described the adherence to the different guidelines using the following qualitative descriptors:
deficient, not adequately reported, generally poor, suboptimal, poor, medium, low, poor to moderate, lack of CONSORT adherence, bad, far from satisfactory, lack of standard reporting, improvement over the years has been minor, weak, quality of the articles varied substantially, insufficient, missed reporting some important factors, deficiencies in reporting, inconsistent, needs to be improved, inadequate, there is a need for improvement in quality of reporting, overall adherence is low.
A summary of the quantitative assessment of adherence to guidelines is presented in .
Table 2 Summary of the included studies’ conclusions
The level of adherence to all included reporting guidelines was 87.9% of all guidelines combined showing a need for improvement in reporting. Factors associated with poor adherence to CONSORT guideline included trials with significantly positive results, trials with the categorical outcome, trials conducted in North America compared to Europe, and trials funded by nonindustry source. A summary of factors associated with adherence standards is summarized in . Several factors were associated with better reporting standards relating to authors, study design, outcome specifications, year of publication (recent years of publications are associated with better reporting standards), journal, funding source, and study/author country.
Table 3 Factors associated with reporting quality of articles using the CONSORT guideline
Factors associated with improved adherence to reporting guidelines
Author factors
The included studies reported that the expertise of the author team, for example, an epidemiologist, improved the quality of reporting the study. In addition, having multiple authors also improved reporting quality.
Study factors
Study design with detailed methods including allocation concealment, randomization, specific outcome measures, sample size and power calculations, acknowledgment of limitations and sources of bias, larger sample size, registration of clinical trials, pharmacological interventions, and detailed statistical analysis plan were associated with better reporting and adherence to reporting guidelines. Year of publication was also associated with adherence in which the more recently published articles had increased adherence.
Journal factor
Publications in journals endorsing reporting guidelines have better adherence to these guidelines than articles published in journals that do not endorse such guidelines. In addition, journals’ impact factor, medical journals, and journals with restriction on the number of words per article also had articles with better reporting standards. Publication in a general medical journal was associated with better reporting quality than a specialty journal.
Ethics and funding factors
Articles that reported ethical approval, participants’ consent, and the source of funding were associated with improved adherence to reporting guidelines.
Country of study factors
Geographic location of the study has an impact on the quality of reporting and adherence to reporting guidelines, for example, studies reported from Europe had better reporting standards compared to studies from North America. Studies reported from China had lower adherence to guidelines than elsewhere indicating geographical variations may directly or indirectly impact the level of adherence to reporting guidelines in the medical literature.
Quality assessment of included studies
For each included systematic review, we performed a quality assessment using the modified AMSTAR/OQAQ score. provides the total score out of 20 for each study. The scores varied from 9 to 20. The average score for all the included studies is 16.14. The lowest scores were related to items 5 and 6 of the quality assessment related to the availability of the primary studies’ characteristics similar to a previously reported study.Citation7 Items 5 and 6 were evaluated if there was information on included and excluded studies provided and if the characteristics of included studies provided, respectively.
Table 4 Reporting quality of the 124 included systematic reviews, assessed by the modified AMSTAR/OQAQ (10 items, score out of 20)
Discussion
The medical literature is paramount to the progression of the understanding of health and disease and the establishment of priorities and recommendations for prevention, diagnosis, treatment, and measurement of outcomes. To implement research findings, transparent and consistent reporting standards are needed to help make informed decisions. Such standards have been set by the CONSORT working group and others for the past 2 decades with the aim of improving the reporting standards in biomedical research. It is expected that the introduction of new change to the current practice will take time to adopt and disseminate. However, the uptake of the widely available guidelines has been less than ideal. We define suboptimal and less than ideal as <100%. The whole idea of a systematic review is to have completely transparent methods reported, so everyone can follow and reproduce the results. Inherently, systematic reviews are meant to be a more rigorous study design. This allows them to produce meaningful results than individual studies. Thus, when reviews fail to adhere to reporting guidelines, it calls into question the consistency of their results. Given the weight that systematic reviews have in the scientific community, it is imperative that we hold reviews to a high standard.
Five years ago, we investigated the level of adherence to reporting standards in the medical literature, and we identified 86% of the systematic reviews conducted on the level of adherence to reporting guidelines of the medical literature to be less than ideal.Citation7 Since our previous scoping review, many new revisions and updates to reporting guidelines have been introduced. Currently, there are 358 reporting guidelines on the EQUATOR Network websiteCitation16 for many study types that are freely available. However, endorsement of reporting guidelines by journals still remains low.
Among all the factors that can improve the reporting quality, such as author factors, study factors, journal factors, ethics and funding factors, and country of study factors, author factors as well as their limitations have been studied in other researches. The author factors were the number of the authors of the publication and the level of expertise in the different research methods. Multiple authorships were shown to be an important determinant of the impact of the research being produced and its likelihood of being cited.Citation17 The complexity and cost of medical research today requires multiple levels of expertise in various disciplines as well as accountability and oversight by study team members, institutions, and funding bodies. It is known that the number of authors per article has increased over the past few decadesCitation18,Citation19 with a concern posed to question the roles of multiple authors and the most senior academics holding senior authorship at the expense of others in the team.Citation20 Other studies have reported that the research produced by teams rather than single authors was impactful and more frequently cited, at least in certain fields.Citation21 It is likely that multiple authorships arising from collaborative efforts have advantages of producing good quality impactful research; however, multiple authorships also have limitations and may not be feasible at every setting due to geographical limitations or strict timeline to follow as bringing more authors is time-consuming.Citation22 In this review, we found that having multiple authorships is important to have publications with better adherence to reporting guidelines. However, the role of each author and the hierarchy of authorship should be clarified for successful collaborations and research impact as discussed earlier.
Study factors that improved adherence to reporting guidelines included well-designed, detailed study methods and adequately powered studies. Study results could be altered regarding trial designs, qualities, and methods.Citation23 Therefore, guidelines such as CONSORT statement that is designed for randomized control trials (RCTs), STROBE guideline for observational studies, and PRISMA guideline for systematic reviews were invented accordingly based on different study designs. RCTs are also considered as the highest level of primary evidence in the clinical practice, and therefore it is vital that these trials are reported according to the expected standards.Citation24
Other factors reported that might improve the level of adherence to reporting guidelines included journals endorsing these guidelines. The Internal Committee of Medical Journal Editors (ICMJEs) recognized the importance of reporting guidelines in ensuring study details that are described adequately to be evaluated appropriately and encouraged journals to request these reporting standards from authors.Citation25 The EQUATOR Network has valuable resources and tool kits to assist authors and journal editors to adopt the reporting guidelines and provide case studies of journals endorsing the guidelines. Since journals that endorsed reporting guidelines often ask authors to submit a completed checklist regarding the guidelines, it improves the quality of reporting for those journals endorsing these guidelines. Yet, not all journals currently endorse the guidelines. According to the CONSORT website, there are 585 journals that endorse CONSORT,Citation26 while there are about 30,000 journals indexed in PubMed.Citation27 While not all of these indexed journals publish RCTs, many of them do publish them, but do not adhere to CONSORT guidelines.Citation27
The EQUATOR Network also has tool kits for ethics boards and study sponsors to ensure that the reporting guidelines are considered when these agencies review research submissions for ethical approval or funding requests. It is therefore important that all stakeholders take part in the use and dissemination of the reporting guidelines to enhance the quality of medical research and biomedical literature.
Limitations
The included studies are limited to only eight of the reporting guidelines, and therefore the current study lacks the generalizability to other guidelines that may have a better adherence standard. In addition, there was no comparison between studies to ensure that they are using qualitative descriptors such as “inadequate” or “suboptimal” with the same operational definition. The studies do not provide sufficient information regarding the operationalization of qualitative descriptors to allow us to adequately compare descriptors across studies.
In addition, the study was limited to systematic reviews that present with its own set of limitations. The most notable limitation is the low mean score on the quality assessment since each systematic review follows different reporting guidelines or does not follow guidelines at all and the lack of detailed data on the included studies’ characteristics. Furthermore, a quantitative analysis was not conducted, as not all included studies provided relevant data. Strict inclusion criteria may have allowed a quantitative analysis. However, for the sake of a more representative sample, such criteria were not implemented.
The inclusion of studies in English only is also a limitation to a selected section of the medical literature and did not include other reporting guidelines that may be in use in other languages.
Despite the limited scope of inclusion criteria and quality limitation of the included studies, this review provides an insight into the limited uptake of reporting guidelines and calls for exploring barriers to such uptake. Future studies may include broad surveys of authors, journal editors, funding agencies, ethics boards, and readers to solicit opinions and understanding of the role of reporting guidelines in the medical research and literature.
Conclusion
Current adherence to reporting guidelines in the medical literature is suboptimal. However, there are factors associated with better reporting upon which we can develop strategies for better reporting. Reporting guidelines are an imperative tool in the endeavor to improve the consistency of reporting in the medical literature. However, the suboptimal uptake and correct usage of reporting guidelines demonstrate the need for further emphasis in the scientific community to encourage the use of reporting guidelines. The responsibility for improving the transparency, quality, and reproducibility of medical literature lies with all stakeholders from the research participants to regulatory authorities and everyone in between including authors, readers, educators, funders, academic and health care institutions, editors, peer reviewers, and guideline developers. Future studies may include broad surveys of authors, journal editors, funding agencies, ethics boards, and readers to solicit opinions and understanding of the role of reporting guidelines in the medical research and literature.
Data sharing statement
Unpublished study data are available upon request.
Author contributions
Contributed to the conception and design of the study, development of data extraction forms, search strategy, analysis of results, manuscript writing, and final review of the manuscript: YJ, NS, IS, CL, HS, and GL. Contributed to the methodological design, critical revision, and final review of the manuscript: MB, LZ, BB, MW, LPFA, IN, AL, LM, MM, YC, GS, MAHL, JDA, and LT. Substantially contributed to the conception and design of the study, critical revision, and final approval of the manuscript: ZS. All the authors read and approved the final manuscript. All the authors consented and approved the manuscript for publication. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- NCBI [database on the Internet]PUBMED2017 Available from: https://www.ncbi.nlm.nih.gov/pubmedAccessed June 16, 2018
- SimeraIAltmanDGMoherDSchulzKFHoeyJGuidelines for reporting health research: the EQUATOR network’s survey of guideline authorsPLoS Med200856e13918578566
- AltmanDGSimeraIHoeyJMoherDSchulzKEQUATOR: reporting guidelines for health researchLancet200837196191149115018395566
- SchulzKFAltmanDGMoherDCONSORT GroupCONSORT 2010 statement: updated guidelines for reporting parallel group randomised trialsBMJ2010340c33220332509
- BernsteinAMSongMZhangXProcessed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by timePLoS One2015108e013595926305323
- AlexanderDDWeedDLMillerPEMohamedMARed meat and colorectal cancer: a quantitative update on the state of the epidemiologic scienceJ Am Coll Nutr201534652154325941850
- SamaanZMbuagbawLKosaDA systematic scoping review of adherence to reporting guidelines in health care literatureJ Multidiscip Healthc2013616918823671390
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementBMJ2009339b253519622551
- LiGMbuagbawLSamaanZState of reporting of primary biomedical research: a scoping review protocolBMJ Open20177e014749
- von ElmEAltmanDGEggerMSTROBE InitiativeThe strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studiesLancet200737095961453145718064739
- MoherDCookDJEastwoodSOlkinIRennieDStroupDFImproving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analysesLancet199935491931896190010584742
- BossuytPMReitsmaJBBrunsDEStandards for Reporting of Diagnostic AccuracyThe STARD statement for reporting studies of diagnostic accuracy: explanation and elaborationClin Chem200349171812507954
- KilkennyCBrowneWJCuthillICEmersonMAltmanDGImproving bioscience research reporting: the ARRIVE guidelines for reporting animal researchPLoS Biol201086e100041220613859
- HusereauDDrummondMPetrouSConsolidated health economic evaluation reporting standards (CHEERS) statementCost Eff Res Allocation2013116
- StroupDFBerlinJAMortonSCMeta-analysis of observational studies in epidemiology: a proposal for reportingJAMA2000283152008201210789670
- EQUATOR [home page on the Internet] Available from: http://www.equator-network.orgAccessed June 16, 2018
- ThelwallMSudPNational, disciplinary and temporal variations in the extent to which articles with more authors have more impact: Evidence from a geometric field normalised citation indicatorJ Inf20161014861
- SchrockJBKraeutlerMJMcCartyECTrends in authorship characteristics in the American journal of sports medicine, 1994 to 2014Am J Sports Med20164471857186027159311
- GeminianiAErcoliCFengCCatonJGBibliometrics study on authorship trends in periodontal literature from 1995 to 2010J Periodontol2013855e136e14324215205
- DrenthJHMultiple authorship: the contribution of senior authorsJAMA199828032192219676660
- WuchtySJonesBFUzziBThe increasing dominance of teams in production of knowledgeScience200731658271036103917431139
- BozemanBFayDSladeCPResearch collaboration in universities and academic entrepreneurship: the-state-of-the-artJ Technol Transf2013381167
- MoherDPhamBJonesADoes quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses?Lancet199835291286096139746022
- AtkinsDBestDBrissPAGRADE Working GroupGrading quality of evidence and strength of recommendationsBMJ20043287454149015205295
- ICMJERecommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical JournalsCiteseer20161211710.1080/08941920.2016.1150542
- CONSORT [webpage on the Internet]Consort Endorsers2018 Available from: http://www.consort-statement.org/about-consort/endorsersAccessed June 16, 2018
- NIH [webpage on the Internet]US National Library of Medicine2018 Available from: https://www.nlm.nih.gov/bsd/serfile_addedinfo.htmlAccessed June 16, 2018
- AdieSCONSORT compliance in surgical randomized trials: possible solutionsAnn Surg2013261549324932
- AdieSMaDHarrisIANaylorJMCraigJCQuality of conduct and reporting of meta-analyses of surgical interventionsAnn Surg2015261468569425575252
- AghaRALeeS-YJeongKJLFowlerAJOrgillDPReporting quality of observational studies in plastic surgery needs improvement: a systematic reviewAnn Plast Surg2015765585589
- AghaRAFowlerAJLimbCImpact of the mandatory implementation of reporting guidelines on reporting quality in a surgical journal: a before and after studyInt J Surg20163016917227112835
- AguiarPMBritoGDCorrerCJLyraDPStorpirtisSExploring the quality of systematic reviews on pharmacist interventions in patients with diabetes: an overviewAnn Pharmacother201448788789624692605
- AguiarPMLimaTMStorpirtisSSystematic review of the economic evaluations of novel therapeutic agents in multiple myeloma: what is the reporting quality?J Clin Pharm Ther201641218919727009796
- Al FalehKAl-OmranMReporting and methodologic quality of Cochrane Neonatal review group systematic reviewsBMC Pediatr200993819534780
- Al-NamankanyAAAshleyPMolesDRParekhSAssessment of the quality of reporting of randomized clinical trials in paediatric dentistry journalsInt J Paediatr Dent200919531832419320912
- AlvarezFMeyerNGourraudPAPaulCCONSORT adoption and quality of reporting of randomized controlled trials: a systematic analysis in two dermatology journalsBr J Dermatol200916151159116519681881
- AnttilaHMalmivaaraAKunzRAutti-RämöIMäkeläMQuality of reporting of randomized, controlled trials in cerebral palsyPediatrics200611762222223016740868
- AreiaMSoaresMDinis-RibeiroMQuality reporting of endoscopic diagnostic studies in gastrointestinal journals: where do we stand on the use of the STARD and CONSORT statements?Endoscopy201042213814720140830
- AugestadKMBerntsenGLassenKStudy Group of Research Quality in Medical Informatics and Decision Support (SQUID)Standards for reporting randomized controlled trials in medical informatics: a systematic review of CONSORT adherence in RCTs on clinical decision supportJ Am Med Inf Assoc20121911321
- BalasubramanianSPWienerMAlshameeriZTiruvoipatiRElbourneDReedMWStandards of reporting of randomized controlled trials in general surgery: can we do better?Ann Surg2006244566366717060756
- Bath FJOVEBathPMQuality of full and final publications reporting acute stroke trialsStroke2000291022032210
- BerezaBGMachadoMEinarsonTRAssessing the reporting and scientific quality of meta-analyses of randomized controlled trials of treatments for anxiety disordersAnn Pharmacother2008421402140918728102
- BianZXMoherDDagenaisSImproving the quality of randomized controlled trials in Chinese herbal medicine, part IV: applying a revised CONSORT checklist to measure reporting qualityZhong Xi Yi Jie He Xue Bao20064323324216696907
- Biondi-ZoccaiGLotriontoMAbbateATestaLCompliance with QUOROM and quality of reporting of over-lapping meta-analyses on the role of acetylcysteine in the prevention of contrast associated nephropathyBMJ2006332753519920416428249
- Borg DebonoVZhangSYeCThe quality of reporting of RCTs used within a postoperative pain management meta-analysis, using the CONSORT statementBMC Anesthesiol2012121322762351
- BousquetPJCalderónMADemolyPThe consolidated standards of reporting trials (CONSORT) statement applied to allergen-specific immunotherapy with inhalant allergens: A global allergy and asthma European network (GA2LEN) articleJ Allergy ClinImmunol20111271495656.e1e11
- BramhallMFlorez-VargasOStevensRBrassACruickshankSQuality of methods reporting in animal models of colitisInflamm Bowel Dis20152161248125925989337
- CairoFSanzIMatesanPNieriMPagliaroUQuality of reporting of randomized control trials to implant in dentistryJ Clin Periodontol20123920220622533957
- CapiliBAnastasiJKGeigerJNAdverse event reporting in acupuncture clinical trials focusing on painClin J Pain2010261434820026952
- CavadasVBrancoFCarvalhoFLOsórioLGomesMJSilva-RamosMThe quality of reporting of randomized controlled trials in pelvic organ prolapseInt Urogynecol J20112291117112521484364
- ChoiJJunJHKangBKKimKHLeeMSEndorsement for improving the quality of reports on randomized controlled trials of traditional medicine journals in Korea: a systematic reviewTrials20141542925373427
- ChowersMYGottesmanBSLeiboviciLPielmeierUAndreassenSPaulMReporting of adverse events in randomized controlled trials of highly active antiretroviral therapy: systematic reviewJ Antimicrob Chemother200964223925019477890
- CookDALevinsonAJGarsideSMethod and reporting quality in health professions education research: a systematic reviewMed Educ201145322723821299598
- DaitchVBabichTSingerPLeiboviciLQuality of reporting nutritional randomized controlled trials in patients with cystic fibrosisJ Pediatr Gastroenterol Nutr201663226526926881412
- DasiFNavarro-GarcíaMMJiménez-HerediaMEvaluation of the quality of publications on randomized clinical trials using the consolidated standards of reporting trials (CONSORT) statement guidelines in a Spanish tertiary hospitalJ Clin Pharmacol20125271106111421593281
- DelaneyMMeyerECserti-GazdewichCA systematic assessment of the quality of reporting for platelet transfusion studiesTransfusion201050102135214420497518
- DeMauroSBGiacconeAKirpalaniHSchmidtBQuality of reporting of neonatal and infant trials in high-impact journalsPediatrics20111283e639e64421859916
- de VriesTWvan RoonENLow quality of reporting adverse drug reactions in paediatric randomised controlled trialsArch Dis Child201095121023102620551194
- DiasSMcNameeRVailAEvidence of improving quality of reporting of randomized controlled trials in subfertilityHum Reprod200621102617262716793995
- EthgenMBoutronLStegPGRoyCRavaudPQuality of reporting internal and external validity data from randomized controlled trials evaluating stents for percutaneous coronary interventionBMC Med Res Methodol20099242419358717
- EyawoOLeeC-WRachlisBMillsEJReporting of noninferiority and equivalence randomized trials for major prostaglandins: a systematic survey of the ophthalmology literatureTrials20089696919055743
- FanF-FXuQSunQZhaoSJWangPGuoXRAssessment of the reporting quality of randomized controlled trials on treatment of coronary heart disease with traditional Chinese medicine from the Chinese journal of integrated traditional and Western medicine: a systematic reviewPLoS One201491e8636024489719
- FarrokhyarFChuRWhitlockRThabaneLA systematic review of the quality of publications reporting coronary artery bypass grafting trialsCan J Surg200750426627717897515
- FidalgoBMRCrabbDPLawrensonJGMethodology and reporting of diagnostic accuracy studies of automated perimetry in glaucoma: evaluation using a standardised approachOphthalmic Physiol Opt201535331532325913874
- FlemingPSSeehraJPolychronopoulouAFedorowiczZPandisNA PRISMA assessment of the reporting quality of systematic reviews in orthodonticsAngle Orthod201383115816322720835
- FontelaPSPant PaiNSchillerIDendukuriNRamsayAPaiMQuality and reporting of diagnostic accuracy studies in TB, HIV and malaria: evaluation using QUADAS and STARD standardsPLoS One2009411e775319915664
- FreemanKSzczepuraAOsipenkoLNon-invasive fetal RHD genotyping tests: a systematic review of the quality of reporting of diagnostic accuracy in published studiesEur J Obstet Gynecol Reprod Biol20091422919819081172
- FroudREldridgeSDiaz OrdazKMarinhoVCCDonnerAQuality of cluster randomized controlled trials in oral health: a systematic review of reports published between 2005 and 2009Community Dent Oral Epidemiol201240suppl 131422369703
- FungAEPalankiRBakriSJDepperschmidtEGibsonAApplying the CONSORT and STROBE statements to evaluate the reporting quality of neovascular age-related macular degeneration studiesOphthalmology2009116228629619091408
- GagnierJJDeMeloJBoonHRochonPBombardierCQuality of reporting of randomized controlled trials of herbal medicine interventionsAm J Med20061199800.e1e11
- GaoJDengGHuYQuality of reporting on randomized controlled trials on recurrent spontaneous abortion in ChinaTrials20151617217225896786
- GianolaSGaspariniMAgostiniMSurvey of the reporting characteristics of systematic reviews in rehabilitationPhys Ther201393111456146623744458
- GohariFBaradaranHRTabatabaeeMQuality of reporting randomized controlled trials (RCTs) in diabetes in Iran; a systematic reviewJ Diabetes Metab Disord20151513627610356
- GulinJENRoccoDMGarcía-BournissenFQuality of reporting and adherence to ARRIVE guidelines in animal studies for Chagas disease preclinical drug research: a systematic reviewPLoS Negl Trop Dis2015911e000419426587586
- HalpernSHDaraniRDouglasMJWightWYeeJCompliance with the CONSORT checklist in obstetric anaesthesia randomised controlled trialsInt J Obstet Anesth200413420721415477048
- HemelsMEHVicenteCSadriHMassonMJEinarsonTRQuality assessment of metaanalyses of RCTs of pharmacotherapy in major depressive disorderCurr Med Res Opin200420447748415119985
- HerdanARothRGrassDImprovement of quality of reporting in randomised controlled trials to prevent hypotension after spinal anaesthesia for caesarean sectionGynecol Surg2011812112721654900
- HuangDJinXGaoJQuality evaluation of randomized controlled trials reports of laparoscopy compared with open colorectal resection for colorectal cancerExpert Rev Anticancer Ther201515672773226004141
- HuiDArthurJDalalSBrueraEQuality of the supportive and palliative oncology literature: A focused analysis on randomized controlled trialsSupport Care Cancer20122081779178521935717
- JunhuaZHongcaiSXiumeiGMethodology and reporting quality of systematic review/meta-analysis of traditional Chinese medicineJ Altern Complement Med200713879780517983335
- KarpouzisFBonelloRQuality of reporting of randomised controlled trials in chiropractic using the CONSORT checklistMan Ther20162419
- KiehnaENStarkeRMPouratianNDumontASStandards for reporting randomized controlled trials in neurosurgeryJ Neurosurg2010114228028521054137
- KimKHKangJWLeeMSLeeJ-DAssessment of the quality of reporting in randomised controlled trials of acupuncture in the Korean literature using the CONSORT statement and STRICTA guidelinesBMJ Open201447e005068
- KoberTTrelleSEngertAReporting of randomized controlled trials in Hodgkin lymphoma in biomedical journalsJ Natl Cancer Inst200698962062516670387
- LaddBOMcCradyBSManuelJKCampbellWImproving the quality of reporting alcohol outcome studies: effects of the CONSORT statementAddict Behav20103566066620207490
- LeeS-YTeohPJCammCFAghaRACompliance of randomized controlled trials in trauma surgery with the CONSORT statementJ Trauma Acute Care Surg201375456257224064867
- LeeSYSagooHWhitehurstKCompliance of systematic reviews in plastic surgery with the PRISMA statementJAMA Facial Plast Surg201618210110526719993
- LiJYZhangYFSmithGSQuality of reporting of randomized clinical trials in tai chi interventions-a systematic reviewEvid Based Complement Alternat Med2011201138324519351709
- LiJ-LGeLMaJCQuality of reporting of systematic reviews published in “evidence-based” Chinese journalsSyst Rev20143585824906805
- LiJLiuZChenRThe quality of reports of randomized clinical trials on traditional Chinese medicine treatments: a systematic review of articles indexed in the China National Knowledge Infrastructure database from 2005 to 2012BMC Complement Altern Med20141436236225256890
- LiuDJinJTianJYangKQuality assessment and factor analysis of systematic reviews and meta-analyses of endoscopic ultrasound diagnosisPLoS One201510113
- LiuLQMorrisPJPengelLHMCompliance to the CONSORT statement of randomized controlled trials in solid organ transplantation: a 3-year overviewTranspl Int20132630030623279054
- LiuXTZhangXWenSPengLHongQKangDImpact of the Consolidated Standards of Reporting Trials (CONSORT) checklist on reporting of randomized clinical trials in traditional Chinese medicineJ Evid Based Med2015819220826334556
- LiuYZhangRHuangJReporting quality of systematic reviews/meta-analyses of acupuncturePLoS One2014911e11317225397774
- LiuYZhaoXMaiYAdherence to ARRIVE guidelines in Chinese journal reports on neoplasms in animalsPLoS One201611e015465727182788
- LuJGaryKWCopolilloAWardJNiemeierJPLapaneKLRandomized controlled trials in adult traumatic brain injury: a review of compliance to CONSORT statementArch Phys Med Rehabil20159670271425497515
- LuLZengJChenYQuality of reporting in randomized controlled trials conducted in China on the treatment of cancer painExpert Rev Anticancer Ther20111187187721707284
- MaBGuoJQiGEpidemiology, quality and reporting characteristics of systematic reviews of traditional Chinese medicine interventions published in Chinese journalsPLoS One20116e2018521633698
- MaBQiGQLinXTWangTChenZMYangKHEpidemiology, quality, and reporting characteristics of systematic reviews of acupuncture interventions published in Chinese journalsJ Altern Complement Med20121881381722924413
- MarshmanZFaridFThe quality of reporting of randomised controlled trials in dental public healthCommunity Dent Health20102725325621473363
- McCormickFCvetanovichGLKimJMAn assessment of the quality of rotator cuff randomized controlled trials: utilizing the Jadad score and CONSORT criteriaJ Shoulder Elbow Surg2013221180118523510746
- MillerERoposchAUlerykEDoriaASJuvenile idiopathic arthritis of peripheral joints. Quality of reporting of diagnostic accuracy of conventional MRI1Acad Radiol20091673975719427982
- Moberg-MogrenENelsonDLEvaluating the quality of reporting occupational therapy randomized controlled trials by expanding the CONSORT criteriaAm J Occup Ther20066022623516596926
- MoherDSampsonMCampbellKAssessing the quality of reports of randomized trials in pediatric complementary and alternative medicineBMC Pediatr2002122021
- MontanéEVallanoAVidalXAguileraCLaporteJ-RReporting randomised clinical trials of analgesics after traumatic or orthopaedic surgery is inadequate: a systematic reviewBMC Clin Pharmacol2010102220067642
- MontgomeryAAAstinMPPetersTJReporting of factorial trials of complex interventions in community settings: a systematic reviewTrials20111217921771302
- NicolauILingDTianLLienhardtCPaiMMethodological and reporting quality of systematic reviews on tuberculosisInt J Tuberc Lung Dis2013171160116923809432
- Norton-MabusJCNelsonDLReporting of randomized controlled trials in occupational therapy and speech therapy: evaluation using an expansion of the consort statementOccup Particip Health2008286471
- NtalaCBirmpiliPWorthAAndersonNHSheikhAThe quality of reporting of randomised controlled trials in asthma: a systematic reviewPrim Care Respir J20132241742424248328
- PanicNLeonciniEDe BelvisGRicciardiWBocciaSEvaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analysesPLoS One2013812e8313824386151
- ParsonsNRHiskensRPriceCLAchtenJCostaMLA systematic survey of the quality of research reporting in general orthopaedic journalsJ Bone Joint Surg20119311541159
- PatelMXCollinsSHellierJBhatiaGMurrayRMThe quality of reporting of phase II and III trials for new antipsychotics: a systematic reviewPsychol Med201445346747925065545
- PiggottMMcGeeHFeuerDHas CONSORT improved the reporting of randomized controlled trials in the palliative care literature? A systematic reviewPalliat Med200418323814982205
- PéronJPondGRGanHKQuality of reporting of modern randomized controlled trials in medical oncology: a systematic reviewJ Natl Cancer Inst201210498298922761273
- PetersJPMHooftLGrolmanWStegemanIReporting quality of systematic reviews and meta-analyses of otorhinolaryngologic articles based on the PRISMA statementPLoS One201510111
- PlintACMoherDMorrisonADoes the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic reviewMed J Aust200618526326716948622
- PradySLRichmondSJMortonVMMacPhersonHA systematic evaluation of the impact of STRICTA and CONSORT recommendations on quality of reporting for acupuncture trialsPLoS One200832e157718270568
- PratoomsootCSruamsiriRDilokthornsakulPChaiyakunaprukNQuality of reporting of randomised controlled trials of herbal interventions in ASEAN plus six countries: a systematic reviewPLoS One2015101e10868125633206
- RaoABrückKMethvenSQuality of reporting and study design of CKD cohort studies assessing mortality in the elderly before and after STROBE: a systematic reviewPLoS One201611116
- RiceDBKlodaLAShrierIThombsBDReporting completeness and transparency of meta-analyses of depression screening tool accuracy: a comparison of meta-analyses published before and after the PRISMA statementJ Psychosom Res201687576927411753
- RiosLPOdueyungboAMoitriMORahmanMOThabaneLQuality of reporting of randomized controlled trials in general endocrinology literatureJ Clin Endocrinol Metab2008933810381618583463
- RikosDDardiotisETsivgoulisGZintzarasEHadjigeorgiouGMReporting quality of randomized-controlled trials in multiple sclerosis from 2000 to 2015, based on CONSORT statementMult Scler Relat Disord2016913513927645361
- SchwarzFIglhautGBeckerJQuality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositisClin Periodontol201239suppl 126372
- ScottPOttFEggerMLowNCompleteness of reporting in randomized controlled trials of 3 vaccinesPediatr Infect Dis J2012311286129422935870
- ShawyerACPembertonJKantersDAlnaqiAAAFlageoleHQuality of reporting of the literature on gastrointestinal reflux after repair of esophageal atresia-tracheoesophageal fistulaJ Pediatr Surg2015501099110325783329
- SheaBBoersMGrimshawJMHamelCBouterLMDoes updating improve the methodological and reporting quality of systematic reviews?BMC Med Res Methodol200661716412232
- SheaBBouterLMGrimshawJMScope for improvement in the quality of reporting of systematic reviews. From the Cochrane Musculoskeletal GroupJ Rheumatol20063391516267878
- StevelyADimairoMToddSAn investigation of the shortcomings of the CONSORT 2010 statement for the reporting of group sequential randomised controlled trials: a methodological systematic reviewPLoS One201510120
- StrechDSoltmannBWeikertBBauerMPfennigAQuality of reporting of randomized controlled trials of pharmacologic treatment of bipolar disorders: a systematic reviewJ Clin Psychiatry2011721214122121294992
- TanWKWigleyJShantikumarSThe reporting quality of systematic reviews and meta-analyses in vascular surgery needs improvement: a systematic reviewInt J Surg2014121262126525448643
- ThabaneLChuRCuddyKDouketisJWhat is the quality of reporting in weight loss intervention studies? A systematic review of randomized controlled trialsInt J Obes20073115541559
- TunisASMcInnesMDFHannaREsmailKAssociation of study quality with completeness of reporting: have completeness of reporting and quality of systematic reviews and meta-analyses in major radiology journals changed since publication of the PRISMA statement?Radiology201326941342623824992
- TurnerLShamseerLAltmanDGConsolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journalsCochrane Database Syst Rev201211MR00003023152285
- Vigna-TagliantiFVineisPLiberatiAFaggianoFQuality of systematic reviews used in guidelines for oncology practiceAnn Oncol20061769170116461333
- WalleserSHillSRBeroLACharacteristics and quality of reporting of cluster randomized trials in children: reporting needs improvementJ Clin Epidemiol2011641331134021775103
- WangGMaoBXiongZYThe quality of reporting of randomized controlled trials of traditional Chinese medicine: a survey of 13 randomly selected journals from mainland ChinaClin Ther2007291456146717825697
- WangPXuQSunQFanFFGuoXRGuoFAssessment of the reporting quality of randomized controlled trials on the treatment of diabetes mellitus with traditional Chinese medicine: a systematic reviewPLoS One201387e7058623894675
- WanggeGKlungelOHRoesKCde BoerAHoesAWKnolMJRoom for improvement in conducting and reporting non-inferiority randomized controlled trials on drugs: a systematic reviewPLoS One2010510e1355021048948
- WeingärtnerVDargatzNWeberCPatient reported outcomes in randomized controlled cancer trials in advanced disease: a structured literature reviewExpert Rev Clin Pharmacol2016982182926959869
- WeirCRStaggersNLaukertTReviewing the impact of computerized provider order entry on clinical outcomes: the quality of systematic reviewsInt J Med Inform201281421923122342868
- WenJRenYWangLThe reporting quality of meta-analyses improves: a random sampling studyJ Clin Epidemiol20086177077518411041
- WillisBQuigleyMThe assessment of the quality of reporting of meta-analyses in diagnostic research: a systematic reviewBMC Med Res Methodol20111116316322151233
- YaoACKhajuriaACammCFEdisonEAghaRThe reporting quality of parallel randomised controlled trials in ophthalmic surgery in 2011: a systematic reviewEye2014281341134925214001
- ZafarAKhanGISiddiquiMAThe quality of reporting of diagnostic accuracy studies in diabetic retinopathy screening: a systematic reviewClin Experiment Ophthalmol200836653754218954316
- ZhangZ-WEpidemiology, quality, and reporting characteristics of systematic reviews and meta-analyses of observational studies published in Chinese journalsBMJ Open201563446455
- ZhaoXZhenZGuoJAssessment of the reporting quality of placebo-controlled randomized trials on the treatment of type 2 diabetes with traditional Chinese medicine in mainland China: a PRISMA-compliant systematic reviewMedicine2016953e252226817893
- ZhengSLChanFTMacleanEJayakumarSNabeebaccusAAReporting trends of randomised controlled trials in heart failure with preserved ejection fraction: a systematic reviewOpen Heart201632e00044927547434
- ZhongYZhouWJiangHQuality of reporting of two-group parallel randomized controlled clinical trials of multi-herb formulae: a survey of reports indexed in the Science Citation Index ExpandedEur J Integr Med201134e309e316
- ZintzarasEKitsiosGDPapathanasiouAARandomized trials of dopamine agonists in restless legs syndrome: a systematic review, quality assessment, and meta-analysisClin Ther201032222123720206780
- ZintzarasEPapathanasiouAAZiogasDCVoulgarelisMThe reporting quality of studies investigating the diagnostic accuracy of anti-CCP antibody in rheumatoid arthritis and its impact on diagnostic estimatesBMC Musculoskelet Disord20121311322730931
- ZiogasDCZintzarasEAnalysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statementAnn Epidemiol200919749450019523596
