Abstract
Dementia is a clinical syndrome of widespread progressive deterioration of cognitive abilities and normal daily functioning. These cognitive and behavioral impairments pose considerable challenges to individuals with dementia, along with their family members and caregivers. Four primary dementia classifications have been defined according to clinical and research criteria: 1) Alzheimer’s disease; 2) vascular dementias; 3) frontotemporal dementias; and 4) dementia with Lewy bodies/Parkinson’s disease dementia. The cumulative efforts of multidisciplinary healthcare teams have advanced our understanding of dementia beyond basic descriptions, towards a more complete elucidation of risk factors, clinical symptoms, and neuropathological correlates. The characterization of disease subtypes has facilitated targeted management strategies, advanced treatments, and symptomatic care for individuals affected by dementia. This review briefly summarizes the current state of knowledge and directions of dementia research and clinical practice. We provide a description of the risk factors, clinical presentation, and differential diagnosis of dementia. A summary of multidisciplinary team approaches to dementia care is outlined, including management strategies for the treatment of cognitive impairments, functional deficits, and behavioral and psychological symptoms of dementia. The needs of individuals with dementia are extensive, often requiring care beyond traditional bounds of medical practice, including pharmacologic and non-pharmacologic management interventions. Finally, advanced research on the early prodromal phase of dementia is reviewed, with a focus on change-point models, trajectories of cognitive change, and threshold models of pathological burden. Future research goals are outlined, with a call to action for social policy initiatives that promote preventive lifestyle behaviors, and healthcare programs that will support the growing number of individuals affected by dementia.
Introduction
The clinical syndrome of dementia as a progressive deterioration of cognitive abilities and functional impairments has been recognized for over a century; however it has only been understood within the context of its current characterization in the past few decades. In the early 20th century, a shift in the conceptualization of mental illness away from causation towards disease etiology occurred, with dementia being conceived as a direct effect of age-related processes. Alzheimer’s disease (AD) as a progressive neurodegenerative disorder did not emerge as a distinct entity until the mid-20th century, which led to the recognition of multiple disease states with unique etiologies. Contemporary dementia research has progressed rapidly on multiple fronts, including epidemiology, pathology, diagnosis, and treatment. As a result, four primary dementia groupings have been defined according to clinical and research criteria: 1) Alzheimer’s disease (AD; including mixed-AD); 2) vascular dementias (VaD; including large and small vessel disease); 3) frontotemporal dementias (FTD; including Pick’s disease, progressive nonfluent aphasia, and semantic dementia); and 4) dementia with Lewy bodies (DLB; including Parkinson’s disease dementia [PDD]). The cumulative efforts of multidisciplinary research teams across the world have advanced our understanding of dementia beyond basic descriptions of clinical symptoms and neuropathological correlates, towards a more complete elucidation of risk factors and underlying pathobiological disease mechanisms and, importantly, have led to targeted treatments, management, and care.
The needs of individuals affected by dementia are extensive, and the management of the clinical syndrome is complex, extending beyond traditional bounds of medical practice. Whereas medicine has historically focused on the effects of disease on the patient, dementia care requires a broader focus extending to family members, caregivers, and support networks. This broad-based approach to the care and management of dementia involves consideration of the quality of life for affected individuals to a greater extent than many other disease states. Dementia is characterized by significant impairments in multiple cognitive domains, functioning, and behavior, and places a tremendous burden not only on individuals, but also on society. Early detection and management may prevent overuse of costly healthcare resources and allow affected individuals and caregivers time to prepare for future medical, financial, and emotional challenges. Many of the most effective management approaches are nonpharmacologic, and provided by healthcare professionals from multiple disciplines. We are now entering an era of dementia care that will be based upon the identification of early disease markers and potentially modifiable risk factors, along with the application of novel diagnostic tools, treatment modalities, and care practices.
In this review, we briefly summarize the current state of knowledge and directions of research for dementia epidemiology, its clinical presentation, multidisciplinary approaches to care, treatment options, management challenges, and future research goals.
Population aging and dementia epidemiology
Population aging and increased life expectancy has become a worldwide epidemiological phenomenon. Global projections for the number of older individuals (≥65 years) are expected to increase from 420 million in 2000, to nearly 1 billion by 2030, with a corresponding increase in the proportion of older individuals rising from 7% to 12%.Citation1,Citation2 Developing countries will see the greatest rise in absolute numbers of older individuals, and will contribute to worldwide population aging with an increase from 59% to 71%.Citation3 Dementia is strongly associated with increasing age, and consequently neurodegenerative disorders are anticipated to pose significant challenges to public healthcare systems worldwide in the coming decades.
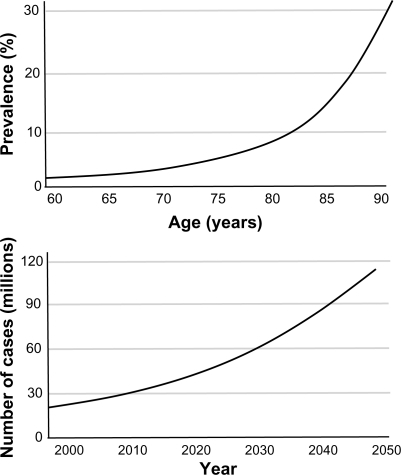
The prevalence of dementia is also rising, particularly AD, which is the most common form of dementia accounting for approximately 60% of all cases.Citation4,Citation5 Worldwide, the global prevalence of dementia is estimated to be 3.9% in individuals over the age of 60 years, with regional prevalence rates of 1.6% in Africa, 4.0% in China and Western Pacific Regions, 4.6% in Latin America, 5.4% in Western Europe, and 6.4% in North America.Citation3 Greater than 25 million people in the world are currently affected by dementia syndromes, with most individuals suffering from AD and vascular dementia (VaD) ().Citation4–Citation6 Approximately 5 million new cases are estimated to occur each year, and the number of individuals with dementia is expected to double every 20 years ().Citation2,Citation3,Citation6
Figure 1 Worldwide subtypes of late onset dementia (≥65 years).
Copyright © 2007, Elsevier Inc. Adapted with permission from Brookmeyer et al.Citation6
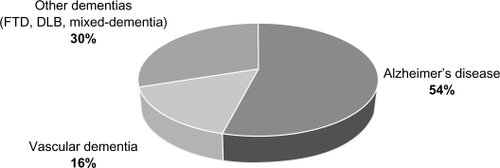
Figure 2 Global prevalence of Alzheimer’s disease as a function of age and total number of worldwide cases per year.
Copyright © 2006, Lancet Publishing Group. Adapted with permission from Ferri et al.Citation3
Risk factors, clinical presentation, and diagnosis of dementia
The clinical syndrome of dementia generally follows three primary expressions.Citation7–Citation10 First, a neuropsychological component consisting of a range of cognitive impairments, often including: memory impairments (declarative and procedural memory problems), aphasias (either receptive or expressive language difficulties), apraxias (inability to carry out directed coordination of movements despite intact sensory and motor nervous systems), agnosias (inability to recognize specific elements of an individual’s environment or self), attentional difficulties (including sustained and divided attention), and executive functioning impairments (including difficulties with abstraction, cognitive flexibility, inhibition, planning, organizing, and adaptation to novel stimuli). The second primary expression of this syndrome is a neuropsychiatric element with associated symptoms and behavioral disturbances. These psychiatric features are present in a substantial proportion of affected individuals, and are commonly referred to as behavioral and psychological symptoms of dementia (BPSD). Common BPSD disturbances include depression, paranoid ideation, delusions, hallucinations, aggression, and wandering.Citation11 The third primary clinical expression includes deficits in activities of daily living (ADL).Citation8 In the early stages of dementia, impaired instrumental activities of daily living (IADL) may be manifest including self-neglect of diet, personal hygiene, and common household tasks. Towards the later stages of dementia, basic activities of daily living (BADL) are often impaired, presenting with obvious problems in dressing, eating, and bathing.Citation12,Citation13
This triad of features is common to most dementias, with the differentiation of subtypes based on clinical presentation, the presence of comorbid symptoms, and other aspects of the individual’s history and examination.Citation7,Citation8,Citation14 Individuals with dementia tend to present to specialist healthcare services (eg, neurologist, neuropsychologist, memory disorders clinic) only when symptoms begin to interfere with everyday activities and functioning. Depending upon the stage of disease progression, the patient is often unable to provide an accurate history and may deny any presence of impairments. Therefore, a detailed history from a reliable primary informant (eg, family, caregiver) is essential to provide collateral information of previous baseline functioning, symptom onset, and supportive evidence of changes in cognitive and behavioral functioning.Citation15 Depending on the dementia subtype, there is wide variability in rates of decline from person to person and in the rapidity with which the disease process develops. Nevertheless, all dementias are degenerative and progressive. Early detection and the differential diagnosis of dementia subtype, disease complexity, and sequelae requires skilled clinical judgment and is based on multiple sources of information (see ).Citation16
Figure 3 Differential diagnostic considerations for dementia.
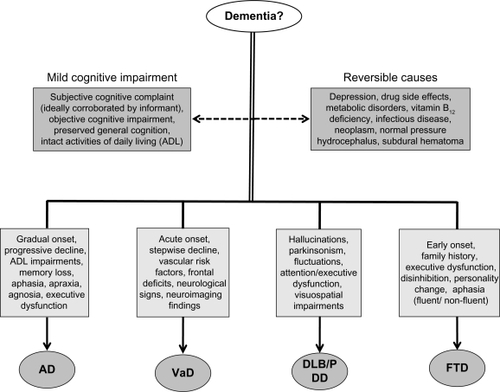
Mild cognitive impairment
Clinical AD is often preceded by a prodromal phase commonly referred to as mild cognitive impairment (MCI).Citation17 Despite several operational definitions, a diagnosis of MCI is generally applied to individuals with a primary complaint and objective demonstration of cognitive impairments in one or more domains (usually memory difficulties), but with relatively preserved ADL.Citation9,Citation17 Debate continues regarding the most useful way to operationalize the clinical and research criteria for a diagnosis of MCI.Citation18–Citation24 Most MCI classifications require a subjective memory complaint, informant corroboration of memory difficulties, relatively intact ADL, and normal or mild problems in nonmemory cognitive functioning.Citation9 More stringent classifications specify unique cutoff points for the severity of memory impairment on standardized neuropsychological tests (eg, performance <1.5 SD below the mean).Citation25 The clinical utility of MCI as a diagnostic classification is evidenced by estimates of increased risk for conversion to AD.Citation24,Citation26,Citation27 While there are considerable individual differences in trajectories of change, in general, individuals with MCI progress to AD at a rate of 10% to 25% per year, compared with healthy elderly controls who progress at a rate of approximately 1% to 2% per year.Citation9,Citation18,Citation24,Citation28
Individuals with MCI tend to demonstrate impaired performance on neuropsychological tests of memory.Citation9 This is in part a reflection of diagnostic criteria such that individuals with mild impairment in other cognitive domains may be missed. The pattern of memory decline observed in MCI appears to reflect a degradation of information in memory storage and retrieval, rather than an impairment of information encoding.Citation25 The storage deficit is demonstrated in the loss of information over time, with minimal or no improvement in performance when assessed using tests of recognition. This pattern of decline is generally consistent with that observed for individuals with AD, and is thought to be evidence that MCI is a preclinical stage of AD.
Recent research has revealed early changes associated with MCI including difficulties with executive functioning and increased apathy.Citation9,Citation29–Citation31 Individuals with MCI have also been found to have difficulties dealing with financial matters, including problems related to cash management and bill payment.Citation32 Given the overall high level of functioning, individuals with these difficulties tend to remain undetected, thereby making them more vulnerable for potential future decline.Citation29,Citation31 Therefore, sensitive measures of memory, executive functioning, and personality shifts may help to improve prediction of conversion from MCI to more severe forms of dementia.Citation9,Citation29,Citation31
Alzheimer’s disease
Risk factors
AD is the most common form of dementia, accounting for an estimated two-thirds of dementia cases in individuals aged 65 years or older.Citation4,Citation5 Age is the most consistent risk factor for AD, presumably due to lifelong exposure to various forms of neural damage, including minor vascular events, white matter disease, and inflammation.Citation5,Citation7,Citation10,Citation18 Other potential risk factors include: level of education (higher education potentially conferring increased brain and cognitive reserve), gender (increased risk for female sex), family history of dementia (particularly for first degree relatives), genetic factors (familial forms of AD are associated with mutations in three autosomal dominant genes: presenilin 1 (PS-1), presenilin 2 (PS-2), and amyloid precursor protein (APP), which are thought to work through increased production of Aβ peptides; and polymorphisms of apolipoprotein E, particularly Apoε4 which is associated with 2 to 3 times (heterozygotes) and 6 to 8 times (homozygotes) increased risk of developing AD), vascular risk factors (including hypertension, hypercholesterolemia, diabetes mellitus), mood disorders (particularly depression), and psychosocial lifestyle factors (including reduced intellectual, physical, recreational, and social engagement).Citation7,Citation33
Clinical features
AD presents with an insidious onset and progresses gradually and inexorably.Citation34 Therefore, identifying specific time points corresponding to initial symptom onset is often difficult.Citation28 The diverse clinical features of cognitive impairment in AD reflect dysfunction of multiple regions of the cerebral cortex. Memory decline is commonly the presenting complaint in AD, and represents a cardinal feature of the disease. The memory changes associated with AD are characterized as storage deficits, with rapid forgetting and poor delayed memory recall. In mild-AD, core episodic memory impairments are observed, reflecting early medial temporal lobe and limbic involvement.Citation8,Citation35 For example, AD individuals may have difficulty recalling details of recent events, personal conversations, or specific elements of a task they may be performing (eg, preparing a meal). Often, they will ask the same question multiple times and may deny their repeated line of questioning.Citation8 Individuals with AD also tend to confabulate and generate inaccurate responses to questions.Citation36 On neuropsychological tests of word recall, AD patients produce more intrusions of incorrect word responses and inaccurately recognize words as being part of the original list (false positive responses) compared with individuals with other subtypes of dementia.Citation9 In general, deficits in declarative memory (episodic memory) are prominent at the disease outset, with more severe memory impairments (semantic memory) emerging later in the disease course.Citation35,Citation37
Orientation to time and place are also disrupted early in the disease, probably because these abilities require effective learning and encoding of information from recent memory. As the disease progresses, orientation worsens to include problems identifying familiar places, family members, or other people well known to the individual.Citation9
Procedural memory may also decline as the disease worsens, resulting in reduced ADL. Problems may include dressing or using common utensils and can be characterized as an ideomotor apraxia. On testing, these maladaptive behaviors are commonly seen when AD individuals use their finger as a toothbrush rather than pantomiming holding the toothbrush as requested.Citation14
Language impairment, although generally preserved early in the disease, is also a prominent clinical feature. Initial signs of language dysfunction often include reduced expressive language (conversational output), word-finding difficulties, and limited vocabulary. Category fluency (eg, naming animals or furniture) tends to be more impaired than semantic fluency (eg, naming words that begin with a particular letter), perhaps because category fluency is dependent on access to temporallobe semantic stores.Citation38,Citation39 As the disease progresses, language difficulties become more pervasive and nonfluent. Spoken language may become less meaningful with an increase in comprehension difficulties, eventually resulting in global aphasia late in the course of illness.Citation14
Although memory decline and language impairment are prominent findings in AD, involvement of other cognitive domains is also common. Visuospatial dysfunction may manifest as impaired driving ability, getting lost, and difficulty producing drawings of figures. Problems with mathematical calculations may impair the individual’s ability to use money and balance their finances. Executive dysfunction results in deficits in problem solving, abstraction, reasoning, decision making, and judgment, particularly for novel situations. For example, AD individuals often display difficulty executing complex tasks, such as planning a vacation or negotiating a business deal.Citation8
In addition to cognitive impairments, behavioral and psychiatric symptoms are common in AD. Depression rates vary by severity and disease stage, and can occur in up to 50% of AD individuals.Citation40 Early in the disease course, depression may be attributed to awareness of the individual’s cognitive changes. In the later stages, features related to apathy may present, but are often confused with depression, particularly by caregivers. Apathy is associated with significant caregiver distress and greater use of healthcare services in AD.Citation41 Psychosis generally occurs later in the disease course. Delusions are predominantly paranoid in nature, with fears of personal harm or mistreatment, theft of personal property (usually related to financial matters), and marital infidelity. Hallucinations are less common than delusions, and tend to be visual. Other behavioral symptoms include agitation, wandering, and sleep disturbances. The primary treatable behavioral and psychiatric symptoms in AD include psychosis, agitation, depression, anxiety, and insomnia.Citation42
Diagnosis
The diagnosis of AD is based on identification and evaluation of features of the individual’s history and clinical exam that are suggestive of the disease, while excluding other causes of dementia through the use of laboratory tests and neuroimaging. The two most commonly used diagnostic criteria for AD are those of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Revised (DSM-IV-TR),Citation43 and those created by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) joint task force ().Citation44 The DSM-IV-TR criteria require an insidious onset with progressive decline of cognitive function that results in impairment of social and occupational functioning, along with memory impairment and at least one other cognitive deficit that cannot be attributed to other psychiatric, neurological, or systemic disease. The NINCDS-ADRDA criteria classify AD according to three levels of diagnostic certainty: definite, probable, and possible. A definite diagnosis of AD is restricted to individuals who meet the clinical criteria for probable AD and have corroborating neuropathological evidence of the disease (by biopsy or autopsy). Probable AD is the most definitive level of diagnostic certainty, without pathological confirmation. Probable AD requires a progressive decline of memory, along with at least one other cognitive domain that is confirmed through clinical examination and neuropsychological testing. The individual must have a preserved level of consciousness, with exclusion of other conditions that may account for the symptoms. Additional features supportive of a probable AD diagnosis include: progressive decline of language functions, praxis, visual recognition, and impaired ADL, with normal results on laboratory tests (eg, cerebrospinal fluid examination), electroencephalogram, and neuroimaging. A diagnosis of possible AD is appropriate when the clinical course of cognitive decline is atypical, when focal neurological findings are present, or when comorbid disorders exist that may account for the dementia-like symptoms. The NINCDS-ADRDA criteria are widely used for research and clinical diagnosis, and have been shown to have a predictive accuracy of up to 90%.Citation35,Citation45,Citation46
Table 1 Summary of DSM-IV and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for the diagnosis of Alzheimer’s disease (AD)
Vascular dementia
Risk factors
VaD is a clinical syndrome of acquired cognitive and functional impairments resulting from the effects of cerebrovascular disease. Due to the multiple etiologies of cerebrovascular disease, VaD can be characterized across a range of neurological and neuropsychological features that vary in presentation by onset (insidious or abrupt) or course (progressive, remitting, or static) of the dementia syndrome.Citation47 Alternate definitions of VaD and a lack of universally accepted diagnostic criteria make it difficult to accurately estimate global prevalence rates. Nevertheless, general trends have been observed with prevalence estimates of 1% to 4% in individuals over 65 years, and worldwide prevalence rates of 15% to 22%.Citation5,Citation48 The association of stroke with cognitive impairment and vascular dementia has been demonstrated in a number of epidemiological, clinical, and neuropathologic studies.Citation49–Citation51 Comorbid disorders leading to hypoxemia (seizures, cardiac arrhythmia) have been shown to be potential risk factors for dementia in stroke patients.Citation51 Other clinical factors associated with a higher risk of VaD include increasing age, lower education, cortical atrophy, previous vascular events, and atherosclerosis.Citation50,Citation51 The association between hypertension and cognitive impairment remains a widely investigated area of research. Most longitudinal studies have found a significant association between elevated blood pressure and the occurrence of dementia 10 to 15 years later. In this association, diastolic pressure appears to be of greater importance than systolic pressure.Citation52 A history of coronary artery disease, myocardial infarction, diabetes mellitus, smoking, and hyperlipidemia are additional risk factors for VaD.Citation51–Citation53 The underlying mechanism by which cerebrovascular risk factors lead to cognitive impairment and dementia remains to be fully elucidated. Most studies suggest that cerebrovascular risk factors are associated with an increased prevalence of cerebral white matter abnormalities, whole brain atrophy, and silent stroke.Citation54 Associations have also been found between the extent of brain atrophy or white matter hyperintensity volumes and decreased cognitive abilities. Therefore, growing evidence suggests that cerebrovascular disease, even in the absence of clinical symptoms, may lead to subsequent cognitive impairment.Citation55–Citation57
Clinical features
Vascular dementia is a diagnostic classification used to describe a constellation of clinical features, including cognitive and functional impairments.Citation58 Three vascular syndromes are commonly cited in the literature: multi-infarct dementia, strategic single infarct dementia, and small vessel disease with dementia.
Multi-infarct dementia (also known as post-stroke dementia) is the most common form of vascular dementia.Citation59 It is attributable to multiple infarcts with occlusion, usually in large vessels, that cause cortical and subcortical infarcts. Occlusion of these arteries occurs as a result of atherosclerotic thrombosis or cardiac embolization, and typically has an abrupt onset with stepwise progression. Multi-infarct dementia is characterized by variable impairment across several cognitive domains.Citation60 Individuals may present with severe deficits in certain domains, while other cognitive abilities may be spared and remain relatively intact. With recurrent stroke episodes, greater areas of cognitive functioning become impaired and the dementia syndrome emerges. VaD individuals often present with lateralized sensorimotor changes, including hemiparesis, hemisensory loss, visual field disturbances, and pathological reflex asymmetries. Agnosia and apraxia may also be part of the clinical features in multi-infarct dementia.Citation60
In strategic single infarct dementia, ischemic damage is focal and involves functionally important cortical and subcortial regions including the thalamus, basal ganglia, angular gyrus, and frontal white matter.Citation54 For example, an infarct in the angular gyrus may result in clinical features such as fluent aphasia, alexia with agraphia, and spatial disorientation. Single lesions in the carotid, anterior, middle, and posterior cerebral arteries are known to give rise to clinical dementia symptoms with a number of neurological and neurobehavioral features.Citation51 Clinical evidence of stroke may or may not be present, but the onset of dementia is generally abrupt with a stepwise decline in cognitive functioning.
Small vessel disease with dementia refers to a syndrome in which occlusion of small vessels causes lesions of subcortical structures, including the thalamus, basal ganglia, internal capsule, and sub-hemispheric white matter. Disruption of these structures results in a form of subcortical dementia. This state is characterized by insidious onset of symptoms including psychomotor slowing, memory impairments, changes in speed, and neuropsychiatric features such as depression and apathy. Neurological features include parkinsonism, ataxia, and urinary incontinence.Citation61 Individuals with small vessel disease with dementia often present with slow, progressive, dementia-like features, often without clinical symptoms of stroke. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is thought to be the hallmark disease associated with this clinical syndrome.Citation62
Diagnosis
Several clinical criteria currently exist for the diagnosis of VaD dementia. The Hachinski Ischemic Scale (HIS) is a commonly used set of criteria for VaD that is easily applied in clinical practice.Citation63 The HIS is based on 13 features used to obtain a total ischemic score. The current modified HIS emphasizes a subset of clinical symptoms as a means of distinguishing VaD from AD.Citation64 The general features of the scale include: abrupt onset, prior history of stroke, focal neurological signs, focal neurological symptoms, stepwise deterioration, somatic complaints, emotional ability, and history of hypertension. Despite a sensitivity and specificity of 70% to 80% in separating VaD from AD, the HIS is limited in its clinical use by not integrating neuroimaging information into the diagnostic process. The DSM-IV-TR approach utilizes clinical evidence of stroke-based signs from the neurological examination or neuroimaging evidence for infarcts as the primary basis for a diagnosis of VaD.Citation43 These criteria do not require that the dementia be temporally associated with the occurrence of stroke, which in effect decreases its specificity and clinical utility. Another definition of VaD is based on the World Health Organization’s International Statistical Classification of Diseases, 10th Revision (ICD-10), which requires a temporal link between a stroke event and dementia, along with signs of stroke on neurological examination or imaging evidence of an infarct.Citation65 A final diagnostic classification for VaD is based on the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Neurosciences (NINDS-AIREN). These criteria are the most widely used for VaD in clinical trials research and emphasize the heterogeneity of vascular syndromes and white matter lesions. The NINDS-AIREN criteria require neuroimaging evidence of lesions in key areas involved in VaD (frontal, temporal, or parietal cortex, thalamus, basal ganglia), along with critical abnormal white matter signals (>25% of total white matter volume).Citation47
Frontotemporal dementia
The clinical and pathological understanding of frontotemporal dementia (FTD) has undergone considerable changes in the past decade. Frontotemporal lobar degeneration (FTLD) is a descriptive term that encompasses a variety of clinical syndromes associated with nonAlzheimer pathology and arises from degeneration of the anterior hemispheres.Citation66 This heterogeneous group of clinical syndromes includes frontotemporal dementia (FTD), FTD with motor neuron disease (FTD/MND), progressive nonfluent aphasia (PNFA), semantic dementia (SD), and progressive apraxia.Citation66 The most commonly occurring syndrome is frontotemporal dementia (FTD) which is characterized by profound alterations in personality and behavior, and is associated with bilateral atrophy of the frontal and anterior temporal lobes.Citation67 Panels of expert international working groups have developed a number of clinical diagnostic criteria for FTD.Citation67–Citation69 The characteristic feature of FTD is a slowly progressive dementia that presents with either predominant behavioral or language disturbances ().Citation70 FTD tends to have a younger age of onset than other dementias, typically between 45 to 65 years, and approximately 20% to 30% of cases are familial and may be associated with mutations in the progranulin or MAPT gene.Citation71 Prevalence studies of FTD are variable, with estimates ranging between 3.6 to 15.0 per 100,000 individuals.Citation72
Table 2 Common clinical presentations of frontotemporal dementia
Of the two FTD clinical presentations, the more common form is that of a behavioral disturbance, which is associated with profound changes in personality. Typical behavioral changes include apathy, lack of initiation, disinhibition (verbal, physical, and sexual disinhibition), perseveration, tactlessness, lack of social graces, emotional blunting, and loss of insight. FTD individuals tend to be unaware of or unconcerned about the social, occupational, and financial consequences of their behavioral changes.Citation73 Cognitive deficits tend to occur in the domains of attention, abstraction, shifting, planning and problem solving, reflecting an executive dysfunction.Citation9
The less common presentation of FTD is that of a progressive and relatively isolated language disturbance. This language presentation can either be that of a difficulty with expression and naming, suggestive of a primary progressive aphasia (usually a nonfluent aphasia), or that of difficulty with word meaning, consistent with a semantic dementia.Citation68 Progressive nonfluent aphasia presents with a gradual disturbance of expressive language with effortful speech production, phonological and grammatical errors, and word retrieval difficulties, ultimately followed by later mutism. Reading and writing can also be impaired. Other cognitive functions are usually spared and these individuals have relatively preserved social skills early in the disease.Citation9 Semantic dementia presents with progressive impairment of comprehension of words and naming in the context of fluent, effortless and grammatically correct speech output. Memory functions are relatively preserved, along with word repetition, reading skills, and mathematical abilities.Citation9
The different clinical presentations of FTD are thought to be associated with the initial focal pattern of cortical atrophy of the frontal lobes. However, as the disease progresses a more global pattern of cognitive impairment emerges.Citation68 On examination, early signs include the presence of a grasp, snout, or palmomental reflex. Further, disinhibition and perseverative behaviors are often observed. The presence of additional neurologic abnormalities may suggest early motor neuron disease (MND) or parkinsonism. There are no widely accepted bedside cognitive or behavioral tests for FTD, and measures of global cognition like the Mini-Mental State Examination (MMSE) are insensitive to change in behavior, aphasia detection, and evaluation of executive function. If FTD is suspected, a more extensive neuropsychological battery should be administered and the individual should be monitored closely.
Dementia with Lewy bodies
Dementia with Lewy bodies (DLB) usually presents in older adulthood between the ages of 60 and 90 years. The epidemiology of DLB remains to be fully elucidated along with potential risk factors for the disease, but to date no significant gender or ethnic differences in prevalence have been identified.Citation74 DLB is a neurodegenerative disorder, thus progressive and functionally disabling cognitive impairment is a central feature. Individuals with DLB exhibit a combination of cortical and subcortical impairments with significant attentional deficits and executive and visuospatial dysfunction.Citation9 Compared to AD, there may be relative sparing of memory functions especially in the early stages of DLB.Citation75 A recent international consortium has resulted in revised criteria for the clinical diagnosis of DLB, incorporating new information about the core clinical features of the disease. These core features include fluctuating cognition, recurrent visual hallucinations, and spontaneous parkinsonism.Citation76
Fluctuations in cognitive function with pronounced variations in attention and alertness can be difficult to evaluate and operationalize, but corroborative reports from primary caregivers can help to clarify these essential clinical features (eg, daytime drowsiness, prolonged staring spells, periods of marked confusion, disorganized speech).Citation76 Cognitive fluctuations are reported across varying time periods, but generally take place for a minimum of 30 minutes and can last as long as several days.Citation9
Visual hallucinations in DLB tend to be recurrent, vivid, and well-formed. The hallucinations tend to emerge early in the disease course, and are therefore a useful diagnostic feature. The content of the hallucinations usually consist of humans or animals, but can be more abstract in nature. Visual illusions and delusions, which typically have a paranoid quality, are also commonly observed. Auditory, tactile, or olfactory hallucinations are rare. Functional neuroimaging studies have demonstrated altered patterns of activation in the visual cortex, along with increased number of Lewy bodies in the anterior and medial temporal lobe of individuals with DLB who report hallucinations.Citation77 Other common neuropsychiatric features include depression, apathy, and anxiety. Agitation and aggressive behavior tend to occur late in the course of the disease.
Extrapyramidal signs, including bradykinesia, facial masking, and rigidity, are the most frequent signs of parkinsonism in DLB. Resting tremor is distinctly uncommon, and parkinsonism is usually bilateral and occurs with the onset of dementia. There is often more axial rigidity and facial masking in DLB than is typically seen in idiopathic Parkinson’s disease.Citation76
An important suggestive clinical feature of DLB includes rapid eye movement (REM) sleep behavioral disturbances. Affected individuals often act out their dreams by screaming and kicking, which can cause injuries to themselves and those around them. The dreams tend to have a chasing or attacking theme, and their content usually matches the exhibited behavior. REM sleep behavior disturbances often begin years before the onset of other cognitive or motor symptoms.Citation78
Other suggestive features of DLB include severe neuroleptic sensitivity, and low dopamine transporter uptake in the basal ganglia on functional neuroimaging. Additional supportive features that commonly occur in DLB include repeated falls and syncope, transient, unexplained loss of consciousness, severe autonomic dysfunction, hallucinations in other modalities, systematized delusions, relative preservation of medial temporal lobe structures on structural neuroimaging, reduced occipital activity on functional neuroimaging, prominent slow wave activity on electroencephalogram, and low uptake myocardial scintigraphy.Citation76
Multidisciplinary team approach to dementia care
An integrated multidisciplinary approach to diagnosing and managing dementia is highly recommended in clinical practice because no single healthcare specialty has the expertise to deal with the complex range of cognitive, physical, social, and emotional problems associated with dementia. Managing dementia presents unique challenges to the practicing clinician, and effective care hinges on a collaborative team approach. This approach relies less on standard pharmacologically based medical practice, and more on the integration of therapies from a wide range of healthcare providers and community professionals. To implement this approach effectively, the clinician must understand the disease processes involved and the clinical presentation of dementia, and also appreciate the caregiving experience and how it affects patient care. The clinician must be informed about the resources available to meet the medical, social, and emotional needs of the patient in the context of their family and community. Using a multidisciplinary team approach can be complex, but it benefits both the patient and the clinician. It allows clinicians to focus on the issues most relevant to their area of expertise, and it facilitates management of patient problems that are likely to require valuable healthcare resources.Citation15
Multidisciplinary team structure
The best approach to the care of individuals affected by dementia includes support from multiple sources. These may be integrated, parallel, or a combination of both. It is ideal for patients and caregivers to seek a blend of multidisciplinary services that come from various healthcare providers, social service agencies, and professionals from outside the field of healthcare. Multidisciplinary teams vary in their composition and may be structured either formally or informally. Team construction is usually dictated by resources, including time, availability, finances, and geographic location. Regardless of structure, successful teams are characterized by a shared commitment to quality care and an appreciation for the contributions of each team member.Citation79,Citation80
Multidisciplinary teams involved in dementia care tend to be based primarily on the availability of service resources, in addition to the social and cultural context of the community. Team members often include neurologists, geriatricians, neuropsychologists, nurse practitioners, physical/occupational therapists, nutritionists, and social workers.Citation15
The neurologist or geriatrician’s primary responsibility is the diagnosis of dementia using validated criteria and practice guidelines. Following diagnosis, a prognosis is determined and a treatment plan is formulated to address the disease and underlying symptoms.Citation15 When diagnostic uncertainty exists, referral to a subspecialist may be useful to refine the diagnosis and help develop a plan of care. Dementia subspecialists may include behavioral neurologists, geriatric psychiatrists, or neuropsychologists. The role of a neuropsychologist is to interpret quantitative cognitive performance data based on the results of psychometric testing. Many neuropsychologists also provide ongoing therapy and support to the dementia patient, and their family members.Citation9
The nurse’s primary role is to assess and manage the dementia patient and their caregivers’ responses to the disease process. This includes monitoring symptom presentation, responding to medication issues, providing education and relevant information to family members, and assisting them in preparing for disease progression. Dementia frequently results in immobility, decreased physical conditioning, loss of muscle strength and tone, and poor coordination. Impaired ambulation is common and increases the risk of falls and injury. Physical therapists can assist dementia patients to optimize their physical conditioning and maintain safe mobility, helping to prolong independent living and the need for institutionalization.Citation81
Another important factor in maintaining the dementia patient’s home independence is their ability to manage ADL. Occupational therapists help the patient and their caregivers to adapt to the patient’s diminished ability to deal with challenges faced in daily living. Use of devices to assist with toileting, eating, dressing, and home management can be recommended and demonstrated by an occupational therapist. As patient independence is enhanced, caregiver burden decreases.Citation82 Cognitive impairment related to dementia also places the patient at increased risk for malnutrition and dehydration. In later, more severe stages of dementia, the patient often experiences sensory-motor impairments with ensuing loss of ability to swallow or eat independently. Weight loss then becomes a significant concern as the disease progresses. Comorbid conditions common to older individuals such as diabetes and coronary heart disease also make dietary management more challenging. Dietary intake also extends beyond medical concerns, and becomes an important quality of life consideration.Citation83
Social workers base their practice on the philosophy that the individual is part of a larger system, and they are prepared to assist the patient and their family in identifying and acquiring the resources needed to manage the burden of chronic illness.Citation84 In nearly all cases, patients with dementia and their caregivers will require access to social services including community programming, respite care, financial services, counseling support groups, and crisis management. Therefore, it is often recommended that a referral to a social worker be initiated at the time of formal diagnosis of dementia.Citation15 As the disease progresses, increasing levels of support will be required. The social worker is able to help anticipate future needs, facilitate the acquisition of services, and navigate the patient and their caregivers through the extended healthcare system. For individuals with dementia, the home environment has been found to promote a sense of personhood, normalcy and continuity in the face of disconnection that is often experienced as a result of multiple losses in functional, cognitive, and social domains of their lives.Citation85 Consequently, ‘aging in place’ is considered to be the preferred choice for many older adults to help maintain independence.Citation86,Citation87 However, individuals with dementia often require care within a structured, institutionalized environment. Caregivers’ reasons for placement into institutionalized care are diverse and often include: 1) the need for more skilled care and more assistance, 2) the caregivers’ health, 3) the presence of behavioral and psychological symptoms of dementia, 4) caregiver burden, and 5) use of community-based services.Citation88 Given the practical and emotional challenges involved in transitioning from a home environment to an institutionalized care facility, the social worker can help guide the dementia patient and their family members through this difficult process.
Managing and treating dementia
Current clinical practice encourages open discussion of the diagnosis of dementia with patients and their caregivers to facilitate early implementation of treatment strategies, and to allow families to plan for the future. An integrated approach involving both pharmacological and psychosocial strategies is essential for effective care and management. This approach often includes community support services, professional groups and associations, specialized dementia clinics, and geriatric outreach services.
Pharmacologic treatment of dementia
Two classes of drugs are currently approved for the symptomatic treatment of dementia: acetylcholinesterase inhibitors (AChEI) and N-methyl-D-aspartic acid (NMDA) receptor antagonists (). These pharmacologic treatments do not target the underlying pathogenesis of dementia, but rather help to improve or maintain function following neuronal damage.Citation89
Table 3 Pharmacologic treatments for dementia
The development of AChEIs emerged from a pathophysiologic model of AD known as the amyloid cascade hypothesis. In this model, it is proposed that accumulation of Aβ in the brain is the primary force driving AD pathogenesis, with the rest of the disease process resulting from an imbalance between Aβ production and clearance. Within the cascade, a dysregulation in amyloid precursor protein (APP) processing by β- and γ-secretases initiates the pathogenic events leading to aggregation of Aβ, specifically Aβ42, an insoluble fragment that deposits in plaques. The formation of these amyloid plaques further instigates pathological events, including the formation of neurofibrillary tangles (NFTs) from hyperphosphorylation of the tau protein, disruption of synaptic connections, reduction in neurotransmitters, death of tangle-bearing neurons, harmful oxidative processes, damaging inflammatory responses, and the eventual emergence of the clinical symptoms of dementia.Citation90 Although the amyloid cascade hypothesis has gained wide acceptance in the field of dementia research, it remains controversial. Emerging evidence shows that many other neuropathological processes may be involved, including the theory that the central mechanism is the hyperphosphorylation of the tau protein. More recent data from multidisciplinary research has suggested the importance of vascular risk factors (eg, smoking, obesity, hypercholesterolemia) and vascular morbidity (eg, hypertension, diabetes, silent brain infarcts, and white matter lesions) in the pathology of dementia.Citation5,Citation91,Citation92
Pharmacotherapy for AD
There are four currently approved AChEIs for the treatment of AD: tacrine, donepezil, rivastigmine, and galantamine. All of these drugs are approved for mild to moderate AD, with the exception of donepezil, which is approved for mild to severe AD.Citation89 Head-to-head clinic trials comparing the effectiveness of each of the AChEIs are rare, thus making it difficult to determine which is best for the treatment of patients with AD. Tacrine has an inconvenient dosing schedule (four doses daily), and significant side effects (hepatotoxicity), and is rarely used in current clinical practice.Citation91 The other three AChEIs appear to be relatively equivalent in terms of efficacy and are associated with symptomatic improvement in cognitive function in patients with mild to moderate AD. Improvement generally lasts for 6 to 12 months, followed by a gradual decline in most, but not all, cases.Citation89
There is some controversy on the duration of use of AChEIs in the treatment of patients with AD. Some clinicians feel that once a patient has reached the stage of severe AD, AChEI use should be discontinued. Others feel that AChEIs may still be useful for managing symptoms in severe AD, but this remains an active and open area of clinical investigation. Despite this controversy, AChEIs represent the primary form of treatment for AD.
Memantine is currently the only NMDA antagonist approved to treat AD. The rationale behind the use of memantine is the glutamatergic excitotoxicity theory, which stipulates that an abnormal and sustained increase in glutamate may lead to neuronal degeneration. Memantine, an NMDA receptor, is a low-to-moderate uncompetitive antagonist that blocks glutamate binding to its receptor, thereby decreasing neuronal excitotoxicity and slowing the progression of AD.Citation93 Memantine has been approved for the treatment of all stages of AD, but in clinical practice is generally used in moderate-to-severe AD. Administered twice daily, compliance is a common issue with memantine, as it is difficult for patients with AD to maintain their dosage schedule. Memantine is a symptomatic treatment for AD, and has been shown to improve cognitive function and slow the rate of decline in moderate-to-severe cases. The duration and extent of cognitive efficacy is approximately 6 months, and may be greater.Citation94 Additional studies are needed to determine whether the efficacy of memantine in patients with moderate-to-severe AD extends beyond 6 months.
Pharmacotherapy for non-AD dementias
Vascular dementia can be treated by the use of donepezil, rivastigmine, and galantamine in patients with probable and possible VaD.Citation47 Several clinical trials have shown that antihypertensive therapy can help to prevent the onset of VaD in certain individuals.Citation95
DLB is a form of dementia that is not easily treated through the use of pharmacotherapy. Rivastigmine has shown some improvement in DLB patients, and low-dose clonazepam has been used for sleep disturbances. Psychostimulants, including levodopa and dopamine agonists, have been shown to improve cognition, apathy, and psychomotor slowing. Management of cognitive fluctuations in DLB has been difficult. Atypical neuroleptics have also been reported to be helpful for symptoms of delusions and agitation, and selective serotonin reuptake inhibitors (SSRIs) can be effective for the treatment of depression and anxiety in DLB patients.Citation96
Frontotemporal dementia is another challenging form of the disease where management and treatment strategies have been inconsistent and disappointing to date. Few large-scale, clinical trials for the treatment of FTD have been conducted, although several smaller trials have been carried out. In these studies, rivastigmine and SSRIs have been shown to improve behavioral symptoms of FTD, but cognitive improvement has been limited.Citation97,Citation98
Goals of pharmacotherapy for dementia
At the initiation of pharmacologic treatment for dementia, the goals of therapy should be clearly established with the patient and their caregivers. AChEIs and memantine are not as effective as drug medications for other medical conditions, and it is important to clarify that they do not alter the eventual course of the disease. The treatment options available mainly stabilize the patient, delaying subsequent decline, with some individuals experiencing transient symptomatic improvements. Clinicians must set realistic goals that take into account the modest benefits that can be expected in various cognitive, behavioral, emotional, and functional domains.
Nonpharmacologic treatment of dementia
Over the past 3 decades, interest has grown in the use of non-pharmacologic treatment approaches, alone or in combination with pharmacotherapy, for the treatment and management of dementia. Several therapies have been suggested including cognitive-based therapies, psychosocial therapies, physical therapies, and sensorial therapies. These strategies may be implemented in a variety of settings (home, institutional care facility) and treatment modalities (individual or group-based therapy). The clinical efficacy of nonpharmacologic treatments remains to be fully evaluated, but it is understood that the primary goal of these approaches is to improve the quality of life for individuals with dementia.
Cognitive-based interventions
Cognitive interventions for dementia rely on the plasticity hypothesis, which theorizes that the brain is able to achieve neural and functional improvements by reorganizing its constituent elements and internal network connectivity according to environmental constraints.Citation99
Cognitive training and cognitive rehabilitation are the two most commonly applied nonpharmacologic strategies to implement in the early stages of dementia. These interventions involve targeted practice and training of specific cognitive domains, with a primary emphasis on memory, attention, and executive functions. Cognitive rehabilitation differs from cognitive training in that it is a more individualized therapeutic approach, and its goals are to enhance functioning in everyday life, rather than improve performance on cognitive tasks. A recent Cochrane review examined the efficacy of cognitive training and rehabilitation in patients with early stage AD and VaD in five domains from nine randomized controlled trials including participant scores on cognitive screening measures, neuropsychological tests, self-reported functioning, informant ratings of functioning, and reactions to memory impairments and behavioral difficulties. While some positive results were found in specific cognitive domains, overall improvements were limited and there were no significant differences between cognitive training and control groups.Citation100
Psychosocial therapies
Psychosocial therapy is an intervention that is intended to enhance self-esteem, well-being, social and communication skills, and to decrease behavioral disturbances.
Reminiscence therapy is a common psychosocial intervention in dementia care. Initially introduced in the 1980s, this approach is based on evocation and discussion between the dementia patient and trusted individuals to think about and revisit past events, experiences, and activities. It often involves the use of objects and supports (eg, photos, personal belongings, music) to help trigger specific memories. Very few randomized controlled trials have assessed reminiscence strategies in dementia. A recent Cochrane meta-analysis concluded that reminiscence therapy resulted in significant improvements in behavioral functioning, as well as cognitive and depressive symptoms, compared with no-treatment and social-contact control groups, with sustained effects 4 to 6 weeks after cessation of the intervention.Citation101
Validation therapy is another form of psychosocial intervention for the treatment of dementia. The goal of validation therapy is to promote and stimulate communication skills in the dementia patient, and to provide the individual with insight into their external reality. A recent Cochrane review found little evidence supporting this form of intervention, but noted that the limited information from randomized controlled trials is insufficient to draw reliable conclusions about the efficacy of validation therapy in dementia.Citation102
Physical activity therapy
Physical activity can be used as a therapeutic approach in a wide range of target populations including healthy aging adults and individuals with dementia. The benefits of physical activity have been demonstrated in terms of mood, quality of life, falls, cardiovascular function, and disability rates.Citation103–Citation106 Regular exercise can also slow down or help prevent functional decline associated with aging, improve muscle mass, arterial compliance, energy metabolism, cardiovascular fitness, and overall functional capacity.Citation107
An individual’s overall physical fitness is composed of several components including cardiorespiratory and muscular fitness, flexibility, and balance. Cardiorespiratory fitness, also known as aerobic training, is a condition in which the cardiovascular and respiratory systems function together to ensure that adequate oxygen is supplied to working muscles to produce energy. A high level of cardiorespiratory fitness permits continuous physical activity without a decline in performance and allows for rapid recovery. It is also believed that cardiovascular fitness can improve cognitive capacities. Animal research has demonstrated beneficial effects of aerobic physical activity on cognition, through physiological processes involved in increased cerebral blood flow, oxygen extraction, and glucose utilization.Citation108 It has therefore been suggested that physical activity may act as a protective factor for cognitive decline in older adults.Citation109
A recent Cochrane review examined the effects of physical activity (aerobic exercise training or physical activity of any length of time) on cognition, function, and behavior in individuals with dementia. The review also evaluated caregiver outcomes and healthcare service use. No significant results were observed but numerous methodological factors limited the potential findings of the meta-analysis.Citation110 More studies with homogeneous populations, in terms of dementia subtype and severity, are required to fully investigate the role of physical activity in dementia. Moreover, several key questions about physical activity remain to be clarified, including how long the effects of exercise last after cessation of training, and how much exercise is required to exert a beneficial effect.
Managing and treating BPSD
BPSD occur in individuals with any of the dementia subtypes and include a heterogeneous range of psychological reactions, psychiatric symptoms, and behavioral disturbances. BPSD can cause serious complications often leading to increased emergency department visits, caregiver distress and illness, increased financial costs to society, early institutionalization, and diminished quality of life.Citation12,Citation13,Citation81,Citation111,Citation112 Therefore, effective management of BPSD in dementia is an important treatment consideration.
BPSD can generally be separated into behavioral excesses, wherein the occurrence of the behavior is problematic (eg, physical and verbal aggression, agitation, anxiety, disinhibition, depression, aberrant motor behavior, irritability, hallucinations, and delusions) and behavior deficits, wherein the nonoccurrence of the behavior causes the complications and challenges (eg, apathy leading to withdrawal from social interactions, cessation of self-care including eating and dressing, and sleep disturbances ().Citation11,Citation113,Citation114 The most frequently occurring BPSD are apathy, depression, and anxiety.Citation115,Citation116 BPSD can occur at any stage along the dementia continuum, but certain symptoms have been found to be more common in mild dementia (eg, depression, anxiety, irritability, and apathy), while others occur more frequently in later stages of the disease process (eg, aberrant vocalizations, delusions, hallucinations, and disinhibition).Citation117–Citation119 Agitation and aggression are among the most challenging BPSD for caregivers, and along with psychosis and depression are the leading predictors of institutionalization.Citation120 When present, inappropriate sexual behaviors have been rated by caregivers as the most difficult symptom of BPSD to manage.Citation121,Citation122
Table 4 Neuropsychiatric features of behavioral and psychological symptoms of dementia
A variety of biological, psychosocial, and environmental factors have been proposed to explain the development of BPSD. Psychosocial models of BPSD include: Need-Driven Dementia Compromised Model, Progressively Lowered Stress Threshold Model, and the Biopsychosocial Model of Dementia.
Need-Driven Dementia Compromised Model
Within the context of the Need-Driven Dementia Compromised Model, BPSD is considered to be the most integrated and meaningful response possible by an individual with dementia to their environment.Citation123 According to Algase, BPSD arise due to the pursuit of a goal or as an expression of a need by the individual with dementia. It is also suggested that the presence, severity, and type of BPSD is a reflection of the interaction between salient background factors which are somewhat stable individual characteristics (eg, gender, personality type, general health), and proximal factors which are more fluid or fluctuating aspects of the immediate physical and social environment, as well as the dynamic or changing needs and internal states of the person with dementia (eg, temperature, excessive noise, hunger, fear). Seen this way, the Need-Driven Dementia Compromised Model defines BPSD as meaningful and potentially useful behaviors that can direct and inform appropriate caregiver responses. The primary goal is therefore not to eliminate the behaviors completely, but rather to understand them and enable caregivers to more readily and accurately meet the needs of the person with dementia.Citation123
Progressively Lowered Stress Threshold Model
The Progressively Lowered Stress Threshold Model posits that persons with dementia are increasingly less able to manage stress as the disease progresses.Citation124 This reduction in their capacity to tolerate stress results in anxious behaviors as an attempt to alleviate perceived stress. According to Hall and Buckwalter, BPSD (referred to as catastrophic reactions) occur if the stress is unrelieved through either adaptation of the environment or appropriate responses from caregivers or both.Citation124
Biopsychosocial model of dementia
The biopsychosocial model of dementia is a theoretical framework developed to illustrate the role of psychosocial factors within the context of biological processes and to increase understanding of the factors that may lead to improvement or deterioration in dementia.Citation125 This model suggests that in both biological and psychosocial domains there are fixed factors, which are not amenable to change (eg, age, education, gender, personality traits), and tractable factors, which are more flexible and adaptive (eg, pain, physical and social environment, level of physical and social activity). The trajectory of dementia is presented as a process, beginning with aging, early organic change, initial cognitive impairments, formal diagnosis of dementia, and then moves progressively towards dependency, institutionalization, end of life care, and eventual death. In the biopsychosocial model, the fixed and the tractable factors will simultaneously influence the point at which the symptoms of dementia begin, the nature and speed of the deterioration, and the effectiveness and appropriateness of interventions used by caregivers to assist persons with dementia. The primary aim of this model is to increase caregivers’ ability to identify ways to improve the quality of life and reduce excess disability (often by reducing BPSD) for the person with dementia.Citation125
Environmental factors
Environmental factors that have been found to contribute to the development of BPSD can be divided into two subgroups: the physical environment (eg, architectural features, interior design features, and sensory attributes such as lighting and noise levels) and the social environment (eg, optimizing the mix of residents, social stimulation, and sensory stimulation). Physical environmental factors that are precursors to BPSD include inadequate lighting, confusing surroundings, excessive noise/stimulation, and poorly designed care environments.Citation120,Citation126 Social environmental factors that have been found to contribute to the presence of BPSD include a lack of daily routine, excessive demands from caregivers, distressing behavior of others, loneliness/boredom, and social isolation.Citation120
Person-centered care for BPSD
Many management and treatment interventions for BPSD are underpinned by a person-centered care approach. An important aspect of the person-centered approach is the therapeutic benefit of positive interpersonal interactions. The conditions that influence positive change include having a therapist who provides unconditional positive regard, is empathetic, and is genuine or congruent within the client–therapist relationship.Citation127 This person-centered approach has been adopted and applied to dementia individuals in long-term care settings.Citation128 It involves recognizing and identifying the personal preferences and needs of dementia patients as a way to guide caregiving, thus enabling the individualization of care plans and routines. Additionally, a central feature of person-centered care is the recognition that all human life is grounded in relationships, and that individuals with dementia need an enriched social environment that fosters opportunities for personal growth while compensating for their cognitive and functional impairments.Citation128,Citation129
The key elements of person-centered care include 1) knowing the person as an individual; 2) providing care that is meaningful to the person in ways that respect their values, preferences, and needs; 3) viewing patients as biopsychosocial beings; 4) enabling the development of consistent and trusting caregiving relationships; 5) emphasizing freedom of choice and individually defined risk taking; 6) promoting emotional and physical comfort; and 7) involving patients’ family, friends, and social networks in care decisions.Citation129
Findings from empirical studies demonstrate that the application of person-centered care results in reduced neuroleptic medication use and agitation.Citation130,Citation131 Despite the widespread recognition and application of person-centered care for individuals with dementia, many studies to date are conceptual or anecdotal, highlighting the need for more research on this treatment approach.Citation132
Nonpharmacological treatments for BPSD
Nonpharmacological treatments for BPSD can be grouped into two categories: 1) indirect interventions aimed at decreasing BPSD through indirect work with caregivers or the environment, and 2) direct interventions targeted directly at individuals to decrease BPSD.
Indirect interventions
Caregiver training
The aim of caregiver training is to increase formal and informal caregiver understanding of BPSD, and improve skills in managing and responding to problem behaviors. Education and training programs have been found to be effective in the reduction of BPSD in both nursing home environments, and the community.Citation133,Citation134 Multidisciplinary team approaches and individualized treatment plans developed according to the unique needs of the individual with dementia, combined with caregiver problem-solving techniques are key features of successful education and training interventions.Citation135,Citation136
Environmental adaptations
When BPSD appear to be triggered by environmental factors, interventions aimed at modifying the individual’s environment may be the most effective means to address the behaviors. Key environmental features include: 1) architectural elements, such as room layout and size (eg, smaller social spaces); 2) interior design features, such as furniture type and arrangement (eg, homelike furnishing, familiar objects, and personalized rooms); and 3) sensory attributes (eg, noise, lighting, and visual accessibility). Adaptations of these environmental features have been found to reduce anxiety, increase a sense of emotional well-being, and enhance social interaction for individuals with dementia.Citation126,Citation132,Citation137–Citation139
Direct interventions
Sensory therapy
Various sensory-based therapies have been developed for use with dementia populations, including music therapy, light therapy, and Snoezelen therapy. Music therapy is defined as the use of music or musical elements (voice, sound, rhythm) by a qualified music therapist. This nonpharmacologic approach is believed to promote nonverbal communication, relationships, learning, expression, and to improve emotional, social, and cognitive functioning through increased quality of life. Music therapy can be applied on an individual or group level, and may be active or receptive. Several randomized controlled trials of music therapy in dementia have recently been conducted, and significant positive effects have been found in the reduction of BPSD. Specific improvements were found on indices of anxiety and depression for up to 8 weeks after discontinuation of music therapy.Citation140–Citation142 Background music has also been shown to be effective in reducing agitation.Citation143,Citation144
The complexity of BPSD in dementia suggests that a multi-factorial and integrated approach is required for appropriate intervention and management. Approaches that do not take into consideration the complex biological, psychosocial, psychological, and environmental factors will likely produce results that are sporadic, inconsistent and short lived. Consequently, a comprehensive and integrated approach, which addresses the needs of the individual with dementia, the caregivers, and the physical and psychosocial environment is highly recommended.
Empirical research on dementia: toward facilitating early detection of those at risk
Considerable effort has been devoted to better understanding change in cognitive functioning during the prodromal phase of dementia.Citation145 This research focus is well intentioned given the protracted nature of neuropathological change in dementia, coupled with the fact that effective treatment pharmacotherapy remains elusive.Citation146 Ultimately, the success of potential interventions will be further augmented through the early detection of those at risk. In this spirit, we briefly summarize some recent empirical findings of preclinical cognitive deficits in AD, and discuss some potentially fruitful avenues for future research.
Characterizing cognitive decline during the prodromal phase of dementia
A recent meta-analysis examined 47 studies comprising more than 1200 AD cases and 9000 controls.Citation147 Among the primary foci, the meta-analysis examined preclinical AD impairment across multiple cognitive domains, as well as the moderating impact of the length of the prodromal period on the magnitude of the preclinical AD effect. Consistent with expectation, larger prodromal deficits were observed for studies characterized by a time interval of less than 3 years between cognitive assessment and clinical diagnosis, relative to studies with average follow-up periods greater than 3 years. For example, episodic memory differences between cases and controls were characterized by a large effect (1.12 SD units) within 3 years of diagnosis, but a moderate effect (0.76 units) for studies with follow-up intervals greater than 3 years between testing and diagnosis. Another critical finding from the meta-analysis concerned the pattern of deficits as a function of cognitive domain. Consistent with the etiology of AD, large magnitude deficits in episodic memory were observed for AD cases relative to controls. Large-magnitude cognitive deficits were also observed, however, for measures of executive functioning, processing speed, and global cognition, consistent with the notion that deficits in multiple cognitive domains characterize the prodromal phase of dementia.
On the surface, detecting such large-magnitude deficits would represent seemingly positive news vis-à-vis early detection of those at risk of dementia. However, despite the large magnitude differences in mean performance across multiple cognitive outcomes, considerable overlap (>40%) remained across performance distributions for the preclinical AD vs nondemented groups, with this overlap severely impairing task sensitivity for detecting those at risk of dementing.Citation147 Further to the point, it is arguably unrealistic to expect nonoverlapping distributions of cognitive performance many years before diagnosis given the large interindividual variability in both preclinical AD cases, and those who will remain without dementia.Citation148–Citation150 Numerous factors can result in cognitive impairment with increasing age, leading to false-positive detection including psychiatric, metabolic, immunological, and circulatory conditions.Citation151–Citation154 Moreover, some individuals who will convert to dementia show accelerated decline only in close proximity to diagnosis, leading to false negatives.Citation149
Change-point models of onset and rate of cognitive decline before dementia diagnosis
Given the problem of overlapping distributions for groups of preclinical AD and nondemented controls, an obvious question concerns what can be done to improve classification of those at risk? Based on recent findings from our research group, we argue that facilitating early disease identification and targeting successful intervention requires moving beyond static mean-difference comparisons at one point in time to consideration of the relative onset and rate of cognitive decline across a continuum of cognitive measures.Citation155 To examine these issues, Thorvaldsson and colleagues employed data from two population-based Swedish longitudinal studies of aging.Citation155 Participants from the age-heterogeneous Kungsholmen Project (Stockholm, Sweden) were aged 75 years or older and completed up to 5 measurement occasions (417 dementia cases and 249 non-cases). The Gerontological and Geriatric Population Studies (Gothenburg, Sweden) included participants who were all initially 70 years of age and who were followed for up to 13 retest occasions spanning 30 years (113 dementia cases and 272 noncases). A series of linear mixed change-point models (see Hall, Lipton, Sliwinski, and Stewart, 2000Citation156) were fit to numerous cognitive outcomes. These piecewise models estimated two slopes, permitting the knot of the spline (the change point) to vary across models in 1-month increments. Prior to the knot, time was parameterized as a function of chronological age (years since birth from each individual measurement). In contrast, following the knot, time was specified as a function of time to dementia diagnosis (years to diagnosis from each individual measurement). A profile likelihood method was then employed to select the best fitting change point spanning a range from 1 month to 10 years prior to diagnosis (eg, 3.1 years before diagnosis, 3.2 years before diagnosis, 3.3 years before diagnosis). These systematic model fit comparisons facilitated the identification of the average transition point that best differentiated between normative age-related cognitive decline and accelerated decline during the preclinical phase.
Thorvaldsson et al reported that the earliest preclinical deficits, around 9 years before diagnosis, were observed for cognitive correlates of dementia pathology (episodic memory) as well as more executively demanding tasks (eg, visuospatial ability, processing speed).Citation155 Notably, however, as nondemented elderly individuals also exhibited decline on these tasks, the relative magnitude of cognitive changes for the preclinical cases was only modestly incremented. Consistent with expectation, tasks that exhibited the earliest onset of cognitive decline did not also exhibit the fastest rate of change. Rather, the most precipitous estimates of decline were observed for tasks that are at least somewhat resistant to age-related decline such as category fluency – a knowledge-based task that incorporates both fluid and crystallized abilities. Such patterns suggest that tasks incorporating both fluid and well-preserved crystallized abilities may facilitate discrimination between preclinical cases vs noncases, but only in close proximity to incident diagnosis.
Onset versus rate of change
In light of these findings underscoring that onset and rate of change in the prodromal period vary as a function of cognitive domain, another critical question concerns which characteristic to focus on for increasing sensitivity of detecting those at risk of dementing. As with most questions, the answer depends on whether an individual’s emphasis concerns clinical practice or basic research. A general practitioner interested in the health of an individual patient should place more emphasis on early detection, and thus differentially focus on onset. Based on our findings, this would mean targeting early behavioral changes in process-based measures of cognition (eg, episodic memory) that exhibit impairments up to 10 or more years prior to diagnosis. Of course, clinical detection of such onset in change requires knowledge of baseline performance and repeated within-person assessments, and even then may prove difficult to detect as normative age-related changes are also typically observed for these same measures. In the Thorvaldsson et al study, for example, episodic memory decline in the prodromal phase was only accelerated by a factor of 2 relative to decline observed during normal aging.Citation155
In contrast, those focusing on more basic research (eg, targeting individuals at greatest-risk of converting to AD for use in clinical trials research, identifying candidates for aggressive pharmacological intervention) may differentially focus on accelerated rate of change (rather than onset) for select cognitive domains. In particular, our findings suggest that verbal fluency may be a particularly important measure for increasing sensitivity of detection for those at risk of dementing. Verbal fluency shows very little age-related change, but when change is finally manifest during the prodromal period within 6 years of diagnosis, performance decline accelerates by a factor of 5 or more relative to normal aging. Indeed, clinical trials research might consider adopting a multivariate approach where at-risk participants are selected on the basis of both onset (individuals exhibit early change in episodic memory consistent with AD etiology) as well as change (individuals with episodic deficit who also show accelerated decline in verbal fluency); such an approach is likely to improve sensitivity of identifying those at greatest risk of converting.
Preclinical cognitive deficits and threshold models
Fundamental tenets of threshold models of cognitive impairment are consistent with both the Thorvaldsson et al findings, demonstrating inflection points that differentiate between normative age-related cognitive decline vs more accelerated decline during the prodromal period in proximity to diagnosis, and the Bäckman et al meta-analysis demonstrating that cognitive function is more strongly affected in proximity to diagnosis.Citation147,Citation155 Further, the concepts of cognitive reserve as well as compression of morbidity are both consistent with initial trajectories of change that are gradual, followed by more pronounced cognitive decline in proximity to diagnosis.Citation157–Citation159 For example, Stern’s notion of cognitive reserve was developed to facilitate explanation on the modest association between the amount of underlying AD pathology and observed behavioral deficit.Citation159 Cognitive reserve is said to reflect the amount of neuropathology that can be accrued prior to reaching a critical threshold beyond which cognitive deficits are manifest.Citation159 Educational attainment in particular has been viewed as an index of cognitive reserve, with recent findings clearly demonstrating the aforementioned threshold effects. Hall et al, for example, showed that those with more years of education (12+) did not exhibit cognitive decline onset until close proximity to dementia diagnosis relative to a low-educated group (less than 7 years) who would subsequently dement.Citation157 However, upon reaching their threshold for pathology burden, the rate of cognitive impairment was steeper for the high-educated group. Thus, later disease onset appears to be associated with greater disease burden, manifest as accelerated cognitive loss (ie, steeper prodromal slopes) once decline occurs.
Future research
In light of the meta-analytic findings by Bäckman et al, it is apparent that prospective identification is not facilitated through solitary use of cognitive measures that exhibit large mean differences between dementia cases and controls years before diagnosis.Citation147 This is because the distributions between the two groups still overlap to a large extent (>40%) for individual cognitive domains such as episodic memory or executive functioning. To increase sensitivity of early detection, Small et al suggest that future research incorporates the following improvements.Citation145 First, these authors emphasize that multifactorial prediction models are required to improve early detection. Evidence suggests that early detection is improved considerably upon inclusion not only of episodic memory tasks, but also indicators of executive functioning and semantic memory.Citation160,Citation161 Next, in service of better differentiating between cases and controls, future research should also supplement such cognitive markers with other markers linked to dementia incidence. These multivariate models and their corresponding interactions could include biomarkers such as brain volumetrics, amyloid deposition, and white-matter alterations, as well as genetic markers, subjective memory complaints, social isolation, and lifestyle factors such as lack of intellectual engagement or low physical activity levels.Citation149,Citation162–Citation169 A key challenge for future research will be to ascertain whether specific combinations of these biological, social, cognitive, genetic, and clinical markers increase identification of those at risk of dementia. To the extent that protective characteristics (eg, high education, active lifestyle) mask the early signs of AD and delay the clinical onset, effective multivariate (multistage) approaches for facilitating detection will be essential given the reduced time window between onset of preclinical decline and subsequent diagnosis.Citation170 However, an alternate view is that delaying the clinical manifestation of AD by just 2 years could significantly reduce the total number of AD cases as well as helping to lower healthcare costs.Citation171 This latter view underscores the critical need to identify sensitive early indicators of those at risk, and then to target interventions such as health or behavior promotion strategies (eg, physical or cognitive lifestyle interventions), which may successfully delay (or prevent) onset of dementia. Finally, given the importance of understanding the natural history of AD progression, more sophisticated longitudinal designs should be adopted for the study of cognitive change during the preclinical period through to dementia onset. In particular, including multiple measurement occasions before diagnosis is required to better identify the onset of cognitive decline during the prodromal period, as well as to quantify the magnitude of acceleration. Multiwave datasets and linear mixed models may also identify whether select dementia correlates increase or decrease in importance in proximity to diagnosis.Citation145 Such differentiation may help differentiate between disease risk factors vs precipitating factors.
Summary: toward a paradigm shift in dementia care for the 21st century
The preceding review highlights the many advances over the past few decades that aid our understanding of dementia epidemiology and its clinical presentation. It outlines various multidisciplinary treatments, including pharmacological and nonpharmacological treatment, and addresses significant management challenges. Demographic projections underscore the potential burden that the various dementias pose for healthcare in the coming 20 years. Dementia prevalence will continue to increase in concert with increased life expectancy and improved medical care. Unequivocally, clear advances have been made in dementia diagnosis, identification of associated risk factors, as well as management and care. However, effective pharmacological treatments that target the underlying neuropathology and alter the disease course remain elusive.
At least in the near term, we can argue that dementia is as much about social policy and governance as it is about medicine or neuropsychology. Given the progressive nature of the disease, it will be necessary to target preventive as opposed to reactive interventions. Improved policy initiatives should target public education documenting specific risk factors for dementia from early-and mid-life, as well as lifestyle interventions (eg, health or behavior promotion strategies) that can potentially delay or prevent the disease. In the United States, for example, delaying the clinical manifestation of dementia by 2 years would reduce the total number of AD cases by approximately 600,000.Citation171 Based on increasing prevalence projections in Canada, the Alzheimer Society of Canada (2010) Rising Tide report projects the total economic burden of dementia to double each decade, increasing from approximately $15 billion (CAD) per year in 2008 to $153 billion (CAD) per year by 2038.Citation172 Clearly, delaying the onset of dementia by even several years would have considerable impact on both disease prevalence as well as associated healthcare expenditures. To realize such benefits, policy makers should place continued emphasis on dementia research, as well as renewed focus on public education initiatives (eg, increased as opposed to decreased emphasis on physical education programs in primary and secondary school) that promote protective and enduring lifestyle behaviors.
Acknowledgements
Jacob Grand was supported by a Frederick Banting and Charles Best Doctoral Research fellowship from the Canadian Institutes for Health Research (CIHR). Sienna Caspar acknowledges support by a Joseph-Armand Bombardier Canada Graduate Scholarships Program from the Social Sciences and Humanities Research Council. Stuart MacDonald was supported by a Career Investigator Scholar Award from the Michael Smith Foundation for Health Research (MSFHR).
Disclosure
The authors declare no conflicts of interest.
References
- From the Centers for Disease Control and PreventionPublic health and aging: trends in aging – United States and worldwideJAMA3192003289111371137312636453
- WimoAWinbladBAguero-TorresHvon StraussEThe magnitude of dementia occurrence in the worldAlzheimer Dis Assoc DisordApr-Jun2003172636712794381
- FerriCPPrinceMBrayneCGlobal prevalence of dementia: a Delphi consensus studyLancet1217200536695032112211716360788
- LoboALaunerLJFratiglioniLPrevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research GroupNeurology20005411 Suppl 5S4S910854354
- QiuCKivipeltoMvon StraussEEpidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward interventionDialogues Clin Neurosci200911211112819585947
- BrookmeyerRJohnsonEZiegler-GrahamKArrighiHMForecasting the global burden of Alzheimer’s diseaseAlzheimers Dement720073318619119595937
- AlvaGPotkinSGAlzheimer disease and other dementiasClin Geriatr Med11200319476377615024811
- GeldmacherDSDifferential diagnosis of dementia syndromesClin Geriatr Med22004201274315062485
- ManningCBeyond memory: neuropsychologic features in differential diagnosis of dementiaClin Geriatr Med22004201455815062486
- KesterMIScheltensPDementia: the bare essentialsPract Neurol820099424125119608778
- MirakhurACraigDHartDJMcLlroySPPassmoreAPBehavioural and psychological syndromes in Alzheimer’s diseaseInt J Geriatr Psychiatry11200419111035103915481075
- SteinbergMCorcoranCTschanzJTRisk factors for neuropsychiatric symptoms in dementia: the Cache County StudyInt J Geriatr Psychiatry9200621982483016955439
- LyketsosCGLopezOJonesBFitzpatrickALBreitnerJDeKoskySPrevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health studyJAMA9252002288121475148312243634
- GrossmanHBergmannCParkerSDementia: a brief reviewMt Sinai J Med11200673798599217195884
- CrooksEAGeldmacherDSInterdisciplinary approaches to Alzheimer’s disease managementClin Geriatr Med2200420112113915062491
- CamicioliRDistinguishing Different DementiasCanadian Review of Alzheimer’s Disease and Other Dementias200693411
- PetersenRCStevensJCGanguliMTangalosEGCummingsJLDeKoskySTPractice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of NeurologyNeurology5820015691133114211342677
- BrooksLGLoewensteinDAAssessing the progression of mild cognitive impairment to Alzheimer’s disease: current trends and future directionsAlzheimers Res Ther2010252820920147
- PalmerKWangHXBackmanLWinbladBFratiglioniLDifferential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen ProjectAm J Psychiatry32002159343644211870008
- WinbladBPalmerKKivipeltoMMild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive ImpairmentJ Intern Med92004256324024615324367
- ForlenzaOVChiuEMild cognitive impairment: a concept ready to move on?Curr Opin Psychiatry11200821652953218852557
- ForlenzaOVDinizBSGattazWFDiagnosis and biomarkers of predementia in Alzheimer’s diseaseBMC Med12222010818921176189
- ForlenzaOVDinizBSNunesPVMemoriaCMYassudaMSGattazWFDiagnostic transitions in mild cognitive impairment subtypesInt Psychogeriatr1220092161088109519691909
- HaroutunianVHoffmanLBBeeriMSIs there a neuropathology difference between mild cognitive impairment and dementia?Dialogues Clin Neurosci200911217117919585952
- FeldmanHHJacovaCMild cognitive impairmentAm J Geriatr Psychiatry8200513864565516085780
- FeldmanHLevyARHsiungGYA Canadian cohort study of cognitive impairment and related dementias (ACCORD): study methods and baseline resultsNeuroepidemiologySep-Oct200322526527412902621
- HsiungGYDonaldAGrandJOutcomes of cognitively impaired not demented at 2 years in the Canadian Cohort Study of Cognitive Impairment and Related DementiasDement Geriatr Cogn Disord2006225–641342016966831
- MinatiLEdgintonTBruzzoneMGGiacconeGCurrent concepts in Alzheimer’s disease: a multidisciplinary reviewAm J Alzheimers Dis Other DemenApr-May20092429512119116299
- ReadyREOttBRGraceJCahn-WeinerDAApathy and executive dysfunction in mild cognitive impairment and Alzheimer diseaseAm J Geriatr PsychiatryMar-Apr200311222222812611752
- ArnaizEAlmkvistONeuropsychological features of mild cognitive impairment and preclinical Alzheimer’s diseaseActa Neurol Scand Suppl2003179344112603249
- NelsonAPO’ConnorMGMild cognitive impairment: a neuropsychological perspectiveCNS Spectr12008131566418204415
- GriffithHRBelueKSicolaAImpaired financial abilities in mild cognitive impairment: a direct assessment approachNeurology211200360344945712578926
- PlassmanBLWilliamsJWJrBurkeJRHolsingerTBenjaminSSystematic review: factors associated with risk for and possible prevention of cognitive decline in later lifeAnn Intern Med832010153318219320547887
- FeldmanHHWoodwardMThe staging and assessment of moderate to severe Alzheimer diseaseNeurology200565Suppl 3S10S17
- DuboisBFeldmanHHJacovaCResearch criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteriaLancet Neurol820076873474617616482
- TallbergIMAlmkvistOConfabulation and memory in patients with Alzheimer’s diseaseJ Clin Exp Neuropsychol4200123217218411309671
- JacovaCKerteszABlairMFiskJDFeldmanHHNeuropsychological testing and assessment for dementiaAlzheimers Dement1020073429931719595951
- CerhanJHIvnikRJSmithGETangalosECPetersenRCBoeveBFDiagnostic utility of letter fluency, category fluency, and fluency difference scores in Alzheimer’s diseaseClin Neuropsychol22002161354211992224
- VlietECManlyJTangMXMarderKBellKSternYThe neuropsychological profiles of mild Alzheimer’s disease and questionable dementia as compared to age-related cognitive declineJ Int Neuropsychol Soc720039572073212901778
- LyketsosCGLeeHBDiagnosis and treatment of depression in Alzheimer’s disease. A practical update for the clinicianDement Geriatr Cogn Disord2004171–2556414564126
- FarcnikKPersykoMSAssessment, measures and approaches to easing caregiver burden in Alzheimer’s diseaseDrugs Aging200219320321512027778
- AmanoNInuzukaSOgiharaTBehavioral and psychological symptoms of dementia and medical treatmentPsychogeriatrics6200992454919604324
- Association APDiagnostic and statistical manual of mental disordersRevised 4th edWashington, DC2000
- McKhannGDrachmanDFolsteinMKatzmanRPriceDStadlanEMClinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s DiseaseNeurology719843479399446610841
- GalaskoDHansenLAKatzmanRClinical-neuropathological correlations in Alzheimer’s disease and related dementiasArch Neurol919945198888958080388
- StoreyESlavinMJKinsellaGJPatterns of cognitive impairment in Alzheimer’s disease: assessment and differential diagnosisFront Biosci5120027e155e18411991855
- RomanGCTatemichiTKErkinjunttiTVascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International WorkshopNeurology219934322502608094895
- KalariaRNMaestreGEArizagaRAlzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factorsLancet Neurol920087981282618667359
- SavvaGMStephanBCEpidemiological studies of the effect of stroke on incident dementia: a systematic reviewStroke12010411e41e4619910553
- PinkstonJBAlekseevaNGonzalez ToledoEStroke and dementiaNeurol Res10200931882483119723451
- PotluriRNatalwalaAUppalHHeunRDifferent risk factors in vascular dementia and ischaemic strokeNeuroepidemiology20093218019001801
- SharpSIAarslandDDaySSonnesynHBallardCHypertension is a potential risk factor for vascular dementia: systematic reviewInt J Geriatr Psychiatry12292010
- DuronEHanonOHypertension, cognitive decline and dementiaArch Cardiovasc Dis32008101318118918477946
- DeCarliCThe role of cerebrovascular disease in dementiaNeurologist520039312313612808409
- BlackSGaoFBilbaoJUnderstanding white matter disease: imagingpathological correlations in vascular cognitive impairmentStroke32009403 SupplS48S5219064767
- EnglundENeuropathology of white matter lesions in vascular cognitive impairmentCerebrovasc Dis200213Suppl 2111511901237
- Kearney-SchwartzARossignolPBracardSVascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaintsStroke420094041229123619246701
- AggarwalNTDecarliCVascular dementia: emerging trendsSemin Neurol22007271667717226743
- RomanGCVascular dementia may be the most common form of dementia in the elderlyJ Neurol Sci11152002203–204710
- RomanGCDefining dementia: clinical criteria for the diagnosis of vascular dementiaActa Neurol Scand Suppl20021786912492785
- ChuiHCSubcortical ischemic vascular dementiaNeurol Clin82007253717740vi17659187
- BenistySHernandezKViswanathanADiagnostic criteria of vascular dementia in CADASILStroke3200839383884418258841
- HachinskiVPreventable senility: a call for action against the vascular dementiasLancet912199234088206456481355217
- SmallGWRevised Ischemic Score for diagnosing multi-infarct dementiaJ Clin Psychiatry12198546125145174066617
- Organization WHICD-10 Classifications of Mental and Behavioral Disorder: Clinical Descriptions and Diagnostic GuidelinesGenevaWorld Health Organization1992
- SnowdenJNearyDMannDFrontotemporal lobar degeneration: clinical and pathological relationshipsActa Neuropathol720071141313817569065
- NearyDSnowdenJSGustafsonLFrontotemporal lobar degeneration: a consensus on clinical diagnostic criteriaNeurology121998516154615549855500
- McKhannGMAlbertMSGrossmanMMillerBDicksonDTrojanowskiJQClinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s DiseaseArch Neurol11200158111803180911708987
- Clinical and neuropathological criteria for frontotemporal dementiaThe Lund and Manchester GroupsJ Neurol Neurosurg Psychiatry419945744164188163988
- ArvanitakisZUpdate on frontotemporal dementiaNeurologist12010161162220065792
- PiguetOBrooksWSHallidayGMSimilar early clinical presentations in familial and non-familial frontotemporal dementiaJ Neurol Neurosurg Psychiatry12200475121743174515548495
- RatnavalliEBrayneCDawsonKHodgesJRThe prevalence of frontotemporal dementiaNeurology611200258111615162112058088
- Graff-RadfordNRWoodruffBKFrontotemporal dementiaSemin Neurol22007271485717226741
- McKeithIDementia with Lewy bodiesHandb Clin Neurol20078453154818808969
- TiraboschiPSalmonDPHansenLAHofstetterRCThalLJCorey-BloomJWhat best differentiates Lewy body from Alzheimer’s disease in early-stage dementia?Brain32006129Pt 372973516401618
- McKeithIGDicksonDWLoweJDiagnosis and management of dementia with Lewy bodies: third report of the DLB ConsortiumNeurology1227200565121863187216237129
- HardingAJBroeGAHallidayGMVisual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobeBrain22002125Pt 239140311844739
- FermanTJBoeveBFSmithGEDementia with Lewy bodies may present as dementia and REM sleep behavior disorder without parkinsonism or hallucinationsJ Int Neuropsychol Soc1120028790791412405541
- VerheyFRJollesJPondsRWDiagnosing dementia: a comparison between a monodisciplinary and a multidisciplinary approachJ Neuropsychiatry Clin NeurosciWinter19935178858428140
- KeoughJHuebnerRATreating dementia: the complementing team approach of occupational therapy and psychologyJ Psychol72000134437539110908071
- ColerickEJGeorgeLKPredictors of institutionalization among caregivers of patients with Alzheimer’s diseaseJ Am Geriatr Soc719863474934983722665
- GauglerJEKaneRLKaneRANewcomerREarly community-based service utilization and its effects on institutionalization in dementia caregivingGerontologist4200545217718515799982
- CaronCDDucharmeFGriffithJDeciding on institutionalization for a relative with dementia: the most difficult decision for caregiversCan J AgingSummer200625219320516821201
- ActonGJWinterMAInterventions for family members caring for an elder with dementiaAnnu Rev Nurs Res20022014917912092509
- AminzadehFDalzielWBMolnarFJGarciaLJSymbolic meaning of relocation to a residential care facility for persons with dementiaAging Ment Health5200913348749619484613
- CutchinMPThe process of mediated aging-in-place: a theoretically and empirically based modelSoc Sci Med920035761077109012878107
- GitlinLNConducting research on home environments: lessons learned and new directionsGerontologist10200343562863714570959
- BuhrGTKuchibhatlaMClippECCaregivers’ reasons for nursing home placement: clues for improving discussions with families prior to the transitionGerontologist22006461526116452284
- BirksJCholinesterase inhibitors for Alzheimer’s diseaseCochrane Database Syst Rev20061CD00559316437532
- HardyJAHigginsGAAlzheimer’s disease: the amyloid cascade hypothesisScience410199225650541841851566067
- SaddichhaSPandeyVAlzheimer’s and non-alzheimer’s dementia: a critical review of pharmacological and nonpharmacological strategiesAm J Alzheimers Dis Other DemenApr-May200823215016118332476
- QuerfurthHWLaFerlaFMAlzheimer’s diseaseN Engl J Med1282010362432934420107219
- RogawskiMAWenkGLThe neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s diseaseCNS Drug RevFall20039327530814530799
- ReisbergBDoodyRStofflerASchmittFFerrisSMobiusHJMemantine in moderate-to-severe Alzheimer’s diseaseN Engl J Med432003348141333134112672860
- In’t VeldBARuitenbergAHofmanAStrickerBHBretelerMMAntihypertensive drugs and incidence of dementia: the Rotterdam StudyNeurobiol AgingMay-Jun200122340741211378246
- JosifSGrahamKDiagnosis and treatment of dementia with Lewy bodiesJAAPA52008215222618556885
- PerryRJMillerBLBehavior and treatment in frontotemporal dementiaNeurology620015611 Suppl 4S46S5111402151
- BoxerALBoeveBFFrontotemporal dementia treatment: current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studiesAlzheimer Dis Assoc DisordOct-Dec2007214S79S8718090429
- LovdenMBackmanLLindenbergerUSchaeferSSchmiedekFA theoretical framework for the study of adult cognitive plasticityPsychol Bull72010136465967620565172
- ClareLWoodsRTMoniz CookEDOrrellMSpectorACognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementiaCochrane Database Syst Rev20034CD00326014583963
- WoodsBSpectorAJonesCOrrellMDaviesSReminiscence therapy for dementiaCochrane Database Syst Rev20052CD00112015846613
- NealMBriggsMValidation therapy for dementiaCochrane Database Syst Rev20033CD00139412917907
- NetzYWuMJBeckerBJTenenbaumGPhysical activity and psychological well-being in advanced age: a meta-analysis of intervention studiesPsychol Aging6200520227228416029091
- SpirdusoWWCroninDLExercise dose-response effects on quality of life and independent living in older adultsMed Sci Sports Exerc62001336 SupplS598S608 discussion S609–S510.11427784
- ChangJTMortonSCRubensteinLZInterventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trialsBMJ3202004328744168015031239
- KeysorJJDoes late-life physical activity or exercise prevent or minimize disablement? A critical review of the scientific evidenceAm J Prev Med102003253 Suppl 212913614552936
- LemuraLMvon DuvillardSPMookerjeeSThe effects of physical training of functional capacity in adults. Ages 46 to 90: a meta-analysisJ Sports Med Phys Fitness3200040111010822903
- ChurchillJDGalvezRColcombeSSwainRAKramerAFGreenoughWTExercise, experience and the aging brainNeurobiol AgingSep-Oct200223594195512392797
- SwainRAHarrisABWienerECProlonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the ratNeuroscience200311741037104612654355
- ForbesDForbesSMorganDGMarkle-ReidMWoodJCulumIPhysical activity programs for persons with dementiaCochrane Database Syst Rev20083CD00648918646158
- SchreinzerDBallabanTBrannathWComponents of behavioral pathology in dementiaInt J Geriatr Psychiatry2200520213714515660411
- EdellWSTunisSLAntipsychotic treatment of behavioral and psychological symptoms of dementia in geropsychiatric inpatientsAm J Geriatr PsychiatrySummer20019328929711481138
- De MedeirosKRobertPGauthierSThe Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementiaInt Psychogeriatr9201022698499420594384
- Vilalta-FranchJLopez-PousaSTuron-EstradaASyndromic association of behavioral and psychological symptoms of dementia in Alzheimer disease and patient classificationAm J Geriatr Psychiatry5201018542143220220583
- RobertPHVerheyFRByrneEJGrouping for behavioral and psychological symptoms in dementia: clinical and biological aspects. Consensus paper of the European Alzheimer disease consortiumEur Psychiatry11200520749049616310680
- SteinbergMShaoHZandiPPoint and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County StudyInt J Geriatr Psychiatry2200823217017717607801
- FeldmanHScheltensPScarpiniEBehavioral symptoms in mild cognitive impairmentNeurology41320046271199120115079026
- LopezOLBeckerJTSweetRAPsychiatric symptoms vary with the severity of dementia in probable Alzheimer’s diseaseJ Neuropsychiatry Clin NeurosciSummer200315334635312928511
- FinkelSIBehavioral and psychologic symptoms of dementiaClin Geriatr Med11200319479982415024813
- GauthierSCummingsJBallardCManagement of behavioral problems in Alzheimer’s diseaseInt Psychogeriatr5201022334637220096151
- OnishiJSuzukiYUmegakiHBehavioral, psychological and physical symptoms in group homes for older adults with dementiaInt Psychogeriatr32006181758616388705
- TuckerIManagement of inappropriate sexual behaviors in dementia: a literature reviewInt Psychogeriatr8201022568369220226113
- AlgaseDLBeckDKolanowskiAWhallABerentSRichardsKNeed-driven dementia-compromised behavior: an alternative view of disruptive behaviorAm J Alzheimers Dis19961161019
- HallGRBuckwalterKCProgressively lowered stress threshold: a conceptual model for care of adults with Alzheimer’s diseaseArch Psychiatr Nurs121987163994063426250
- SpectorAOrrellMUsing a biopsychosocial model of dementia as a tool to guide clinical practiceInt Psychogeriatr9201022695796520561384
- KaneRALong-term care and a good quality of life: bringing them closer togetherGerontologist6200141329330411405425
- KirschenbaumHCarl Rogers’ Life and Work: An Assessment on the 100th Anniversary of His BirthJournal of Counseling and Development2004821116124
- BoiseLWhiteDThe family’s role in person-centered care: practice considerationsJ Psychosoc Nurs Ment Health Serv52004425122015182046
- TalericoKAO’BrienJASwaffordKLPerson-centered care. An important approach for 21st century health careJ Psychosoc Nurs Ment Health Serv11200341111216
- FosseyJBallardCJuszczakEEffect of enhanced psychosocial care on antipsychotic use in nursing home residents with severe dementia: cluster randomised trialBMJ412006332754475676116543297
- ChenowethLKingMTJeonYHCaring for Aged Dementia Care Resident Study (CADRES) of person-centred care, dementia-care mapping, and usual care in dementia: a cluster-randomised trialLancet Neurol420098431732519282246
- EdvardssonDWinbladBSandmanPOPerson-centred care of people with severe Alzheimer’s disease: current status and ways forwardLancet Neurol420087436236718339351
- DeudonAMaubourguetNGervaisXNon-pharmacological management of behavioural symptoms in nursing homesInt J Geriatr Psychiatry12200924121386139519370714
- TeriLLogsdonRGUomotoJMcCurrySMBehavioral treatment of depression in dementia patients: a controlled clinical trialJ Gerontol B Psychol Sci Soc Sci71997524P159P1669224439
- Cohen-MansfieldJNonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critiqueAm J Geriatr PsychiatryFall20019436138111739063
- TurnerSBehavioural symptoms of dementia in residential settings: a selective review of non-pharmacological interventionsAging Ment Health32005929310415804626
- McAllisterCLSilvermanMACommunity formation and community roles among persons with Alzheimer’s disease: a comparative study of experiences in a residential Alzheimer’s facility and a traditional nursing homeQual Health Res1199991658510558359
- SloanePDMitchellCMPreisserJSPhillipsCCommanderCBurkerEEnvironmental correlates of resident agitation in Alzheimer’s disease special care unitsJ Am Geriatr Soc719984678628699670873
- ZeiselJSilversteinNMHydeJLevkoffSLawtonMPHolmesWEnvironmental correlates to behavioral health outcomes in Alzheimer’s special care unitsGerontologist10200343569771114570966
- RaglioABellelliGTraficanteDEfficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementiaAlzheimer Dis Assoc DisordApr-Jun200822215816218525288
- HolmesCKnightsADeanCHodkinsonSHopkinsVKeep music live: music and the alleviation of apathy in dementia subjectsInt Psychogeriatr12200618462363016805928
- GuetinSPortetFPicotMCEffect of music therapy on anxiety and depression in patients with Alzheimer’s type dementia: randomised, controlled studyDement Geriatr Cogn Disord2009281364619628939
- SvansdottirHBSnaedalJMusic therapy in moderate and severe dementia of Alzheimer’s type: a case-control studyInt Psychogeriatr12200618461362116618375
- GerdnerLATop cited papers in International Psychogeriatrics: 4. Effects of individualized vs classical “relaxation” music on the frequency of agitation in elderly persons with Alzheimer’s disease and related disordersInt Psychogeriatr8200921466767119366486
- SmallBJMacDonaldSWSIserLBackmanLMemory and cognitive performance in preclinical Alzheimer’s Disease and preclinical Vascular DiseaseE DereAENadelLHustonJPHandbook of Episodic MemoryNew YorkElsevier2008537552
- WinbladBKilanderLErikssonSDonepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled studyLancet41200636795161057106516581404
- BackmanLJonesSBergerAKLaukkaEJSmallBJCognitive impairment in preclinical Alzheimer’s disease: a meta-analysisNeuropsychology7200519452053116060827
- BackmanLJonesSSmallBJAguero-TorresHFratiglioniLRate of cognitive decline in preclinical Alzheimer’s disease: the role of comorbidityJ Gerontol B Psychol Sci Soc Sci72003584228236
- PalmerKBackmanLWinbladBFratiglioniLDetection of Alzheimer’s disease and dementia in the preclinical phase: population based cohort studyBMJ212003326738324512560271
- HultschDFHertzogCDixonRASmallBJMemory change in the agedNew YorkCambridge University Press1998
- BackmanLForsellYEpisodic memory functioning in a community-based sample of old adults with major depression: utilization of cognitive supportJ Abnorm Psychol5199410323613708040505
- CalvaresiEBryanJB vitamins, cognition, and aging: a reviewJ Gerontol B Psychol Sci Soc Sci112001566P327P33911682586
- WilsonRSBeckettLABarnesLLIndividual differences in rates of change in cognitive abilities of older personsPsychol Aging6200217217919312061405
- FahlanderKWahlinAAlmkvistOBackmanLCognitive functioning in Alzheimer’s disease and vascular dementia: further evidence for similar patterns of deficitsJ Clin Exp Neuropsychol9200224673474412424648
- ThorvaldssonVMacdonaldSWFratiglioniLOnset and rate of cognitive change before dementia diagnosis: findings from two Swedish population-based longitudinal studiesJ Int Neuropsychol Soc1201117115416221083966
- HallCBLiptonRBSliwinskiMStewartWFA change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s diseaseStat Med615–3020001911–121555156610844718
- HallCBDerbyCLeValleyAKatzMJVergheseJLiptonRBEducation delays accelerated decline on a memory test in persons who develop dementiaNeurology1023200769171657166417954781
- SmithGEPankratzVSNegashSA plateau in pre-Alzheimer memory decline: evidence for compensatory mechanisms?Neurology710200769213313917620545
- SternYCognitive Reserve: Theory and ApplicationsNew York, NYTaylor & Francis2007
- SmallBJHerlitzAFratiglioniLAlmkvistOBackmanLCognitive predictors of incident Alzheimer’s disease: a prospective longitudinal studyNeuropsychology719971134134209223145
- ChenPRatcliffGBelleSHCauleyJADeKoskySTGanguliMPatterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community studyArch Gen Psychiatry9200158985385811545668
- JagustWGitchoASunFKuczynskiBMungasDHaanMBrain imaging evidence of preclinical Alzheimer’s disease in normal agingAnn Neurol4200659467368116470518
- SpulberGNiskanenEMacDonaldSWhole brain atrophy rate predicts progression from MCI to Alzheimer’s diseaseNeurobiol Aging920103191601160518829136
- RoweCCAckermanUBrowneWImaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanismLancet Neurol220087212913518191617
- HuangJAuchusAPDiffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer’s diseaseAnn N Y Acad Sci22007109725926417413027
- SmallBJRosnickCBFratiglioniLBackmanLApolipoprotein E and cognitive performance: a meta-analysisPsychol Aging12200419459260015584785
- FratiglioniLPaillard-BorgSWinbladBAn active and socially integrated lifestyle in late life might protect against dementiaLancet Neurol620043634335315157849
- CroweMAndelRPedersenNLJohanssonBGatzMDoes participation in leisure activities lead to reduced risk of Alzheimer’s disease? A prospective study of Swedish twinsJ Gerontol B Psychol Sci Soc Sci92003585P249P25514507930
- RovioSKareholtIHelkalaELLeisure-time physical activity at midlife and the risk of dementia and Alzheimer’s diseaseLancet Neurol11200541170571116239176
- GroberEHallCBLiptonRBZondermanABResnickSMKawasCMemory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s diseaseJ Int Neuropsychol Soc3200814226627818282324
- JormAProspects for the prevention of dementiaAustralian Journal of Ageing200221913
- Canada Alzheimer SocietyRising Tide: The Impact of Dementia on Canadian Society2010 www.alzheimer.ca/english/rising_tide/rising_tide_summary.htm. Accessed January 23, 2011.