Abstract
Objectives
We aimed to evaluate the efficacy, safety, and diagnostic utility of transbronchial cryobiopsy (TBCB) in diagnosing diffuse parenchymal lung diseases (DPLDs) in an Egyptian population and to identify common DPLD pathologies among them.
Methods
This prospective interventional study enrolled 25 Egyptian patients presenting to the Main Alexandria University Hospital who had clinical and radiological features of DPLD, but insufficient elements to achieve definite features of usual interstitial pneumonia on chest high-resolution computed tomography. Twelve patients were subjected to TBCB and 13 to forceps transbronchial lung biopsy (TBLB).
Results
The diagnostic yield was significantly higher among the TBCB group (83.3%), and increased to 100% with clinicopathological correlation vs the TBLB group (38.5%, P=0.041). Granulomatous diseases (24%, either sarcoidosis or hypersensitivity pneumonitis) were the commonest pathology, followed by malignancy (12%) in both groups. TBCB sizes were 2.5–5 mm vs 1-3 mm in TBLB (P<0.001), with preserved tissue architecture (91.7% vs 38.5%, respectively; P=0.011). Only 8.3% were complicated by insignificant bleeding grade 2 after TBCB, but no pneumothorax was detected.
Conclusion
TBCB is a safe, tolerable procedure with high diagnostic yield for evaluating DPLD with indefinite usual interstitial pneumonia pattern on high-resolution computed tomography.
Introduction
Diffuse parenchymal lung diseases (DPLDs) are a heterogeneous group of disorders that predominantly affect the lung parenchyma and vary widely in etiology, clinicoradiological presentation, histopathologic features, clinical course, and prognosis.Citation1,Citation2 Among DPLDs, idiopathic pulmonary fibrosis (IPF) is the commonest form and carries a bad prognosis.Citation3 However, a definite usual interstitial pneumonia (UIP) pattern is detected in only 50% of patients on high-resolution computed tomography (HRCT), while the remaining 50% of cases require histological diagnosis by surgical lung biopsy (LB).Citation4
Surgical LB is the gold method to identify UIP histologically from, other forms of idiopathic interstitial pneumonias (IIPs) and other interstitial lung diseases (ILDs) as granulomatous diseases affecting the lungs and ILD secondary to known causes that can mimic IPF.Citation3,Citation5 However, surgical LB is associated with potential morbidity and significant risk of mortality despite being low.Citation6,Citation7 Bronchoscopy is commonly employed in the diagnostic evaluation of DPLD, but transbronchial LB (TBLB) specimens have low diagnostic yield,Citation8 due to small specimens and associated artifacts (especially crush artifact)Citation9 rendering specimens inadequate and nondiagnostic fragments.
Cryobiopsy (CB), a new bronchoscopic biopsy method, has recently demonstrated superior diagnostic yield to conventional forceps biopsy,Citation10,Citation11 due to larger tissue specimens extracted with the freeze–thaw cycle. Preliminary data has shown that this technique is safe and the occurrence of pneumothorax and bleeding are comparable to regular forceps biopsies.Citation10,Citation11 In the current study, we aimed to evaluate the efficacy, safety, and diagnostic utility of transbronchial CB (TBCB) in diagnosing DPLD other than radiologically definite UIP in an Egyptian population. Further, we aimed to identify common DPLD pathologies among this population.
Methods
Design
This prospective interventional study enrolled 25 Egyptian patients who had clinical and radiological features of DPLD, but insufficient elements to achieve definite features of UIP patterns on chest HRCT according to current international consensus criteria.Citation4 Patients were randomly divided into two groups: one subjected to TBCB (12 patients) and the other subjected to ordinary forceps TBLB (13 patients). The study was conducted in the Main Alexandria University Hospital, Egypt between December 2015 and October 2016, approved by the local ethics committee of Alexandria University, and adhered Declaration of Helsinki principles. All patients signed a formal consent to undergo bronchoscopic procedures and participate.
Population
Patients with DPLD who had features of possible UIP patterns or patterns inconsistent with UIP on HRCT defined in American Thoracic Society–European Respiratory Society–Japanese Respiratory Society–Latin American Thoracic Association guidelinesCitation4 were recruited. All patients were discussed in a multidisciplinary conference (including pulmonology, radiology, pathology, and oncology) before being assigned to bronchoscopy and biopsy. Among eligible patients, those with systolic pulmonary arterial pressure (sPAP) >40 mmHg, coagulopathy (ie, platelet count <70,000/µL, international normalized ratio ≥1.5), FEV1 <0.8 L or FVC <50% of predicted normal value, diffuse bullous disease, hemodynamic instability, and severe hypoxemia uncorrected by oxygen therapy were excluded from the study. Further, all patients with UIP patterns on HRCT (ie, subpleural- and basal-predominant, honeycombing with or without peripheral traction bronchiectasis or bronchiolectasis)Citation4 were excluded from the study.
All patients in both groups were subjected to detailed history-taking, including age, sex, smoking status, comorbidities, occupational and exposure history, and full clinical examination. Spirometry was performed before the procedure. Arterial blood gases were requested if indicated. Echocardiography was performed for assessment of sPAP using a modified Bernoulli equation (sPAP = 4 × tricuspid regurgitation velocity2).
Bronchoscopy
All patients were subjected to fiberoptic bronchoscopy under conscious sedation (midazolam 3–7 mg intravenously) if TBLB were to be taken. Bronchoalveolar lavage (BAL) was collected from all patients. Briefly, 100 mL sterile saline as 5×20 aliquots was injected through the working channel in the selected affected segment, then aspirated and put in sterile containers for culture, sensitivity, differential cell count, and cytology.Citation12 TBLBs had been taken from selected segments previously chosen according to HRCT results. Four to six biopsies were taken and fixed in formaldehyde for histopathological evaluation. During bronchoscopy, all patients were fully monitored for oxygen saturation (SaO2), electrocardiography, and blood pressure. Oxygen therapy was given during the procedure in order to keep SaO2 ≥92%.
Transbronchial cryobiopsy
TBCBs were performed under general anesthesia. Bronchoscopy was introduced through a Portex cuffed endotracheal tube, which allows rapid reentry into the airway after the biopsy. A flexible cryoprobe with a diameter of 2.4 mm was used (ERBE, Tübingen, Germany). The cryoprobe was introduced into the selected area previously chosen according to HRCT with approximately 2 cm from the thoracic wall under radiological guidance using real-time fluoroscopy (mobile C-arm; Siemens, Munich, Germany). The cryoprobe was cooled to a temperature of about –77 °C in its position using carbon dioxide (Erbokryo CA; ERBE, Tübingen, Germany) for 6 seconds, then retracted with frozen lung tissue attached. Both probe and bronchoscope were removed en bloc from the airway.Citation13 Extended probes and frozen specimens were submerged in room-temperature saline, then the bronchoscope was reintroduced into the airway to clear bleeding by wedging into the biopsied subsegment for 3 minutes. A Fogarty catheter was used to control bleeding when it occurred. Two to four biopsies were taken for histopathological evaluation. All patients were followed up for 24 hours after the procedure as plain chest X-rays were obtained.
Histopathological evaluation
All biopsies collected were fixed in 4% buffered formalin. For each case, 5 μm–thick sections were obtained from formalin-fixed paraffin-embedded blocks. These sections were examined under light microscopy after staining using H&E. Slides were examined using different magnifications for various histopathological features, including size and adequacy of specimen, presence of emphysema, atelectasis, interstitial, and intra-alveolar inflammatory infiltrates, interstitial fibrosis and distribution, bronchiolar epithelial lining, and inflammation.
Cytopathology of BAL
Samples were centrifuged at 600 xg, the supernatant discarded, and cell pellets smeared on glass slides. Slides were immediately immersed in 95% ethanol for fixation, then stained using H&E. All samples were examined under light microscopy to assess sample adequacy, the nature of the cell population, and configuration.
Data analysis
Qualitative data are described using numbers and percentages, while quantitative data are described using means ± SD or range and medians according to the normality of distribution of the data. Fisher’s exact, χ2, MonteCarlo correction, Student's t, and Mann-Whitney tests were used as appropriate. SPSS version 20.0 (IBM, Armonk, NY, USA) was used for all statistical analysis. Significance of the obtained results was judged at the 5% level (ie, P<0.05 was considered significant and P>0.05 nonsignificant).
Results
shows the baseline characteristics of all patients. Twelve patients underwent TBCB, while 13 were subjected to TBLB. In both groups, females predominated (>65%), with mean age of 45.58±9.21 and 41.54±15.19 years, respectively, with no statistically significant difference. The majority of patients (69.2% and 75% of TBLB and TBCB groups, respectively) showed restrictive patterns on spirometry without significant differences when FVC, FEV1, and FEV1/FVC were compared. Median SaO2 in room air was 97% (87%–97%) and 95% (86%–97%) in the TBLB and TBCB groups, respectively, with a statistically significant difference (P=0.009, ). All patients in both groups presented with exertional dyspnea and dry or productive cough. Five patients (20%) had fever, while three (12%) had chest pain. The median duration of symptoms was 60 days in the TBLB group vs 120 days in the TBCB group, with no statistically significant difference (P=0.054).
Table 1 Baseline characteristics of patients studied
HRCT findings
shows the radiological findings seen on HRCT. Ground-glass opacity and interstitial thickening were the most commonly seen abnormalities in both groups, followed by consolidation and nodules, with no statistically significant difference. Mediastinal/hilar lymphadenopathy was not uncommon in the TBCB group and significantly higher (P=0.028). Mediastinal/hilar lymphadenopathy was seen in cases of sarcoidosis, malignancy, nonspecific interstitial pneumonia (NSIP), and hypersensitivity pneumonitis. However, only two of eight patients had significant lymph nodes >1 cm, sampled with conventional transbronchial needle aspiration, and were not diagnostic.
Table 2 Radiological findings on HRCT in both groups
Bronchoalveolar lavage
shows predominant cellular components of BAL collected and its correlations with the final diagnosis. BAL in cases of ILD secondary to connective disease and hypersensitivity pneumonitis was mainly lymphocytic, while BAL of follicular bronchiolitis and cryptogenic organizing pneumonia was predominantly neutrophilic (). BAL results were mainly neutrophilic in cases with an indefinite diagnosis in the TBLB group.
Table 3 Final diagnosis and predominant cellular BAL findings in each
TBLB and TBCB
The size of biopsies was 1–3 mm with a mean of 1.46±0.66 mm for TBLBs vs 2.5–5 mm, with a mean of 3.88±1.19 mm for TBCBs (P<0.001). Also, adequate well-preserved tissue architecture of TBCBs was seen in eleven patients (91.7%) vs only five (38.5%) in the TBLB group (P=0.011). There was a statistically significant difference between the groups regarding for final diagnosis, whereas ten patients (83.3%) had definite diagnoses in the TBCB group vs five (38.5%) in the TBLB group (P=0.041).
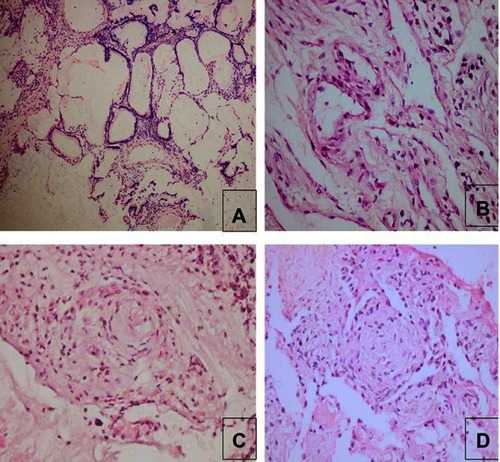
In the TBLB group, noncaseating granuloma, mostly sarcoidosis, was commonest (15.4%, and ), with 7.7% showing nonspecific patterns of ILD considered secondary to connective tissue disease (systemic sclerosis) based on clinical history and HRCT pattern improving the diagnostic yield of TBLB to 46.2%. In the TBCB group, hypersensitivity pneumonitis was the commonest diagnosis (25%) followed by malignancy (16.7%), metastatic mucinous carcinoma from the ovary or pulmonary adenocarcinoma ( and ). However, a specific diagnosis could not be reached in two patients (16.7%), as the biopsy showed only interstitial fibrosis. They were referred to as ILD secondary to connective tissue disease (rheumatoid arthritis) and ILD secondary to occupational exposure, based on clinical and radiological data.
Figure 1 Histopathological examples of those with proved diagnosis in forceps transbronchial lung biopsy group.
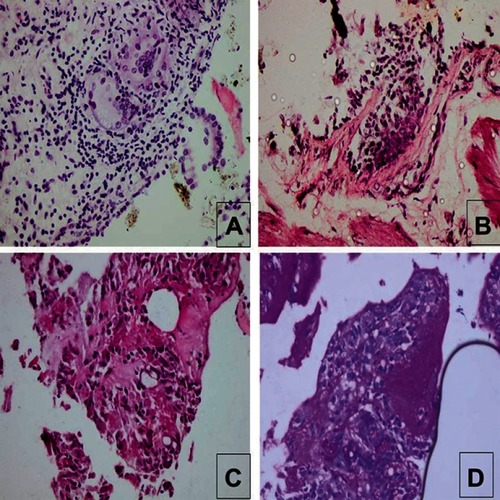
Figure 2 Histopathological examples of those with proved diagnosis in transbronchial cryobiopsy group.
Safety
We did not encounter any complications after TBLB, and only one patient (8.3%) had mild bleeding after TBCB that was controlled intraoperatively with segmental blockage using the Fogarty catheter. There were no detected cases of pneumothorax immediately or 24 hours after any of the procedures. There was no statistically significant difference between the groups on occurrence of postprocedure complications (P=0.480).
Discussion
The study found a high diagnostic yield of TBCB (83.3%) in diagnosing DPLD other than UIP patterns on HRCT in an Egyptian population and confirmed the safety of this procedure (only 8.3% had complications in the form of mild controllable bleeding). Further, this study highlights the value of multidisciplinary approach in diagnosing cases with indefinite histopathological features, which improved the diagnostic yield of TBCB to 100%.
Previous studies and interpretation of results
Kropski et alCitation13 and Bondue et alCitation14 studied the validity of TBCB for evaluation of DPLD, and found that the overall diagnostic yield was 80%. Pajares et alCitation10 conducted a randomized clinical trial that compared conventional forceps vs cryoprobe transbronchial biopsy, and reported a higher diagnostic yield in the TBCB group vs conventional forceps TBLB (51.3% vs 29.1%, respectively). Fruchter et alCitation15 reported that definite and probable clinicopathological consensus diagnosis was possible in 70% and 28% of patients, respectively, while a diagnosis could not be established in only 2% of patients. The current results are in agreement with these studies. The significant difference in the diagnostic yield between TBCB and forceps TBLB in the current study and other studies can be explained mainly by two factors: biopsy sizeCitation15,Citation16 and crush artifacts caused by regular forceps.Citation9
Based on the multidisciplinary ie, clinicopathological radiological review, two cases (16.7%) in the TBCB group were referred to as connective tissue–associated ILD and ILD secondary to occupational exposure, as the pathologist could not specify the subtype of the ILD. This in turn improved the diagnostic yield of TBCB from 83.3% to 100%. Further, on multidisciplinary review, the diagnostic yield of TBLB increased from 38.5% to 46.2% with the addition of the case of systemic sclerosis–associated ILD. A multidisciplinary approach is widely recommended in the latest guidelines to reach final diagnosis.Citation4,Citation17
El-Hoffy et alCitation18 found granulomatous diseases were common in their Egyptian cohort (32% of studied cases), being the second-commonest diagnosis after IIPs (46%). Further, Bondue et alCitation14 reported a high percentage of granulomatous diseases in a Belgian population (34%) and NSIP (20%). These results are in agreement with ours, as granulomatous diseases were the commonest pathology seen. However, malignancy was the second-commonest diagnosis, followed by cryptogenic organizing pneumonia and NISP. On the contrary, Kropski et alCitation13 found UIP/IPF to be the commonest diagnosis among their patients. This difference could be attributed to exclusion of UIP/IPF from the current study based on HRCT findings, as recommended, due to high sensitivity of HRCT in diagnosing definite UIP/IPF.Citation4 Further, the currently studied population were younger and the majority female, rather than old men denoting a different studied population.
Regarding BAL differential cell count, the current study findings were in accordance with Jara-Palomares et alCitation19 and Efared et al,Citation20 who found that mixed alveolitis was the commonest pattern in ILD, but lymphocytic alveolitis was common in sarcoidosis and extrinsic allergic alveolitis. Accordingly, BAL is helpful in diagnosing DPLD, and must be always associated with other diagnostic methods; however, LB is still needed to confirm the diagnosis in many circumstances.Citation20,Citation21
Clinical implications
Obtaining an LB using a cryoprobe has the potential to change practice dramatically in the evaluation of patients with DPLD with atypical imaging findings. Tomassetti et alCitation22 found that bronchoscopic LB had diagnostic yield of 91% versus 98% for surgical LB, and recommended that future guidelines should implement CB in the diagnostic algorithm of ILD. Further, TBLB could be still useful if sarcoidosis, infection, pulmonary alveolar proteiniosis, and lymphangitis carcinomatosis are suspected.Citation23 This improved diagnostic yield could be due to centrilobular accentuation with involvement of terminal and respiratory bronchiole (as in respiratory bronchiolitis, infection and organizing pneumonia) or distributed along the lymphatic route (as in sarcoidosis and lymphangitis carcinomatosis).Citation24–Citation26 Further, TBCB can be considered a safe procedure, whereas in the current study mild bleeding occurred in 8.3%, but no pneumothorax. These complications were less common than reported in other studies.Citation10,Citation13–Citation15,Citation27 This could be attributed to negative pulmonary hypertension, which is the most important factor predispose to bleeding during CB,Citation28 avoiding biopsy from the central lung zoneCitation28 and avoiding closeness to the chest wall.
Limitations
The current study had some limitations. Firstly, the sample was relatively small. Secondly, all patients with CBs had to be under general anesthesia and endotracheally intubated. Other research has been conducted with sedation without intubation,Citation15 with a diagnostic yield of 70%. However, this is against the recently published international guidelinesCitation22 that were followed in the current study. Further, intubation ensured rapid reintroduction of bronchoscope after biopsies and allowed better control of postprocedure bleeding.
Conclusion
TBCB had a good diagnostic yield (83%) that increased to 100% when applying a multidisciplinary approach. Further, TBCB was associated with high safety and tolerability, and it is possible to be applied in low-resource areas with experienced hands. TBLB is still associated with good diagnostic yield in cases of sarcoidosis and lymphangitis carcinomatosis.
Acknowledgment
The authors are grateful to the nursing staff in the bronchoscopic unit and anesthetic staff for their cooperation and continuous willingness to share in this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- BT Society, SO Committee. The diagnosis, assessment and treatment of diffuse parenchymal lung disease in adults. Introduction. Thorax. 1999;54(Suppl 1):S1–S28. doi:10.1136/thx.54.suppl_1.s1 11006787
- Ryu JH, Olson EJ, Midthun DE, Swensen SJ. Diagnostic approach to the patient with diffuse lung disease. Mayo Clin Proc. 2002;77(11):1221–1227. doi:10.4065/77.11.122112440558
- Travis WD, Costabel U, Hansell DM, et al. An official American thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi:10.1164/rccm.201308-1483ST24032382
- Wells AU. The revised ATS/ERS/JRS/ALAT diagnostic criteria for idiopathic pulmonary fibrosis (IPF) - practical implications. Respir Res. 2013;14(1):S2. doi:10.1186/1465-9921-14-1923734820
- Ryu JH, Daniels CE, Hartman TE, Yi ES. Diagnosis of interstitial lung diseases. Mayo Clin Proc. 2007;82(8):976–986. doi:10.4065/82.8.97617673067
- Krasna MJ, White CS, Aisner SC, Templeton PA, McLaughlin JS. The role of thoracoscopy in the diagnosis of interstitial lung disease. Ann Thorac Surg. 1995;59(2):348–351. doi:10.1016/0003-4975(94)00844-w7847948
- Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J. 2001;17(2):175–179.11334116
- Poletti V, Patelli M, Poggi S, Bertanti T, Spiga L, Ferracini R. Transbronchial lung biopsy and bronchoalveolar lavage in diagnosis of diffuse infiltrative lung diseases. Respiration. 1988;54(Suppl 1):66–72. doi:10.1159/0001954793231906
- Berbescu EA, Katzenstein AL, Snow JL, Zisman DA. Transbronchial biopsy in usual interstitial pneumonia. Chest. 2006;129(5):1126–1131. doi:10.1378/chest.129.5.112616685001
- Pajares V, Torrego A, Puzo C, Lerma E, Gil De Bernabé MA, Franquet T. Transbronchial lung biopsy using cryoprobes. Arch Bronconeumol. 2010;46(3):111–115. doi:10.1016/j.arbres.2009.09.01219939546
- Yarmus L, Akulian J, Gilbert C, et al. Cryoprobe transbronchial lung biopsy in patients after lung transplantation: a pilot safety study. Chest. 2013;143(3):621–626. doi:10.1378/chest.12-229023328889
- Meyer KC, Raghu G, Baughman RP, et al. An official American thoracic society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–1014. doi:10.1164/rccm.201202-0320ST22550210
- Kropski JA, Pritchett JM, Mason WR, et al. Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLoS One. 2013;8(11):e78674. doi:10.1371/journal.pone.007867424265706
- Bondue B, Pieters T, Alexander P, et al. Role of transbronchial lung cryobiopsies in diffuse parenchymal lung diseases: interest of a sequential approach. Pulm Med. 2017;(2017):6794343.28512583
- Fruchter O, Fridel L, El Raouf BA, Abdel-Rahman N, Rosengarten D, Kramer MR. Histological diagnosis of interstitial lung diseases by cryo-transbronchial biopsy. Respirology. 2014;19(5):683–688. doi:10.1111/resp.1229624750376
- Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21(5):834–841. doi:10.1111/resp.1277026991519
- American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ers executive committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi:10.1164/ajrccm.165.2.ats0111790668
- El-Hoffy MM, Mohalal A, Abd-Elhamid AE, Abd-Elazim H. High resolution multi-detector row computed tomography in imaging of interstitial lung diseases. Alexandria J Med. 2008;44(2):1–7.
- Jara-Palomares L, Martín-Juan J, Gómez-Izquierdo L, Cayuela-Domínguez A, Rodríguez-Becerra E, Rodríguez-Panadero F. Bronchoalveolar lavage findings in patients with diffuse interstitial lung diseases: prospective study of cohort of 562 patients. Arch Bronconeumol. 2009;45(3):111–117. doi:10.1016/j.arbres.2008.04.00519286112
- Efared B, Ebang-Atsame G, Rabiou S, et al. The diagnostic value of the bronchoalveolar lavage in interstitial lung diseases. J Negat Results Biomed. 2017;16(1):4. doi:10.1186/s12952-017-0069-028245857
- Meyer KC, Raghu G. Bronchoalveolar lavage for the evaluation of interstitial lung disease: is it clinically useful? Eur Respir J. 2011;38:761–769. doi:10.1183/09031936.0006950921540304
- Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193(7):745–752. doi:10.1164/rccm.201504-0711OC26562389
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British thoracic society in collaboration with the thoracic society of Australia and New Zealand and the Irish thoracic society. Thorax. 2008;63(Suppl 5):v1–v58. doi:10.1136/thx.2008.10169118757459
- Descombes E, Gardiol D, Leuenberger P. Transbronchial lung biopsy: an analysis of 530 cases with reference to the number of samples. Monaldi Arch Chest Dis. 1997;52(4):324–329.9401359
- Gilman MJ, Wang KP. Transbronchial lung biopsy in sarcoidosis. An approach to determine the optimal number of biopsies. Am Rev Respir Dis. 1980;122(5):721–724. doi:10.1164/arrd.1980.122.5.7217447156
- Glaspole IN, Wells AU, du Bois RM. Lung biopsy in diffuse parenchymal lung disease. Monaldi Arch Chest Dis. 2001;56(3):225–232.11665502
- Cascante JA, Cebollero P, Herrero S, et al. Transbronchial cryobiopsy in interstitial lung disease: are we on the right path? J Bronchology Interv Pulmonol. 2016;23(3):204–209. doi:10.1097/LBR.000000000000029227261937
- Poletti V, Hetzel J. Transbronchial cryobiopsy in diffuse parenchymal lung disease: need for procedural standardization. Respiration. 2015;90(4):275–278. doi:10.1159/00043931326384323
