Abstract
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may offer a safer alternative to their oral counterparts for the management of osteoarthritis. Diclofenac sodium topical solution with dimethyl sulfoxide (TDiclo) was evaluated in five randomized, controlled trials and is indicated for treatment of the signs and symptoms associated with osteoarthritis of the knee. Three studies showed that TDiclo is superior to placebo and vehicle control with respect to pain, physical function, and perception of osteoarthritis symptoms. Two studies showed that benefits are similar to those of oral diclofenac, with one study demonstrating statistical equivalence. The most common adverse event associated with TDiclo in these studies was dry skin. Incidences of gastrointestinal adverse events and abnormal levels of liver enzymes were lower with TDiclo compared with oral diclofenac in active-controlled studies. Based on these studies, TDiclo represents a practical, evidence-based option for the management of osteoarthritis of the knee.
Introduction
Osteoarthritis is the most common form of arthritic disease. It is estimated that 27 million US adults suffer from osteoarthritis, more than nine million of whom have symptomatic osteoarthritis of the knee.Citation1 The prevalence of osteoarthritis is expected to rise as the population ages. Osteoarthritis has a high economic burden, with costs attributable to treatment and the effects of disability and comorbidity, and indirect costs, such as loss of earnings.Citation2
Because there are currently no disease-modifying therapies for osteoarthritis, modern management aims to relieve pain and improve function and health-related quality of life.Citation3–Citation8 Pharmacological management of osteoarthritis usually begins with acetaminophen and progresses to traditional nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) or cyclo-oxygenase-2 (COX-2) selective NSAIDs. Most patients with osteoarthritis are likely to receive oral traditional NSAIDs or COX-2 inhibitors; however, the associated gastrointestinal, renal, and cardiovascular adverse effects of these agents can potentially limit long-term use.Citation9–Citation15 Topical formulations of NSAIDs, recently approved by the US Food and Drug Administration (FDA) for the treatment of osteoarthritis, have the potential to offer a safe alternative to oral NSAIDs due to decreased systemic exposure to the active molecule.Citation16
Several international and national guidelines for the management of osteoarthritis are available,Citation3–Citation8 all of which recommend a similar, multifaceted approach toward treatment for individual patients that involves lifestyle changes as well as pharmacological therapy. These guidelines primarily recommend acetaminophen as first-line therapy for most patients. However, traditional nonselective and COX-2 selective NSAIDs are a mainstay in osteoarthritis management due to their well established efficacy.Citation3–Citation8 Topical analgesics, including topical NSAIDs, are recommended as an alternative therapeutic option in several guidelines, particularly for patients who are at risk for serious gastrointestinal or cardiovascular adverse events, such as elderly patients.Citation3,Citation5,Citation6,Citation17
In 2008, the UK-based National Institute for Clinical Excellence (NICE) published guidance recommending that topical NSAIDs, along with acetaminophen, should be the first pharmacological options in the management of osteoarthritis pain (after nonpharmacological treatment alternatives). NICE prioritizes the use of topical NSAIDs before the addition of oral nonselective or COX-2 selective NSAIDS, or opioid analgesics. The use of oral nonselective or COX-2 selective NSAIDs, starting at the lowest effective dosage and administered with gastroprotective agents regardless of the patient’s risk for gastrointestinal adverse events, is recommended as a second-line treatment.Citation8 The NICE guidelines highlight a move to consider topical NSAIDs as first-line therapy.
Diclofenac is one of the most frequently used NSAIDs in the treatment of osteoarthritis and is the active ingredient in each of the several topical NSAIDs approved for use in the US. Among the several approved topical diclofenac formulations, diclofenac sodium gel 1% and diclofenac sodium topical solution 1.5% (TDiclo) are approved for the treatment of osteoarthritis.Citation18,Citation19 Diclofenac sodium gel is approved for the treatment of the pain of osteoarthritis in joints amenable to topical treatment, whereas TDiclo is approved for the treatment of the signs and symptoms of osteoarthritis of the knee. The purpose of this article is to review currently available data for the TDiclo formulation.
Diclofenac sodium topical solution with DMSO
Each 1 mL of TDiclo contains 16.05 mg diclofenac sodium. TDiclo solution also contains 45.5% dimethyl sulfoxide (DMSO) vehicle, which can result in enhanced penetration of active drug through the skin.Citation20 The mechanism of action of DMSO is complex, including denaturation of proteins and interaction with the polar head groups of some lipid bilayers to loosen the packing geometry of the skin.Citation21,Citation22 In addition, it may facilitate drug partitioning from the formulation into the solvent within the skin tissue.Citation22
Efficacy studies
Five double-blind, randomized, clinical trials of TDiclo have been published ().Citation23–Citation27 Three trials were placebo- controlled and/or vehicle/active-controlled.Citation23–Citation25 One trial included a topical diclofenac arm, an oral diclofenac arm, a topical plus active control (oral diclofenac) arm, a placebo control, and a vehicle control,Citation26 and one trial was an equivalence study comparing topical diclofenac with oral diclofenac.Citation27
Table 1 Overview of randomized, double-blind clinical trials of diclofenac sodium topical solution
Patient demographics
All patients included in the five studies were adults with primary osteoarthritis of the knee based on standard radiological criteria (defined by joint space narrowing and marginal osteophytes in the medial, lateral, and patellofemoral compartments).Citation28 The total number of patients in each study is shown in . The mean age of the included patients was approximately 60–65 years, and approximately 60%–70% in each study were female. Patients applied TDiclo around the affected knee at a dose of 40 drops (about 1.2 mL) four times dailyCitation23–Citation26 or 50 drops (about 1.5 mL) three times daily in the equivalence study.Citation27 Concomitant NSAIDs and other analgesics were not allowed during the studies. Use of rescue acetaminophen was permitted in all studies except the equivalence study,Citation27 with up to four 325 mg tablets per day allowed in three studies,Citation24–Citation26 and up to two 325 mg tablets four times daily allowed in the other study.Citation23 Low-dose aspirin was allowed for cardiovascular prophylaxis in all studies.
In three of the five studies, patients were required to have experienced a flare of pain after washout of previous therapy (). Pain flares were defined as an increase on the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index pain subscale of ≥2 points and 25%, score of ≥2 (moderate) on at least one of the five questions of the pain subscale, and baseline total pain score ≥6.Citation24 The WOMAC Osteoarthritis Index is a validated questionnaire consisting of 24 questions (five about pain, 17 about physical function, and two about stiffness).
Outcome measures
Efficacy variables in the studies included measures of pain, physical function, and patients’ perception of their osteoarthritis symptoms and/or overall health (). It is important to note that this core set of outcome measures complies with the recommendations of the Outcome Measures in Arthritis Clinical Trials (OMERACT) III,Citation29 the Osteoarthritis Research Society International (OARSI),Citation30 and the Group for the Respect of Ethics and Excellence in Science.Citation31 The WOMAC Osteoarthritis Index pain score, the WOMAC Osteoarthritis Index physical function score, and the Patient Global Assessment (PGA) score were coprimary efficacy variables in three of the five studies. One of the remaining studies utilized the Patient Overall Health Assessment (POHA) score instead of the PGA as the third coprimary endpoint. The PGA question asks patients about their osteoarthritis in the target knee during the past 48 hours, whereas the POHA question asks patients how they rated their overall state of health in the past 48 hours in relation to their osteoarthritis knee and its treatment. The fifth study evaluated only the WOMAC pain score as a primary endpoint and evaluated all other scales as a part of its secondary endpoints (). Secondary efficacy variables included the WOMAC stiffness subscale and pain on walking (the first question in the WOMAC pain assessment). All questions were scored on a five-point Likert scale, except in the equivalence study, in which questions were scored on a 100 mm visual analog scale.
Efficacy vs DMSO vehicle or placebo
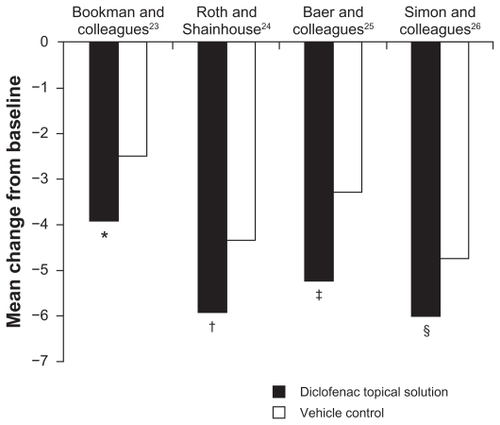
In all four placebo-controlled or vehicle-controlled studies, TDiclo showed statistically significant improvements vs placebo and/or vehicle in WOMAC pain score (). Mean improvement in scores for TDiclo vs vehicle control in each of the four studies was −3.9 vs −2.5 (P = 0.023),Citation23 −5.9 vs −4.3 (P = 0.001),Citation24 −5.2 vs −3.3 (P = 0.003),Citation25 and −6.0 vs −4.7 (P = 0.009).Citation26 The mean percent improvement in WOMAC pain score from baseline to final assessment with TDiclo was 42.9%,Citation23 45.7%,Citation24 40.0%,Citation25 and 45.5%.Citation26 In contrast, the percent change with vehicle or placebo ranged between 26.0% and 36.4%.
Figure 1 Improvement from baseline to final assessment in WOMAC pain score with diclofenac sodium topical solution and vehicle control. Pain score was based on five questions scored on a scale of 0 to 4.
Abbreviation: WOMAC, Western Ontario and McMaster Universities.
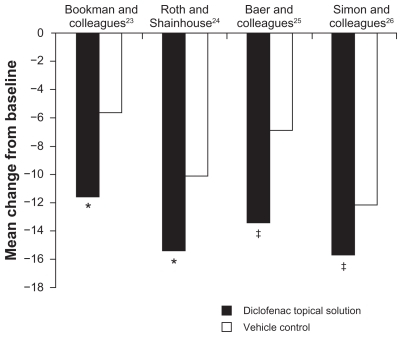
Statistically significant improvements in WOMAC physical function compared with vehicle and/or placebo were also shown in all four studies (). Mean change in scores for TDiclo vs vehicle control in each of the four studies was −11.6 vs −5.7 (P = 0.002),Citation23 −15.4 vs −10.1 (P = 0.002),Citation24 −13.4 vs −6.9 (P = 0.001),Citation25 and −15.8 vs −12.1 (P = 0.026).Citation26 Improvements with TDiclo corresponded to a percent improvement from baseline to final assessment of 39.3%,Citation23 36.7%,Citation24 32.8%,Citation25 and 37.9%,Citation26 compared with 17.1%–29.6% for vehicle or placebo.
Figure 2 Improvement from baseline to final assessment in WOMAC physical function score with diclofenac sodium topical solution and vehicle control. Physical function score was based on 17 questions scored on a scale of 0 to 4.
Abbreviation: WOMAC, Western Ontario and McMaster Universities.
Patients who used TDiclo also reported better overall health status as assessed by the POHA or PGA, with a statistically significant difference vs vehicle and/or placebo in all studies ().Citation23–Citation26 Mean change from baseline to final assessment in PGA for TDiclo vs vehicle control in each of the three studies assessing PGA as a primary outcome was −1.3 vs −0.9 (P = 0.003),Citation24 −1.3 vs −0.7 (P = 0.0001),Citation25 and −1.36 vs −1.07 (P = 0.018).Citation26 For TDiclo, the percent improvement was 42.2%,Citation24 41.9%,Citation25 and 43.6%,Citation26 compared with 21.9%–34.2% for vehicle or placebo. Bookman et al assessed PGA as a secondary variable and showed similar results.Citation23 After 4 weeks of treatment, TDiclo was associated with a significantly better mean PGA score (6.7) compared with vehicle control and placebo (7.8 in both groups, P = 0.039).Citation23 A single study utilizing POHA showed a mean change from baseline to final assessment for TDiclo vs vehicle control of −0.95 vs −0.65 (P = 0.016).Citation26
Table 2 Patient perception of osteoarthritis symptoms and overall health status as assessed by the PGA or POHA, after treatment with diclofenac sodium topical solution, vehicle control, or placebo
Greater improvements in WOMAC stiffness score with TDiclo vs vehicle and/or placebo were also observed; all improvements were statistically significant (P < 0.05) other than the comparison with placebo in the study by Simon et al.Citation26 Statistically significant (P < 0.05) improvements compared with vehicle and/or placebo were also seen for pain on walking.Citation23–Citation25
Efficacy vs oral diclofenac
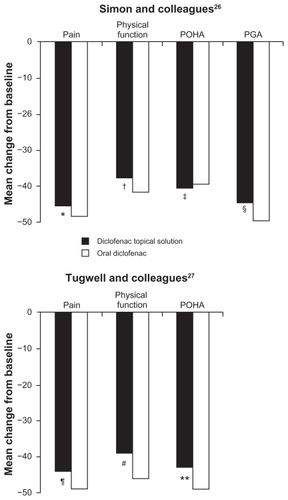
Two studies of TDiclo have compared efficacy of the solution vs oral diclofenac.Citation26,Citation27 One of the studies also included placebo and vehicle control arms, as described above.Citation26 In the oral diclofenac arm, patients received diclofenac 100 mg once daily in a extended-release formulation (Novopharm Ltd, Stouffville, ON). The efficacy of TDiclo was equivalent to that of oral diclofenac, and no statistically significant differences were observed between the two treatments in a post hoc analysis. The mean changes in outcomes measurement scores for TDiclo vs oral diclofenac were −6.0 vs −6.4 for WOMAC pain score, −15.1 vs −17.5 for WOMAC physical function score, −0.95 vs −0.88 for POHA, and −1.36 vs −1.42 for PGA. These differences were not statistically significant. Percent improvements for these outcome measures with TDiclo ranged from 38% to 45%, and with oral diclofenac ranged from 39% to 48% (). In addition to the oral diclofenac arm, this study also included a treatment arm in which patients received both oral diclofenac and the topical solution (). The combination did not produce greater improvements compared with oral diclofenac alone.
Figure 3 Percent change from baseline to final assessment in efficacy variables with diclofenac sodium topical solution and oral diclofenac.
Abbreviations: PGA, Patient Global Assessment; POHA, Patient Overall Health Assessment.
The second active comparator study was designed as an equivalence trial ().Citation27 Oral diclofenac was administered at a dose of 50 mg three times daily. The coprimary efficacy variables were WOMAC pain, WOMAC physical function, and PGA, measured on a 100 mm visual analog scale. To demonstrate equivalence between TDiclo and oral diclofenac, two-sided 95% confidence intervals (CIs) were calculated for the difference between treatments in the mean change in score for each of the coprimary efficacy variables. The treatments were considered equivalent if the CIs were in the range of −75 to 75 mm for WOMAC pain score (±15 mm for each of the five questions in the WOMAC pain subscale), −255 to 255 mm for WOMAC physical function score (±15 mm for each of the 17 questions in the WOMAC physical function subscale), and −20 to 20 mm for PGA. These limits were based on the minimal clinically important difference in osteoarthritis trials that was previously established by a panel of expert rheumatologists.Citation32 The primary analysis was performed in the per protocol population, as is typical for equivalence studies.
The 95% CIs for the three coprimary efficacy parameters were well within the predefined limits; therefore, the analysis demonstrated equivalence of TDiclo and oral diclofenac. The difference between treatments in mean change in score from baseline was 13.3 mm (95% CI: −8.6–35.2) for WOMAC pain score, 71.0 mm (95% CI: −2.4–144.5) for WOMAC physical function score, and 4.3 mm (95% CI: −1.2–9.8) for PGA. TDiclo treatment resulted in a 44% improvement in pain, 39% improvement in physical function, and 43% improvement in PGA ().Citation26,Citation27 No statistically significant differences were seen between treatments in the per protocol data set. Similar efficacy results were seen for stiffness and pain on walking.
Safety data
Plasma levels of diclofenac
The concentration of diclofenac reached in plasma is considerably lower after topical application compared with oral administration. In a study of a single application of 1 mL TDiclo to one knee in healthy volunteers, the maximum plasma concentration was 11.8 ± 4.2 ng/mL (standard deviation) after 24–48 hours.Citation33 In another single-dose study in healthy volunteers, the maximum plasma concentration was 8.1 ± 5.9 ng/mL approximately 10 hours after an application of 40 drops (approximately 1.2 mL) to each knee.Citation19 Following application of 40 drops to each knee four times daily for 7 days in healthy volunteers, maximum plasma concentration at steady state was 19.4 ± 9.3 ng/mL.Citation19 In contrast, following administration of oral diclofenac 50 mg every 8 hours in a separate study, maximum plasma concentration at steady state was 2270 ng/mL.Citation34
Tolerability and adverse events vs oral diclofenac
The incidence of adverse events occurring in the two randomized, controlled studies comparing TDiclo with oral diclofenac is shown in .Citation26,Citation27 Both studies included a dermatological assessment of the knee to evaluate application site reactions,Citation26,Citation27 and the equivalence study included a statistical comparison of adverse event incidence between the study groups.Citation27 Most adverse events occurring in patients treated with TDiclo were application site reactions. Dry skin was by far the most common, occurring in 27% of patients treated with TDiclo in the equivalence studyCitation27 and in 18.2% of patients in the other active controlled studyCitation26 (). In the equivalence study, a statistically significant higher incidence of dry skin, rash, pruritus, and vesiculobullous rash occurred with TDiclo compared with oral diclofenac ().Citation27 More patients withdrew due to skin-related adverse events in the TDiclo group than in the oral diclofenac group (10% vs 0.3%, P < 0.0001).Citation27 A much lower rate of study discontinuation due to application site reactions (3.2%) was seen in the TDiclo arm of the other active-controlled study.Citation26 The dry skin seems to be in part related to the DMSO vehicle, because it was also observed in patients treated with vehicle control.Citation23–Citation26 In clinical practice, emollients may be used after the topical solution has dried in order to alleviate skin dryness; however, this practice was not specifically evaluated during the course of the study, so it is not known to what extent emollients will alleviate these application site reactions.
Table 3 Adverse events in studies comparing diclofenac sodium topical solution with oral diclofenacCitation26,Citation27
Gastrointestinal adverse events occurred more frequently with oral diclofenac in both active-controlled studies (). In the study reported by Simon et al,Citation26 gastrointestinal adverse events occurred in 23.8% of patients receiving oral diclofenac vs 6.5% of patients receiving TDiclo. In the equivalence study, gastrointestinal events occurred in 48% of patients receiving oral diclofenac vs 35% of patients receiving TDiclo (P < 0.05). Specific gastrointestinal adverse events for which a statistically significant difference between groups was shown included abdominal pain, diarrhea, dyspepsia, flatulence, and nausea (). There was no significant difference between the study groups with respect to constipation, melena, and vomiting. The proportion of patients with a gastrointestinal adverse event classified as severe was greater in the oral diclofenac group (21.3%) compared with the TDiclo group (7.4%), and the same pattern was observed for abdominal pain, dyspepsia, and diarrhea.Citation27
Significantly more patients withdrew due to gastrointestinal adverse events in the oral diclofenac group than in the TDiclo-group (16% vs 6%, respectively, P < 0.0001). Interestingly, a much lower incidence of gastrointestinal adverse events was reported with TDiclo in the three randomized, placebo-and/or vehicle-controlled studiesCitation23–Citation25 compared with the active-controlled studies. This is consistent with a systematic review of oral NSAID trials that showed a higher incidence of gastrointestinal adverse events in active comparator trials compared with placebo-controlled trials.Citation35
In addition, laboratory parameters changed from normal to abnormal levels in a greater number of patients receiving oral diclofenac compared with TDiclo in both active- controlled studies (). In the equivalence study, a statistically significant difference between treatment groups was observed in elevations in aspartate aminotransferase, alanine aminotransferase, and hemoglobin levels. However, there was no difference between study groups in the incidence of hypertension or cardiovascular events in either study.Citation26,Citation27
Long-term safety
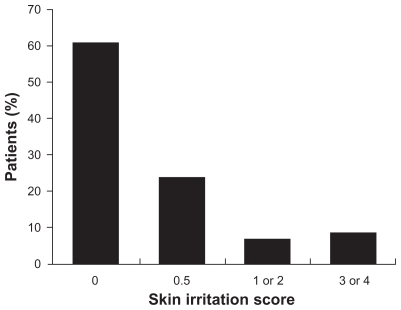
TDiclo was well tolerated in controlled clinical trials of 6–12 weeks’ duration. However, because patients with osteoarthritis of the knee are likely to use topical NSAIDs over the course of many years, it is important to gain an understanding of long-term safety reflecting use in clinical practice. An open-label, prospective study evaluated the safety of TDiclo for up to 52 weeks.Citation36 The study was conducted at outpatient centers and included 793 patients aged 35–85 (mean 62.5) years with radiologically confirmed osteoarthritis of the knee. Patients applied 40 drops of TDiclo to the knee four times daily. The most common adverse event reported was dry skin at the application site (25.3% of patients), followed by contact dermatitis (13.0%) and contact dermatitis with vesicles (9.5%). However, dermatological assessment of the application site showed that most patients had a normal skin irritation score ().Citation36
Figure 4 Skin irritation scores in a long-term, open-label study of diclofenac sodium topical solution.Citation36
In this open-label, long-term study, gastrointestinal adverse events occurred in 12% of patients, including abdominal pain in 2.3%, gastroesophageal reflux in 1.6%, diarrhea in 1.4%, dyspepsia in 1.4%, and nausea in 1.1%. A total of 28 cases of hypertension were reported (3.5% of patients).Citation36 However, most of these patients had hypertension at study enrolment; only 13 (1.6%) were considered to be true cases of new-onset or worsening hypertension. Other cardiovascular adverse events that occurred in ≥0.5% of patients were peripheral edema (1.4%), angina/chest pain (0.8%), palpitation (0.6%), myocardial infarction (0.5%), arrhythmia (0.5%), deep vein thrombosis (0.5%), and vasodilatation (0.5%). Prolonged exposure to TDiclo with DMSO resulted in few changes in hemoglobin, aspartate aminotransferase, alanine aminotransferase, or creatinine values. A change from normal to abnormal levels occurred in 3.2% of patients for hemoglobin, 6.4% for aspartate aminotransferase, 7.3% for alanine aminotransferase, and 4.2% for creatinine.Citation36
DMSO-related adverse reactions
Preclinical studies of DMSO have resulted in rare reports of ocular lens abnormalities with high-dose exposure in nonprimate species,Citation37 although studies in humans have found no ocular toxicity even with high doses applied topically or directly to the eye.Citation38–Citation40 In the controlled trial reported by Simon et al,Citation26 there was no increase in eye abnormalities in the TDiclo groups or the vehicle control group compared with placebo. In the long-term safety study, cataracts were reported in 18 patients (3% of those who received an ocular examination); seven patients developed a new cataract, whereas progression of an existing cataract was reported in 11 patients.Citation36 These cataract event rates appeared to be somewhat lower than seen in natural history studies.Citation41–Citation45 Another adverse reaction that may be attributed to DMSO is a garlic taste or odor, due to exhalation of dimethyl sulfide, a metabolite of DMSO. However, a low incidence of taste perversion (0.6%–3.7%) and halitosis (0%–5%) was reported in all studies reported here.Citation23–Citation27,Citation36
Comments and conclusion
Oral nonselective NSAIDs have shown to be effective and are widely used in the treatment of osteoarthritis, but these agents are associated with a risk of serious gastrointestinal and cardiovascular adverse events.Citation9 These events, which can be fatal and may occur without warning, are the basis of the boxed warning in the prescribing information required by the FDA and the required risk evaluation and mitigation strategy program, which includes a medication guide.Citation9,Citation46 When determining a pain management plan in patients with osteoarthritis, minimizing risk is a priority.Citation9
TDiclo may provide analgesia similar to oral NSAIDs with significantly lower dosage and systemic absorption of the active drug. Lower systemic concentrations may therefore result in less risk of systemic adverse events, including the gastrointestinal events discussed above. At this time, the prescribing information for topical NSAIDs also includes the classwide boxed warning regarding gastrointestinal and cardiovascular events.Citation18,Citation19 Based on the summary of data reported here, by adopting topical NSAID treatment as the first method of intervention for osteoarthritis, healthcare professionals may be able to decrease the risk of adverse events without compromising the efficacy of treatment.
TDiclo demonstrated efficacy in osteoarthritis of the knee similar to oral diclofenac in large, well-designed, randomized, controlled trials. Dry skin was the most frequent adverse event observed, but systemic NSAID class-related adverse events and laboratory abnormalities were considerably less with TDiclo compared with oral diclofenac. Topical NSAIDs have not been included in some clinical guideline recommendations because of a lack of data concerning overall efficacy. As more data about topical NSAIDs are published, health organizations will have new opportunities to evaluate these data as they update their existing recommendations. Clinical studies to date show that TDiclo represents a practical and evidence-based option for the management of osteoarthritis of the knee.
Disclosure
PF is an employee of Covidien, the distributor of Pennsaid® (diclofenac sodium topical solution 1.5% w/w) in the US. SR serves as a consultant and speaker for Covidien, and is a current stakeholder in Transdel Pharmaceuticals.
References
- LawrenceRCFelsonDTHelmickCGNational Arthritis Data WorkgroupEstimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part IIArthritis Rheum2008581263518163497
- BittonRThe economic burden of osteoarthritisAm J Manag Care200915Suppl 8S230S23519817509
- American College of Rheumatology Subcommittee on Osteoarthritis GuidelinesRecommendations for the medical management of osteoarthritis of the hip and knee: 2000 updateArthritis Rheum20004391905191511014340
- JordanKMArdenNKDohertyMStanding Committee for International Clinical Studies Including Therapeutic Trials ESCISITEULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT)Ann Rheum Dis200362121145115514644851
- ZhangWMoskowitzRWNukiGOARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelinesOsteoarthritis Cartilage200816213716218279766
- TannenbaumHBombardierCDavisPRussellASThird Canadian Consensus Conference GroupAn evidence-based approach to prescribing nonsteroidal antiinflammatory drugs. Third Canadian Consensus ConferenceJ Rheumatol200633114015716331802
- ChouRHelfandMPetersonKDanaTRobertsCComparative Effectiveness and Safety of Analgesics for Osteoarthritis. Comparative Effectiveness Review No. 4Rockville, MDAgency for Healthcare Research and Quality September Available from: http://www.effectivehealthcare.ahrq.gov/reports/final.cfmAccessed May 5, 2010
- National Collaborating Centre for Chronic ConditionsOsteoarthritis: National Clinical Guideline for Care and Management in AdultsLondon, UKRoyal College of Physicians2008 Available from: http://www.nice.org.uk/nicemedia/pdf/cg059fullguideline.pdfAccessed May 5, 2010
- RothSHNonsteroidal antiinflammatory drug gastropathy: we started it, why don’t we stop it?J Rheum20053271189119115996051
- AntmanEMBennettJSDaughertyAFurbergCRobertsHTaubertKAAmerican Heart AssociationUse of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart AssociationCirculation2007115121634164217325246
- Hippisley-CoxJCouplandCLoganRRisk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysisBMJ200533175281310131616322018
- KearneyPMBaigentCGodwinJHallsHEmbersonJRPatronoCDo selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trialsBMJ200633275531302130816740558
- MamdaniMJuurlinkDNKoppANaglieGAustinPCLaupacisAGastrointestinal bleeding after the introduction of COX 2 inhibitors: ecological studyBMJ200432874531415141615138157
- MooreRADerrySMcQuayHJCyclo-oxygenase-2 selective inhibitors and nonsteroidal anti-inflammatory drugs: balancing gastrointestinal and cardiovascular riskBMC Musculoskelet Disord200787317683540
- HarirforooshSJamaliFRenal adverse effects of nonsteroidal anti-inflammatory drugsExpert Opin Drug Saf20098666968119832117
- MooreRADerrySMcQuayHJTopical agents in the treatment of rheumatic painRheum Dis Clin North Am200834241543218638684
- American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older PersonsPharmacological management of persistent pain in older personsJ Am Geriatric Soc200957813311346
- Voltaren Gel (diclofenac sodium topical gel) 1% Prescribing informationChadds Ford, PAEndo Pharmaceuticals Inc2009
- Pennsaid (diclofenac sodium topical solution) 1.5% w/w Prescribing informationHazelwood, MOMallinckrodt Inc2010
- HewittPGPobleteNWesterRCMaibachHIShainhouseJZIn vitro cutaneous disposition of a topical diclofenac lotion in human skin: effect of a multi-dose regimenPharm Res19981579889929688049
- WiechersJWThe barrier function of the skin in relation to percutaneous absorption of drugsPharm Weekbl Sci19891161851982694089
- WilliamsACBarryBWPenetration enhancersAdv Drug Deliv Rev200456560361815019749
- BookmanAAWilliamsKSShainhouseJZEffect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trialCMAJ2004171433333815313991
- RothSHShainhouseJZEfficacy and safety of a topical diclofenac solution (Pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trialArch Intern Med2004164182017202315477437
- BaerPAThomasLMShainhouseZTreatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial [ISRCTN53366886]BMC Musculoskelet Disord200564416086839
- SimonLSGriersonLMNaseerZBookmanAAZev ShainhouseJEfficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritisPain2009143323824519380203
- TugwellPSWellsGAShainhouseJZEquivalence study of a topical diclofenac solution (Pennsaid®) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trialJ Rheumatol200431102002201215468367
- AltmanRDHochbergMMurphyWAJWolfeELequesneMAtlas of individual radiographic features in osteoarthritisOsteoarthritis Cartilage19953Suppl A3708581752
- BellamyNKirwanJBoersMRecommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT IIIJ Rheumatol19972447998029101522
- AltmanRBrandtKHochbergMDesign and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshopOsteoarthritis Cartilage19964421724311048620
- Group for the Respect of Ethics and Excellence in Science (GREES): osteoarthritis sectionRecommendations for the registration of drugs used in the treatment of osteoarthritisAnn Rheum Dis19965585525578774185
- BellamyNCaretteSFordPMOsteoarthritis antirheumatic drug trials. III. Setting the delta for clinical trials – results of a consensus development (Delphi) exerciseJ Rheumatol19921934514571578462
- HuiXHewittPGPobleteNMaibachHIShainhouseJZWesterRCIn vivo bioavailability and metabolism of topical diclofenac lotion in human volunteersPharm Res19981510158915959794502
- KienzlerJLGoldMNollevauxFSystemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteersJ Clin Pharmacol2010501506119841157
- RochonPABinnsMALitnerJAAre randomized control trial outcomes influenced by the inclusion of a placebo group? A systematic review of nonsteroidal antiinflammatory drug trials for arthritis treatmentJ Clin Epidemiol199952211312210201651
- ShainhouseJZGriersonLMNaseerZA long-term, open-label study to confirm the safety of topical diclofenac solution containing dimethyl sulfoxide in the treatment of the osteoarthritic kneeAm J Ther201017656657620216203
- RubinLFToxicologic update of dimethyl sulfoxideAnn N Y Acad Sci19834116106576723
- BrobynRDThe human toxicology of dimethyl sulfoxideAnn N Y Acad Sci19752434975061055563
- GarciaCAOcular toxicology of dimethyl sulfoxide and effects on retinitis pigmentosaAnn N Y Acad Sci198341148516349496
- ShirleyHHLunderganMKWilliamsHJSpruanceSLLack of ocular changes with dimethyl sulfoxide therapy of sclerodermaPharmacotherapy1989931651682755866
- TaylorHRMuñozBThe incidence and progression of lens opacitiesAust N Z J Ophthalmol19911943533561789977
- Italian-American Cataract Study GroupIncidence and progression of cortical, nuclear, and posterior subcapsular cataractsAm J Ophthalmol199411856236317977575
- LeskeMCChylackLTJrHeQIncidence and progression of cortical and posterior subcapsular opacities: the Longitudinal Study of Cataract. The LSC GroupOphthalmology199710412198719939400756
- LeskeMCChylackLTJrWuSYIncidence and progression of nuclear opacities in the Longitudinal Study of CataractOphthalmology199610357057128637678
- KleinBEKleinRLeeKEIncidence of age-related cataract: the Beaver Dam Eye StudyArch Ophthalmol199811622192259488275
- US Food and Drug AdministrationPublic Health Advisory – FDA announces important changes and additional warnings for COX-2 selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm150314.htmAccessed August 11, 2010