Abstract
Purpose
Predisposition to acute illness from COVID-19 is suggested to correlate with cigarette smoking as it augments the risk of developing cardiovascular and respiratory illnesses, including infections. However, the effects of smoking on COVID-19 symptoms are not well described and controversial. In this study, we aim to explore the associations between smoking and COVID-19 symptoms.
Subjects and Methods
A cross-sectional study using the Ministry of Public Health (MoPH), Qatar database was administered to a Qatari population with confirmed COVID-19 disease who filled in pre-defined phone-call questionnaire between 27th February 2020 and 31st December 2020. We analyzed 11,701 non-vaccinated COVID-19 individuals (2952 smokers and 8749 non-smokers) with confirmed RT-PCR test results. The association of smoking and the presence of symptoms as well as patient characteristics was calculated using Pearson’s Chi-square and Fisher’s exact tests, adjusting for potential covariates.
Results
Compared with the non-smokers, symptomatic COVID-19 infection is significantly higher in smokers. In addition, we found fever as the most common symptom developed in COVID-19 patients followed by cough, headache, muscle ache, and sore throat. As compared to other symptoms, association of smoking with chills and abdominal pain was less evident (P < 0.05 and P < 0.001, respectively). However, both groups showed similar rates of developing cough.
Conclusion
In conclusion, smoking is associated with COVID-19 symptoms frequency in non-vaccinated patients; nevertheless, further investigations are necessary to understand the mechanism of this association which could generate new targets for the management of COVID-19 in smoker patients.
Introduction
Coronavirus disease 2019 (COVID-19), is a severe respiratory illness that is highly infectious, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).Citation1 It is evident that SARS-CoV-2 infection leads to multi-organ disease, with a high prevalence of acute respiratory distress syndrome (ARDS) and digestive tract complications.Citation2 COVID-19 has a wide range of clinical symptoms ranging from asymptomatic to multi-organ dysfunction, and is classified into mild, moderate, severe, and critical based on the severity of its manifestation.Citation3 The most common symptoms of COVID-19 include fever, cough, diarrhoea, and fatigue.Citation3 Moreover, aged individuals as well as those who are immunocompromised, comorbid patients, and smokers are at a higher risk of casualty.Citation4,Citation5
Smoking is a known to trigger for respiratory tract inflammation, allergy, epithelial cells permeability, mucus development, and defective muco-ciliary clearance.Citation6 Earlier studies reported that smoking practices elevate the risk of several bacterial and viral respiratory infections and correlates with worst outcomes in infected individuals.Citation7–Citation9 Smoking is a well- recognized risk factor for various respiratory and cardio-metabolic diseases, including chronic obstructive pulmonary disease (COPD) and bronchial asthma.Citation10,Citation11 In comparison to non-smokers, acute or chronic smokers can develop infection, tuberculosis, bacterial pneumonia, ARDSCitation12–Citation14 with severe complicationsCitation14–Citation16 and present with several co-morbidities, including emphysema, atherosclerosis, and immune dysregulation,Citation17 thus, promoting the onset and progression of COVID-19. Nevertheless, the role of smoking in COVID-19 remains controversial and understated.Citation14,Citation18–Citation21 As some studies demonstrated an association between smoking and COVID-19 severity,Citation16,Citation22 while others failed to report such a correlation.Citation23,Citation24 As such, a previous report across acute care hospitals and associated outpatient clinics found smoking as an independent risk factor for COVID-19 hospitalization,Citation25 another meta-analysis described smoking as a risk factor for the development and progression of severe COVID-19.Citation26,Citation27 In contrast, a study by Farsalinos et al,Citation20 found smoking prevalence relatively lower in COVID-19 patients.
Although, the effects of smoking on COVID-19 symptoms vary and are not well-described in literature, especially in Qatar, despite the high prevalence of smoking; we herein explored the correlation between smoking and COVID-19 symptoms in the population of Qatar. Our study pointed out that smoking is associated with symptomatic COVID-19 infection. The results of this study can help in understanding the correlation between smoking and COVID-19 symptoms, however, further studies are needed to investigate the mechanism behind this association.
Materials and Methods
Sample Collection
This is a matched cross-sectional study conducted using the Ministry of Public Health (MoPH), Qatar database. MoPH gathers de-identified information from all confirmed COVID-19 patients and stores the data in their electronic database. Between 27th February 2020 and 31st December 2020, a total of 105,745 patients were confirmed to have COVID-19 and were included in the database. Our study included all adult patients (≥18 years) with RT-PCR-confirmed COVID-19 infection. The study was approved by the MoPH institutional review board (IRB: ERC-826-3-2020) with waiver of informed consent and Qatar University IRB. The authors had no direct contact with any of the participants.
All patients filled a pre-defined phone-call based forms that included “yes/no” questions about smoking status, COVID-symptoms, and comorbidities. COVID-19 symptoms included in the questionnaire were fever, chills, cough, shortness of breath, headache, muscle ache, sore throat, diarrhoea, and abdominal pain. Smoking status was defined as either active smoker or non-smoker with no further classification based on the number of cigarettes. All non-smokers were considered with no previous history of smoking. Subjects who identified themselves as smokers were matched in a 1:3 ratio with non-smokers based on age, gender, nationality, and known comorbidities. A total of 2952 smokers were extracted from the database and were matched with 8749 non-smokers. None of these patients had received any dose of the COVID-19 vaccine.
Subjects were excluded if COVID-19 was confirmed by methods other than RT-PCR, had active or prior cancer, were immunocompromised, were receiving immunosuppressive drugs or had missing demographic data (age, gender, nationality, smoking status, and symptoms).
Statistical Analysis
Pearson’s Chi-square and Fisher’s exact tests were used to compare the presence of symptoms and the characteristics between the two groups. Continuous variables were expressed as mean ± standard deviation (SD) while categorical variables were presented by the count and percentage. All statistical analyses were carried out using Stata Statistical Software: Release 16 (College Station, TX: StataCorp LLC). Matching was performed using SPSS Fuzzy Case Control Matching extension. Two-tailed P-values of less than 0.05 was considered statistically significant.
Results
This study included 11,701 patients, of whom 2952 were smokers and 8749 were non-smokers. The two groups were matched based on age, gender, nationality, and comorbidities. Hence, both groups had similar characteristics. The mean age of the study population was 34.5 years with the vast majority being males (97.3%). The most common nationality in this study was Indian which comprises around 25% of the population, followed by Qatari, Nepalese, and Bangladeshi. Diabetes and cardiovascular diseases were the most frequent comorbidities in the study population, each affecting around 6% of the patients. The rest of the comorbidities were rare. Four subjects from the smokers group underwent surgeries previously which was significantly higher than the non-smokers group, where only two had prior surgeries (P < 0.05) ().
Table 1 Baseline Characteristics of Study Participants Based on Their Smoking Status. Data are Presented as Mean ± Standard Deviation or as Number of Subjects (Percentage)
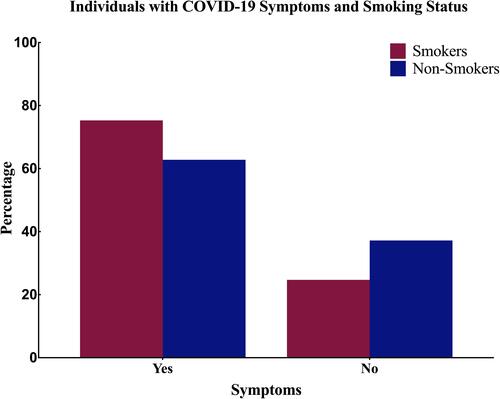
As indicated in , our data revealed that a significantly higher proportion of smokers developed symptomatic COVID-19 infection (75.3%) compared to the non-smokers (62.8%; P < 0.001), indicating that smokers have a risk ratio of 1.2 (95% confidence interval: 1.17–1.23) to develop symptoms related to COVID-19 infection when compared to non-smokers ().
Table 2 The COVID-19 Symptoms Based on Patients’ Smoking Status. Data are Presented as Number of Subjects (Percentage)
Figure 1 Bar chart showing the percentage of COVID-19 patients who experienced any COVID-19 related symptoms stratified based on their smoking status.
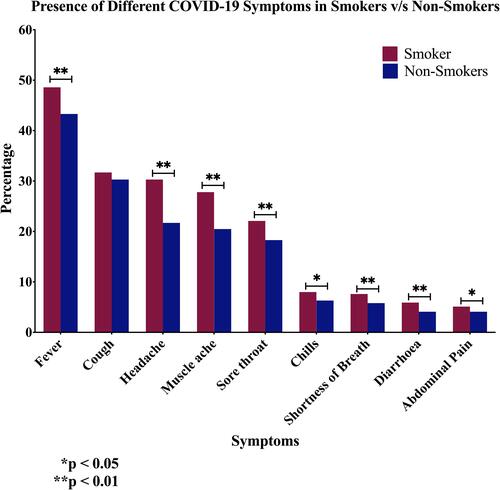
On the other hand, and as demonstrated in , the type of symptoms experienced in relation with smoking status show that fever is the most frequent symptom affecting 45% of confirmed COVID-19 cases, followed by cough, headache, muscle ache, and sore throat. Less frequent symptoms, present in less than 10% of patients, include chills, shortness of breath, diarrhoea, and abdominal pain. Smoking is associated with a significantly higher rate of developing all types of symptoms except cough, as both groups showed similar rates of developing cough (). On the other hand, the association of smoking with chills and abdominal pain was less evident when compared to the rest of the symptoms (P < 0.05 and P < 0.001; respectively) ().
Discussion
In this investigation we explored for the first time the correlation between smoking and COVID-19 symptoms frequency in the population of Qatar. Our data revealed that current smoking is associated with a risk of developing symptoms suggestive of COVID-19, as well as a greater symptom burden, indicating an impact of smoking on COVID-19 disease frequency. Additionally, amongst patients positive for COVID-19, smokers had a higher symptom burden in comparison to non-smokers.
In our cohort, 97% of COVID-19 patients are males and 3% are females, which concurs with recent studies of COVID-19 infection.Citation28–Citation31 Furthermore, in this study, the estimated median age for patients is 34 years which is concordant with previous studies among COVID-19 patients in other countriesCitation32,Citation33 as well as in Qatar and Saudi Arabia.Citation29,Citation30,Citation34,Citation35 As compared to other parts of the world, COVID-19 is found to affect younger populations in the Gulf Region.Citation29,Citation30,Citation34 Such a trend is observed in our study, nevertheless, the age-groups effected with COVID-19 range from 15 to 87 years, indicating disease susceptibility across these ages. High infection and severity rates in adult patients are found to be associated with smoking, as seen in previous studies.Citation36,Citation37
Approximately 15% of COVID-19 patients investigated here exhibited various comorbidities as reported in previous studies.Citation27,Citation29,Citation38 In this study, we found that both smokers and non-smokers had diabetes (6%), followed by cardiovascular disease (6%) and asthma (1%). This is in concordance with other reports which showed diabetes and/or hypertension as the most common comorbidities.Citation27,Citation29,Citation32,Citation34,Citation39–Citation41 However, our findings revealed that smoking had no significant association with diabetes and cardiovascular comorbidities. Along with cardiac and chronic respiratory diseases, lung and chronic kidney diseases are reported as risk factors associated with worse outcomes in COVID-19 patients.Citation42,Citation43
Similar to our data, various cohort studies revealed an association between smoking and COVID-19 disease comorbidity, incidence and mortality.Citation14,Citation16,Citation22,Citation27 In this context, smoking is known to damage the vascular endothelial,Citation44 a characteristic feature in COVID-19 pathophysiology. A meta-analysis of 13 published Chinese studies pointed out older age (age > 65 years), male gender, and smoking were risk factors for disease progression in COVID-19 patients.Citation45 On the other hand, a recent meta-analyses showed significant correlation between smoking and COVID-19 progression.Citation27,Citation46 Another meta-analysis based on 15 studies also reported concordant data where 22% of current and 46% of former smokers had severe COVID-19 complications.Citation14 A recent study by Almazeedi et alCitation47 was performed based on a cohort of 1096 COVID-19 patients in Kuwait and they reported that age > 50 years, smoking, elevated C-reactive protein (CRP) and prolactin levels as well as a quick sequential organ failure assessment (qSOFA) score greater than 0 as risk factors associated with COVID-19 severity. Likewise, a study on 10,713 COVID-19 patients from Brazil also reported smoking and pulmonary disease to augment the risk of COVID-19 severity.Citation48 In addition to studies carried out in larger cohorts, studies in small cohorts also showed similar results.Citation49,Citation50 Recently, Archie et alCitation51 postulated a plausible role of smoking on cerebrovascular and neurological dysfunction in COVID-19 patients. Recently, Jiménez-RuizCitation11 performed a systematic review and meta-analysis and reported that smoking is a risk factor for a severe COVID-19 infection with a plausibility of developing a more critical condition. Similarly, another investigation showed a clear association between smoking and disease severity, with approximately 3% of deceased patients being smokers.Citation52 Moreover, a meta-analysis that included 7 studies found that smokers had twice the risk of developing COVID-19 severity.Citation53
Although, smoking may not essentially elevate the risk of developing COVID-19, the underlying mechanisms involving the biological and inflammatory signaling pathways induced by COVID-19 infection can be severe for smokers.Citation21 Likewise, earlier research showed increased susceptibility to COVID-19 infection is attributed to the overexpression of one of the key SARS-CoV-2 receptors, angiotensin converting enzyme 2 (ACE2) receptor.Citation4,Citation17,Citation54 Smoking increases susceptibility to COVID-19 infection via the triggering of peripheral nicotinic acetylcholine receptors (nAChRs) present in various organ systems.Citation55 Nicotine disrupts homeostasis of the renin angiotensin system (RAS), and overexpresses the ACE/angiotensin (ANG)-II/ANG II type 1 receptor axis, resulting in cardiovascular and pulmonary diseases.Citation55 Hence, nicotine can affect the nAChRs and enhance the SARS-CoV-2 host entry.Citation56 Moreover, studies reported that smokers with diabetes, cardiovascular disease, COPD, asthma or cancer can enhance the expression pattern of ACE2; hence, they are more susceptible to develop COVID-19 infection and have worst prognosis.Citation57,Citation58 Diabetes can overexpress ACE2 expression via hyperinsulinemia along with other mechanisms.Citation59 During diabetes, the function of Treg cells is compromised and expression of tumor necrosis factor-alpha (TNF-α) is reduced, further enhancing infections.Citation59,Citation60 On the other hand, cardiovascular patients are also prone to COVID-19 disease severity. In hypertensive patients, ACE2 modulators (ACE1 inhibitors, ANG blockers, and thiazolidinediones) are often prescribed to treat hypertension and can overexpress ACE2;Citation39 a risk factor for the onset of COVID-19 disease. Likewise, the viral capsid of SARS-CoV-2 binds to the ACE2 surface and activates TMPRSS2, (Transmembrane serine protease 2, an androgen-responsive gene highly expressed in men), thus, increased TMPRSS2 expression in men increases their proneness to COVID-19 disease.Citation61 Furthermore, it has been demonstrated that cigarette exposure can increase the expression pattern of ACE2 and TMPRSS2 in patients with COPD and idiopathic pulmonary fibrosis (IPF);Citation17,Citation62–Citation66 in this context, it is important to highlight that COPD is reported as a major risk factor for COVID-19 infection.Citation53,Citation67 Smokers suffering from cancer are also found to be at a higher risk of developing COVID-19 infection due compromised immunity, thus providing an effectual environment for viral replication.Citation68
On the contrary, while several studies reported an association between disease severity and smoking,Citation16,Citation22 few investigations found no difference or a negative correlation between smoking and COVID-19 severity.Citation23,Citation24 Moreover, a recent review reported that current smokers are at a comparatively lower risk of developing COVID-19 as compared to never smokers, however, these findings aim to analyse certain factors that could make the interpretation of these results more complex.Citation19 Hence, it is necessary to conduct interventional studies in the general population and in patients post-COVID-19 disease. In addition to investigations analysing the correlation between smoking and COVID-19 severity, reports on behavioural changes in tobacco consumption during the pandemic found varying data. While, in USA there was an increase by 41% in tobacco consumption during the onset of COVID-19,Citation69 in Italy, smokers had considered quitting.Citation70 However, the same study found that former smokers and never smokers wanted to start smoking.Citation70 In China and England as well, there was an increase in smoking among smokers and relapsed smoking habits among former smokers.Citation71,Citation72 As of currently, both epidemiological/clinical evidence and in-silico findings indicate that COVID-19 infection is a nAChR disease that could be prevented and may be controlled by nicotine.Citation56,Citation73 Smoking was suggested to exert a protective measure as its nicotine acts as a nAChR agonist and competes with the SARS-COV-2 for the binding site, hence leading to a decline in accessible viral adhesion sites.Citation74 Nevertheless, the plausibility of nicotine displaying protective effect against COVID-19 can be partially masked by smoking-related toxicity. In addition, abrupt cessation of nicotine consumption when smokers are hospitalized need further validation in laboratory studies using nicotine-based pharmaceutical products. Considering the limited and contradicting past investigations available on this topic, we hypothesized a strong association of smoking with the risk of developing respiratory diseases. However, coughing, which is the major respiratory depression symptom was reported in both smokers and non-smokers nearly equally, with only a non-statistically significant difference of 1.4% more for smokers. Moreover, significant differences were reported in other symptoms such as fever, muscle ache, abdominal pain, diarrhoea, headaches, and shortness of breath, all of which are also symptoms of nicotinic overstimulation of the cholinergic systemCitation75 which is activated by the nicotine in cigarettes and triggers COVID-19 disease.Citation76,Citation77
Although, our present study included a large sample size and points out a strong link between smoking and symptoms development among COVID-19 patients, there are several factors limiting the interpretation of our investigation. The present results remain limited by the available information. In particular, detailed data on possible sources of exposure to smoking are lacking. The data was self-reported by patients via phone-call based forms. The patients reported their symptoms through this form. Additionally, there was no classification based on the number of packs smoked per day. Thus, heavy smokers and light smokers were grouped together. Moreover, patients who reported as non-smokers are assumed to have no history of smoking and thus, we believe that further studies are needed to investigative the consequences and underlying mechanisms of smoking and its association with COVID-19 infection and its frequency in patients. In addition to cigarette smoking, effects of waterpipe and electronic cigarette use on COVID-19 transmission, clinical progression and frequency of COVID-19 should be further studied. Lastly, the association between COPD frequency and COVID-19 should be also analysed.
Conclusion
In conclusion, our present study demonstrates an association between smoking and COVID-19 symptoms frequency in non-vaccinated patients. While we believe that further studies in a larger cohort are required to clearly determine the association between smoking and COVID-19 symptoms which can help in controlling COVID-19 induced morbidity and mortality especially in smoker patients. Nevertheless, and based on our present study it is evident that discontinuing of smoking practices should be contemplated as a part of the strategies to address COVID-19 infection management in the international arena, as smoking increases both the likelihood of symptomatic disease and the frequency of disease.
Ethics Approval and Consent to Participate
Patient consent was waived as this was a matched cross-sectional study conducted using the Ministry of Public Health (MoPH), Qatar database. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Ministry of Public Health (IRB: ERC-826-3-2020).
Acknowledgments
We would like to thank Qatar’s Ministry of Public Health for providing the data used in this study. Also, we would like to thank the Department of Population Medicine at College of Medicine, Qatar University for their support provided throughout the study.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New Engl J Med. 2020;382(16):1564–1567. doi:10.1056/NEJMc2004973
- Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). In: Statpearls [Internet]. StatPearls Publishing; 2020.
- Wang Y, Wang Y, Chen Y, Qin QJ. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J Med Virol. 2020;92(6):568–576. doi:10.1002/jmv.25748
- Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19). J Clin Med. 2020;9(3):841. doi:10.3390/jcm9030841
- Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar AJC. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020;12(3):e7355. doi:10.7759/cureus.7355
- Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018;15(5):1033. doi:10.3390/ijerph15051033
- Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4(1):12. doi:10.1186/1617-9625-4-12
- Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. 1996;67(10 Suppl):1050–1054. doi:10.1902/jop.1996.67.10s.1050
- Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206–2216. doi:10.1001/archinte.164.20.2206
- Eapen MS, Sharma P, Moodley YP, Hansbro PM, Sohal SS. Dysfunctional immunity and microbial adhesion molecules in smoking-induced pneumonia. Am J Respir Crit Care Med. 2019;199(2):250–251. doi:10.1164/rccm.201808-1553LE
- Jiménez-Ruiz CA, López-Padilla D, Alonso-Arroyo A, Aleixandre-Benavent R, Solano-Reina S, de Granda-orive JI. COVID-19 and smoking: a systematic review and meta-analysis of the evidence. Arch Bronconeumol. 2021;57 Suppl 1:21–34. doi:10.1016/j.arbres.2020.06.024
- Han L, Ran J, Mak Y-W, et al. Smoking and influenza-associated morbidity and mortality: a systematic review and meta-analysis. Epidemiology. 2019;30(3):405–417. doi:10.1097/EDE.0000000000000984
- Lawrence H, Hunter A, Murray R, Lim W, McKeever T. Cigarette smoking and the occurrence of influenza–systematic review. J Infect. 2019;79(5):401–406. doi:10.1016/j.jinf.2019.08.014
- Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15(5):e0233147. doi:10.1371/journal.pone.0233147
- Zhang -J-J, Dong X, Cao -Y-Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi:10.1111/all.14238
- Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
- Smith JC, Sausville EL, Girish V, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev Cell. 2020;53(5):514–529.e513. doi:10.1016/j.devcel.2020.05.012
- Vardavas CI, Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi:10.18332/tid/119324
- Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction. 2021;116(6):1319–1368. doi:10.1111/add.15276
- Farsalinos K, Angelopoulou A, Alexandris N, Poulas K. COVID-19 and the nicotinic cholinergic system. Eur Respir J. 2020;56(1):2001589. doi:10.1183/13993003.01589-2020
- Leung JM, Sin DD. Smoking, ACE-2, and COVID-19: ongoing controversies. Eur Respir J. 2020;56:2001759. doi:10.1183/13993003.01759-2020
- Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–1038. doi:10.1097/cm9.0000000000000775
- Chen X, Tong J, Xiang J, Hu J. Retrospective study on the epidemiological characteristics of 139 patients with novel coronavirus pneumonia on the effects of severity. Chongqing Med. 2020;3:1–9.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/s0140-6736(20)30183-5
- Killerby ME, Link-Gelles R, Haight SC, et al. Characteristics associated with hospitalization among patients with COVID-19 — metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):790–794. doi:10.15585/mmwr.mm6925e1
- Karanasos A, Aznaouridis K, Latsios G, et al. Impact of smoking status on disease severity and mortality of hospitalized patients with COVID-19 infection: a systematic review and meta-analysis. Nicotine Tob Res. 2020;22(9):1657–1659. doi:10.1093/ntr/ntaa107
- Zhang H, Ma S, Han T, et al. Association of smoking history with severe and critical outcomes in COVID-19 patients: a systemic review and meta-analysis. Eur J Integr Med. 2021;43:101313. doi:10.1016/j.eujim.2021.101313
- Omar SM, Musa IR, Salah SE, Elnur MM, Al-Wutayd O, Adam I. High mortality rate in adult COVID-19 inpatients in Eastern Sudan: a Retrospective Study. J Multidiscip Healthc. 2020;13:1887–1893. doi:10.2147/jmdh.S283900
- Shaikh F, Aldhafferi N, Buker A, et al. Comorbidities and risk factors for severe outcomes in COVID-19 patients in Saudi Arabia: a Retrospective Cohort Study. J Multidiscip Healthc. 2021;14:2169–2183. doi:10.2147/JMDH.S317884
- Al Kuwari HM, Abdul Rahim HF, Abu-Raddad LJ, et al. Epidemiological investigation of the first 5685 cases of SARS-CoV-2 infection in Qatar, 28 February–18 April 2020. BMJ Open. 2020;10(10):e040428. doi:10.1136/bmjopen-2020-040428
- Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi:10.1136/bmj.m1996
- Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi:10.1164/rccm.202002-0445OC
- Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80(6):e14–e18. doi:10.1016/j.jinf.2020.03.005
- Alsofayan YM, Althunayyan SM, Khan AA, Hakawi AM, Assiri AM. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. 2020;13(7):920–925. doi:10.1016/j.jiph.2020.05.026
- Alyami MH, Naser AY, Orabi MAA, Alwafi H, Alyami HS. Epidemiology of COVID-19 in the Kingdom of Saudi Arabia: an Ecological Study. Front Public Health. 2020;8(506). doi:10.3389/fpubh.2020.00506
- Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience. 2020;42(2):505–514. doi:10.1007/s11357-020-00186-0
- Joly BS, Siguret V, Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med. 2020;46(8):1603–1606. doi:10.1007/s00134-020-06088-1
- AlJishi JM, Alhajjaj AH, Alkhabbaz FL, et al. Clinical characteristics of asymptomatic and symptomatic COVID-19 patients in the Eastern Province of Saudi Arabia. J Infect Public Health. 2021;14(1):6–11. doi:10.1016/j.jiph.2020.11.002
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/s0140-6736(20)30566-3
- Baradaran A, Ebrahimzadeh MH, Baradaran A, Kachooei AR. Prevalence of comorbidities in COVID-19 patients: a systematic review and meta-analysis. Arch Bone Jt Surg. 2020;8(Suppl1):247–255. doi:10.22038/abjs.2020.47754.2346
- Khamis F, Al-Zakwani I, Al Naamani H, et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020;13(7):906–913. doi:10.1016/j.jiph.2020.06.002
- Khan A, Althunayyan S, Alsofayan Y, et al. Risk factors associated with worse outcomes in COVID-19: a retrospective study in Saudi Arabia. East Mediterr Health J. 2020;26(11):1371–1380. doi:10.26719/emhj.20.130
- Abohamr SI, Abazid RM, Aldossari MA, et al. Clinical characteristics and in-hospital mortality of COVID-19 adult patients in Saudi Arabia. Saudi Med J. 2020;41(11):1217–1226. doi:10.15537/smj.2020.11.25495
- Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–515. doi:10.1161/atvbaha.113.300156
- Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi:10.1016/j.jinf.2020.04.021
- Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi:10.1093/ntr/ntaa082
- Almazeedi S, Al-Youha S, Jamal MH, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi:10.1016/j.eclinm.2020.100448
- Soares RCM, Mattos LR, Raposo LM. Risk factors for hospitalization and mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103(3):1184–1190. doi:10.4269/ajtmh.20-0483
- Zhao Z, Chen A, Hou W, et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. 2020;15(7):e0236618. doi:10.1371/journal.pone.0236618
- Mohsin FM, Tonmon TT, Nahrin R, et al. Association between smoking and COVID-19 severity: evidence from Bangladesh. J Multidiscip Healthc. 2021;14:1923–1933. doi:10.2147/JMDH.S317603
- Archie SR, Cucullo L. Cerebrovascular and neurological dysfunction under the threat of COVID-19: is there a comorbid role for smoking and vaping? Int J Mol Sci. 2020;21(11):3916. doi:10.3390/ijms21113916
- Ujjan ID, Devrajani BR, Ghanghro AA, Shah SZA. The clinical and demographical profile of Coronavirus illness: the tale of Tablighi Jamaat and Zaireen in Quarantine/Isolation center at Sukkur and Hyderabad. Pak J Med Sci. 2020;36(Covid19–s4):S12–s16. doi:10.12669/pjms.36.COVID19-S4.2829
- Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92(10):1915–1921. doi:10.1002/jmv.25889
- Leung JM, Yang CX, Tam A, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5):2000688. doi:10.1183/13993003.00688-2020
- Oakes JM, Fuchs RM, Gardner JD, Lazartigues E, Yue X. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2018;315(5):R895–r906. doi:10.1152/ajpregu.00099.2018
- Changeux JP, Amoura Z, Rey FA, Miyara M. A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. C R Biol. 2020;343(1):33–39. doi:10.5802/crbiol.8
- Yilin Z, Yandong N, Faguang JJB. Role of angiotensin-converting enzyme (ACE) and ACE2 in a rat model of smoke inhalation induced acute respiratory distress syndrome. Burns. 2015;41(7):1468–1477. doi:10.1016/j.burns.2015.04.010
- Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75(11):2829–2845. doi:10.1111/all.14429
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi:10.1007/s00392-020-01626-9
- Sardu C, D’Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi:10.2337/dc20-0723
- Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging. 2020;12(11):10087–10098. doi:10.18632/aging.103415
- Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther - Methods Clin Dev. 2020;18:1–6. doi:10.1016/j.omtm.2020.05.013
- Cruz T, López-Giraldo A, Noell G, et al. Multi-level immune response network in mild-moderate Chronic Obstructive Pulmonary Disease (COPD). Respir Res. 2019;20(1):152. doi:10.1186/s12931-019-1105-z
- Kim WJ, Lim JH, Lee JS, Lee S-D, Kim JH, Oh Y-M. Comprehensive analysis of transcriptome sequencing data in the lung tissues of COPD subjects. Int J Genomics. 2015;2015:206937. doi:10.1155/2015/206937
- McDonough JE, Ahangari F, Li Q, et al. Transcriptional regulatory model of fibrosis progression in the human lung. JCI Insight. 2019;4(22):e131597. doi:10.1172/jci.insight.131597
- Pardo A, Gibson K, Cisneros J, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2(9):e251. doi:10.1371/journal.pmed.0020251
- Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. 2020;167:105941. doi:10.1016/j.rmed.2020.105941
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
- Kowitt SD, Cornacchione Ross J, Jarman KL, et al. Tobacco quit intentions and behaviors among cigar smokers in the United States in response to COVID-19. Int J Environ Res Public Health. 2020;17(15):5368. doi:10.3390/ijerph17155368
- Caponnetto P, Inguscio L, Saitta C, Maglia M, Benfatto F, Polosa R. Smoking behavior and psychological dynamics during COVID-19 social distancing and stay-at-home policies: a survey. Health Psychol Res. 2020;8(1):9124. doi:10.4081/hpr.2020.9124
- Sun Y, Li Y, Bao Y, et al. Brief report: increased addictive internet and substance use behavior during the COVID-19 pandemic in China. Am J Addict. 2020;29(4):268–270. doi:10.1111/ajad.13066
- Patwardhan P. COVID-19: risk of increase in smoking rates among England’s 6 million smokers and relapse among England’s 11 million ex-smokers. BJGP Open. 2020;4(2):bjgpopen20X101067. doi:10.3399/bjgpopen20X101067
- Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2021;93(2):1045–1056. doi:10.1002/jmv.26389
- Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P, Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis. 2020;11:2040622320935765. doi:10.1177/2040622320935765
- Smith EW, Smith KA, Maibach HI, Andersson PO, Cleary G, Wilson D. The local side effects of transdermally absorbed nicotine. Skin Pharmacol. 1992;5(2):69–76. doi:10.1159/000211021
- Berlin I, Thomas D, Le Faou A-L, Cornuz J. COVID-19 and smoking. Nicotine Tob Res. 2020;22(9):1650–1652. doi:10.1093/ntr/ntaa059
- Alexandris N, Lagoumintzis G, Chasapis CT, et al. Nicotinic cholinergic system and COVID-19: in silico evaluation of nicotinic acetylcholine receptor agonists as potential therapeutic interventions. Toxicol Rep. 2021;8:73–83. doi:10.1016/j.toxrep.2020.12.013