Abstract
Peripartum cardiomyopathy is a rare cause of heart failure that occurs during late pregnancy or in the early postpartum period. Delays in diagnosis may occur as symptoms of heart failure mimic those of normal pregnancy. The diagnosis should be considered in any pregnant or postpartum woman with symptoms concerning for heart failure. If there are clinical concerns, labs including N-terminal pro-BNP should be checked, and an echocardiogram should be ordered to assess for systolic dysfunction. Prompt medical treatment tailored for pregnancy and lactation is essential to prevent adverse events. Outcomes are variable, including complete recovery, persistent myocardial dysfunction with heart failure symptoms, arrhythmias, thromboembolic events, and/or rapid deterioration requiring mechanical circulatory support and cardiac transplantation. It is essential that care is provided as part of a multidisciplinary cardio-obstetrics team including obstetrics, cardiology, maternal fetal medicine, anesthesiology, and nursing. All women with peripartum cardiomyopathy should have close follow-up with a cardiologist, although optimal duration of medical therapy following complete recovery is unknown. Women considering a subsequent pregnancy require preconception counseling and close collaboration between obstetrics and cardiology throughout pregnancy.
Introduction
Peripartum cardiomyopathy (PPCM) is a rare and idiopathic form of systolic heart failure affecting childbearing women towards the end of pregnancy or in the early postpartum period.
Figure 1 Presentation, risk factors, diagnosis, and management of peripartum cardiomyopathy (PPCM).
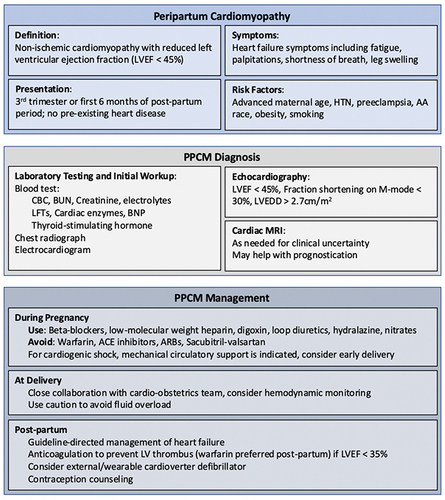
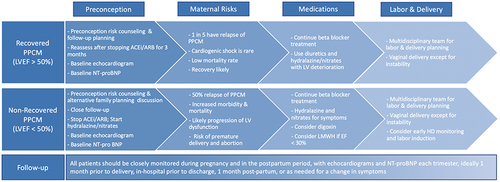
Figure 2 Subsequent pregnancy for patients with history of PPCM. The risks and recommendations vary based on the patient’s LVEF recovery status. The risks are much higher in those with non-recovered PPCM (LVEF <50%) and pregnancy should be discouraged in these patients.
The 2019 Heart Failure Association of the European Society of Cardiology Working Group defines PPCM as
an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause is found.Citation1
Echocardiography is essential for the diagnosis, and diagnostic criteria require that the left ventricular ejection fraction (LVEF) is <45%, with or without left ventricular dilatation ().Citation1–3
In the United States, reported incidence ranges from 1 in 900 to 1 in 4000 and this number is likely increasing given the increased recognition of the disease, rise in maternal age, and increase in multifetal pregnancies due to assisted reproductive techniques.Citation4–6 Incidence is four times higher in African American women than in white women, and lowest in Hispanic women.Citation5,Citation7–9 Even though less than 15% of the US population is African American, in two recent US studies African American women accounted for nearly half of PPCM cases.Citation5,Citation10 Global estimates of the incidence of PPCM vary widely throughout the world, as high as 1 in 96 deliveries in parts of Nigeria and as low as 1 in 20,000 deliveries in Japan.Citation11–13
In this article, we aim to review the data regarding the pathogenesis, typical presentation, risk factors, recommendations for diagnosis, and the management of PPCM, including counseling considerations for breastfeeding and subsequent pregnancies.
Pathogenesis and Presentation
The precise pathogenesis of PPCM remains unknown, with prior hypotheses related to autoimmune processes, nutritional deficiencies, and hemodynamic stress of pregnancy.Citation14–17 Currently, the development of PPCM is understood with a “two-hit” model, where a vascular insult caused by hormonal effects of late pregnancy and the early postpartum period induce cardiomyopathy in patients with an underlying genetic predisposition.Citation18–23 Some studies have suggested the vascular insult may be due to a prolactin-mediated oxidative stress on the myocardium.Citation24 Given the association of PPCM with pre-eclampsia, it is also thought that placental angiogenic factors could provide a shared pathophysiologic mechanism.Citation25,Citation26 Furthermore, it has been shown that certain anti-adrenergic and anti-sarcomeric protein antibodies are more common in PPCM, which could suggest an additional autoimmune mechanism for PPCM.Citation27,Citation28
The typical clinical presentation involves typical signs and symptoms of heart failure including shortness of breath on exertion, orthopnea, paroxysmal nocturnal dyspnea, edema, and chest tightness. On physical examination, patients typically demonstrate tachypnea, tachycardia, elevated jugular venous pressure, pulmonary rales, and peripheral edema.Citation29 More severe, but uncommon presentations include serious arrhythmias, cardiac arrest, thromboembolic complications, and cardiogenic shock.Citation30,Citation31 Between 50% and 80% of the affected women recover systolic function to a normal LVEF of ≥50%, with the majority of the recovery occurring in the first 6 months.Citation32,Citation33 Partial recovery is defined as improving LVEF by at least 10%. However, some women will have serious complications including arrhythmias, thromboembolism, and cardiogenic shock in some instances requiring mechanical support or cardiac transplantation.Citation5,Citation13,Citation32,Citation34 Medical management centers around standard treatment for heart failure with reduced ejection fraction, with special attention to increased risk of thromboembolism in these women, adverse effects of some medications on the fetus for women who are still pregnant, and risk of lethal arrhythmias in the postpartum period. Timing and mode of delivery should be discussed with the patient and carefully planned by a multidisciplinary cardio-obstetrics team, including obstetrics, cardiology, maternal fetal medicine, anesthesiology, and nursing.Citation35 There is risk of relapse with subsequent pregnancies, and thus close collaboration and follow-up by cardiology and obstetrics is essential even in patients who have recovered systolic function.
Risk Factors
The incidence of PPCM is higher in women of African descent. Possible reasons for this include genetic predisposition, higher incidence of pre-eclampsia in these women, and regional nutritional deficiencies; however, racial and ethnic disparities in outcomes also exist and are likely related to systemic inequities and racism.Citation36,Citation37 While PPCM occurs in approximately 1 in 100 live births in Nigeria, the rate is approximately 1 in 10,000 in Denmark and 1 in 20,000 in Japan.Citation11,Citation12,Citation38 In the United States, several statewide studies have reported PPCM occurring 3 to 4 times as often in African American women compared with white women, and the rates are lowest in Hispanic women.Citation5,Citation7–9
Hypertension and pre-eclampsia are strongly associated with PPCM. A meta-analysis of 22 studies of PPCM reported that pre-eclampsia was present in 22% of the women with PPCM as compared to 5% of the other pregnancies, and that other hypertensive disorders were present in 37% of the women with PPCM as compared to 13% of all pregnancies.Citation39,Citation40 The majority of women with pre-eclampsia do not develop PPCM, which requires a decreased systolic function for diagnosis. Rather, hypertension and pre-eclampsia lead to predominant diastolic dysfunction.
PPCM also appears to be associated with increasing maternal age, with one-half of cases occurring in women over 30 years of age with an adjusted odds ratio of 1.8 compared to those younger than 30 years.Citation9,Citation29 Furthermore, one study found that women over 40 years of age had an odds ratio of 10 of developing PPCM compared to women under 20 years of age.Citation5 This trend is reflected in an analysis of US data that showed an incidence of 1 in 1200 of PPCM in women 20–29 years of age and 1 in 270 in women greater than 40 years of age.Citation5 Multigestational pregnancies are also shown to increase the risk of PPCM with a threefold risk reported for twin gestations.Citation41 In the United States, 7–14.5% of the women with PPCM occur in the setting of a multigestational pregnancy.Citation41–43
Multiple serum biomarkers have been associated with PPCM. Serum prolactin level has been shown to be significantly elevated at baseline in patients with PPCM compared to controls. While the baseline prolactin level has not been shown to be prognostic, the failure of this to decrease over time is associated with lack of PPCM recovery.Citation44 Similarly, the persistent elevation of markers of inflammation and oxidative stress such as oxidized low-density lipoprotein (ox-LDL) and interferon-gamma (INF-g) have also been associated with lack of recovery while elevations of C-reactive protein (CRP), white blood cell (WBC) count and monocyte to high-density lipoprotein ratio (MHR) may be predictive of persistent systolic dysfunction.Citation45,Citation46 Additionally, early post-partum serum biomarkers of vascular endothelial dysfunction have also been prognostic. Relaxin-2 is associated with greater recovery and less ventricular remodeling, while soluble Fms-like tyrosine kinase (sFlt1) is associated with disease progression and poorer prognosis.Citation26
Multiple studies have identified a genetic component to PPCM. In fact, approximately 16% of the patients report a family history of HF.Citation47 A study assessing 43 genes with a known association with dilated cardiomyopathy in patients with PPCM showed a positive association in 15%.Citation21 Specific associated genes include a gene for the sarcomeric protein titin (TTN), a mutation near the vascular homeostasis parathyroid hormone-like hormone (PTHLH) gene, as well as the dystrophic gene.Citation21 Additional genes include the beta-myosin heavy chain (MYH7), a sodium voltage-gated channel alpha subunit 5 (SCN5A), and myosin binding protein C (MYBPC3).Citation48 Truncating variants of the TTN gene have been shown to carry a worse prognosis in PPCM. Given our understanding, genetic testing may be a useful screening tool in patients with a family history of PPCM.Citation49 However, the genetic origin of PPCM is most likely incompletely penetrant since most women do not have a family history of PPCM and do not always develop recurrent PPCM in subsequent pregnancies.Citation3
Closer surveillance of women at high risk during pregnancy is essential as earlier detection of PPCM can lead to more appropriate treatment and safer delivery as well as postpartum care.
Diagnosis
Due to under-recognition of PPCM and the overall similarity in signs and symptoms of normal pregnancy with those of heart failure, there are frequent delays in diagnosis. PPCM is a diagnosis of exclusion, and it is essential that the patient is evaluated for possible pre-existing heart disease. Lab testing should include checking levels N-terminal pro-BNP (NT-pro BNP), which do not change significantly during pregnancy but will be markedly elevated in PPCM. The electrocardiogram is often normal but may show nonspecific abnormalities.Citation50,Citation51 An echocardiogram is essential in any suspected case of PPCM. In addition to systolic dysfunction, the echocardiogram may demonstrate left ventricular dilatation, functional mitral and/or tricuspid regurgitation, right ventricular dysfunction, pulmonary hypertension, and atrial enlargement.Citation52,Citation53 The left ventricular apex should be clearly visualized to evaluate for intracardiac thrombus. If echocardiogram is inadequate, cardiac magnetic resonance imaging should be considered for a more accurate measure of systolic function and chamber measurements.Citation54 Notably, gadolinium is avoided during pregnancy. If there is concern for an alternative diagnosis that would require different management, endomyocardial biopsy may be considered but is rarely needed. Further research is needed to address knowledge gaps regarding the optimal diagnostic strategy, which may include a combination of genetic testing, serum biomarkers, laboratory values, and imaging findings.
Management
Very few studies of management have been performed specifically in patients with peripartum cardiomyopathy. As a result, the treatment of PPCM is derived from a combination of expert opinion as well as recommendations for other forms of systolic heart failure. The guidelines advise that standard treatment for systolic heart failure is indicated for patients with PPCM, with special consideration for fetal safety in pregnant patients and for safety of newborns in breast-feeding mothers.Citation53,Citation55,Citation56 The first-line therapy of volume management is dietary sodium restriction, but loop diuretics can be used safely in patients with peripheral or pulmonary edema.Citation57 Beta-blockers, hydralazine/nitrates, and digoxin can also be used safely during pregnancy.Citation22,Citation52,Citation58,Citation59 Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ACEi/ARBs), sacubitril-valsartan, sodium glucose co-transporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists should be avoided during pregnancy.Citation59–62 Heart failure medications that are compatible with breastfeeding include evidence based beta-blockers, certain ACEi such as captopril or enalapril and mineralocorticoid inhibitors. ()
Table 1 Peripartum Cardiomyopathy Medications and Safety in Pregnancy and with Lactation
In patients who develop acute decompensated heart failure, nitroglycerine may be necessary and is preferred to nitroprusside owing to the potential risk of cyanide toxicity in the latter. There is one study that suggested β-agonists (ie dobutamine) in patients with PPCM and severe left ventricular dysfunction (LVEF ≤25%) may be detrimental and precipitate the need for left ventricular assist device or transplantation.Citation63 However, more studies are needed. Bromocriptine is a dopamine (D2) agonist that inhibits prolactin production and is a promising drug under investigation in the REBIRTH study for treatment in women with PPCM.Citation64 At present, it may be reasonable in patients with cardiogenic shock or severe LV dysfunction (EF <25%).Citation65–68 As it suppresses lactation, implications for breastfeeding should be discussed prior to initiation.
After delivery, cardiac medications are continued indefinitely in the presence of persistent cardiac dysfunction.Citation22 Optimal duration of treatment is unknown after systolic recovery. However, expert opinion suggests that in low-risk patients with fully normalized myocardial function for at least a year, medications could be slowly weaned in a stepwise approach with frequent clinical assessment and echocardiographic monitoring, potentially every 3–6 months. All patients who have recovered function should continue with annual clinic assessment by a cardiologist. We discuss some special consideration in treatment and counseling of patients with PPCM below.
Anticoagulation
Pregnant women and those in the early post-partum period are at increased risk of thrombosis due to the hypercoagulable state of pregnancy.Citation69 Furthermore, patients with PPCM have been found to have an increased incidence of left ventricular thrombi when compared to other forms of cardiomyopathy.Citation5,Citation13,Citation51,Citation70 Thus, anticoagulation may be considered in the setting of severe left ventricular dysfunction in the peripartum period to 8 weeks postpartumCitation53,Citation55 Anticoagulation is also recommended in patients who receive bromocriptine, given its increased risk of thromboembolism, stroke and myocardial infarction.Citation60 There are no established data on whether therapeutic or prophylactic anticoagulation should be used. Unfractionated and low-molecular-weight heparin can be used during pregnancy and lactation. Warfarin should not be used during pregnancy but is safe with lactation.Citation71 The novel anticoagulants are currently avoided as they have not been sufficiently studied during pregnancy or lactation.
Labor and Delivery
Multidisciplinary coordination by a cardio-obstetrics team of experts, including obstetrics, cardiology, maternal fetal medicine, anesthesiology, and nursing, is essential in timing and mode of delivery.Citation35,Citation72 It is recommended that hemodynamically stable patients are safe to continue pregnancy with a preference for vaginal delivery. According to American Heart Association (AHA) and European Society of Cardiology (ESC) guidelines, cesarean delivery may be considered in cases of acute heart failure, but is associated with increased risks of thromboembolism, infection, and hemorrhage.Citation60,Citation73 Invasive hemodynamic monitoring during labor and in the early post-partum period should be considered in patients with unstable hemodynamics.Citation22
Lactation
Most medications required for treatment of PPCM can be given safely with breastfeeding. The 2010 European Society of Cardiology statement on PPCM recommended against breastfeeding due to the postulated link between prolactin and PPCM.Citation3 However, subsequent IPAC data have not shown breastfeeding to be associated with adverse outcomes and a small study from the United States suggests breastfeeding may be associated with a higher chance of recovery.Citation24,Citation74 Given this and the known benefits of breastfeeding to the infant, many experts recommend breastfeeding in those stable enough to do so.Citation75–77
Sudden Death Prevention
Both cardioversion and defibrillation are safe in pregnancy and should be performed as indicated in any patient.Citation78 Fetal monitoring can be considered in non-emergent settings to assess for secondary fetal arrhythmias.Citation79 Early implantation of an ICD is usually discouraged as most patients recover to an LVEF ≥ 35% within 6 months.Citation60 Both the AHA and ESC recommend consideration of wearable cardioverter defibrillators (WCDs) in these patients as they could also be used to bridge to an ICD in patients that do not recover.Citation53,Citation55,Citation60,Citation80 A recent study examining the use of WCDs in pregnancy found that arrhythmia detection remained excellent despite the altered anatomy and physiology, but since no shocks were delivered in these patients, the efficacy of these devices remains unclear.Citation81
Contraception
Contraception should be discussed with all women with PPCM prior to discharge and should be emphasized by the patient’s cardiologist and obstetrician.Citation82 Estrogen-containing contraceptives should be avoided if possible in the early postpartum setting given the risk of thromboembolism in the setting of systolic dysfunction. Safe and effective choices include progesterone-releasing subcutaneous implants or the Mirena intrauterine device, with tubal ligation and vasectomy as options for those not considering future pregnancy.
Subsequent Pregnancies
Patient counseling regarding subsequent pregnancies is essential. LVEF before a subsequent pregnancy is the strongest predictor of outcomes, with substantially worse outcomes in women whose ventricular function fails to normalize (LVEF ≥50%) ().Citation83,Citation84 Prior to pregnancy, teratogenic heart failure medications should be discontinued for at least 3 months and systolic function reassessed to ensure it remains normal prior to determining a patient as having recovered ventricular function. Even with normalized systolic function, subsequent pregnancies carry risk of relapse and persistent cardiac dysfunction.Citation84 Assessing for subclinical ventricular dysfunction with stress testing and echocardiographic strain imaging may help identify patients at higher risk for relapse.Citation85–88 Although there is not enough data, prophylactic use of beta-blockers during subsequent pregnancies should be considered in women with recovered systolic function. Women should be thoroughly counseled about the risks of subsequent pregnancy. Those choosing to become pregnant should be closely monitored during pregnancy and for 6 months postpartum with serial echocardiograms and clinical exams by a cardiologist.Citation22,Citation89
Conclusion
PPCM should be considered in any pregnant or postpartum patient with symptoms concerning for heart failure. Although uncommon, it is a serious medical condition that affects women of childbearing age throughout the world. The etiology of PPCM is not fully understood, but both genetic susceptibility and hormonal influences play major role. In patients with clinical symptoms and an elevated NT-pro-BNP level, an echocardiogram is essential for assessment of systolic function. Multidisciplinary collaboration within a cardio-obstetrics team is necessary for prompt treatment, delivery planning, and postpartum care, and may prevent adverse outcomes. Development of more specific diagnostic criteria such as specific imaging findings, serum biomarker level, and/or genetic markers could help clinicians initiate more timely treatment. Given the rarity and underdiagnosis of PPCM, research collaboration among multiple centers is needed to answer important questions and define optimal management strategies. Significant gaps in knowledge remain, including the optimal anticoagulation strategy, timing of ICD implantation, frequency of imaging as well as more precise evaluation with strain imaging and stress testing for women who recover their systolic function, management of subsequent pregnancy, and long-term medical management after myocardial recovery.
Disclosure
The authors report no conflicts of interest in this work.
References
- Bauersachs J, König T, van der Meer P, et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019;21(7):827–843. doi:10.1002/ejhf.1493
- Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283(9):1183–1188. doi:10.1001/jama.283.9.1183
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi:10.1093/eurjhf/hfq120
- Gunderson EP, Croen LA, Chiang V, Yoshida CK, Walton D, Go AS. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol. 2011;118(3):583–591. doi:10.1097/AOG.0b013e318229e6de
- Kolte D, Khera S, Aronow WS, et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. J Am Heart Assoc. 2014;3(3):e001056.
- Mielniczuk LM, Williams K, Davis DR, et al. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006;97(12):1765–1768. doi:10.1016/j.amjcard.2006.01.039
- Brar SS, Khan SS, Sandhu GK, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100(2):302–304. doi:10.1016/j.amjcard.2007.02.092
- Harper MA, Meyer RE, Berg CJ. Peripartum cardiomyopathy: population-based birth prevalence and 7-year mortality. Obstet Gynecol. 2012;120(5):1013–1019. doi:10.1097/AOG.0b013e31826e46a1
- Kao DP, Hsich E, Lindenfeld J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. JACC Heart Fail. 2013;1(5):409–416. doi:10.1016/j.jchf.2013.04.011
- Krishnamoorthy P, Garg J, Palaniswamy C, et al. Epidemiology and outcomes of peripartum cardiomyopathy in the United States: findings from the Nationwide Inpatient Sample. J Cardiovasc Med. 2016;17(10):756–761. doi:10.2459/JCM.0000000000000222
- Karaye KM, Ishaq NA, Sa’idu H, et al. Incidence, clinical characteristics, and risk factors of peripartum cardiomyopathy in Nigeria: results from the PEACE Registry. ESC Heart Fail. 2020;7(1):235–243.
- Kamiya CA, Kitakaze M, Ishibashi-Ueda H, et al. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders. Results from the Japanese Nationwide survey of peripartum cardiomyopathy. Circ J. 2011;75(8):1975–1981. doi:10.1253/circj.CJ-10-1214
- Sliwa K, Petrie MC, van der Meer P, et al. Clinical presentation, management, and 6-month outcomes in women with peripartum cardiomyopathy: an ESC EORP registry. Eur Heart J. 2020;41(39):3787–3797. doi:10.1093/eurheartj/ehaa455
- Fett JD. Viral infection as a possible trigger for the development of peripartum cardiomyopathy. Int J Gynaecol Obstet. 2007;97(2):149–150. doi:10.1016/j.ijgo.2007.01.012
- Fett JD, Ansari AA, Sundstrom JB, Combs GF. Peripartum cardiomyopathy: a selenium disconnection and an autoimmune connection. Int J Cardiol. 2002;86(2–3):311–316.
- Ansari AA, Fett JD, Carraway RE, Mayne AE, Onlamoon N, Sundstrom JB. Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol. 2002;23(3):301–324. doi:10.1385/CRIAI:23:3:301
- Lamparter S, Pankuweit S, Maisch B. Clinical and immunologic characteristics in peripartum cardiomyopathy. Int J Cardiol. 2007;118(1):14–20. doi:10.1016/j.ijcard.2006.04.090
- Fett JD, Sundstrom BJ, Etta KM, Ansari AA. Mother-daughter peripartum cardiomyopathy. Int J Cardiol. 2002;86(2–3):331–332.
- Pearl W. Familial occurrence of peripartum cardiomyopathy. Am Heart J. 1995;129(2):421–422. doi:10.1016/0002-8703(95)90032-2
- van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, et al. Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J. 2014;35(32):2165–2173. doi:10.1093/eurheartj/ehu050
- Ware JS, Li J, Mazaika E, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med. 2016;374(3):233–241. doi:10.1056/NEJMoa1505517
- Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(2):207–221. doi:10.1016/j.jacc.2019.11.014
- Goli R, Li J, Brandimarto J, et al. Genetic and phenotypic landscape of peripartum cardiomyopathy. Circulation. 2021;143(19):1852–1862. doi:10.1161/CIRCULATIONAHA.120.052395
- Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600. doi:10.1016/j.cell.2006.12.036
- Honigberg MC, Cantonwine DE, Thomas AM, Lim KH, Parry SI, McElrath TF. Analysis of changes in maternal circulating angiogenic factors throughout pregnancy for the prediction of preeclampsia. J Perinatol. 2016;36(3):172–177. doi:10.1038/jp.2015.170
- Damp J, Givertz MM, Semigran M, et al. Relaxin-2 and soluble Flt1 levels in peripartum cardiomyopathy: results of the multicenter IPAC study. JACC Heart Fail. 2016;4(5):380–388. doi:10.1016/j.jchf.2016.01.004
- Haghikia A, Kaya Z, Schwab J, et al. Evidence of autoantibodies against cardiac troponin I and sarcomeric myosin in peripartum cardiomyopathy. Basic Res Cardiol. 2015;110(6):60. doi:10.1007/s00395-015-0517-2
- Liu J, Wang Y, Chen M, et al. The correlation between peripartum cardiomyopathy and autoantibodies against cardiovascular receptors. PLoS One. 2014;9(1):e86770. doi:10.1371/journal.pone.0086770
- Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58(7):659–670. doi:10.1016/j.jacc.2011.03.047
- Puri A, Sethi R, Singh B, et al. Peripartum cardiomyopathy presenting with ventricular tachycardia: a rare presentation. Indian Pacing Electrophysiol J. 2009;9(3):186–189.
- Carlson S, Gravely A, Adabag S. Trajectory of left ventricular ejection fraction among individuals eligible for implantable cardioverter-defibrillator. Pacing Clin Electrophysiol. 2021;44(5):800–806. doi:10.1111/pace.14168
- McNamara DM, Elkayam U, Alharethi R, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015;66(8):905–914. doi:10.1016/j.jacc.2015.06.1309
- Irizarry OC, Levine LD, Lewey J, et al. Comparison of clinical characteristics and outcomes of peripartum cardiomyopathy between African American and Non-African American Women. JAMA Cardiol. 2017;2(11):1256–1260. doi:10.1001/jamacardio.2017.3574
- Sliwa K, Mebazaa A, Hilfiker-Kleiner D, et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): eURObservational research programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur J Heart Fail. 2017;19(9):1131–1141. doi:10.1002/ejhf.780
- Davis MB, Arendt K, Bello NA, et al. Team-based care of women with cardiovascular disease from pre-conception through pregnancy and postpartum. J Am Coll Cardiol. 2021;77(14):1763–1777. doi:10.1016/j.jacc.2021.02.033
- Carland C, Panelli DM, Leonard SA, et al. Association of neighborhood income with clinical outcomes among pregnant patients with cardiac disease. Reprod Sci. 2022;29(10):3007–3014. doi:10.1007/s43032-022-00978-z
- Olanipekun T, Abe T, Effoe V, et al. Racial and ethnic disparities in the trends and outcomes of cardiogenic shock complicating peripartum cardiomyopathy. JAMA Network Open. 2022;5(7):e2220937. doi:10.1001/jamanetworkopen.2022.20937
- Ersbøll AS, Johansen M, Damm P, Rasmussen S, Vejlstrup NG, Gustafsson F. Peripartum cardiomyopathy in Denmark: a retrospective, population-based study of incidence, management and outcome. Eur J Heart Fail. 2017;19(12):1712–1720. doi:10.1002/ejhf.882
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125(1):5–12. doi:10.1097/AOG.0000000000000564
- Ford ND, Cox S, Ko JY, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization — United States, 2017–2019. MMWR Morb Mortal Wkly Rep. 2022;71(17):585–591. doi:10.15585/mmwr.mm7117a1
- Bello N, Rendon ISH, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62(18):1715–1723. doi:10.1016/j.jacc.2013.08.717
- Amos AM, Jaber WA, Russell SD. Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J. 2006;152(3):509–513. doi:10.1016/j.ahj.2006.02.008
- Goland S, Bitar F, Modi K, et al. Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail. 2011;17(5):426–430. doi:10.1016/j.cardfail.2011.01.007
- Forster O, Hilfiker-Kleiner D, Ansari AA, et al. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2008;10(9):861–868. doi:10.1016/j.ejheart.2008.07.005
- Ekizler FA, Cay S. A novel marker of persistent left ventricular systolic dysfunction in patients with peripartum cardiomyopathy: monocyte count- to- HDL cholesterol ratio. BMC Cardiovasc Disord. 2019;19(1):114. doi:10.1186/s12872-019-1100-9
- Sanusi M, Momin ES, Mannan V, et al. Using echocardiography and biomarkers to determine prognosis in peripartum cardiomyopathy: a systematic review. Cureus. 2022;14(6):e26130. doi:10.7759/cureus.26130
- Zagelbaum NK, Bhinder J, Gupta CA, Frishman WH, Aronow WS. Peripartum cardiomyopathy incidence, risk factors, diagnostic criteria, pathophysiology, and treatment options. Cardiol Rev. 2020;28(3):148–155.
- Koziol KJ, Aronow WS, Giuliani E, Nunziata A. Peripartum cardiomyopathy: current understanding of pathophysiology, diagnostic workup, management, and outcomes. Curr Probl Cardiol. 2023;48(8):101716.
- Morales A, Painter T, Li R, et al. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation. 2010;121(20):2176–2182. doi:10.1161/CIRCULATIONAHA.109.931220
- Honigberg MC, Elkayam U, Rajagopalan N, et al. Electrocardiographic findings in peripartum cardiomyopathy. Clin Cardiol. 2019;42(5):524–529. doi:10.1002/clc.23171
- Tibazarwa K, Lee G, Mayosi B, Carrington M, Stewart S, Sliwa K. The 12-lead ECG in peripartum cardiomyopathy. Cardiovasc J Afr. 2012;23(6):322–329. doi:10.5830/CVJA-2012-006
- Arany Z, Elkayam U. Peripartum Cardiomyopathy. Circulation. 2016;133(14):1397–1409. doi:10.1161/CIRCULATIONAHA.115.020491
- Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134(23):e579–e646. doi:10.1161/CIR.0000000000000455
- Pierce T, Hovnanian M, Hedgire S, Ghoshhajra B. Imaging of cardiovascular disease in pregnancy and the peripartum period. Curr Treat Options Cardiovasc Med. 2017;19(12):94. doi:10.1007/s11936-017-0593-8
- Bauersachs J, Arrigo M, Hilfiker-Kleiner D, et al. Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2016;18(9):1096–1105. doi:10.1002/ejhf.586
- Heidenreich PA, Bozkurt B, Aguilar D; Writing Committee Members, ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. 2022;28(5):e1–e167. doi:10.1016/j.cardfail.2022.02.010
- Bello NA, Bairey Merz CN, Brown H, et al. Diagnostic cardiovascular imaging and therapeutic strategies in pregnancy: JACC focus seminar 4/5. J Am Coll Cardiol. 2021;77(14):1813–1822. doi:10.1016/j.jacc.2021.01.056
- Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7(5):961–967. doi:10.1161/CIRCEP.114.001517
- Halpern DG, Weinberg CR, Pinnelas R, Mehta-Lee S, Economy KE, Valente AM. Use of medication for cardiovascular disease during pregnancy. J Am Coll Cardiol. 2019;73(4):457–476. doi:10.1016/j.jacc.2018.10.075
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi:10.1093/eurheartj/ehy340
- Hanssens M, Keirse MJ, Vankelecom F, Van Assche FA. Fetal and neonatal effects of treatment with angiotensin-converting enzyme inhibitors in pregnancy. Obstet Gynecol. 1991;78(1):128–135.
- Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451–456. doi:10.1016/0002-9343(94)90172-4
- Stapel B, Kohlhaas M, Ricke-Hoch M, et al. Low STAT3 expression sensitizes to toxic effects of β-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart J. 2017;38(5):349–361. doi:10.1093/eurheartj/ehw086
- McNamara DM. Randomized Evaluation of Bromocriptine in Myocardial Recovery Therapy for Peripartum Cardiomyopathy (REBIRTH) [Internet]. clinicaltrials.gov; Report No.: NCT05180773; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT05180773. Accessed April 28, 2023.
- Sliwa K, Blauwet L, Tibazarwa K, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121(13):1465–1473. doi:10.1161/CIRCULATIONAHA.109.901496
- Seghda TAA, Boro T, Bambara JE, et al. Prognosis of peripartum cardiomyopathy in sub-Saharan Africa (Burkina Faso South-West PPCM register). J Cardiol Cardiovasc Med. 2020;5(2):109–113. doi:10.29328/journal.jccm.1001096
- Haghikia A, Podewski E, Libhaber E, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108(4):366. doi:10.1007/s00395-013-0366-9
- Hilfiker-Kleiner D, Haghikia A, Berliner D, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J. 2017;38(35):2671–2679. doi:10.1093/eurheartj/ehx355
- Moiz B; Department of Pathology and Laboratory Medicine, the Aga Khan University Hospital, Karachi, Pakistan. A review of hemostasis in normal pregnancy and puerperium. Natl J Health Sci. 2017;2(3):123–127. doi:10.21089/njhs.23.0123
- Goland S, Modi K, Bitar F, et al. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail. 2009;15(8):645–650. doi:10.1016/j.cardfail.2009.03.008
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012:1728.
- Davis MB, Walsh MN. Cardio-Obstetrics. Circulation. 2019;12(2):e005417. doi:10.1161/CIRCOUTCOMES.118.005417
- Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135(8):e50–e87. doi:10.1161/CIR.0000000000000458
- Koczo A, Marino A, Jeyabalan A, et al. Breastfeeding, cellular immune activation, and myocardial recovery in peripartum cardiomyopathy. JACC Basic Transl Sci. 2019;4(3):291–300. doi:10.1016/j.jacbts.2019.01.010
- Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;30(364):k5287. doi:10.1136/bmj.k5287
- Victora CG, Bahl R, Barros AJD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi:10.1016/S0140-6736(15)01024-7
- Nguyen B, Jin K, Ding D, Remuzzi G. Breastfeeding and maternal cardiovascular risk factors and outcomes: a systematic review. PLoS One. 2017;12(11):e0187923. doi:10.1371/journal.pone.0187923
- Jeejeebhoy FM, Zelop CM, Lipman S, et al. Cardiac arrest in pregnancy: a scientific statement from the American Heart Association. Circulation. 2015;132(18):1747–1773. doi:10.1161/CIR.0000000000000300
- Honigberg MC, Givertz MM. Arrhythmias in peripartum cardiomyopathy. Card Electrophysiol Clin. 2015;7(2):309–317. doi:10.1016/j.ccep.2015.03.010
- Elkayam U, Goland S, Pieper PG, Silversides CK. High-risk cardiac disease in pregnancy: part II. J Am Coll Cardiol. 2016;68(5):502–516. doi:10.1016/j.jacc.2016.05.050
- Olic JJ, Stöllberger C, Schukro C, et al. Usage of the wearable cardioverter-defibrillator during pregnancy. IJC Heart Vasc. 2022;1(41):101066. doi:10.1016/j.ijcha.2022.101066
- Lindley KJ, Bairey MCN, Davis MB, et al. Contraception and reproductive planning for women with cardiovascular disease. J Am Coll Cardiol. 2021;77(14):1823–1834. doi:10.1016/j.jacc.2021.02.025
- Elkayam U, Tummala PP, Rao K, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344(21):1567–1571. doi:10.1056/NEJM200105243442101
- Joseph MS, Davis MB. Counseling women with peripartum cardiomyopathy about subsequent pregnancies. Curr Treat Options Cardio Med. 2021;23(6):41. doi:10.1007/s11936-021-00915-4
- Fett JD, Fristoe KL, Welsh SN. Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet. 2010;109(1):34–36. doi:10.1016/j.ijgo.2009.10.011
- Barbosa MM, Freire CMV, Nascimento BR, et al. Rest left ventricular function and contractile reserve by dobutamine stress echocardiography in peripartum cardiomyopathy. Rev Port Cardiol. 2012;31(4):287–293. doi:10.1016/j.repc.2012.02.002
- Yucel E, Davis EF, Scott N, Lewis GD, DeFaria YD. Exercise ventricular reserve among women with a history of peripartum cardiomyopathy. JACC Case Rep. 2021;3(15):1649–1653.
- Davis MB, Rameez R, Joseph MS. Cardiac function in women with peripartum cardiomyopathy. JACC Case Rep. 2021;3(15):1654–1655. doi:10.1016/j.jaccas.2021.07.003
- Elkayam U. Risk of subsequent pregnancy in women with a history of peripartum cardiomyopathy. J Am Coll Cardiol. 2014;64(15):1629–1636. doi:10.1016/j.jacc.2014.07.961
