Abstract
Objective
To compare patient experience in a real-life population of people living with HIV (PLWH) who received pharmaceutical care (PC) based on the Capacity-Motivation-Opportunity (CMO) model versus the traditional model.
Methods
Prospective cohort study in PLWH receiving either CMO-based PC or traditional PC in Spain between October 2019 and June 2021 (24 weeks), performed by the pharmacy department of 14 Spanish hospitals. Participants were adult patients with a clinical diagnosis of HIV treated with antiretrovirals who had been monitored in the participating hospital pharmacies for >1 year. Patient experience (IEXPAC questionnaire), clinical outcomes (cholesterol, triglycerides, HDL, glycated haemoglobin, and blood pressure), adherence to treatment, virologic control and patient satisfaction were determined.
Results
Patient experience in the CMO group at week 24 was significantly better (7.6 vs 6.9) than in the traditional group, with a higher mean improvement. Adherence was better in the CMO group, particularly with regard to concomitant medications (53.2% to 91.7%, p<0.001); no changes were observed in the traditional group. Patient satisfaction improved in the CMO group vs the traditional group (48 vs 44, p<0.001).
Conclusion
To our knowledge, this is the first study to compare CMO vs traditional methodology. The CMO model showed an overall improvement in real-life patient experience, satisfaction, and adherence to treatment compared to the traditional methodology.
Plain Language Summary
In recent years, several authors have agreed that the classic definition of pharmaceutical care has already “hit the ceiling” and needs to be rethought, rather than changed, leading the way to rethink the definition of this activity so that it is much more in line with the times and the needs of patients. This study was conducted to compare and measure the patient experience and clinical impact of a new methodology of pharmaceutical care applied to people living with HIV versus the traditional model. To this end, a prospective multicenter study was developed to evaluate this question. The generation of new high quality evidence is essential to incorporate a new concept and methodology of pharmaceutical care and for it to become the gold standard in routine practice.
Introduction
Human immunodeficiency virus (HIV) infection compromises CD4 cells and renders people living with HIV (PLWH) susceptible to other infections and some types of cancers.Citation1 According to the World Health Organization (WHO), the numbers of PLWH rose to 37.7 million worldwide in 2020,Citation1 of which 73% were receiving treatment.Citation2 New infections were estimated at 1.5 million,Citation1 and the proportion of elderly people infected with HIV also increased.Citation3
Antiretroviral treatment (ART) increases life expectancy in these patients and HIV has become a chronic infection for most of them.Citation4,Citation5 As a consequence, patients with HIV develop other comorbidities earlier than their HIV-negative counterparts.Citation4,Citation6 Some of these comorbidities are associated with age, which may lead to polypharmacy.Citation5,Citation7
Many studies over the years have suggested that HIV, ART treatment, or both, increase the risk of metabolic disorders,Citation8–10 which explains why clinical outcomes such as cholesterol, high-density lipoproteins (HDL), triglycerides, glycated haemoglobin A1c (HbA1c) and blood pressure have been assessed in PLWH.
In this complex setting, individualised pharmaceutical care (PC) is crucial. This concept, established in the 1990s, has traditionally focused on the medication administered to individuals.Citation11
Although different studies suggest that PC improves adherence, suppression of viral load, and improvement of CD4-T lymphocytes, it should be noted that most studies have a high risk of bias. New professional roles of clinical pharmacists are emerging in recent years.Citation12
However, in 2017, a new model based on Capacity-Motivation-Opportunity (CMO) pillars changed this paradigm, strengthening the idea that the patient and their circumstances, in terms of complexity, adherence or polypharmacy, needed to be at the centre of the model and managed according to a multidisciplinary and multidimensional perspective.Citation13,Citation14
To test this new model, patient activation,Citation15,Citation16 adherence to ART,Citation17,Citation18 adherence to concomitant medication,Citation15,Citation18 clinical outcomesCitation16,Citation17,Citation19 and patient experienceCitation20 were assessed in PLWH treated using the CMO model. Studies showed that this approach positively influenced these variables, and that both patients and healthcare professionals generally agreed on the benefit of the pharmacy intervention.Citation17
Although the CMO model has been successfully applied in single and multicentre studies,Citation16–20 no direct comparison with the traditional model has been made.
In this study, we applied the CMO model in real-life patients treated in 14 centres across Spain and assessed their experience, clinical outcomes, adherence, viro-immunological control, and satisfaction with the PC they received compared with the traditional approach, which does not include patient stratification or motivational interviews. To our knowledge, this is the first direct comparison of the CMO vs the traditional model.
Methods
Study Design and Participants
PLWH were prospectively recruited between October 2019 and June 2021 in 14 centres (specified in the acknowledgement section) across Spain. Patients were 18 years of age or more, clinically diagnosed with HIV infection (including hepatitis B or C virus coinfection), receiving ART, attending PC visits in a participating hospital pharmaceutical department for ≥1 year before the beginning of the study, and eligible to sign the informed consent. Patients were excluded if they were unable to complete the study questionnaires. No patients were paid for their participation in the study.
Patients were evaluated and followed up until November 2021. The study was carried out according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and the Declaration of Helsinki.Citation21 The study protocol and any other information requiring pre-approval was reviewed and approved by the research centre ethics committee Comité de Ética de Investigación Sevilla Sur (#0159-N-19).
Intervention
Thirteen participating hospitals (14 in all) that had been using the traditional model before the study were randomised to continue using the same model or to implement the CMO model. Participating centres were matched by size and number of patients to balance recruitment and activities among those using the 2017 CMO modelCitation13 and those using the traditional model.Citation11,Citation22,Citation23 Seven hospitals were included in each group. For analysis, patients were grouped according to whether they received PC according to the CMO method (CMO group) or the traditional method (traditional group).
In brief, the CMO model initially stratified the patients into three levels of priority according to their demographic, social, health, cognitive, functional, clinical services utilisation, and medication-related variables. Each patient received intensive PC corresponding to the predetermined interventions for each level of care, including training and education, individual pharmacotherapeutic monitoring and coordination of healthcare team members. Every level of priority required certain interventions and frequency of visits, plus the interventions of the previous level, eg the interventions and frequency offered to priority 2 patients corresponded to those of priority 1 plus priority 2.Citation13 According to the PC model, the interventions were led by hospital pharmacists, but agreed and carried out jointly with the multidisciplinary team in charge of the clinical follow-up of the patients.
Interestingly, during the face-to-face visit to the Hospital Pharmacy Service, a motivational interview was conducted for each patient. In each interview, pharmacotherapeutic objectives were established or re-evaluated, in consensus with the rest of the medical team taking care of the patient at all times.
Lastly, all patients received permanent contact tools (web, phone, email, etc.) with study pharmacists to resolve any incident or doubt related to their treatment at any time during the study.
Traditional PC intervention does not include patient stratification or the use of motivational interviews or modern technologies. The follow-up performed was the traditional one, according to the classic guidelines for the follow-up of this type of patients, more based on identifying, preventing and solving drug-related problems.Citation11,Citation22,Citation23
Outcomes
Outcomes were measured at the beginning (baseline) and at the end (24 weeks) of the study. Patient experience was evaluated using the IEXPAC questionnaire that was developed to measure the experience of patients with chronic illnesses with healthcare and social care professionals and servicesCitation24 and was administered by technical support staff to avoid bias. IEXPAC survey comprises two parts. The first one (global IEXPAC) consists of 11 items that are given a score of 1 (never) to 5 (always) depending on their frequency. The sum of the items scores ranges from 11 to 55, and this is converted to a global score between 0 and 10. The second part comprises four conditional questions that are assigned a score of 0 to 10 (conditional IEXPAC).
Secondary variables included measurement of individual clinical outcomes (cholesterol [mg/dL], blood pressure [mm Hg], triglycerides [mg/dL], HDL [mg/dL], and HbA1c [%]), adherence to treatment (both primary adherence and secondary adherence to ART and concomitant medication), viro-immunological control (CD4 cell counts [cells/µL], CD4/CD8 ratio, viral load [copies/mL]) and satisfaction.
Dispensing records were analysed, and the Simplified Adherence Medication Questionnaire (SMAQ) questionnaireCitation25 and the Morisky-Green questionnaireCitation26 were used to assess adherence to ART and concomitant medication, respectively. Baseline and end-of-treatment ART, concomitant treatment, primary and secondary adherence were assessed. Adherence is defined as “the process by which patients take their medications as prescribed”.Citation27
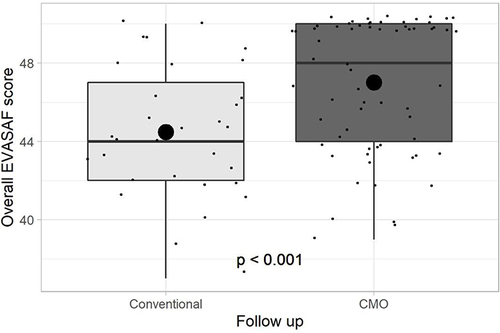
Patient satisfaction was measured using the EVASAF questionnaire, a survey completed by patients about the PC that they received at the hospital.Citation28
Statistical Analysis
Qualitative values were described as frequencies and percentages based on the population size, and quantitative values were based on the total number of patients (n), median and interquartile range (IQ range, percentile 75–25). Since no previous standard for calculating sample size was available in the literature, this was set to at least 1% of the patients receiving treatment in each centre, which translated to ≥ 7 patients/centre.
To analyse the relationship between the different time points and the clinical variables, each outcome was described using frequencies and analysed with ordinal logistic regressions, except for HDL values that were calculated with binomial logistic regressions. For each parameter, the group, time, and interaction group-time coefficients were included in the models. Interaction coefficients were used to compare if a change over time in one group differs to that of the other group, indicating a differential effect among groups. These measurements were used for adherence parameters and viro-immunological control. The odds ratio (OR) was also calculated for clinical outcomes.
For patient satisfaction, EVASAF questionnaire distribution and scores were analysed using the Shapiro–Wilk test and the Mann Whitney U-test, respectively. An additional sensitive exploratory analysis using several linear regression models was performed. Total EVASAF score was taken as the dependent variable, and age, level of studies, CD4 cell counts, and baseline viral loads were selected as independent variables that could be confounding factors. Model 1 was a simple analysis; model 2 included adjustment by age and level of studies; model 3, CD4 cell counts and baseline viral loads, and model 4 was an analysis adjusted for these 4 variables.
Data were collected and uploaded to the SEFH REDCap website.
The statistical analysis for the baseline parameters and the evaluation of patient satisfaction with their PC were performed using the program R Studio v. 1.1.456; the program R v.4.1.1 and jamovi v.2.2.2 were used for the statistical analysis of the IEXPAC questionnaire, biochemical and physiological clinical outcomes, adherence, the viro-immunological control, and the exploratory analysis of the evaluation of patient satisfaction with PC.
Results
Participant Characteristics
The baseline characteristics of the study population are described in . We evaluated 158 patients. Seven changed centre during the study and were therefore excluded; a total of 151 patients (117 men and 34 women) were included. The number of patients included in each variable can be found in the Supplementary Figure 1.
Table 1 Baseline Characteristics of Patients Included in the Study
Their median age was 51.35 years (IQR = 14.2). There was heterogeneity between intervention groups, which was considered in the statistical analysis of these results (). There were more patients with undetectable viral load and a CD4 cell count > 200 cells/µL in the CMO group (p<0.001) (). Clinical characteristics and pharmacotherapeutic variables were also evaluated (). At baseline, the CMO group had more patients with blood pressure levels above the normal range (p=0.001). No significant differences were observed between both groups in pharmacotherapeutical characteristics, but the number of concomitant medications was significantly higher in the CMO group (p=0.023), in line with their greater number of comorbidities (as described in Prados-Torres et al)Citation29 (data not shown). No patient had been seen in the emergency room or had been hospitalised before the study (data not shown).
Clinical Outcomes
Pharmacotherapeutic goals for biochemical parameters, hypertension, and glycaemic control after applying CMO or traditional PC models were monitored (). Although reductions were observed after PC intervention in both groups, an ordinal logistic regression analysis showed no significant differences in clinical variables between baseline and week 24 in either the CMO or traditional group, with the exception of a significant increase in blood pressure in the traditional group (p=0.02) (). The 1 point increase in the predictor “time” indicated that patients in the traditional group were 4.10 times more likely to have medium-high blood pressure.
Table 2 Comparison of Clinical Outcomes Before and After the Implementation of the Traditional PC Model or the CMO-Based PC Model
Additionally, after analysing the interaction between the time and groups, a significant difference was observed in the Hba1c and blood pressure values between the CMO and traditional groups (p=0.036), despite the low number of patients ().
Differential experience using the CMO versus traditional methodology in PC.
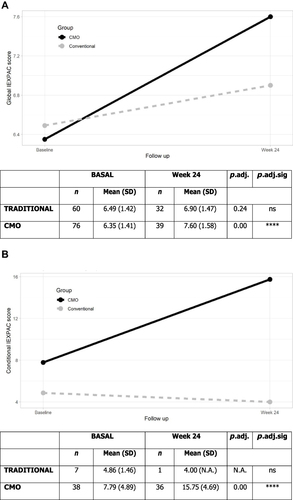
Results of the global IEXPAC questionnaire showed a significant improvement in patient experience with CMO-based PC (). An increase of >1 point was observed from the beginning of treatment to week 24 in the CMO group (adjusted p-value <0.005), while the increase from baseline to end-of-treatment in the traditional group was not significant (adjusted p-value=0.24). The mean value in the CMO group at week 24 was higher than in the traditional group (7.6, SD=1.58 vs 6.9, SD=1.47), although it was not significant (p=0.06, data not shown). Regarding the conditional IEXPAC (), CMO methodology had a positive impact after 24 weeks in PLWH (adjusted p-value <0.005).
Figure 1 Global and conditional IEXPAC at baseline and week 24. (A) Global IEXPAC. (B) Conditional IEXPAC.
End-of Treatment Adherence
Adherence is shown in . Increase in adherence to ART in the traditional group was non-significant, in contrast with the CMO group (p=0.034). Percentages of adherence to concomitant medication rose in the CMO group between baseline and end-of-treatment (p<0.001), while the change observed in the traditional group could not be assessed in terms of significance due to lacking values. Primary adherence was similar in the traditional group but increased in the CMO group (p<0.001). In this case, analysis of the CMO and traditional groups in terms of time and group resulted in significant difference (p=0.017). Secondary adherence results mimicked the trend seen in primary adherence: ie, no differences in the traditional group between both time points (p=0.317) but a significant rise in the CMO group (p<0.001). Again, the interaction value in both groups was significant (p=0.045).
Table 3 Evaluation of Adherence to Treatment in the Two Intervention Groups After 24 Weeks
Viro-Immunological Control
Baseline differences observed in CD4 cell counts and viral loads of the CMO and traditional groups (p<0.001) were addressed in the subsequent statistical analyses ().
Table 4 Viro-Immunological Control
The number of patients with CD4 cell counts > 200 cells/µL and undetectable viral load significantly increased after 24 weeks in the traditional group (71.2% to 91.9%, p=0.002, and 46.9% to 89.2%, p<0.001, respectively), while in the CMO group these values shifted from 98.9% to 98.7% and 96.6% to 98.6% for CD4 cell counts > 200 cells/µL and undetectable viral load at the end of treatment, respectively (). Differences between traditional and CMO groups were only significant in the case of CD4 cell counts (p=0.043). CMO methodology significantly impacted on the CD4/CD8 ratio at the end of treatment (p=0.048), but no other changes were observed.
Patient Satisfaction
Results of the EVASAF questionnaire showed that the score obtained in the CMO group was significantly higher than in the traditional group (48 vs 44, p<0.001) ( and Supplementary Tables 1 and 2). Knowledge about medications and their possible interactions and adverse effects showed the most noticeable differences. Since baseline differences among patients in the CMO and traditional groups may confound the interpretation of this questionnaire, we developed a series of models. Simple analysis of the group showed significant changes between the traditional and the CMO groups (p=0.001). This result persisted after adjustment by age and level of studies (model 2), and by CD4 cell counts and baseline viral loads (model 3), p=0.004 and p=0.043, respectively. However, model 4 showed no difference (p=0.082).
Discussion
In this study, we prospectively compared the impact of two different approaches to PC in PLWH from 14 different centres across Spain. The traditional model emphasises treatment, while the CMO model is patient-focused. We found that CMO methodology improves patients’ experience, adherence, and satisfaction compared with the traditional model.
The IEXPAC questionnaire measures different items that affect the relationship of patients with their treatment and healthcare specialists. The CMO model has previously obtained a high score in each questionnaire level,Citation20 but no comparison with other models has been made. In our study, PLWH experience using the CMO approach PC significantly improved after 24 weeks of follow-up, in contrast with traditional PC. Additionally, conditional IEXPAC significantly improved in the CMO group.
Previous studies have reported that HIV infection and/or ART may trigger metabolic disorders.Citation8 CMO model reduces the cardiovascular risk,Citation19 and it has been recently shown to improve biochemical parameters, such as cholesterol, triglycerides, HDL and blood pressure.Citation16 We found that cardiovascular variables remained unchanged, except for blood pressure and HbA1c. In the traditional group, end-of-treatment blood pressure was more likely to be middle to high. In contrast, the percentage of HbA1c in the normal range in patients treated with the traditional methodology rose significantly, while that of the CMO group remained unchanged. Remarkably, the percentage of patients with normal HbA1c in the CMO group at the beginning of the study was already high (91.3%) and number of patients was low. Significant improvements from this baseline level would be probably easier to observe in another population with lower values and a higher number of patients. This may also be the reason for the difference in CD4 cells > 200 cells/µL in both groups.
Another major concern in PLWH is adherence to treatment, a variable negatively impacted not only for the polypharmacy but by the therapeutic complexity resulting from HIV infection and comorbidities.Citation30–32 In our study, given the baseline characteristics of the population, the CMO arm had a higher percentage of polypharmacy and complexity than the traditional arm. Even under these circumstances, the establishment of PC in PLWH implies an increase in adherence and our results, that showed an increase in primary and secondary adherence, are in line with previous CMO-based PC, supporting the use of this model to improve patient adherence.Citation15,Citation17,Citation18
PLWH satisfaction was measured using the EVASAF questionnaire.Citation28 This survey was developed as a result of pharmacists’ awareness of the impact of their work on patient outcomes and treatment. Our results corroborated that the CMO model, focused on the patients, is superior to the traditional model and increases patient satisfaction. However, this result needs to be viewed with caution, since some variables may have a confounding influence. We found that patients are more satisfied when treated with the CMO compared to the traditional model when age and level of studies are considered, or when CD4 cell counts, and baseline viral loads are taken into account. However, this scenario changes after adjustment of these four factors, highlighting the need for more studies to assess this issue.
Even though the 2017 PC tool based on the CMO model has repeatedly reported advantages for PLWH, it is too complex an instrument. Therefore, a simplified adaptation was recently implemented.Citation33 This new tool has not been tested yet, but given its simplicity, it should be easy to apply and garner greater benefits for HIV patients. In order to implement this methodology, it is not necessary to acquire new technologies or get more staff, it is simply necessary to change the professional approach, more focused on the patient and less on the treatment. It is recommended that specialist pharmacists disseminate this new conception of the profession among the rest of the multidisciplinary team, even among the patients themselves, so that there is a better work and communication dynamic and the best health results are obtained from this work model.
Our study had some limitations. Participation and number of patients were smaller than planned due to the COVID-19 situation and heterogeneity was present at the beginning of the study. Besides, the number of participants for each study outcome fluctuated and, on certain occasions, was too low to draw definitive conclusions. Higher numbers of patients, or a follow-up of more than 24 weeks to allow the patient more time to ascertain the impact and differences between the two models, may shed some light on whether the difference in mean value of these two methodologies, which turned out to be p=0.06 in patient experience, would be significant. Indeed, longer study periods will be necessary to determine whether the effects observed after CMO pharmacist interventions are preserved over time. Despite these limitations, this is the first study to our knowledge to compare the impact of the patient-centred PC with that of traditional treatment-centred PC. Considering its ease of implementation, future research in other countries could validate this model in different health systems.
Conclusions
In conclusion, the CMO PC model, a pharmacist-led intervention based on the stratification of patients according to their specific necessities and their pharmacotherapeutic objectives, and reinforced by motivational interviews and tailored follow-up using the new technological tools, has improved PLWH experience, increased their adherence to treatment and offered other advantages over the traditional model, in line with previous publications, and might be considered as the gold standard in the PC of HIV disease.
Abbreviations
ART, antiretroviral therapy; CMO, capacity, motivation and opportunity; NRTI, nucleoside reverse transcriptase inhibitors; PC, pharmaceutical care; PLWH, People living with HIV.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
R.M.V. has received speaker honoraria from Gilead, Janssen, MSD, and ViiV Healthcare; A.L.L. has received speaker honoraria from Gilead, Janssen, MSD, ViiV Healthcare and Abbvie; E.A.G declares no conflicts of interest; M.M.C has received speaker honoraria from Gilead, Janssen, MSD and ViiV Healthcare; P.D.R declares no conflicts of interest; E.M.C. has received honoraria from Abbvie, AstraZeneca, Merck, Almirall, Celgene, CSL Behring, and Galapagos; M.J.H. declares no conflicts of interest; H.N.A. declares no conflicts of interest; V.A.A. declares no conflicts of interest; M.G.G has received speaker honoraria from Gilead, Janssen, MSD and ViiV Healthcare; L.M.F. declares no conflicts of interest; J.M.M.S. has received consultancy honoraria from MSD, ViiV Healthcare, Gilead and Janssen. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors would like to thank the pharmaceutical care human immunodeficiency virus group of the Sociedad Española de Farmacia Hospitalaria (Spanish Society of Hospital Pharmacists, SEFH) and the participant centres: Hospital Clínico San Carlos (Madrid, Spain), Hospital Universitario de Guadalajara (Guadalajara, Spain), Hospital Universitario Virgen de Valme (Sevilla, Spain), Hospital Clinic i Provincia (Barcelona, Spain), Complejo Hospitalario A Coruña (A Coruña, Spain), Hospital Miguel Servet, Hospital Universitario La Paz (Madrid, Spain), Hospital del Tajo (Madrid, Spain), Hospital de Ciudad Real (Ciudad Real, Spain), Hospital Puerta del Mar (Cádiz, Spain), Hospital de Torrecárdenas (Almería, Spain), Hospital Lozano Blesa (Zaragoza, Spain), Hospital Virgen de la Candelaria (Tenerife, Spain), Hospital Virgen de la Luz (Cuenca, Spain). We also acknowledge Dr Alfonso Picó, Dr Carlos Fernández-Escobar (Medical Science Consulting; Valencia, Spain) and Dr Emilio García (Delos Clinical; Sevilla, Spain) for the statistical support, and Dr Elena Rebollo-Gómez and Dr Vanessa Marfil (Medical Science Consulting; Valencia, Spain) for medical writing support and editing.
Additional information
Funding
References
- World Health Organization. HIV/AIDS; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/hiv-aidss . Accessed September 3, 2022.
- World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact. Web Annex 1: key data at a glance; 2021.
- Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019. HIV Surveillance Supplemental Report; 2021.
- Trickey A, May MT, Vehreschild -J-J; Antiretroviral Therapy Cohort C. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e356. doi:10.1016/S2352-3018(17)30066-8
- Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA. 2013;309:1397–1405. doi:10.1001/jama.2013.2963
- Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–1797. doi:10.1093/cid/ciu701
- Milic J, Russwurm M, Cerezales Calvino A, Brañas F, Sánchez-Conde M, Guaraldi G. European cohorts of older HIV adults: POPPY, AGE(h)IV, GEPPO, COBRA and FUNCFRAIL. Eur Geriatr Med. 2019;10:247–257. doi:10.1007/s41999-019-00170-8
- Ergin HE, Inga EE, Maung TZ, Javed M, Khan S. HIV, antiretroviral therapy and metabolic alterations: a review. Cureus. 2020;12:e8059. doi:10.7759/cureus.8059
- da Cunha J, Maselli LM, Stern AC, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol. 2015;4(2):56–77. doi:10.5501/wjv.v4.i2.56
- d’Ettorre G, Ceccarelli G, Pavone P, et al. What happens to cardiovascular system behind the undetectable level of HIV viremia? AIDS Res Ther. 2016;13:21. doi:10.1186/s12981-016-0105-z
- Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47:533–543.
- Ahmed A, Abdulelah Dujaili J, Rehman IU, et al. Effect of pharmacist care on clinical outcomes among people living with HIV/AIDS: a systematic review and meta-analysis. Res Social Adm Pharm. 2022;18(6):2962–2980. doi:10.1016/j.sapharm.2021.07.020
- Morillo-Verdugo R, Martínez-Sesmero JM, Lázaro-López A, Sánchez-Rubio J, Navarro-Aznárez H, DeMiguel-Cascón M. Development of a risk stratification model for pharmaceutical care in HIV patients. Farm Hosp. 2017;41:346–356. doi:10.7399/fh.2017.41.3.10655
- Morillo-Verdugo R, Calleja-Hernandez MA, Robustillo-Cortes MLA. A new pharmaceutical care concept: more capable, motivated, and timely. Hosp Pharm. 2019;54:348–350. doi:10.1177/0018578719867657
- Morillo-Verdugo R, Robustillo-Cortés MA, Manzano García M, Almeida-González CV. Influence of pharmacist intervention, based on CMO model, to improve activation in HIV patients. Rev Esp Quimioter. 2019;32:40–49. doi:10.1056/NEJM199803263381301
- Morillo-Verdugo R, Robustillo-Cortes MLA, Navarro-Ruiz A, et al. Clinical impact of the capacity-motivation-opportunity pharmacist-led intervention in people living with HIV in Spain, 2019–2020. J Multidiscip Healthc. 2022;15:1203–1211. doi:10.2147/jmdh.S361305
- Cantillana-Suárez MG, Robustillo-Cortés MLA, Gutiérrez-Pizarraya A, Morillo-Verdugo R. Impact and acceptance of pharmacist-led interventions during HIV care in a third-level hospital in Spain using the Capacity-Motivation-Opportunity pharmaceutical care model: the IRAFE study. Eur J Hosp Pharm. 2021;28(e1):e157–e163. doi:10.1136/ejhpharm-2020-002330
- Morillo-Verdugo R, Vélez-Díaz-Pallarés M, Fernández-Pacheco García-Valdecasas M, Fernández-Espínola S, Sánchez-Rubio Ferrández J, Navarro-Ruiz A. Application of the CMO methodology to the improvement of primary adherence to concomitant medication in people living with-HIV. The PRICMO Project. Farm Hosp. 2021;45:247–252.
- Morillo-Verdugo R, Robustillo-Cortes MLA, Martin-Conde MT, et al. Effect of a structured pharmaceutical care intervention versus usual care on cardiovascular risk in HIV patients on antiretroviral therapy: INFAMERICA study. Ann Pharmacother. 2018;52:1098–1108. doi:10.1177/1060028018778045
- Cantillana-Suárez MG, Manzano-García M, Robustillo-Cortés MLA, Morillo-Verdugo R. Evaluation of HIV+ patients experience with pharmaceutical care based on AMO-methodology. Farm Hosp. 2018;42:200–203. doi:10.7399/fh.10947
- Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi:10.1001/jama.2013.281053
- Vlasses PH. Education of manpower for future pharmaceutical care and research. Drug Intell Clin Pharm. 1988;22:800–803. doi:10.1177/106002808802201018
- Schafer JJ, Gill TK, Sherman EM, McNicholl IR, Hawkins B. ASHP guidelines on pharmacist involvement in HIV care. Am J Health Syst Pharm. 2016;73(7):468–494. doi:10.2146/ajhp150623
- Mira JJ, Nuño-Solinís R, Guilabert-Mora M, et al. Development and validation of an instrument for assessing patient experience of chronic illness care. Int J Integr Care. 2016;16:13. doi:10.5334/ijic.2443
- Mendoza-Aguilera M, Ferrando-Piqueres R, Martín TA, et al. Adherencia al tratamiento antirretroviral en pacientes VIH: todavía queda mucho por hacer. Ibero Latin Am J Health Syst Pharm. 2018;28:203–210.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi:10.1097/00005650-198601000-00007
- Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. doi:10.1111/j.1365-2125.2012.04167.x
- Monje-Agudo P, Borrego-Izquierdo Y, Robustillo-Cortés Mde L, Jiménez-Galán R, Almeida-González CV, Morillo-Verdugo RA. Encuesta de valoración de la satisfacción de los pacientes con la Atención Farmacéutica recibida en las consultas de Farmacia hospitalaria [Design and validation of a satisfaction survey with pharmaceutical care received in hospital pharmacy consultation]. Farm Hosp. 2015;39:152–156. Spanish. doi:10.7399/fh.2015.39.3.8366
- Prados-Torres A, Poblador-Plou B, Calderón-Larrañaga A, et al. Multimorbidity patterns in primary care: interactions among chronic diseases using factor analysis. PLoS One. 2012;7:e32190. doi:10.1371/journal.pone.0032190
- Ho IS, Holloway A, Stenhouse R. What do HIV-positive drug users’ experiences tell us about their antiretroviral medication-taking? An international integrated literature review. Addiction. 2020;115(4):623–652. doi:10.1111/add.14857
- Hernández Arroyo MJ, Cabrera Figueroa SE, Sepúlveda Correa R, Valver de Merino Mde L, Iglesias Gómez A, Domínguez-Gil Hurlé A. Impact of a pharmaceutical care program on clinical evolution and antiretroviral treatment adherence: a 5-year study. Patient Prefer Adherence. 2013;7:729–739. doi:10.2147/ppa.S47519
- McNicholl IR, Gandhi M, Hare CB, Greene M, Pierluissi E, Pharmacist-Led A. Program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV-positive patients. Pharmacotherapy. 2017;37(12):1498–1506. doi:10.1002/phar.2043
- Morillo-Verdugo R, Aguilar Pérez T, Gimeno-Gracia M, Rodríguez-González C, Robustillo-Cortes MLA. Simplification and multidimensional adaptation of the stratification tool for pharmaceutical care in people living with HIV. Ann Pharmacother. 2022;10600280221096759. doi:10.1177/10600280221096759