Abstract
Objective
Rapid identification is critical for ischemic stroke due to the very narrow therapeutic time window. The objective of this study was to construct a diagnostic model for the rapid identification of ischemic stroke.
Methods
A mixture population constituted of patients with ischemic stroke (n = 481), patients with hemorrhagic stroke (n = 116), and healthy individuals from communities (n = 2498) were randomly resampled into training (n = 1547, mean age: 55 years, 44% males) and testing (n = 1548, mean age: 54 years, 43% males) samples. Serum corin was assayed using commercial ELISA kits. Potential risk factors including age, sex, education level, cigarette smoking, alcohol consumption, obesity, blood pressure, lipids, glucose, and medical history were obtained as candidate predictors. The diagnostic model of ischemic stroke was developed using a backward stepwise logistic regression model in the training sample and validated in the testing sample.
Results
The final diagnostic model included age, sex, cigarette smoking, family history of stroke, history of hypertension, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, fasting glucose, and serum corin. The diagnostic model exhibited good discrimination in both training (AUC: 0.910, 95% CI: 0.884–0.936) and testing (AUC: 0.907, 95% CI: 0.881–0.934) samples. Calibration curves showed good concordance between the observed and predicted probability of ischemic stroke in both samples (all P>0.05).
Conclusion
We developed a simple diagnostic model with routinely available variables to assist rapid identification of ischemic stroke. The effectiveness and efficiency of this model warranted further investigation.
Introduction
Stroke, the leading cause of long-term disability and mortality, can result in a rapid loss of neurological function due to disturbance of blood supply to the brain.Citation1 It is a heterogeneous disorder with two major subtypes – ischemic and hemorrhagic stroke, with fundamentally different pathogenesis and thus needs different treatment strategies.Citation2 Proper medical treatment of stroke relies on accurate and rapid differentiation between ischemic and hemorrhagic stroke due to a narrow therapeutic time window for the former. Currently, neuroimaging remains the gold standard to distinguish the two subtypes, but it is limited temporallyCitation3 and may thereby lead to missing treatment golden time for stroke, the ischemic subtype in particular. Many efforts have been made to improve the differential diagnosis of ischemic stroke at a very early occurrence. For example, many candidate biomarkers in blood and cerebrospinal fluid have been identified as potential diagnostic markers, such as Glial protein (S100β),Citation4 glial fibrillary acidic protein (GFAP),Citation5 matrix metalloproteinase-9 (MMP-9),Citation6 ubiquitin C-terminal hydrolase-L1 (UCH-L1), nucleoside diphosphate kinase A (NDKA), C-reactive protein (CRP)Citation7 and myelin basic protein (MBP).Citation8 However, none of them achieved sufficient discrimination in clinical settings. In addition, several prediction scores have been developed aiming to clinically differentiate ischemic stroke from stroke patients, such as the Guy’s Hospital score,Citation9 the Siriraj score,Citation10 and the score developed by Besson et al.Citation11 These scores were not sufficient enough to initiate thrombolytic therapy for ischemic stroke without further diagnostics to rule out hemorrhage.Citation12,Citation13 Recently, our group found that serum corin was significantly decreased in patients with stroke and further lower in those with hemorrhagic stroke, compared to healthy controls.Citation14 Corin is a physiological convertase of atrial natriuretic peptide,Citation15 and its circulating levels have also been associated with other cardiovascular disorders, eg, hypertension,Citation16 heart failure,Citation17 and coronary syndrome.Citation18 Circulating corin appears to participate in stroke development and may therefore possess a potential for risk prediction and prognostic assessment of stroke.Citation19 However, no study has examined the effect of circulating corin on the differentiation of stroke subtypes. Here, we aimed to develop a diagnostic model incorporating serum corin with conventional risk factors for differentiation between ischemic and hemorrhagic stroke subtypes. This model could be applied as assistance in the differential diagnosis of ischemic stroke and speed up the initiation of thrombolysis, for patients whose therapeutic window is going to past in particular.
Methods
Participants
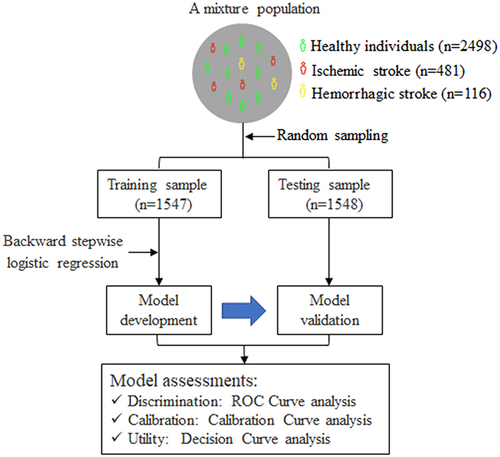
As illustrated in , this study included a mixture population constituted of patients with ischemic stroke (n = 481), patients with hemorrhagic stroke (n = 116), and healthy controls from communities (n = 2498). The detailed methods of study participants’ recruitment have been described previously.Citation14 In brief, a total of 597 patients with hospitalized first-ever stroke were consecutively recruited from January to May 2014. The inclusion criteria were as follows: (1) age ≥22 years; (2) stroke onset within 48 hours confirmed by imaging; (3) willing to sign informed consent by patients or their direct family members. Patients with one of the following were excluded: (1) recurrent stroke; (2) current pregnancy; (3) unable to participate in the follow-up examination. Stroke including ischemic and hemorrhagic subtypes was diagnosed by a trained neurologist and confirmed by brain computed tomography (CT) or magnetic resonance imaging (MRI) scan. A total of 2498 healthy controls who never had cardiovascular diseases were selected from the Gusu cohort study,Citation16 a community-based prospective study including Chinese Han adults in Suzhou city. The study protocols were approved by the Ethics Committee of Soochow University. All participants provided written informed consent. Our study complies with the Declaration of Helsinki.
Figure 1 A flowchart illustrating the selection of study participants and analytical plan.
Measurement of Serum Soluble Corin
As detailed previously,Citation14 levels of serum soluble corin were quantified in the Central Laboratory of the School of Public Health at Soochow University by trained staff who were blind to the clinical characteristics of the study participants, using commercial ELISA kits (R&D Systems, Inc., Minneapolis, USA). All the samples were processed in a duplicate assay. Intra- and inter-assay coefficients of variation were less than 2.7% and 6.3%, respectively.
Collection of Conventional Risk Factors
The methods of data collection of conventional risk factors of stroke have been described in our previous study.Citation14 Briefly, information on demographic characteristics (age and sex), lifestyle risk factors (cigarette smoking and alcohol consumption), metabolic risk factors (obesity, diabetes, dyslipidemia, and hypertension), and medical history was obtained using a structured questionnaire administered by trained staff. Three consecutive blood pressure measurements were measured by trained staff according to a common protocol recommended by the American Heart Association.Citation20 Blood pressure was measured with the participant in a supine or sitting position using a standard mercury sphygmomanometer and appropriate cuff size. The means of the three measurements were recorded as systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively. Fasting plasma glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and serum uric acid were assayed using commercial reagents.
Statistical Analysis
Data were analyzed using R statistical software (version 4.1.3). As shown in , we randomly divided the mixture population into training and testing samples for model development and validation, respectively. The clinical characteristics of study participants were presented according to the two samples.
Model Development
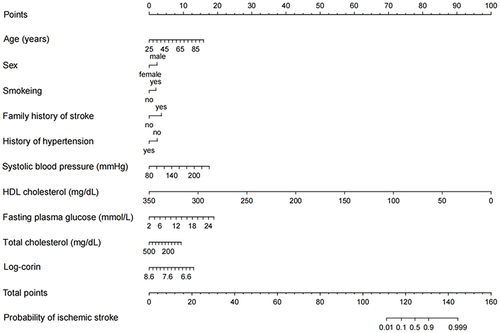
To derive the diagnostic model for the identification of ischemic stroke, we first developed the diagnostic model in the training sample. Easily available conventional risk factors such as age, sex, cigarette smoking, alcohol consumption, family history of stroke, hypertension, diabetes mellitus, SBP, DBP, total cholesterol, triacylglycerol, HDL-C, LDL-C, fasting plasma glucose, and serum uric acid were considered candidate predictors. Of them, a univariable logistic regression model with ischemic stroke as the dependent variable was constructed to select predictors. Variables showing a significant univariable association (P<0.05) were included to fit a multivariable logistic regression model, and backward stepwise selection was applied to select final predictors. Together with serum corin, the selected predictors were used to develop the final prediction model by constructing a multivariable logistic regression model. Serum corin was forced into the model fitting because it was a predictor for stroke independent of conventional risk factors in our previous study.Citation21 To ease the clinical application of the final prediction model, a nomogram was developed using the R package “rms” to determine the predicted probability of being ischemic stroke.
Model Validation
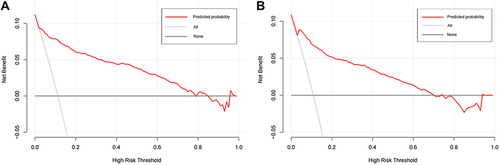
To estimate the performance of the diagnostic model developed in the training sample, discrimination, calibration, and utility of the model were assessed in the testing sample. In specific, the receiver operating characteristic (ROC) curve was drawn with the R package “pROC” to evaluate the discrimination. Calibration curve analysis was applied by plotting the observed probability against the predicted probability to evaluate the calibration. Decision curve analysis (DCA) was conducted with the R package “rmda” to determine the clinical practicability of the prediction model based on the net benefit under different threshold probabilities. These model assessments were also conducted in the testing sample.
Evaluation of the Diagnostic Test
We first determined the cut-off value of the predicted probability by the ROC curve, followed by evaluations of its validity and predictive value. Sensitivity, specificity, Youden’s index, and likelihood ratio were used to assess the validity. Positive and negative predictive values were estimated based on the sensitivity, specificity, and prevalence of ischemic stroke in China.Citation22
Results
Characteristics of Study Participants
A total of 1547 participants in the training sample (mean age: 55 years, 44% men) and 1548 participants in the testing sample (mean age: 54 years, 43% men) were included in the current study. Their clinical characteristics are presented in . Most variables were equally distributed, except for history of CHD (1.23% vs 2.45%), and levels of triglycerides (mean level: 1.55 vs 1.44) between the training and testing samples (all P<0.05).
Table 1 Clinical Characteristics of Study Participants
Development of the Diagnostic Model in the Training Sample
Univariable logistic regression found that age, sex, cigarette smoking, alcohol consumption, family history of stroke, history of hypertension, history of diabetes, SBP, total cholesterol, HDL-C, fasting plasma glucose, and serum uric acid were significantly associated with ischemic stroke (Supplementary Table S1). Of these variables, age, sex, cigarette smoking, family history of stroke, history of hypertension, SBP, total cholesterol, HDL-C, and fasting plasma glucose survived from the multivariable regression with backward stepwise selection. As expected, the prediction performance was significantly improved after adding log-corin, as indicated by the increased Nagelkerke’s R² (from 47.6% to 50.5%), area under the ROC curve (from 0.902 to 0.910, P=0.016) and decreased Akaike information criterion (from 626 to 601), Brier score (from 0.061 to 0.058). Therefore, ten predictors including age, sex, cigarette smoking, family history of stroke, history of hypertension, SBP, total cholesterol, HDL-C, fasting plasma glucose, and Log-corin were included in the final model (). The probability of the diagnostic model developed in the training sample was shown below:
Table 2 The Final Diagnostic Model Developed in the Training Sample
The area under the ROC curve for the diagnostic model was 0.910 (95% CI: 0.884–0.936), suggesting a good discriminatory capacity (). The calibration plots showed an almost perfect overlap between the observed and predicted probability of ischemic stroke (P=0.619), suggesting a good predictive accuracy of the diagnostic model (). The DCA curves showed that the prediction model could be applied clinically if the risk threshold was 1–79% (), suggesting a wide clinical application.
Figure 2 The receiver-operating characteristic curve illustrating the discrimination of the diagnostic model in the training (A) and testing (B) samples.
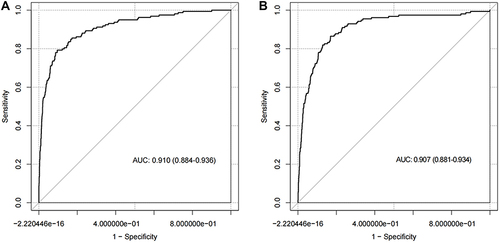
Figure 3 The calibration curve illustrating the agreement between the observed and predicted probability of ischemic stroke determined by the diagnostic model in the training (A) and testing (B) samples.
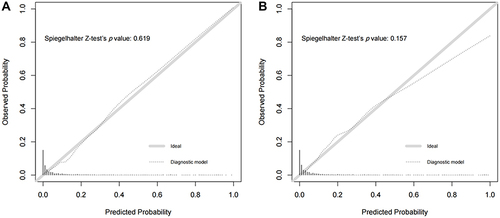
Figure 4 The decision curve for the diagnostic model in the training (A) and testing (B) samples.
Validation of the Diagnostic Model in the Testing Sample
We further conducted an external validation of the diagnostic performance of the model developed in the training sample. The results showed that this diagnostic model also achieved a good discrimination, calibration, and clinical applicability in the testing sample, as suggested by an area under the ROC curve of 0.907 (95% CI: 881–0.934, ), an overlap between the observed and predicted probability of ischemic stroke (P=0.157, ), and a wide range of application between 2% and 70% of the risk of ischemic stroke determined by the DCA curves (), respectively.
Application and Evaluation of the Diagnostic Test
To ease the clinical application of the diagnostic model, we drew a nomogram that can be used to manually obtain predicted probability of ischemic stroke from the final diagnostic model (). Supplementary Table S2 shows the validity and predictive value of the diagnostic test obtained from the final diagnostic model. The ROC curve analysis showed that the cut-off value of the diagnostic score was 0.190, with a sensitivity of 79.2% and a specificity of 90.4% in the training sample, with a sensitivity of 72.9% and a specificity of 90.1% in the testing sample as well. A higher diagnostic score over 0.190 indicates a higher probability of being ischemic stroke. This diagnostic test received an ideal Youden’s index, a large positive likelihood ratio and a small negative likelihood ratio in both samples, indicating that the diagnostic test could be reliable. The latest Global Burden of Disease Study showed that the prevalence of ischemic stroke in China was 1700/100,000 in 2019. Based on this prevalence, we estimated the predictive value of the diagnostic test. The positive and negative predictive values were 0.125 and 0.996 in the training sample and 0.113 and 0.995 in the testing sample.
Discussion
Leveraging a large mixture sample including patients with ischemic stroke, patients with hemorrhagic stroke, and healthy individuals, we constructed a simple-to-use diagnostic model to assist rapid identification of ischemic stroke. This diagnostic model can have immediate practical implications as routinely available variables can be easily obtained before admission. Therefore, this model could be used in predicting the risk of ischemic stroke for community members, applied as assistance in the differential diagnosis of ischemic stroke, and used in speeding up the initiation of thrombolysis for patients with neurological symptoms.
Several key risk factors have been established to account for ischemic stroke risk in previous studies. Age and male sex are robust non-modifiable risk factors and influence the epidemiology, pathophysiology, and treatment efficacy for ischemic stroke. The risk of ischemic stroke doubles every 10 years after the age of 55, and almost 75% of strokes occur in people aged >65 years.Citation23,Citation24 The male gender was a significantly stronger predictor of ischemic stroke since males have a higher prevalence of cardiovascular conditions such as higher blood pressureCitation25 and stroke.Citation26,Citation27 The EPIC Potsdam StudyCitation28 indicated that almost 60% of ischemic stroke risk could be attributed to hypertension, hypercholesterolemia, and cigarette smoking. There is a strong dose–response relationship between the number of cigarettes smoked and the risk of ischemic stroke, cigarette smoking causes a twofold increase in the risk of ischemic stroke.Citation29 Reducing blood pressure in individuals with hypertension is highly effective in preventing ischemic stroke.Citation30 Family history of stroke, elevated fasting plasma glucose, and low cumulatively averaged HDL-C were associated with an increased IS risk.Citation31–33 In line with previous studies, our study shows that these risk factors are independent risk factors for ischemic stroke. All variables in our final prediction model are related to patient demographics and laboratory measurements, enabling the prediction of ischemic stroke risk using only clinical data. This provides a simpler method for assistance in the clinical diagnosis of ischemic stroke.
Thrombolysis is currently recommended for IS patients within 4.5 hours of stroke onset. However, disappointingly, thrombolysis in patients with acute ischemic stroke is infrequent due to reasons such as high cost, not always being available, and possible contraindications. An alternative approach in acute stroke diagnosis is to identify stroke biomarkers that reflect the body’s response to the damage caused by the different types of stroke. Over 150 candidate biomarkers have been studied. For example, S100β,Citation34 a glial protein, was one of the first molecules suggested as a candidate to aid the diagnosis of ischemic stroke, but its measurement had poor sensitivity.Citation35 GFAPCitation5 was the best candidate to date for differentiating hemorrhage and ischemic stroke. Controversially, it was previously reported that, when measured early (<1 h after stroke onset), serum GFAP did not distinguish hemorrhagic and ischemic stroke.Citation36 The concentration of MMP-9 was reported to peak at 24 h post stroke,Citation6 where the therapeutic window has been passed. Other markers include, but are not limited to, UCH-L1, NDKA, and CRPCitation7 and MBP.Citation8,Citation37 Nevertheless, measurements of candidate biomarkers have usually occurred beyond the time window for thrombolysis and no individual candidate has proven to have adequate performance for use in an acute clinical setting where decisions about an individual patient are being made.Citation38–40 More importantly, none of the biomarkers have entered routine clinical use. There are also several clinical scores to distinguish between hemorrhagic and ischemic stroke, such as the Guy’s Hospital score,Citation9 the Siriraj score,Citation10 and the score developed by Besson et al,Citation11 but these scores are insufficient to initiate treatment for ischemic stroke without further diagnostics to rule out hemorrhage stroke.Citation12 CT, the golden diagnostic method, is very popular now, especially in developed areas. The assistance diagnostic methods including markers and scores mentioned above seemed usefulness in clinical settings and not studied anymore. However, it is not very easy to transport patient timingly to stroke units in undeveloped areas, those with inconvenient traffic in particular. Therefore, we developed a diagnostic model that may help individuals in undeveloped areas.
Corin was thought to be a physiological activator of atrial natriuretic peptides,Citation41 which correspond to the decreased natriuretic peptides in stroke. Its circulating levels have also been associated with other cardiovascular disorders, eg, hypertension, heart failure, and coronary syndrome. Studies have shown that the activity of corin on activation of ANP has been reduced in patients with heart failureCitation17 and corin was associated with atrial fibrillationCitation42 and heart failure.Citation43 These disease states may therefore affect the association between serum corin and stroke. Recently, our group demonstrated that serum corin was significantly decreased in patients with stroke and further lower in those with hemorrhagic stroke, compared to healthy controls.Citation16
Our study is not devoid of limitations. First, although we found that serum corin could predict future risk of ischemic stroke independently of conventional risk factors, including age, sex, cigarette smoking, family history of stroke, blood pressure, lipids, and glucose, serum corin just slightly (but not significantly) improved the prediction performance for ischemic stroke over these conventional risk factors. Second, the study was a case–control study only and a prospective study is needed to confirm the model. We also need to confirm the use of this model with other populations. Third, specific markers that are uniquely response to ischemic stroke are ideal markers for the construction of diagnostic model. However, such markers are rare. In our study, we developed a diagnostic model incorporating serum corin with conventional risk factors. Although these markers are not disease-specific, the performance of the diagnostic model is relatively acceptable.
In summary, we developed a simple diagnostic model with routinely available variables to assist rapid identification of ischemic stroke. The effectiveness and efficiency of this model warranted further investigation.
Abbreviations
AUC, area under the receiver-operating characteristic curve; CI, confidence interval; S100β, glial protein; GFAP, glial fibrillary acidic protein; MMP-9, matrix metalloproteinase-9; UCH-L1, ubiquitin C-terminal hydrolase-L1; NDKA, nucleoside diphosphate kinase A; CRP, C-reactive protein; MBP, myelin basic protein; CT, computed tomography; MRI, magnetic resonance imaging; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Log-corin, Log-transformed corin; ROC, receiver operating characteristic; CHD, coronary heart disease; DCA, decision curve analysis; IQR, interquartile range.
Disclosure
None of the authors have financial associations that might pose a conflict of interest in connection with the submitted article. The datasets used during the current study are available from the corresponding author on a reasonable request.
Acknowledgments
We gratefully acknowledge the cooperation and participation of the members of the Gusu cohort. We especially thank the clinical staff at all participating hospitals for their support and contribution to this project. Without their contribution, this research would not have been possible.
Additional information
Funding
References
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics–2010 update: a report from the American heart association. Circulation. 2010;121:948–954. doi:10.1161/CIRCULATIONAHA.109.192666
- Abdu H, Tadese F, Seyoum G, Milligan C. Comparison of ischemic and hemorrhagic stroke in the medical ward of Dessie referral hospital, northeast Ethiopia: a retrospective study. Neurol Res Int. 2021;2021:9996958. doi:10.1155/2021/9996958
- Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–298. doi:10.1016/S0140-6736(07)60151-2
- Zhou S, Bao J, Wang Y, Pan S. S100β as a biomarker for differential diagnosis of intracerebral hemorrhage and ischemic stroke. Neurol Res. 2016;38:327–332. doi:10.1080/01616412.2016.1152675
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: gfap-thirty-one years (1969–2000). Neurochem Res. 2000;25:1439–1451. doi:10.1023/A:1007677003387
- Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis. 2011;20:47–54. doi:10.1016/j.jstrokecerebrovasdis.2009.10.008
- Hasan N, McColgan P, Bentley P, Edwards RJ, Sharma P. Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review. Br J Clin Pharmacol. 2012;74:230–240. doi:10.1111/j.1365-2125.2012.04212.x
- Matias-Guiu J, Martinez-Vazquez J, Ruibal A, Colomer R, Boada M, Codina A. Myelin basic protein and creatine kinase bb isoenzyme as csf markers of intracranial tumors and stroke. Acta Neurol Scand. 1986;73:461–465. doi:10.1111/j.1600-0404.1986.tb04585.x
- Allen CM. Clinical diagnosis of the acute stroke syndrome. Q J Med. 1983;52:515–523.
- Poungvarin N, Viriyavejakul A, Komontri C. Siriraj stroke score and validation study to distinguish supratentorial intracerebral haemorrhage from infarction. BMJ. 1991;302:1565–1567. doi:10.1136/bmj.302.6792.1565
- Sandercock PA, Allen CM, Corston RN, Harrison MJ, Warlow CP. Clinical diagnosis of intracranial haemorrhage using guy’s hospital score. Br Med J. 1985;291:1675–1677. doi:10.1136/bmj.291.6510.1675
- Bamford J. Clinical examination in diagnosis and subclassification of stroke. Lancet. 1992;339:400–402. doi:10.1016/0140-6736(92)90085-H
- Weir CJ, Murray GD, Adams FG, Muir KW, Grosset DG, Lees KR. Poor accuracy of stroke scoring systems for differential clinical diagnosis of intracranial haemorrhage and infarction. Lancet. 1994;344(8928):999–1002. doi:10.1016/S0140-6736(94)91648-9
- Peng H, Zhu F, Shi J, et al. Serum soluble corin is decreased in stroke. Stroke. 2015;46:1758–1763. doi:10.1161/STROKEAHA.114.008368
- Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–8529. doi:10.1073/pnas.150149097
- Peng H, Zhang Q, Cai X, et al. Association between high serum soluble corin and hypertension: a cross-sectional study in a general population of China. Am J Hypertens. 2015;28:1141–1149. doi:10.1093/ajh/hpv002
- Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;4:114–120. doi:10.1161/CIRCHEARTFAILURE.109.895581
- Peleg A, Ghanim D, Vered S, Hasin Y. Serum corin is reduced and predicts adverse outcome in non-st-elevation acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2013;2:159–165. doi:10.1177/2048872613483588
- Yu R, Han X, Zhang X, Wang Y, Wang T. Circulating soluble corin as a potential biomarker for cardiovascular diseases: a translational review. Clin Chim Acta. 2018;485:106–112. doi:10.1016/j.cca.2018.06.036
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an aha scientific statement from the council on high blood pressure research professional and public education subcommittee. J Clin Hypertens. 2005;7:102–109. doi:10.1111/j.1524-6175.2005.04377.x
- Chen L, Zhang Q, Zhang M, et al. Soluble corin predicts the risk of cardiovascular disease. JACC. 2022;2:490–501. doi:10.1016/j.jacasi.2022.01.004
- Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the gbd 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi:10.1016/j.jacc.2020.11.010
- Yousufuddin M, Young N. Aging and ischemic stroke. Aging. 2019;11:2542–2544. doi:10.18632/aging.101931
- Roy-O’Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology. 2018;159:3120–3131. doi:10.1210/en.2018-00465
- Wiinberg N, Høegholm A, Christensen HR, et al. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8:978–986. doi:10.1016/0895-7061(95)00216-2
- de Miguel-Yanes JM, Jiménez-García R, López-de-Andrés A, et al. The influence of sex on ischemic stroke incidence, therapeutic procedures and in-hospital mortality: results of the Spanish national hospital discharge. Int J Clin Pract. 2021;75:e14984. doi:10.1111/ijcp.14984
- Poorthuis MHF, Algra AM, Algra A, Kappelle LJ, Klijn CJM. Female- and male-specific risk factors for stroke: a systematic review and meta-analysis. JAMA Neurol. 2017;74:75–81. doi:10.1001/jamaneurol.2016.3482
- Weikert C, Berger K, Heidemann C, et al. Joint effects of risk factors for stroke and transient ischemic attack in a German population: the epic Potsdam study. J Neurol. 2007;254:315–321. doi:10.1007/s00415-006-0358-x
- Markidan J, Cole JW, Cronin CA, et al. Smoking and risk of ischemic stroke in young men. Stroke. 2018;49:1276–1278. doi:10.1161/STROKEAHA.117.018859
- Diener HC, Hankey GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage: jacc focus seminar. J Am Coll Cardiol. 2020;75:1804–1818. doi:10.1016/j.jacc.2019.12.072
- Fan M, Lv J, Yu C, et al. Family history, tobacco smoking, and risk of ischemic stroke. J Stroke. 2019;21:175–183. doi:10.5853/jos.2018.03566
- Madsen TE, Long DL, Carson AP, et al. Sex and race differences in the risk of ischemic stroke associated with fasting blood glucose in regards. Neurology. 2021;97:e684–e694. doi:10.1212/WNL.0000000000012296
- Li H, Qian F, Zuo Y, et al. U-shaped relationship of high-density lipoprotein cholesterol and incidence of total, ischemic and hemorrhagic stroke: a prospective cohort study. Stroke. 2022;53:1624–1632. doi:10.1161/STROKEAHA.121.034393
- Takahashi M, Chamczuk A, Hong Y, Jackowski G. Rapid and sensitive immunoassay for the measurement of serum s100b using isoform-specific monoclonal antibody. Clin Chem. 1999;45:1307–1311. doi:10.1093/clinchem/45.8.1307
- Hill MD, Jackowski G, Bayer N, Lawrence M, Jaeschke R. Biochemical markers in acute ischemic stroke. CMAJ. 2000;162:1139–1140.
- Dvorak F, Haberer I, Sitzer M, Foerch C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis. 2009;27:37–41. doi:10.1159/000172632
- Foerch C, Wunderlich MT, Dvorak F, et al. Elevated serum s100b levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38:2491–2495. doi:10.1161/STROKEAHA.106.480111
- Cuadrado E, Rosell A, Colomأ╚ N, et al. The proteome of human brain after ischemic stroke. J Neuropathol Exp Neurol. 2010;69:1105–1115. doi:10.1097/NEN.0b013e3181f8c539
- Lescuyer P, Allard L, Zimmermann-Ivol CG, et al. Identification of post-mortem cerebrospinal fluid proteins as potential biomarkers of ischemia and neurodegeneration. Proteomics. 2004;4:2234–2241. doi:10.1002/pmic.200300822
- Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. 2009;40:e380–e389. doi:10.1161/STROKEAHA.108.528752
- Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J Biol Chem. 2002;277:16900–16905. doi:10.1074/jbc.M201503200
- Chen F, Xia Y, Liu Y, et al. Increased plasma corin levels in patients with atrial fibrillation. Clinica Chimica Acta. 2015;447:79–85. doi:10.1016/j.cca.2015.05.017
- Dong N, Chen S, Yang J, et al. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–211. doi:10.1161/CIRCHEARTFAILURE.109.903849