Abstract
Background
Herpes zoster (HZ) is a skin disease that can also cause virus-infectious peripheral neuropathies. Despite this, there is limited information on patient preferences for seeking medical attention for HZ and zoster-associated pain (ZAP). Our study aimed to evaluate how frequently patients with ZAP choose to visit neurologists for their symptoms.
Methods
This study conducted a retrospective review of electronic health records in three general hospitals from January 2017 to June 2022. Using association rule mining, the study analyzed referral behaviors.
Results
We identified 33,633 patients with 111,488 outpatient visits over 5.5 years. The study found that the majority of patients (74.77–91.22%) visited dermatologists during their first outpatient visit, while only a small percentage (0.86–1.47%) preferred to consult a neurologist. The proportion of patients referred to a specialist during their medical visit varied significantly between different specialties within the same hospital (p <0.05) and even within the same specialty (p<0.05). There was a weak association (Lift:1.00–1.17) of referral behaviors between dermatology and neurology. Across the three hospitals, the average number of visits to a neurologist for ZAP was 1.42–2.49, with an average electronic health record duration of 11–15 days per patient. After consulting with a neurologist, some patients were referred to other specialists.
Conclusion
It was observed that patients with HZ and ZAP tended to visit a variety of specialists, with only a small number seeking the assistance of neurologists. However, from the perspective of neuroprotection, it is the duty of neurologists to provide more means.
Introduction
Herpes zoster (HZ) is caused by the reactivation of latent varicella zoster virus (VZV) in the sensory ganglia, such as cranial nerve ganglia and dorsal root ganglia. Although it presents as a painful vesicular rash, HZ is not just a skin disease. HZ episodes can lead to various complications and sequelae, including, neurological disorders such as limb paralysis, cerebrovascular disorders, cardiovascular disease, and serious scarring.Citation1 Pain is the most common complication, with many cases of acute HZ followed by severe zoster-associated pain (ZAP). This pain may persist for months or even years after the HZ rash heals, which is known as postherpetic neuralgia (PHN). PHN is a type of neuropathic pain caused by damage to the axons and myelin by the VZV.Citation2,Citation3 This condition, along with acute ZAP, imposes a significant economic burden on the healthcare system,Citation4,Citation5 which implies there are currently no effective and safe treatments for PHN.Citation6 VZV infection is a complex medical condition that requires expertise from multiple specialties, including infectious diseases, immunology, dermatology, pain management, and neurology. Although available guidelines suggest effective agents based on current evidence, they do not provide a specific algorithm for management. This leaves the choice of agents and timing of evaluation to the physician’s discretion.Citation7–9 A survey of internists and family practitioners found the median number of yearly patient visits for HZ and PHN was two and four, respectively.Citation10 Unfortunately, only one-quarter of physician respondents referred patients with HZ, and two-thirds referred patients with PHN to a subspecialist. Approximately 3% and just over 20% of respondents reported referring over 10% of their patients with HZ or PHN, respectively, to a subspecialist.Citation10 This highlights the need for improved treatment methods,Citation6 as HZ and PHN continue to be prevalent.Citation11 HZ and ZAP are VZV-infectious peripheral neuropathies or VZV-induced nerve injuries,Citation12 that require attention from neurologists and researchers as an area of unmet clinical need and as an archetype of neuropathic pain.Citation13 This study aimed to assess the frequency of HZ, ZAP, and PHN patients visiting neurologists rather than other specialists.
Materials and Methods
Study Design and Patients
This study is based on EHR, without the direct involvement of patients, and without obtaining informed consent from patients prior to the study. The participants’ data are anonymous, and these changes do not distort the scholarly meaning. This study was approved by the Ethics Review Board of Affiliated Tenth People’s Hospital of Tongji University (SHSY-IEC-4.1/18-203). All procedures involving human participants were performed by the ethical standards of the institutional and national research committee and the 1964 Helsinki Declaration, its later amendments, or comparable ethical standards.
This study was performed with the collaboration of clinics from the following three hospitals in East China: Affiliated Tenth People’s Hospital of Tongji University in Shanghai; Taicang First People’s Hospital in Jiangsu, and Bengbu Second People’s Hospital in Anhui. Randomly select three hospitals from different provinces in Eastern China for research, which represent the characteristics of outpatient diseases in general hospitals in the Yangtze River Basin of Eastern China.
Data Collection
The data were retrieved from electronic health records (EHRs) using the hospital information system for patients with HZ, ZAP, or PHN who were received at one of three general hospitals between January 2017 and June 2022.
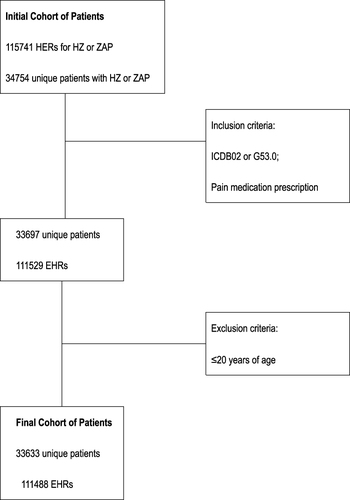
Persons with diagnostic codes for HZ (International Classification of Diseases (ICD),Citation14 Ninth Revision, Clinical Modification codes starting with 053, and Tenth Revision, Clinical Modification codes starting with B02) were categorized as cases with episode start dates based on their earliest recorded diagnosis. Episodes of ZAP were identified using pain medication prescription. PHN was defined by a diagnostic HZ code (B02 or G53.0) or a pain medication prescription during the quarter of the HZ episode or the following quarter. Using ICD diagnostic codes to diagnose zoster was both highly selective (positive predictive value 93%) and sensitive (97.5%).Citation15 Episodes, where individuals were under 20 years of age at the time of diagnosis, were excluded to avoid misclassification of varicella cases as HZ.
Follow-up data for this set of patients were available up to June 2022, covering 1.0−5.5 years. The EHR was a medical record for patients with HZ, ZAP, or PHN who had received the total activities and services of the hospital and medical specialists within a maximum period of 5.5 years.
Data Analyses
Only fields for patient demographics, medical visits, and diagnoses were retrieved. Two steps of data manipulation and analysis were then performed using SPSS 19.0 and SPSS Modeler 18.0 (IBM Corp., Armonk, NY, USA).
Descriptive statistics (eg, means and proportions) were used to present the patient characteristics of the study population as well as HZ-specific information. The relevant medical specialties considered in our study were as follows: dermatology, internal medicine, geriatrics, neurology, pain management, surgery, ophthalmology, otorhinolaryngology, rehabilitation medicine, Chinese traditional medicine, and other specialties (such as gynecology, oncology, and psychiatry).
We utilized association rule mining (ARM) to detect the selection preference of specialists for individual patients during their entire visit and to analyze the external reason for PHN development and the role of neurologists. ARM is used in many fields of study, including not only market research but also medicine and epidemiology. For example, ARM has been applied in areas such as the prediction of acute myocardial infarction,Citation16 studies of ADHD comorbidity,Citation17 and cancer prevention factors.Citation18 The data were formatted as the “shopping basket”, a single outpatient medical insurance number was analyzed to observe the different EHR and medical specialists’ “co-occurrence” situation, analyzing 50% of the frequent items and association rules of the data. To avoid redundant rules, a support threshold was established as 1%, and confidence thresholds were established as 50%.
Except where stated otherwise, the data were presented as the mean ± standard deviation (SD) or % values. Statistical analysis was made by the Kruskal–Wallis H-test and the one-way ANOVA test. The cut-off for statistical significance was set at p<0.05.
Results
Patients Disposition
In total, 33,633 unique HZ patients were received at the hospital for the first time in January 2017. During follow-up (to June 2022), a total of 111,488 EHRs were invoiced for these patients (). The EHR was equally dispersed over the age categories and sexes. Approximately half (51.6%) of the patients were female. Moreover, at the time of first visit to the hospital, 31.5% of the patients were aged between 21 and 49 years, 22.6% were aged between 50 and 59 years, 24.7% were between 60 and 69 years, 12.9% were between 70 and 79 years, and 8.3% were older than 80 years.
Preferred Specialist at a First Medical Visit
Within the three hospitals, the vast majority (74.77−91.22%) of patients first visit a dermatologist, followed by specialists in pain management (3.05−4.25%), and neurology (0.86−1.47%), except for the rehabilitation specialist in the Affiliated Tenth People’s Hospital of Tongji University (). The proportion of patients who were received by a specialist during their initial medical visit had significant differences in the different specialties in the same hospital (p <0.05) and significant differences in the same specialty in the three different hospitals (p <0.05).
Table 1 Patients Referral Behaviors
The number of EHRs per patient ranged from 1 to 36, with an average of 3.57 EHRs per patient. Approximately 1% of the patients needed only one EHR, with fewer than 50% of the patients who needed 10 EHRs or more. The majority of EHRs were invoiced by the dermatologist (50.83−88.65%) within the three hospitals, followed by the specialists in pain management (4.63−8.72%), and geriatrics (0.84−1.56%), except for the rehabilitation specialist in the Affiliated Tenth People’s Hospital of Tongji University, respectively. The average number of EHRs was longest for geriatrics (7.84−11.81 times) and shortest for surgery (1.32−1.66 times). The EHR duration per patient ranged from 1 to 356 days, whereas the average EHR duration was 35.72 days per patient. The average EHR duration was the shortest for surgery (5.63−9.25 days) and the longest for pain management (169.98−195.03 days). The proportion of the number of EHRs, the average numbers of EHRs, and the average EHR duration that patients received to specialists had significant differences in the different specialties in the same hospital (p <0.05) and in the same specialty within the three hospitals (p <0.05), respectively ().
Frequency of Referral Between Different Sub Specialties
A total of 77,855 (69.83%) observations were omitted due to the EHR of only one specialist visit, leaving 33,633 observations for ARM analysis.
After executing the association model, 210 association rules, including 70 rules whose confidence degree is greater than 50%, were obtained. Due to a large number of association rules, it is difficult to elaborate on all the rules. Combined with the purpose of this study, we used only selected meaningful association rules of dermatology and neurology.
When the consequent was dermatology, the ARM was applied and four rules were identified and passed the thresholds of 1% and 50% for the support and confidence parameters, respectively. For the Affiliated Tenth People’s Hospital of Tongji University, the strongest support parameter (9.3%) was between dermatology and ophthalmology, or neurology; the strongest confidence parameter (100.00%) was between dermatology and otorhinolaryngology, or rehabilitation with the highest lift of 1.31; the remainder indicated low associations between dermatology and other specialties. The lift value was 1.07 to 1.31, which implied that there was a weak positive association. The resulting association rules in the other two hospitals are shown in .
Table 2 Top 4 Results of Association Rule Mining of Referral Behaviors
Analysis of Meaningful Association Rules of Neurology
Although a few patients (42−283) initially visited the neurologist in the three hospitals, only a small number received treatment. The number of EHRs was 102−704; the average number of EHRs was only 1.42−2.49; and the average duration of an EHR was only 11−15 days per patient. The majority of patients (348/704− 88/193−64/102) were referred by other specialists to a neurologist for ZAP in the three hospitals, followed by patient referrals for Ramsay Hunt syndrome (152/704−62/193−26/102) or other disorders (5/704−3/193− 1/102). The proportion of patients referred to a neurologist for ZAP or Ramsay Hunt syndrome varied significantly across different specialties within the same hospital (p<0.05) and even within the same departments within the three hospitals (p<0.05), respectively (). Additionally, some patients were referred to other specialists after they consulted with a neurologist (). The proportion of patients referred to other specialists after seeing a neurologist also varied significantly across different specialties within the same hospital (p<0.05) and the same departments in the three hospitals (p<0.05), respectively ().
Table 3 Patients Referral Behaviors in Neurology Specialists
In the field of neurology, four rules were established and met the required thresholds of 1% and 50% for support and confidence parameters, respectively. Upon analyzing data from the Affiliated Tenth People’s Hospital of Tongji University, it was found that the strongest support parameter (1.6%) and confidence parameter (66.67%) existed between neurology and dermatology, ophthalmology, or traditional Chinese medicine (TCM), with the highest lift of 28.10. This suggests a strong positive association between neurology and dermatology, ophthalmology, or TCM. The resulting association rules in the other two hospitals are shown in .
Discussion
Although the HZ lesion might influence the patient’s choice bias, our study found that the majority of patients first visited a dermatologist within one of the three hospitals. Following the dermatologist, patients typically visited a pain management specialist. The only exception was the Affiliated Tenth People’s Hospital of Tongji University, where a significant proportion of patients visited rehabilitation experts as their second choice. The Department of Rehabilitation Medicine at the Affiliated Tenth People’s Hospital of Tongji University has primarily focused on providing neuroprotective treatment for ZAP and PHN. As a result, the majority of outpatients received by the department are those with ZAP or PHN, accounting for more than 60% of their total patient population. Including rehabilitation EHRs in this study may lead to a decrease in the proportion of patients visiting dermatologists. Even with this factor, over 70% of patients sought treatment from a dermatologist at the Affiliated Tenth People’s Hospital of Tongji University. Additionally, there were significant differences in the proportion of patients who saw a specialist during their initial medical visit across different specialties within the same hospital (p<0.05). As the three hospitals are not primary healthcare units, a portion of the diagnosed subjects at the time of their initial medical visit in these hospitals may not start onset patients. In cases where shingles are present on the skin, it seems to be the right choice to visit a dermatologist. VZV is a skin-tropic virus.Citation19 HZ is caused by reactivated-VZV, the virulent progeny virus spreads orthodromically from the ganglion, via the sensory nerve root, to the innervated target tissue (skin, cornea, auditory canal, etc.). During the acute eruptive phase of herpes zoster (HZ), painful vesicles develop in a single dermatome and appear on the skin. These vesicles are typically umbilicated and multiple. The distribution of HZ is usually along the innervating area of one ganglion, although adjacent dermatomes may also be affected.Citation20,Citation21 HZ is generally self-limiting in immunocompetent patients without risk factors for complications,Citation22 and the acute eruptive phase typically lasts for 2–4 weeks.Citation20 Although dermatology is the first department to see a doctor for the first time with HZ,Citation23 the time to control virus outbreaks and deal with vesicles is usually limited to one month.Citation20
Despite its outward appearance, the hallmark of HZ in adulthood is pain. More than 95% of patients over the age of 50 experience acute ZAP.Citation24 Additionally, 60–70% of patients continue to experience persistent pain one month after disease onset, and 40% of patients consider their pain to be severe.Citation24 Except for the dermatologist, the second was a pain management specialist within the three hospitals in this study. This aligns with existing guidelines, which were updated in 2020 to provide comprehensive information on pain management and treatment options for both residents and board-certified specialists.Citation22 In cases of persistent intolerable pain, it is advisable to refer patients to a pain specialist.Citation22 Acute ZAP is caused by the spread of the virus along the axon and the migration of immune cells. This leads to subsequent inflammation and damage to sensory nerves and endings.Citation25–27 The interdisciplinary European consensus-based guidelines on the management of HZ have cautioned that neuroinflammation is partly responsible for painful sensations.Citation28
In the acute phase, it is important to manage neuritic pain. One method proposed in this guideline is to actively intervene in pain management while using antiviral drugs during a rash.Citation22,Citation29 Unlike PHN, acute ZAP should preferentially be treated with systemic analgesics, initially including non-steroidal anti-inflammatory drugs (NSAIDs) and opioids.Citation22 However, these therapies may not completely alleviate acute ZAP.Citation29,Citation30 It is worth noting that the incidence and severity of PHN tend to be higher in patients over the age of 50.Citation31–33 Approximately 20% of patients over the age of 60 years experienced disease complications that persist for more than one year.Citation34 Pain specialists at this stage may employ an analgesic strategy that involves selecting from multiple agents with complementary mechanisms of action, such as gabapentin, pregabalin, tricyclic antidepressants, opioids, and nerve blocks with steroids and local anesthetics, which have demonstrated analgesic activity.Citation35 The latest guidelines emphasize the use of local treatment options, including capsaicin 8% patches and lidocaine 5% medicated plaster.Citation22 According to the 2017 update of a Cochrane review, capsaicin has been found to alleviate pain in all studies.Citation36 The temporary relief of pain is believed to be caused by the deactivation of heat-sensitive epidermal nociceptors that express the Transient Receptor Potential Vanilloid 1 (TRPV1).Citation37 Skin biopsies conducted after prolonged or high-concentration application of capsaicin have shown a significant reduction in intra-epidermal nerve fiber density.Citation38,Citation39 Recent studies suggest that capsaicin-induced Ca2+/calpain-mediated ablation of axonal terminals is required for long-lasting analgesia in a mouse model of neuropathic pain.Citation40,Citation41 However, this is not an optimal choice for the VZV-damaged nerves. Other neuromodulation treatments, such as pulsed radiofrequency and spinal cord electrical stimulation, may also provide pain relief.Citation12 The existence of multiple methods implies that the efficacy of analgesic therapy is limited.Citation12,Citation35 The current analgesic strategy for PHN often falls short, with less than half of patients achieving adequate 50% pain relief.Citation13
Management of HZ is not limited to dermatologists and pain management specialists; but also falls under the purview of neurologists. Guidelines suggest providing sufficient pain medication for the typically severe neuralgiform pain and referring patients with specialized conditions to both a neurologist and a specialist.Citation22 The study found that the number of visits to a neurologist for ZAP and PHN within the three hospitals was only 1.42−2.49, with an average EHR duration of 11−15 days per patient. This suggests that only a small number of patients directly visited neurologists. Approximately 50% of the HZ patients visited with neurologists more than one HER, while only 10% (7879 patients) of the patients experienced 10 or more EHRs. The study found that around 80% of patients diagnosed with ZAP reported inadequate pain treatment, suggesting that our neurologist did not effectively intervene in the treatment of ZAP and PHN. Additionally, the results of the ARM analysis revealed a weaker correlation between the referral behaviors of dermatologists and neurologists. While there was a strong positive association among neurologists and dermatologists, ophthalmologists, or TCM doctors, the majority of patients received by other specialists referred to neurologists were for Ramsay Hunt syndrome, followed by ZAP or other disorders. Other studies have shown that most (>60%) of patients with PHN were referred for specialty care, with 30% of them being referred to neurologists.Citation10 The patterns of medication use were similar across all specialties.Citation10 Approximately 40% of specialties prescribed narcotics, and >90% prescribed antivirals, to the majority of their patients,Citation10 which indicated a lack of alternative therapies. The most surprising result of this study was the portion of patients who visited neurologists were later transferred to other specialists, suggesting that many neurologists may not fully understand ZAP ().
VZV is not only a skin-tropicCitation19 but also a neurotropic virus.Citation42 When the virus reactivates, it is assembled in neuronal cell bodies and transported anterogradely through axons to the nerve ending and epidermis.Citation43 ZAP, especially PHN, is a specific type of neuropathy in which the virulence VZV is transmitted from the neuron to the axon endings,Citation44 causing axon destruction from the inside out.Citation45 As VZV-induced peripheral axonal damage causes ZAP, it raises the question as to why there is no targeted nerve treatment available. The treatment goal for patients with ZAP should not only focus on pain relief; but also on protecting and repairing damaged nerves. However, current guidelines do not address the importance of neuroprotection or promoting nerve repair in these patients. When faced with nerve damage caused by VZV, many neurologists are unsure of how to proceed. Specialists in HZ typically wait for neuropathic pain to develop, then refer patients to pain management specialists, which can make treating nerve injuries more challenging.
Effective pain management is crucial for patients with ZAP and PHN. However, it is not sufficient on its own. In addition to reducing pain, it is important to promote nerve repair and functional recovery. Immediate protection of affected nerves, controlling the inflammatory reaction of nerve axons, and minimizing nerve damage caused by VZV are all necessary. The attending physicians who have initial contact with the patient play a critical role in the early management of HZ and the prevention of PHN. Unfortunately, attending physicians and dermatologists have not fully grasped the importance of early anti-neuroinflammation and neuroprotection in managing ZAP and reducing the likelihood of PHN.Citation46 While ZAP and PHN may require few qualitative and localization diagnoses, effective treatment by neurologists is necessary. Although some patients recognize ZAP as a neuropathic pain and seek out a neurologist, the majority of neurologists do not fully understand this neuropathy. The Department of Rehabilitation Medicine of Affiliated Tenth People’s Hospital of Tongji University has seen a significant influx of patients, highlighting the growing need for effective treatment options.
To address the challenges of ZAP and PHN, it is crucial to adopt mechanism-based approaches. Recent research has suggested a link between ZAP and increased levels of oxygen free radicals and homocysteine (Hcy), as well as reduced levels of vitamins C and D. Firstly, low antioxidant levels may be associated with uncontrolled VZV reactivation, acute nerve damage, and PHN.Citation47–49 Antioxidant therapy may help in preventing both ZAP and PHN.Citation47 Secondly, studies have indicated a high prevalence of Vitamin C deficiency in patients with HZ and PHN, and Vitamin C deficiency independently predicts the development of HZ and PHN.Citation50–53 Vitamin C has been found to enhance the synthesis of beta-endorphin and endomorphin,Citation54–56 both of which have well-known analgesic effects, particularly for neuropathic pain.Citation56,Citation57 Interestingly, at physiological levels, extracellular vitamin C can protect neurons from glutamate excitotoxicity induced by the activation of NMDA receptors.Citation58 Ascorbate present within cells directly prevents excessive nerve stimulation caused by glutamate by scavenging ROS,Citation59 decreasing cell membrane levels of the NMDA receptor, and inhibiting glutamate uptake by decreasing cell surface levels of the neuronal glutamate transporter.Citation60 Furthermore, high-dose Vitamin C has been found to effectively decrease spontaneous pain in PHN.Citation51,Citation61–64 Thirdly, Vitamin D, has immunotropic properties that could contribute to the development of immune-related diseases such as VZV reactivation.Citation65–67 Insufficient levels of Vitamin D are associated with PHN.Citation47,Citation68,Citation69 Vitamin D is essential for the growth, survival, and proliferation of neurons, making it a potential therapeutic option for various neurodegenerative diseases.Citation70–72 In patients with HZ, Vitamin D supplementation may reduce the likelihood of subsequent PHN, indicating that it could have a protective effect against severe neuroinflammationCitation47,Citation73,Citation74 Lastly, in their study, Oskay et al discovered that there is a significant positive correlation between the levels of Hcy in severe HZ rashes and the development of PHN.Citation47 The increased Hcy levels in HZ may indicate a deficiency of Vitamins B6, B12, and folate, along with elevated inflammatory markers and oxidative stress.Citation47,Citation75,Citation76 Neurotropic B vitamins (thiamine, pyridoxine, and cobalamin) play a crucial role in axonal transport, neuron excitability, and neurotransmitter synthesis.Citation77–80 As modulators, they regulate several inflammatory and neural mediators involved in nociceptive and neuropathic pain through various mechanisms. These mechanisms include targeting the activation of the descending pain modulatory system, specific intracellular pathways, anti-inflammatory, antioxidative, antinociceptive, antiallodynic, anti-hyperalgesic, and nerve regenerative effects.Citation81 Therefore, understanding the mechanisms underlying the neuroprotective effect of Vitamins B, C, and D can be significant in the treatment of ZAP and PHN.
VZV-induced damage of afferent fibers and endings plays an important role in the pathogenesis of ZAP. It is possible that the involvement or damage to the structure and function of multiple types of afferent nerves and channels in the periphery, rather than just one type of fibers.Citation44,Citation82 The tips of axons are often far away from the cell soma. Recent studies have shown that axonal mRNA transport and localized translation play important roles in regulating these distant outposts of the cell, allowing them to quickly respond to external factors and maintain axonal homeostasis.Citation83 Localized protein synthesis provides spatiotemporal precision for injury responses and growth decisions at remote positions in nerve axons.Citation84 Approximately 83.3% of ZAP is localized, which suggests that topical treatment could be a viable option.Citation85 The neurotropic B vitamins are essential for maintaining a healthy nervous system.Citation86 Cobalamin, an endogenous neuroprotectant and neurotrophic agent, targets protein synthesis in the distal axonal cytoskeletonCitation87 and scavenges superoxideCitation88 making it a beneficial treatment for neuropathic pain.Citation89 According to several clinical studies, the injection of methylcobalamin locally has shown promising results in treating ZAP and PHN, with an overall effectiveness rate of over 80%.Citation90–95 Additionally, locally injected thiamine has been found to have a significant antipruritic effect.Citation96 These findings suggest that a more targeted approach, focused on protecting the affected nerve, promoting nerve repair, and nourishing the damaged nerve, may be more effective than simply providing analgesia for ZAP and PHN.
The accounting of the public health burden of HZ is incomplete, and changes in the age-specific incidence of HZ further exacerbate knowledge gaps.Citation97 As up to one-third of the population may develop HZ from VZV reactivation and suffer from PHN,Citation98 it is crucial to strengthen both basic and clinical research. However, our understanding of VZV neuropathy is remarkably poor, particularly in terms of patient visits that lack the involvement of neurologists.Citation98
Although 2 vaccines for HZ are currently recommended, the popularization of vaccination still needs time. Neurologists need to be made aware of ZAP and PHN; and urged to give VZV-induced nerve injury the attention it deserves. As medical knowledge and research continue to evolve, it is crucial for medical education to stay up-to-date. As neurologists, we have a responsibility to fully understand this disease and provide patients with timely and effective treatment. The possibility of promoting neural repair strategies based on VZV-induced axonal damage mechanisms is an exciting prospect.
Strengths and Limitations
This study’s strength lies in its unbiased reporting of results, which were based solely on the HER of three hospitals without any influence from the author. The study’s analysis of patients’ visit preferences confirmed the inadequacy of neurologists in treating ZAP. However, the data derived from EHR for the detection of incident HZ and ZAP has limitations. The hospital information system’s EHR data often lacked detailed information on extensive patient characteristics. The study is limited in its ability to examine the potential differences in patient characteristics and treatment practices across hospitals. However, the data from all three hospitals indicates a weak correlation between dermatological and neurological consultations for ZAP or PHN, suggesting these had a certain representation. Additionally, the accuracy of the ICD-9/10-based HZ diagnostic codes used in the study may be limited in their ability to accurately identify cases of HZ. The diagnostic codes commonly used by TCM doctors may not be specific enough to accurately identify cases of HZ, ZAP, and PHN, which could result in slightly lower referral proportions.
Conclusion
Our analysis of association rules revealed that patients with ZAP and PHN tended to have higher rates of visits to dermatology and pain management specialists, as opposed to neurologists. Furthermore, there was less correlation between the referral behaviors of dermatologists and neurologists. Effective management of ZAP and PHN requires multidisciplinary knowledge and involvement. However, it is unfortunate that many neurologists do not actively participate in the treatment of VZV-nerve damage. As experts in anti-neuroinflammation and neuroprotection, it is the duty of neurologists to be actively involved in the treatment of these conditions.
Data Sharing Statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. Full data set available from the corresponding author. However, a reanalysis of the full data needs to be approved by the Ethics Committee of each hospital.
Disclosure
The authors report no conflict of interest in this work.
Acknowledgments
The author thankfulness the invaluable support from Affiliated Tenth People’s Hospital of Tongji University, Taicang Affiliated Hospital of Soochow University, and Bengbu Second People’s Hospital. The authors would like to acknowledge the volunteer actors and patients who made this module possible, including Yingfei Ou (Taicang First People Hospital), Bo Chen (Bengbu Second People’s Hospital), Wei-Zhen Tang, Chaosheng Zhou, Jie Xu, Chao Cheng, and Yaping Zhou (All from Shanghai Tenth People’s Hospital).
Additional information
Funding
References
- Chen LK, Arai H, Chen LY, et al. Looking back to move forward: a twenty-year audit of herpes zoster in Asia-Pacific. BMC Infect Dis. 2017;17:213. doi:10.1186/s12879-017-2198-y
- Lenfant T, L’Honneur AS, Ranque B, et al. Neurological complications of varicella zoster virus reactivation: prognosis, diagnosis, and treatment of 72 patients with positive PCR in the cerebrospinal fluid. Brain Behav. 2022;12:e2455. doi:10.1002/brb3.2455
- Kennedy PGE, Gershon AA. Clinical Features of Varicella-Zoster Virus Infection. Viruses. 2018;10:609. doi:10.3390/v10110609
- Friesen KJ, Chateau D, Falk J, Alessi-Severini S, Bugden S. Cost of shingles: population based burden of disease analysis of herpes zoster and postherpetic neuralgia. BMC Infect Dis. 2017;17:69. doi:10.1186/s12879-017-2185-3
- Crosbie B, Lucey S, Tilson L, Domegan L, Kieran J. Acute herpes zoster and post herpetic neuralgia in primary care: a study of diagnosis, treatment and cost. Eur J Clin Microbiol Infect Dis. 2018;37:627–631. doi:10.1007/s10096-017-3153-y
- Cohen EJ. Commentary on herpes zoster and postherpetic neuralgia. Clin Infect Dis. 2021;73:e3218–e3219. doi:10.1093/cid/ciaa1192
- Glauser TA, Salinas GD, Nevins H, Williamson JC, Wallace MS, Abdolrasulnia M. Communication gaps between physicians and patients with postherpetic neuralgia: results from a national study on practice patterns. J Pain Res. 2011;4:407–415. doi:10.2147/JPR.S27310
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223–230 e223. doi:10.1016/j.jaad.2017.07.054
- Singh P, Silverberg NB, Silverberg JI. Outpatient healthcare utilization and prescribing patterns for herpes zoster in United States adults. Arch Dermatol Res. 2021;313:155–162. doi:10.1007/s00403-020-02085-y
- Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis. 2008;197(Suppl 2):S216–223. doi:10.1086/522153
- Thompson RR, Kong CL, Porco TC, Kim E, Ebert CD, Acharya NR. Herpes zoster and postherpetic neuralgia: changing incidence rates from 1994 to 2018 in the United States. Clin Infect Dis. 2021;73:e3210–e3217. doi:10.1093/cid/ciaa1185
- Gonima Valero E, Mendoza WAS, Sarmiento DA, Amaya S. Analgesic treatment approach for postherpetic neuralgia: a narrative review. J Pain Palliat Care Pharmacother. 2023;1–10. doi:10.1080/15360288.2023.2174632
- Schutzer-Weissmann J, Farquhar-Smith P. Post-herpetic neuralgia - A review of current management and future directions. Expert Opin Pharmacother. 2017;18:1739–1750. doi:10.1080/14656566.2017.1392508
- Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi:10.4161/auto.19496
- Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86:1146–1153. doi:10.4065/mcp.2011.0305
- Lee DG, Ryu KS, Bashir M, Bae JW, Ryu KH. Discovering medical knowledge using association rule mining in young adults with acute myocardial infarction. J Med Syst. 2013;37:9896. doi:10.1007/s10916-012-9896-1
- Tai YM, Chiu HW. Comorbidity study of ADHD: applying association rule mining (ARM) to National Health Insurance Database of Taiwan. Int J Med Inform. 2009;78:e75–83. doi:10.1016/j.ijmedinf.2009.09.005
- Nahar J, Tickle KS, Ali AB, Chen YP. Significant cancer prevention factor extraction: an association rule discovery approach. J Med Syst. 2011;35:353–367. doi:10.1007/s10916-009-9372-8
- Zhang Z, Selariu A, Warden C, et al. Genome-wide mutagenesis reveals that ORF7 is a novel VZV skin-tropic factor. PLoS Pathog. 2010;6:e1000971. doi:10.1371/journal.ppat.1000971
- Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14:192. doi:10.3390/v14020192
- Dayan RR, Peleg R. Herpes zoster - typical and atypical presentations. Postgrad Med. 2017;129:567–571. doi:10.1080/00325481.2017.1335574
- Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18:55–78. doi:10.1111/ddg.14013
- Feli KH, Ediale CE, McMichael AJ. Update in herpes zoster prevention and the role of dermatologists. J Drugs Dermatol. 2019;18:18–22.
- Whitley RJ, Volpi A, McKendrick M, Wijck A, Oaklander AL. Management of herpes zoster and post-herpetic neuralgia now and in the future. J Clin Virol. 2010;48(Suppl 1):S20–28. doi:10.1016/S1386-6532(10)70005-6
- Steain M, Sutherland JP, Rodriguez M, Cunningham AL, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014;88:2704–2716. doi:10.1128/JVI.03445-13
- Kleinschmidt-DeMasters BK, Gilden DH. Varicella-Zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–780. doi:10.5858/2001-125-0770-VZVIOT
- Devor M. Rethinking the causes of pain in herpes zoster and postherpetic neuralgia: the ectopic pacemaker hypothesis. Pain Rep. 2018;3:e702. doi:10.1097/PR9.0000000000000702
- Werner RN, Nikkels AF, Marinovic B, et al. European consensus-based (S2k) guideline on the management of herpes zoster - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 1: diagnosis. J Eur Acad Dermatol Venereol. 2017;31:9–19. doi:10.1111/jdv.13995
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;CD006866. doi:10.1002/14651858.CD006866.pub3
- Peng F, He H, Xia T, Lv S. Comparison of a one- versus two-week treatment with famciclovir upon reductions in pain and occurrence of postherpetic neuralgia in herpes zoster: a randomized open-label trial. Infect Drug Resist. 2023;16:721–726. doi:10.2147/IDR.S385442
- Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E. Incidence of herpes zoster and its complications in Germany, 2005–2009. J Infect. 2015;70:178–186. doi:10.1016/j.jinf.2014.08.018
- Forbes HJ, Thomas SL, Smeeth L, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157:30–54. doi:10.1097/j.pain.0000000000000307
- Ghaznawi N, Virdi A, Dayan A, et al. Herpes zoster ophthalmicus: comparison of disease in patients 60 years and older versus younger than 60 years. Ophthalmology. 2011;118:2242–2250. doi:10.1016/j.ophtha.2011.04.002
- Ultsch B, Siedler A, Rieck T, Reinhold T, Krause G, Wichmann O. Herpes zoster in Germany: quantifying the burden of disease. BMC Infect Dis. 2011;11:173. doi:10.1186/1471-2334-11-173
- Kim J, Kim MK, Choi GJ, Shin HY, Kim BG, Kang H. Pharmacological and non-pharmacological strategies for preventing postherpetic neuralgia: a systematic review and network meta-analysis. Korean J Pain. 2021;34:509–533. doi:10.3344/kjp.2021.34.4.509
- Derry S, Rice AS, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1:CD007393. doi:10.1002/14651858.CD007393.pub4
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi:10.1038/39807
- Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–145. doi:10.1016/s0304-3959(99)00007-x
- Rage M, Van Acker N, Facer P, et al. The time course of CO2 laser-evoked responses and of skin nerve fibre markers after topical capsaicin in human volunteers. Clin Neurophysiol. 2010;121:1256–1266. doi:10.1016/j.clinph.2010.02.159
- Arora V, Campbell JN, Chung MK. Fight fire with fire: neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol Ther. 2021;220:107743. doi:10.1016/j.pharmthera.2020.107743
- Wang S, Wang S, Asgar J, et al. Ca(2+) and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J Biol Chem. 2017;292:8291–8303. doi:10.1074/jbc.M117.778290
- Selariu A, Cheng T, Tang Q, et al. ORF7 of varicella-zoster virus is a neurotropic factor. J Virol. 2012;86:8614–8624. doi:10.1128/JVI.00128-12
- Reichelt M, Zerboni L, Arvin AM. Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J Virol. 2008;82:3971–3983. doi:10.1128/JVI.02592-07
- Grigoryan S, Kinchington PR, Yang IH, et al. Retrograde axonal transport of VZV: kinetic studies in hESC-derived neurons. J Neurovirol. 2012;18:462–470. doi:10.1007/s13365-012-0124-z
- Christensen J, Steain M, Slobedman B, Abendroth A. Varicella-zoster virus glycoprotein I is essential for spread in dorsal root ganglia and facilitates axonal localization of structural virion components in neuronal cultures. J Virol. 2013;87:13719–13728. doi:10.1128/JVI.02293-13
- Jang YH, Lee JS, Kim SL, et al. Do interventional pain management procedures during the acute phase of herpes zoster prevent postherpetic neuralgia in the elderly?: A meta-analysis of randomized controlled trials. Ann Dermatol. 2015;27:771–774. doi:10.5021/ad.2015.27.6.771
- Oskay T, Keskin C, Ozen M. Antioxidant and inflammatory biomarkers in herpes zoster. J Med Virol. 2022;94:3924–3929. doi:10.1002/jmv.27781
- Khazan M, Hedayati M, Robati RM, Riahi SM, Nasiri S. Impaired oxidative status as a potential predictor in clinical manifestations of herpes zoster. J Med Virol. 2018;90:1604–1610. doi:10.1002/jmv.25204
- Khazan M, Nasiri S, Riahi SM, Robati RM, Hedayati M. Measurement of melatonin, indole-dioxygenase, IL-6, IL-18, ferritin, CRP, and total homocysteine levels during herpes zoster. J Med Virol. 2020;92:1253–1259. doi:10.1002/jmv.25484
- Thomas SL, Wheeler JG, Hall AJ. Micronutrient intake and the risk of herpes zoster: a case-control study. Int J Epidemiol. 2006;35:307–314. doi:10.1093/ije/dyi270
- Chen JY, Chang CY, Feng PH, Chu CC, So EC, Hu ML. Plasma vitamin C is lower in postherpetic neuralgia patients and administration of vitamin C reduces spontaneous pain but not brush-evoked pain. Clin J Pain. 2009;25:562–569. doi:10.1097/AJP.0b013e318193cf32
- Chen JY, Chu CC, Lin YS, So EC, Shieh JP, Hu ML. Nutrient deficiencies as a risk factor in Taiwanese patients with postherpetic neuralgia. Br J Nutr. 2011;106:700–707. doi:10.1017/S0007114511000481
- Chen JY, Chang CY, Lin YS, Hu ML. Nutritional factors in herpes zoster, postherpetic neuralgia, and zoster vaccination. Popul Health Manag. 2012;15:391–397. doi:10.1089/pop.2012.1563
- Carr AC, McCall C. The role of vitamin C in the treatment of pain: new insights. J Transl Med. 2017;15:77. doi:10.1186/s12967-017-1179-7
- Yang Z, Copolov DL, Lim AT. Ascorbic acid augments the adenylyl cyclase-cAMP system mediated POMC mRNA expression and beta-endorphin secretion from hypothalamic neurons in culture. Brain Res. 1996;706:243–248. doi:10.1016/0006-8993(95)01135-8
- Wu HY, Mao XF, Tang XQ, et al. Spinal interleukin-10 produces antinociception in neuropathy through microglial beta-endorphin expression, separated from antineuroinflammation. Brain Behav Immun. 2018;73:504–519. doi:10.1016/j.bbi.2018.06.015
- Wang CL, Yang DJ, Yuan BY, Qiu TT. Antiallodynic effects of endomorphin-1 and endomorphin-2 in the spared nerve injury model of neuropathic pain in mice. Anesth Analg. 2017;125:2123–2133. doi:10.1213/ANE.0000000000002318
- Ballaz S, Morales I, Rodriguez M, Obeso JA. Ascorbate prevents cell death from prolonged exposure to glutamate in an in vitro model of human dopaminergic neurons. J Neurosci Res. 2013;91:1609–1617. doi:10.1002/jnr.23276
- May JM. Vitamin C transport and its role in the central nervous system. Subcell Biochem. 2012;56:85–103. doi:10.1007/978-94-007-2199-9_6
- Domith I, Socodato R, Portugal CC, Munis AF, Duarte-Silva AT, Paes-de-Carvalho R. Vitamin C modulates glutamate transport and NMDA receptor function in the retina. J Neurochem. 2018;144:408–420. doi:10.1111/jnc.14260
- Chen JY, Chu CC, So EC, Hsing CH, Hu ML. Treatment of postherpetic neuralgia with intravenous administration of vitamin C. Anesth Analg. 2006;103:1616–1617. doi:10.1213/01.ane.0000246396.64010.ee
- Kim MS, Kim DJ, Na CH, Shin BS. A study of intravenous administration of vitamin C in the treatment of acute herpetic pain and postherpetic neuralgia. Ann Dermatol. 2016;28:677–683. doi:10.5021/ad.2016.28.6.677
- Schencking M, Vollbracht C, Weiss G, et al. Intravenous vitamin C in the treatment of shingles: results of a multicenter prospective cohort study. Med Sci Monit. 2012;18:CR215–224. doi:10.12659/msm.882621
- Kapoor S. Vitamin C for attenuating postherpetic neuralgia pain: an emerging treatment alternative. J Headache Pain. 2012;13:591;author reply 593. doi:10.1007/s10194-012-0476-z
- Chao CT, Chiang CK, Huang JW, Hung KY. Vitamin D is closely linked to the clinical courses of herpes zoster: from pathogenesis to complications. Med Hypotheses. 2015;85:452–457. doi:10.1016/j.mehy.2015.06.027
- Chao CT, Lee SY, Yang WS, et al. Serum vitamin D levels are positively associated with varicella zoster immunity in chronic dialysis patients. Sci Rep. 2014;4:7371. doi:10.1038/srep07371
- Bartley J. Post herpetic neuralgia, schwann cell activation and vitamin D. Med Hypotheses. 2009;73:927–929. doi:10.1016/j.mehy.2009.06.039
- Seetan K, Albashir S, Jarrar B, et al. Assessment of serum vitamin D levels in the serum of patients with postherpetic neuralgia and its correlation to pain severity: a cross-sectional comparative study. Int J Clin Pract. 2021;75:e14750. doi:10.1111/ijcp.14750
- Chen JY, Lin YT, Wang LK, et al. Hypovitaminosis din postherpetic neuralgia-high prevalence and inverse association with pain: a retrospective study. Nutrients. 2019;11:2787. doi:10.3390/nu11112787
- Zhang WY, Guo YJ, Wang KY, Chen LM, Jiang P. Neuroprotective effects of vitamin D and 17ss-estradiol against ovariectomy-induced neuroinflammation and depressive-like state: role of the AMPK/NF-kappaB pathway. Int Immunopharmacol. 2020;86:106734. doi:10.1016/j.intimp.2020.106734
- AlJohri R, AlOkail M, Haq SH. Neuroprotective role of vitamin D in primary neuronal cortical culture. eNeurologicalSci. 2019;14:43–48. doi:10.1016/j.ensci.2018.12.004
- Kalueff AV, Eremin KO, Tuohimaa P. Mechanisms of neuroprotective action of vitamin D(3). Biochemistry (Mosc). 2004;69:738–741. doi:10.1023/b:biry.0000040196.65686.2f
- Powanda MC. Is there a role for vitamin D in the treatment of chronic pain? Inflammopharmacology. 2014;22:327–332. doi:10.1007/s10787-014-0219-7
- Martin KR, Reid DM. Is there role for vitamin D in the treatment of chronic pain? Ther Adv Musculoskelet Dis. 2017;9:131–135. doi:10.1177/1759720X17708124
- Olaso-Gonzalez G, Inzitari M, Bellelli G, Morandi A, Barcons N, Vina J. Impact of supplementation with vitamins B(6), B(12), and/or folic acid on the reduction of homocysteine levels in patients with mild cognitive impairment: a systematic review. IUBMB Life. 2022;74:74–84. doi:10.1002/iub.2507
- Kumar T, Sharma GS, Singh LR. Homocystinuria: therapeutic approach. Clin Chim Acta. 2016;458:55–62. doi:10.1016/j.cca.2016.04.002
- Wang ZB, Gan Q, Rupert RL, Zeng YM, Song XJ. Thiamine, pyridoxine, cyanocobalamin and their combination inhibit thermal, but not mechanical hyperalgesia in rats with primary sensory neuron injury. Pain. 2005;114:266–277. doi:10.1016/j.pain.2004.12.027
- Zhang M, Han W, Zheng J, et al. Inhibition of hyperpolarization-activated cation current in medium-sized DRG neurons contributed to the antiallodynic effect of methylcobalamin in the rat of a chronic compression of the DRG. Neural Plast. 2015;2015:197392. doi:10.1155/2015/197392
- Xu J, Wang W, Zhong XX, Feng Y, Wei X, Liu XG. EXPRESS: methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn. Mol Pain. 2016;12:174480691665708. doi:10.1177/1744806916657089
- Yu CZ, Liu YP, Liu S, Yan M, Hu SJ, Song XJ. Systematic administration of B vitamins attenuates neuropathic hyperalgesia and reduces spinal neuron injury following temporary spinal cord ischaemia in rats. Eur J Pain. 2014;18:76–85. doi:10.1002/j.1532-2149.2013.00390.x
- Paez-Hurtado AM, Calderon-Ospina CA, Nava-Mesa MO. Mechanisms of action of vitamin B1 (thiamine), B6 (pyridoxine), and B12 (cobalamin) in pain: a narrative review. Nutr Neurosci. 2023;26:235–253. doi:10.1080/1028415X.2022.2034242
- Petersen KL, Rice FL, Farhadi M, Reda H, Rowbotham MC. Natural history of cutaneous innervation following herpes zoster. Pain. 2010;150:75–82. doi:10.1016/j.pain.2010.04.002
- Cioni JM, Koppers M, Holt CE. Molecular control of local translation in axon development and maintenance. Curr Opin Neurobiol. 2018;51:86–94. doi:10.1016/j.conb.2018.02.025
- Terenzio M, Koley S, Samra N, et al. Locally translated mTOR controls axonal local translation in nerve injury. Science. 2018;359:1416–1421. doi:10.1126/science.aan1053
- Casale R, Mattia C. Building a diagnostic algorithm on localized neuropathic pain (LNP) and targeted topical treatment: focus on 5% lidocaine-medicated plaster. Ther Clin Risk Manag. 2014;10:259–268. doi:10.2147/TCRM.S58844
- Calderon-Ospina CA, Nava-Mesa MO. B Vitamins in the nervous system: current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther. 2020;26:5–13. doi:10.1111/cns.13207
- Perry G, Zelasko DA, Sayre LM, Smith MA. Oxidative damage to axonal cytoskeletal proteins. Microscopy Microanal. 2020;3:43–44. doi:10.1017/s1431927600007108
- Chan W, Almasieh M, Catrinescu MM, Levin LA. Cobalamin-associated superoxide scavenging in neuronal cells is a potential mechanism for vitamin B(12)-deprivation optic neuropathy. Am J Pathol. 2018;188:160–172. doi:10.1016/j.ajpath.2017.08.032
- Song XS, Huang ZJ, Song XJ. Thiamine suppresses thermal hyperalgesia, inhibits hyperexcitability, and lessens alterations of sodium currents in injured, dorsal root ganglion neurons in rats. Anesthesiology. 2009;110:387–400. doi:10.1097/ALN.0b013e3181942f1e
- Xu G, Xu S, Tang WZ, Xu G, Cheng C, Xu J. Local injection of methylcobalamin combined with lidocaine for acute herpetic neuralgia. Pain Med. 2016;17:572–581. doi:10.1093/pm/pnv005
- Xu XG, Cheng C, Xu G, Tang WZ, Xu J, Xu J. Local administration of methylcobalamin and lidocaine for acute ophthalmic herpetic neuralgia: a single-center randomized controlled trial. Pain Pract. 2016;16:869–881. doi:10.1111/papr.12328
- Xu G, Lv ZW, Feng Y, Tang WZ, Xu GX. A single-center randomized controlled trial of local methylcobalamin injection for subacute herpetic neuralgia. Pain Med. 2013;14:884–894. doi:10.1111/pme.12081
- Xu G, Zhou CS, Tang WZ, et al. Local administration of methylcobalamin for subacute ophthalmic herpetic neuralgia: a randomized, phase III clinical trial. Pain Pract. 2020;20:838–849. doi:10.1111/papr.12909
- Xu G, Xu G, Feng Y, Tang WZ, Lv ZW. Transcutaneous electrical nerve stimulation in combination with cobalamin injection for postherpetic neuralgia: a single-center randomized controlled trial. Am J Phys Med Rehabil. 2014;93:287–298. doi:10.1097/PHM.0000000000000002
- Xu G, Tang W, Zhou C, et al. Pain fluctuations of women with subacute herpetic neuralgia during local methylcobalamin in combination with lidocaine treatment: a single-blinded randomized controlled trial. J Pain Res. 2023;16:1267–1284. doi:10.2147/jpr.S404713
- Xu G, Lv ZW, Xu GX, Tang WZ. Thiamine, cobalamin, locally injected alone or combination for herpetic itching: a single-center randomized controlled trial. Clin J Pain. 2014;30:269–278. doi:10.1097/AJP.0b013e3182a0e085
- Curran D, Kim JH, Matthews S, et al. Recombinant zoster vaccine is efficacious and safe in frail individuals. J Am Geriatr Soc. 2021;69:744–752. doi:10.1111/jgs.16917
- Harpaz R. How little we know herpes zoster. J Infect Dis. 2020;222:708–711. doi:10.1093/infdis/jiz653