Abstract
Purpose
To analyze the adverse events reported in the pharmacovigilance of the health service provider institutions of the municipality of Monteria in the period 2018–2021.
Patients and Methods
Descriptive, cross-sectional, retrospective, and quantitative approach study, the information was analyzed by statistical analysis by multiple correspondence using Orange Data Mining software; the analysis consisted of visual programming to perform interactive data exploration, to identify and differentiate associations or oppositions between different categories in space.
Results
The most frequently reported adverse events were allergic reactions, with 28.5%. Female sex and adult age are the groups most prone to present these events; antibiotics were the pharmacological group that produced the most adverse events with 18.3%; the main errors that produce these events are related to prescription.
Conclusion
Age and sex increase the risk of adverse drug events; most of these events are the product of erroneous prescriptions. The findings presented in this article are useful for pharmacovigilance programs in health institutions.
Introduction
Medications play an important role in health care of people, not only in the treatment but also in the prevention and diagnosis of diseases; however, they also have the disadvantage of causing adverse events.Citation1 These events are considered by the World Health Organization (WHO) as unwanted events that occur during treatment and may or may not be related to the drug.Citation2
Within health care, adverse drug-related events are cataloged as anomalous events in public health.Citation2,Citation3 Similarly, they have become the main cause of hospital readmission, health cost overruns and, in extreme cases, disability and death.Citation4 Therefore, these events are the object of study of pharmacovigilance programs, immersed in patient safety policies.Citation2 Adverse drug-related events represent one of the leading causes of mortality in the world,Citation5 surpassing the mortality caused by traffic accidents and breast cancer in many countries.Citation2 Thus, it has a negative human, clinical and economic impact, associated with erroneous prescriptions, misdiagnosis, overdose, and incorrect route of administration, among others.Citation6,Citation7 A study conducted in Copenhagen found that adverse drug events (ADEs) are considered the third leading cause of death worldwide, of which between 65% and 75% could be avoided if more importance were given to drug-related risks.Citation8,Citation9 Likewise, in Colombia, an investigation on adverse drug reactions (ADRs) found that there is little information on these reactions and that pharmacovigilance programs are deficient.Citation10
Pharmacovigilance programs, which are part of the Compulsory Health Quality Assurance System (SOGCS)Citation11,Citation12 play an important role in patient safety measures, which aim to reduce the risk of suffering an adverse event;Citation12 for this reason the alliance for patient safety, led by the WHO, established among its purposes to promote actions that improve patient safety, encouraging the investigation of adverse events, as an essential element in the improvement of health care.Citation13
Adverse drug events in healthcare institutions (IPS) compromise patient safety and are therefore studied very frequently; however, in many municipalities of Colombia, there are few studies of these events and the pharmacovigilance programs of the IPS are inefficient; this situation hinders information on the true impact of morbidity and mortality related to these events.Citation14,Citation15 From this point of view, it is important for health institutions to systematically record detailed information on adverse drug events presented in their facilities, to allow for their evaluation, taking into account the possibilities of occurrence and analyzing morbidity and mortality rates over time, in order to reduce the risk of suffering an adverse drug event; Therefore, taking into account that pharmacovigilance studies are not constant and continuous, but are necessary to prevent future adverse events, the aim of this study is to analyze the adverse drug events presented in healthcare institutions in the city of Monteria and reported to the National Institute of Health (INS) during 2018–2021.
Materials and Methods
Descriptive, cross-sectional, retrospective, and quantitative approach study conducted through the analysis of databases of adverse event reporting of healthcare institutions in the municipality of Monteria (Córdoba-Colombia) region with approximately 434,960 inhabitants.Citation16 The databases used for the collection of information were obtained from the open data of Colombia’s National Institute of Health (INS) which contains the report of adverse events from 2018 to 2021 and the National Institute of Drug and Food Surveillance (INVIMA)Citation17 with reports from 2021. The adverse drug event reports for the year 2021 were requested from the INVIMA health authority by means of an electronic application under the following number: 20221148664 and in this way the reports were obtained for the health institutions (IPS), health promoting entities (EPS) and social state entities (EPS) in the city of Monteria that reported adverse drug events. The present investigation included the entire population that presented adverse drug events, comprised of 949 cases; however, after taking into account the inclusion and exclusion criteria cases reported from 2018 to 2021 with complete information in the records were included and all cases of patients with duplicate records or incomplete information were excluded. The population size was reduced to 586 cases of interest, which were used in this study. In this study, we carried out a non-probabilistic sampling by convenience; this type of sampling allows the selection of cases of interest for the research. The data was organized into 15 variables, each with multiple categories according to each variable’s importance and research objectives using Microsoft Excel. A statistical analysis by multiple correspondence (MCA) was performed, using Orange data mining software (Bioinformatics Laboratory, Slovenia),Citation18 this analysis is based on visual programming to perform interactive data exploration for a quick qualitative analysis with visualizations; making it possible to clearly identify and differentiate associations or oppositions between the different categories in space, given the relative weight exerted by the modalities on two main axes.Citation19
At all stages of this research, the ethical principles and guidelines established in the Nuremberg Code and the Declaration HelsinkiCitation20 were complied with. For the execution and management of the information of this research, the provisions of Resolution 8430 of 1993, issued by the Ministry of Health,Citation21 were taken as a reference; in which scientific, technical and administrative norms are established, with the purpose of establishing requirements for the development of research activity in health.
Results
The results recorded in this research were obtained using two sources of information: those obtained by request to INVIMA by email with code number: 20221148664 and those obtained from the EAM database for the years 2018–2020 published in the open data of the INS, which contains 15 variables that allowed compliance with the objectives of the research; it is important to note that 40% of incomplete reports were found, which were a limitation for a better analysis of this study.
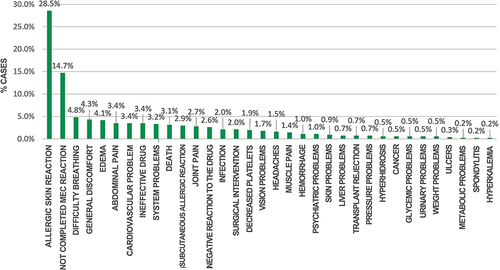
After analyzing the data retrieved from National Institute of Health, we found that out of 586 reports under study, 28.5% (167) patients presented allergic reactions to the drugs. However, 14.7%, ie, 86 of the reports, did not fill-in the complete information regarding the adverse event presented as required by INVIMA’s Suspected Adverse Drug Reaction Report Form (FORAM); Likewise, an important percentage of the reported events analyzed could be associated with the triggering of lethality, represented in 3.1% (18) of the total population. The most frequent adverse drug events (see ) are as follows.
Figure 1 Most frequent adverse events presented in health institutions in Monteria, Colombia, 2018–2021.
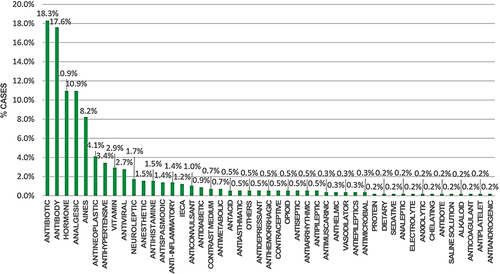
Regarding the pharmacological group, 18.3% of the drugs reported to have caused adverse events corresponded to antibiotics, 17.6% to antibodies and 10.09% to hormones, with these being the most representative percentages in the study (see ).
Figure 2 Therapeutic groups of adverse drug events presented in health institutions in Monteria, Colombia, 2018–2021.
Life course and sex are important data when reporting adverse drug events, these variables were found to be an important factor influencing the risk of adverse events (see ), we found that women were more prone to suffer adverse drug events from youth onwards, being the adult life cycle (25.3%) and old age (15%) the age groups with the highest percentage of reports, this may be due to females receiving more medical assistance from healthcare providers during these studied periods of time.
Figure 3 Adverse drug events by age and sex reported in health institutions in Montería, Colombia, 2018–2021.
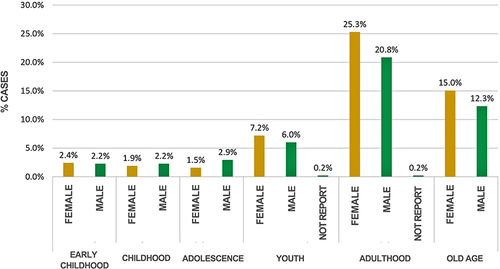
This relationship is very important for health institutions, since medical personnel must take into account the life stage and sex before prescribing a drug, as well as the pharmaceutical and nursing professionals in charge of dispensing and administering drugs, respectively, especially in women of advanced age whose biological modifications over the years reduce the efficacy and effectiveness of drugs within the body; thus, therapeutic failures or adverse drug reactions may occur.
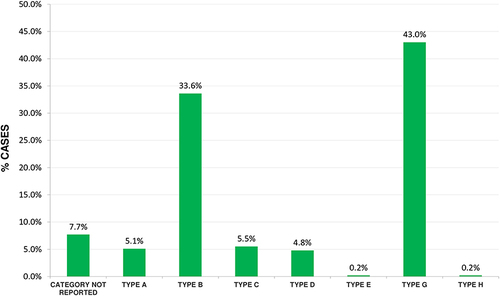
Knowing the category of an adverse drug event or adverse drug reaction makes it possible to determine what type of events are occurring in the healthcare institutions, what damage did they cause to the patient, and how they affect the healthcare system. In this regard, we found that category G (53%) was the most frequent (see ), ie, adverse drug events caused permanent damage to patients, increasing the length of hospital stay and supply demand, therefore causing cost overruns in the health system.
Figure 4 Category of adverse drug events presented in health institutions in Monteria, Colombia, 2018–2021.
The severity of the adverse events defines the patient’s situation within the health institutions, ie, a severity category A-C are those that do not cause harm to the patient but should be kept under medical supervision; in categories D-F, the adverse event does cause harm to the patient, ranging from incapacity to disability, and in the case of category I are events that cause death in the population. Adverse drug events can occur in two different ways: they can be associated with professional practice or can be directly related to the drug due to alterations in the process of absorption, metabolism, distribution, and elimination of the drugs; in the population studied, adverse drug events (see ) are most frequently manifested by adverse drug reactions (84.8%); 7.4% are associated with professional practice, with prescription having the highest percentage (6.1%).
Figure 5 Drug-related problems presented in health institutions in Monteria, Colombia, 2018–2021.
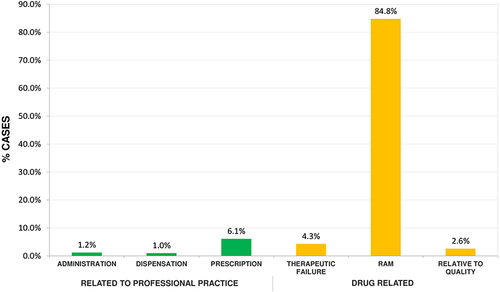
Adverse drug events related to the prescription and administration of drugs are the result of poor drug handling and sometimes derive from long working hours, working conditions, and even poor doctor-patient or doctor-nurse communication.
These are also considered a matter of interest for public health, due to the multiple problems they can cause to the health of the population (disability, invalidity, death), which can bring psychological disorders to patients and increase the costs of the health system.
When grouping the categories of the variables of interest in the study, 3 large groups of the categories of the adverse drug event variables in this study can be clearly identified and differentiated visually, marked with colors to facilitate their identification; Group 1, green: Group 2, and red: Group 3; two components can also be seen, one on the X axis called component 1 and the other on the Y axis called component 2, with positive and negative quadrants; in each of these components and quadrants therapeutic groups, event categories, life cycle, among other representative groups can be found, indicating that there are groups of variables that can be associated with a specific quadrant, because they have similar characteristics (see ).
Figure 6 Differentiated groups. Showing some elements that constitute adverse drug events presented in health institutions in Monteria, Colombia, 2018–2021.
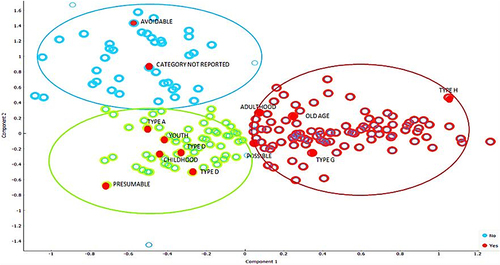
Three groups were established in which the differentiating variables are interrelated and identified, such as age group, severity of the event, type of DRP, among others; the following are the main variables and categories of variables that are most related to each of the groups.
Group 1: within this group, avoidable adverse drug events related to professional practice are identified, such as administration, dispensing and prescription problems. There is no statistically significant difference in relation to the life course; however, it is strongly related to events that have a lack of information in the report of some EAMs.
Group 2: characterized by drug-related problems such as possible adverse drug reactions, the most representative life cycle identified in the study is youth; in this group, the event categories that prevail are the least severe (type A, type B and type C).
Group 3: is also characterized by adverse drug reaction events but differs from group 2 by the proximity in the life course of adulthood and old age. The event category is one of the most severe or serious (type G).
Discussion
Taking into account that adverse drug events occur very frequently in healthcare institutions, this study identified that the adverse drug events that occur continuously are allergic skin reactions; these results also coincide with the study conducted by Obreque, Mellano and Andresen in which the adverse events or adverse reactions that occurred most frequently were skin disorders, manifested in skin allergies;Citation22 However, there are other reactions that are uncommon but are of special interest to the healthcare system, such as adverse drug events that can lead to disability or death.Citation23 Age and sex are considered a risk factor associated with adverse drug events, within the results found in this research it was obtained that women are more prone to suffer adverse drug events, results that coincide with the study by Watson, Caster, Rochon and Ruijer; in which it is expressed that women’s ADRs outnumber men’s ADR reports worldwide.Citation24 Similarly, it was found that people in the life cycle of adulthood and old age are more prone to suffer adverse drug events, which coincides with the study by Collao, Favereau, Miranda and Aceitón, which states that people over 80 years of age have a higher risk of suffering these events.Citation25 In Colombia, the report of studies on population size and volume shows that there are more women than men and that most of the population is adultCitation26 which may be a factor that increases the probability that adverse drug events occur more frequently in adult women; these results may also be associated with the life cycle of women, the different hormonal changes in this sex and the dose of drugs administered according to their body weight.
In relation to the therapeutic group, the drugs that tend to present continuously and with a high percentage of adverse events are antibiotics, followed by antibodies and analgesics; these results are also related to the research by Obreque, Mellano and Andresen, which showed that antibiotics represent 58.3% of the adverse reactions associated with drugs and 22.9% correspond to sedative-analgesics;Citation22 These therapeutic groups are the most widely administered in health care, since they are used as initial treatment for infections and pain; however, due to the large number of bacteria, antibiotic regimens can fail and produce adverse reactions. These results also coincide with the study by Orellana, et al, 2021 which characterized antibiotics and anti-inflammatory drugs as the pharmacological groups with the highest number of adverse reactions in outpatients, representing 12.4% of the people studied;Citation27 in contrast to the study by Ruiz-Ramos et al, 2021 in which the therapeutic group of antithrombotics were responsible for the majority of adverse eventsCitation28, and the study by Machado-Duque et al, 2021 in which the pharmacological groups most involved in medication errors were antidiabetics.Citation29
According to the category of the event or severity of the event, within the findings of this study, the most reported category is category type G with 43%, this being one of the categories that represent a serious risk that can cause harm to the patient; unlike the study of Orellana et al 2021 75.1% of adverse drug events reported category A.Citation27
Likewise, adverse events of drug-related problems known as adverse reactions and adverse events related to professional practice were also found; the most frequently occurring drug-related problems are adverse drug reactions, coinciding with the findings of the studies by Velasco-Gonzalez, Loya-Perez, Navarro-Garcia and Sainz-Gil when describing that most of the adverse drug events are non-serious adverse drug reactions;Citation30 in the case of adverse events associated with professional practice, prescription and administration represent a considerable percentage of 7.3%, unlike adverse drug reactions, these events are considered avoidable; scientific evidence through studies such as the present one have proven that prescribing errors are the most frequent in professional practice, as evidenced by the study of Ferrández et al,Citation31 Garzón et al, Bohórquez-Moreno et al,Citation32 among many other studies that confirm that these prescribing errors are related to the incorrect use of the computerized medical order, work overload, communication failures, among many other factors.
Likewise, it was found that antibiotics are the therapeutic group that produces the most adverse events in the population studied; they are not the ones that generate serious adverse events, as is the case of antibodies, which are little related to the presence of adverse events but have a great impact on serious events and fatal discharges. This may be due to the age group in which these drugs are administered, such as adults, in whom the processes of absorption, metabolism, distribution and elimination of the drugs are affected by the deterioration of their organs. For this reason, it is important to study the adverse events produced by each therapeutic group and to know the physiological conditions with which the patient is admitted in order to avoid drug–drug interactions.
Conclusion
This study found that the adverse drug events that occur most frequently regardless of the level of care in health institutions are allergic skin reactions related mainly to the use of antibiotics; however, special events occur infrequently. Allergic reactions, despite being the most common adverse events related to medicines, have a lower negative impact on mortality risk as an indicator for the health system adverse events related to medicines that can lead to cardiovascular problems.
For this reason, it is very important for health institutions to know which therapeutic groups most generate these events, which could be considered sentinel drugs; therefore, health institutions should have a person in charge of monitoring adverse drug events and report them according to the established forms; It is also necessary to train healthcare personnel in the proper handling of medications and in the humanization of health, in order to achieve good communication with the patient, with the purpose of taking the pertinent data to reduce the presence of adverse events and the morbidity and mortality rates that occur as a result of this public health problem.
Ethics Statement
Ethical approval was not required. The open database of the INS consulted, the only data that were provided about the patients were age and sex, personal data that violated their rights and self-determination of the participants were kept under protection and anonymity, only information of interest to them was obtained. To analyze adverse drug events, this research was carried out considering the current ethical regulations: Declaration of Helsinki. For the execution and management of the information in this research, the provisions of Resolution 8430 of 1993, issued by the Ministry of Health of the Republic of Colombia, which establishes scientific, technical, and administrative standards in order to establish requirements for the development of research activity in health, were considered; in this study, retrospective, non-experimental documentary research techniques and methods were used. No intervention or intentional modification of the biological, physiological, psychological, or social variables of the individuals who participated in the study was carried out; only access to databases was provided for the purpose of analyzing the information and characterizing adverse events related to medicines; for this reason, this exploration can be classified as a risk-free research study.Citation21 The data obtained remain anonymous. The collection of research data complies with national laws, regulations, and social ethics.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors would like to extend their sincere appreciation to the University of Córdoba - Colombia (Grant No. FCS 01-22) for the financial support of this research. The authors also express their deepest gratitude to all participants for their contribution to the study.
References
- Araujo-Rosero O, Guerrero-Lasso P, Matabanchoy-Tulcán S, et al. Systematic review: adverse events and human talent management in the Latin American hospital context. Univ Salud Suplemento1 Especial Psicología y Trabajo. 2021;23(3):351–366. doi:10.22267/rus.212303.249
- Alonso L, Rojas M. Adverse event and public health. Rev Salud Uninorte. 2009;25(1):i–iv.
- Achury D, Rodríguez S, Díaz J, et al. Study of adverse events, factors and periodicity in patients hospitalized in intensive care units. Rev Enferm Glob. 2016;15(42):324–340. doi:10.6018/eglobal.15.2.215791
- World Health Organization. Pharmacovigilance: guarantee of safety in the use of medicines; 2004 [ cited october 1, 2022]. Available from: https://apps.who.int/iris/handle/10665/68862. Accessed November 16, 2023.
- Martin AM, Michael D. Medical error—the third leading cause of death in the US. BMJ. 2016. doi:10.1136/bmj.i2139
- Font I, Climent C, Poveda J. Quality of the pharmacotherapeutic process through medication errors in a tertiary hospital. Rev Farm Hosp. 2008;32(5):274–279. doi:10.1016/S1130-6343(08)75946-4
- Rodríguez R, Gómez B, Diaz M, et al. Hospitalization caused by adverse drug reactions. Correo Científico Méd. 2019;23(1):223–244.
- Auraaen A, Slawomirski L, Klazinga N. The Economics of Patient Safety in Primary and Ambulatory Care: Flying Blind [Internet]. Paris: OECD; 2018. doi:10.1787/baf425ad-en
- Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277(4):307–311.
- Mesa J. Number of adverse effects to medications increases. Rev. Arsenal Terapéutico; 2016 [ cited November 18, 2022]. Available from: https://www.arsenalterapeutico.com/2016/03/28/colombia-crece-numero-de-efectos-adversos-a-medicamentos/. Accessed November 16, 2023.
- Ministry of Health and Social Protection. Mandatory Health Quality Assurance System; 2016 [ cited November 19, 2022]. cited November 19, 2022: https://www.minsalud.gov.co/salud/PServicios/Paginas/sistema-obligatorio-garantia-calidad-SOGC.aspx. Accessed November 16, 2023.
- Colombia. Ministry of social protection. Decree 1011 of 2006. In: Diario oficial 46.230; 2006 [ cited July 29, 2022]. cited July 29, 2022: https://www.minsalud.gov.co/Normatividad_Nuevo/DECRETO%201011%20DE%202006.pdf. Accessed November 16, 2023.
- World Health Organization. Alliance for patient safety: research in patient safety [Internet]; 2022 [ cited May 29, 2022]. Available from: https://www.who.int/es/news-room/fact-sheets/detail/patient-safety. Accessed November 16, 2023.
- Consultorsalud. 14.873 Medication errors were identified in Colombia for 8 years [Internet]; 2022 [ cited July 29, 2022]. Available from: https://consultorsalud.com/informe-especial-14-873-errores-de-medicacion-seidentificaron-en-colombia-durante-8-anos/. Accessed November 16, 2023.
- Panamerican Health Organization. Dissemination body of the collaborating center. Adverse events. [Internet]; 2015 [ cited July 29, 2022]. Available from: http://www.conamed.gob.mx/gobmx/boletin/pdf/boletin3/eventos_adversos.pdf. Accessed November 16, 2023.
- Ibero-American Center for Strategic Urban Development. Monteria; [ cited September 10, 2023]. cited September 10, 2023: https://www.cideu.org/miembro/monteria/. Accessed November 16, 2023.
- Ministry of Health and Social Protection. Adverse Events Associated with Medications. [Internet]; 2022 [ cited July 25, 2022]. cited July 25, 2022: https://www.datos.gov.co/Salud-y-Protecci-n-Social/EVENTOS-ADVERSOS-ASOCIADOS-A-MEDICAMENTOS/my7f-awtq. Accessed November 16, 2023.
- Demsar J, Curk T, Erjavec A, et al. Orange: data mining toolbox in python. J Mach Learn Res. 2013;14:2349–2353.
- Van Der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008;9:2579–2605.
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
- Ministry of Health. Scientific, technical and administrative standards for health research. Resolution 008430. Bogotá; p. 1–12 [Internet]. [ cited September 26, 2022]. Available from: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/RESOLUCION-8430-DE-1993.PDF. Accessed November 16, 2023.
- Obreque K, Mellado R, Andresen M. Risk factors for adverse drug reactions in patients admitted to intensive care units. Rev Méd Chile. 2021;149(9):1258–1266. doi:10.4067/S0034-98872021000901258
- Panamerican Health Organization. Good Pharmacovigilance Practices for the Americas [Internet]; 2021 [ cited February 13, 2021]. Available from: https://www3.paho.org/hq/index.php?option=com_docman&view=download&alias=33513-buenas-pra-cticas-farmacovigilancia-ame-ricas-2010-513&category_slug=documentos-8499&Itemid=270&lang=es. Accessed November 16, 2023.
- Watson S, Caster O, Rochon PA, den Ruijter H. Reported adverse drug reactions in women and men: aggregated evidence from globally collected individual case reports during half a century. eClinicalMedicine. 2019;17:100188. doi:10.1016/j.eclinm.2019.10.001
- Collao J, Favereau R, Miranda R, Aceitón C. Drug related harm in Chilean hospitals: prevalence analysis 2010–2017. Rev Médica Chile. 2019;147(4):416–425. doi:10.4067/S0034-98872019000400416
- Ministry of Health and Social Protection. Health Situation Analysis. Directorate of Epidemiology and Demography. [Internet]; 2019 [ cited November 9, 2022]. cited November 9, 2022: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/PSP/asis-2019-colombia.pdf. Accessed November 16, 2023.
- Orellana EA, Carias A, Cruz W, et al. [Reacciones adversas por antibióticos y antiinflamatorios no esteroides] Adverse reactions by antibiotics and non-steroidal anti-inflammatory drugs. J Pharm Pharmacogn. 2021;10(2):186–195.
- Ruiz-Ramos J, Santolaya-Perrín R, García-Martín M, Sempere-Serrano P, Alonso-Díaz M, Calderón-Hernanz B. Prevalence of adverse drug events in emergency services. FARM-URG multicenter project. Farm Hosp. 2021;45(4):176–179.
- Machado-Duque M, Machado-Alba JE, Gaviria-Mendoza A, et al. Detection of medication errors through a monitoring and minimization program in outpatients in Colombia, 2018–2019. Biomédica. 2021;41(1):79–86. doi:10.7705/biomedica.5544
- Velasco-González V, Loya-Pérez L, Navarro-García E, et al. Reporting of suspected adverse drug reactions by nursing in Spain. An observational-descriptive retrospective study. Enferm Clínica Engl Ed. 2021;31(6): 363–370.
- Ferrández O, Casañ B, Grau S, et al. Analysis of drug-related problems in a tertiary university hospital in Barcelona (Spain). Gac Sanit. 2019;33(4):361–368. doi:10.1016/j.gaceta.2018.01.002
- Garzón González G, Morales M, de Miguel García S, Domínguez J, Pérez D, Herrera M. Descriptive analysis of medication errors reported in primary care: learning from our mistakes. Prim Care. 2020;52(4):233–239. doi:10.1016/j.aprim.2019.01.006
