Abstract
The use of anabolic androgenic steroids (AAS) for strength training and muscle building is a widespread practice among athletes and young individuals. Athletes and bodybuilders are using these substances for various purposes, such as enhancing muscle mass, strengthening their bodies, and enhancing their performances. AAS exert a wide range of physiological effects that result in the activation of central signaling, resulting in adverse effects. Moreover, excessive use of AAS which can be categorized as AAS abuse; is linked to biological and psychological pathologies, which can lead to mortality. Complications arising from steroid abuse involve both cellular and physiological complications. Cellular complications arise when activation of signaling proteins like mTOR, Akt, etc. leads to alteration in protein synthesis pathways, cell cycle, oxidative stress, and apoptosis, contributing to damage at the cellular level. Physiological complications are evident with cardiovascular pathologies, including an altered lipid profile, cardiac hypertrophy, hypogonadism after discontinuation of AAS, and modulation of GABA receptors in the brain, all contributed by the androgen receptor signaling. Clinical complications budding from these altered physiological processes lead to clinical effects like testicular dysfunction, acne, gynecomastia, and neuropsychiatric disorders. Despite potential therapeutic benefits, AAS use is prohibited by the World Anti-Doping Agency (WADA) due to concerns over adverse health effects. This review highlights the molecular mechanisms, physiological processes, and clinical complications arising from the excessive use of AAS among athletes.
Introduction
The world of sports has long been a stage for human achievement, where athletes push their physical and mental limits to attain greatness. In this pursuit, the use of performance-enhancing substances has remained a contentious issue, with anabolic androgenic steroids (AAS) emerging as one of the most widely debated substances in sports circles.Citation1 Anabolic androgenic steroids, derived from the male sex hormone testosterone, have garnered attention due to their potential to significantly alter an athlete’s physical capabilities.Citation2 This literature review delves into the multifaceted landscape of AAS use among athletes, examining both its positive and negative aspects, shedding light on the motivations behind their use, and the subsequent physiological and psychological consequences.
Anabolic androgenic steroids, originally developed for medical purposes such as treating hormone deficiencies and muscle-wasting diseases, have found a different path in the realm of sports.Citation3 Athletes often seek their effects, which include increased muscle mass, strength, and endurance, with the aim of gaining a competitive edge. The allure of improved performance and the desire to achieve remarkable athletic feats has led to the illicit use of AAS by athletes across various disciplines.Citation4
The allure of AAS for athletes predominantly lies in the potential positive impacts on performance. The heightened ability to build lean muscle mass and increase strength can contribute to enhanced athletic performance.Citation5 AAS can accelerate recovery from intense training, enabling athletes to train more frequently and intensely. This heightened training capacity can translate to improved skill development and performance outcomes.Citation6 Moreover, the psychological boost resulting from these physical improvements can enhance an athlete’s self-confidence and self-esteem, which are crucial for optimal performance under pressure.Citation7
While the potential benefits of AAS are enticing, the negative aspects of their use cannot be overlooked. The misuse of AAS in sports can lead to a range of adverse health effects.Citation8 Physiologically, AAS can disrupt the body’s natural hormone balance, leading to a plethora of complications such as cardiovascular issues, liver damage, and reproductive system abnormalities.Citation9 Moreover, the misuse of AAS can also impact an athlete’s credibility and tarnish the integrity of sports. The unlevel playing field created by doping can undermine the principles of fair competition, eroding the essence of sportsmanship.Citation10
Athletes turn to AAS for a variety of reasons, often influenced by the intense pressures to succeed in a hyper-competitive sporting landscape.Citation11 The pursuit of fame, financial rewards, and national pride can push athletes to seek shortcuts to greatness. Additionally, the fear of falling behind peers who might be using AAS can create a sense of necessity, fuelling the prevalence of their misuse. The use of AAS in sports raises significant ethical and legal dilemmas.Citation12 The breach of fair play and the inherent risks associated with AAS consumption challenge the integrity of sports. Organizations such as the World Anti-Doping Agency (WADA) have established stringent regulations and testing protocols to curb the misuse of AAS and maintain a level playing field.Citation13 Athletes found to be using AAS face not only disqualification but also damage to their reputation and potential legal consequences.Citation14,Citation15
The issue of AAS use among athletes is a complex matter with far-reaching consequence.Citation16 While the allure of enhanced performance through AAS is undeniable, the potential detrimental effects on health, integrity, and fairness within sports cannot be understated.Citation17 This literature review will delve into the nuanced layers of AAS use among athletes, dissecting the motivations that drive their use and the subsequent impact on physiological and psychological well-being. By comprehensively understanding the multifaceted nature of AAS use, stakeholders in the world of sports can make informed decisions to preserve the authenticity and sanctity of athletic competition while safeguarding the health and dignity of athletes.Citation18
Anabolic Steroids
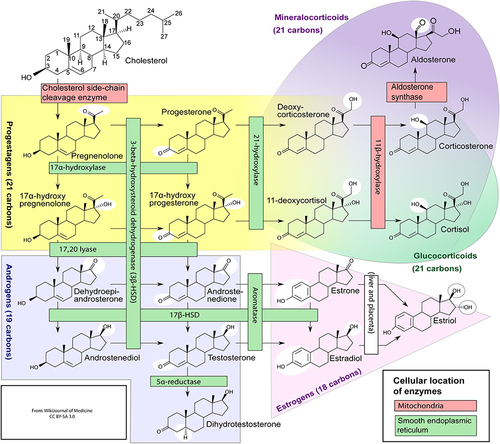
Athletes use anabolic steroids to increase their strength and muscular mass. These substances are also known as anabolic-androgenic steroids.Citation19 Despite their negative reputation, anabolic steroids may be used therapeutically. These drugs might lower the damage that happens to muscles during a hard workout. That could help athletes bounce back faster from a workout. They might be able to exercise harder and more often.Citation20 Anabolic-androgenic steroids, sometimes known as “steroids” or “androgens”, are the most abused Performance Enhancing Drugs (PEDs).Citation21,Citation22 These compounds are synthetic analogs of the male sex hormone testosterone. In both males and females, they increase the growth of skeletal muscle (anabolic effects) and the development of male sexual traits (androgenic effects).Citation23 The steroids are generally formed biologically from cholesterol and then interconverted, as shown in . Besides naturally occurring steroids, synthetic steroids do exist and are widely used among athletes.Citation24
Figure 1 Various steps involved in the biosynthesis of steroid hormones.
Anabolic steroids are classified as controlled substances in numerous countries, including Australia, Argentina, Brazil, Canada, the United Kingdom, and the United States.Citation26 Despite this, there remains a readily accessible global supply of steroids for non-medicinal use. This accessibility is because, in many countries, anabolic steroids can be legally purchased without a prescription.Citation27,Citation28 Consequently, foreign distributors often operate within the legal framework of their nations when supplying these substances to international customers via online platforms and email orders. The majority of hormone products circulating in the European market originate from countries within the European Union and Russia, with occasional contributions from Thailand, Turkey, Egypt, India, and Pakistan.Citation29–31 Similarly, significant volumes of anabolic steroids in the United States are sourced from Mexico, Russia, Romania, and Greece.Citation32
In the United Kingdom, the Misuse of Drugs Act classifies anabolic steroids under Schedule IV Part 2.Citation24 This includes most anabolic steroids as well as clenbuterol (an adrenoreceptor stimulant) and human growth hormone.Citation33 While there are no restrictions on possessing these substances within medicinal products for self-administration, individuals have faced intent-to-supply charges for possessing large quantities without a prescription. Importing and exporting anabolic steroids requires a Home Office license, with exceptions for small quantities for legitimate purposes.Citation34
Regarding doping control in human sports, the International Olympic Committee (IOC) Medical Commission designated anabolic steroids as a banned class in 1974.Citation35 The term “anabolic agents” emerged in the 1990s to encompass substances like clenbuterol and other β2-agonists, which also possess anabolic activity and are subject to out-of-competition testing.Citation36 The World Anti-Doping Agency (WADA) was established in 1999 under the IOC’s initiative, uniting various organizations and governments against doping in sports.Citation37 WADA’s regulations and technical documents, including those concerning anabolic steroids, are consistently evolving, and accessible on the WADA website (http://www.wada-ama.org/en/).
Misuse of anabolic steroids extends beyond sports to society, with adults and adolescents seeking cosmetic benefits such as muscle growth. Anabolic steroid abuse rates vary, with around 5% use among gym-goers and 25–50% among competitive bodybuilders.Citation38–40 Estimating the true UK-wide usage is challenging, but the British Medical Association’s report indicates widespread use.Citation41 The United States also faces high prevalence rates.Citation42
Within sports drug control, anabolic steroids are both performance enhancers and health hazards. In 2006, among 198,143 urine samples analyzed by WADA-accredited labs, 2% contained prohibited substances, with 45% of adverse findings linked to anabolic steroids.Citation35 Frequent steroids include testosterone, nandrolone, stanozolol, and methandienone. Detecting testosterone use is complex due to its endogenous production. The urge for success drives competitors to enhance performance despite the risk of penalties, even though some claim WADA’s statistics do not fully reflect steroid doping in top-level athletics.Citation34,Citation43,Citation44
Anabolic steroids’ chemical structures and activity have undergone modifications to amplify anabolic effects and minimize androgenic ones.Citation45 While some steroids have been withdrawn in several countries, they remain available in others for medical use, such as methandienone, methyltestosterone, oxandrolone, and stanozolol. In the United Kingdom, licensed products include testosterone and its esters, nandrolone (as the decanoate ester), mesterolone, and oxymetholone (restricted to named patients). Some countries limit boldenone and trenbolone to veterinary purposes, yet athletes and bodybuilders sometimes administer these anabolic steroids.Citation34
Methenolone acetate, methandrostenolone, oxandrolone, oxymetholone, and stanozolol are some of the orally administered steroids in use among athletes. Some intramuscularly used steroids are boldenone undecenoate, methenolone acetate, nandrolone decanoate, Sustanon 250®, testosterone enanthate, and testosterone cypionate.Citation46 Anabolic-androgenic steroids are the best-studied class of PEDs that can boost a user’s confidence and strength, leading users to overlook the severe, long-lasting, and in some cases, irreversible damage they can cause.Citation47
Population Burden of AAS Abuse
Anabolic androgenic abuse gained popularity due to its impact on physique and performance in sports like bodybuilding, weightlifting, baseball, football, cycling, wrestling, and many others to improve their performance.Citation48 The gain in muscle mass and strength contributes to aesthetic appeal and therefore use of androgenic anabolic steroids paved the way to the events of abuse. Among Americans with ages ranging from 13 to 50 years, 2.9–4.0 million people have reported using AAS. Almost 1 million people utilizing AAS within this community have reportedly developed AAS dependence.Citation49 The use of AAS is likely to be observed in gym members practicing weightlifting followed by the number of people working in the private sector and beyond 25 years of age. AAS abuse is more evident in Western regions as compared to Africa and Asia. The report explains that this high likelihood of AAS abuse can be attributed to the concept of “muscularity” in those culture.Citation50,Citation51 However, other than the cultural norms, factors that are associated with AAS abuse, especially in gym members include weightlifting, the use of supplementary vitamins, special diets, and social exposure to people who regularly use AAS for similar purposes.Citation52 Accurate estimation of steroid-associated gynecomastia is crucial for effective healthcare planning and tailored treatment approaches. The studies identify indicators of steroid usage, assesses responses to surgical and nonsurgical management, and compares preoperative and intraoperative data between different groups. The results highlight the significance of monitoring steroid consumption in gynecomastia cases, which often goes underestimated due to social stigma and misdiagnosis.Citation53,Citation54 The use of AAS not only impacts sports performance but also poses significant health risks and psychological consequences, highlighting the importance of comprehensive education, awareness, and intervention strategies to address the widespread issue of AAS abuse.Citation8
Uses and Recommendations of AAS
Since use of drugs is generally regulated, AAS has also been subject to approval from FDA,Citation55 where it has been indicated for primary hypogonadism, delayed puberty in boys, hypogonadotropic hypogonadism, gonadotropin and luteinizing hormone-releasing hormone (LHRH) deficiency, pituitary-hypothalamic axis (HPA-axis) dysfunction originating from various tumors.Citation56 AAS is also used for the treatment of physiological deficits arising from anatomical abnormalities like cryptorchidism, orchitis, testicular torsion, vanishing testis syndrome. Patients with a history of orchiectomy, Klinefelter syndrome, or ongoing therapy with chemotherapeutic agents, alcohol abuse, and heavy metal poisoning are also treated with AAS.Citation57
Testosterone is also used as an adjunct treatment for certain malignant conditions bone marrow stimulation in leukemia and aplastic anemia, however, these treatments are non-FDA approved.Citation58 Similarly, kidney failure, growth failure, stimulation of appetite, muscle mass in malignancy, and acquired immunodeficiency syndrome are also treated with AAS.Citation59 Furthermore, it’s important to note that while AAS has legitimate medical uses as mentioned, its potential for misuse and abuse, along with the associated health risks, underscores the critical necessity for responsible medical supervision and comprehensive patient education regarding the proper and safe use of these substances.Citation60
Molecular Mechanisms of AAS
Increased size and muscle strength are desirable for aesthetic appeal however, at a cellular level, the use of AAS will lead to activation of multiple pathways that result in the increase in size of muscles and strength. Since these pathways have different outcomes in other types of cells, they may, therefore, elicit a different outcome response.Citation61 Androgens increase both the size and strength of skeletal muscle via diverse mechanisms. AAS binds to and activates androgen receptors in nuclei resulting in transcription of the associated genes.Citation62,Citation63 These genes include transcription factors specific to muscles, structural proteins, microRNAs, and enzymes. Activation of these proteins also results in cellular cross-talk with other signaling molecules as well which include Akt, myostatin, IGF-I, and Notch signaling.Citation62 Akt is a central signaling molecule therefore, its activation can also lead to unwanted physiological effects. The metabolic effects of anabolic androgen are reflected by their effect on muscles where they increase Ca2+ uptake and modulate kinase activities.Citation62 While IGF-1 signaling is responsible for cellular growth functions, its receptor, IGFR, is a tyrosine kinase receptor and activates common downstream signaling molecules including Akt, leading to the activation of S6K1, which translates into protein synthesis and growth. The cellular growth and strength are therefore contributed by both activation of downstream Akt and S6K1 and activation of nuclear receptors.Citation64
Anabolic androgenic steroids (AAS) are synthetic derivatives of testosterone that are commonly used to enhance athletic performance and promote muscle growth. These compounds exert their effects primarily by binding to androgen receptors within cells, leading to various physiological responses.Citation65 The molecular mechanisms of AAS involve interactions at the cellular and molecular levels, influencing gene expression, protein synthesis, and cellular signaling pathways.Citation66 Several studies have investigated the molecular mechanisms of AAS. For instance, research has shown that AAS usage can induce changes in gene expression in muscle cells, contributing to muscle growth and potentially muscle memory. This suggests that AAS might lead to the retention of myonuclei in muscle tissue, which could contribute to long-term muscle adaptations.Citation66,Citation67
AAS has also been found to influence cardiac function and the central nervous system.Citation68 Chronic use of AAS has been associated with ventricular repolarization disturbances and disruptions in areas of the central nervous system, leading to behavioral changes.Citation69 The precise molecular mechanisms underlying these effects are still being studied, and more research is needed to fully understand these complexities. At the cellular level, AAS impacts protein synthesis by upregulating the expression of various genes related to muscle growth and repair. Additionally, AAS may modulate calcium homeostasis and cardiac contraction in heart cells, potentially affecting cardiac function.Citation70
Physiological Mechanisms of AAS
Effects on Muscles
The growth effects of AAS are exerted by the promotion of protein synthesis through gene transcription as described earlier. The binding of AAS to its receptor results in the displacement of glucocorticoids from their receptors.Citation71 Consequently, protein degradation is inhibited as glucocorticoids are responsible for the degradation of protein in the cell.Citation72 Among these anabolic steroids, stanozolol’s mechanism of action has been hypothesized to be influenced by a significant increase in type I muscle fiber size which provides enough strength for the athletes to exercise longer, consequently resulting in type II fiber hypertrophy.Citation73 Testosterone injections of 600 mg dose on the other hand have shown a greater increase in fat-free mass in individuals who were not exposed to exercise intervention as compared with those who did resistance exercise with placebo treatment.Citation74 These effects are demonstrated for the increase in muscle mass mainly while no such effects have been observed in muscle concentrations of creatine.
Contrary to these findings, AAS doping in athletes in a dose-dependent manner has exhibited an increase in capillary density, muscle fiber area, myonuclei density, and lean body mass, thereby leading to the conclusion that AAS supplementation increases lean leg mass, muscle fiber size and improvement in muscle strength.Citation75 These studies showing long-term administration of AAS’s enhancement effects in human skeletal muscle morphology and physical performance.Citation76 The effects of anabolic androgenic steroids (AAS) on muscle growth involve intricate physiological mechanisms. AAS promotes protein synthesis through gene transcription and inhibits protein degradation by displacing glucocorticoids from their receptors.Citation77 Notably, distinct AAS like stanozolol may influence type I muscle fiber size, enhancing endurance, while testosterone injections exhibit differential fat-free mass increases based on exercise exposure.Citation78 Despite debates, AAS supplementation demonstrates the potential to augment muscle mass, fiber size, and overall strength, particularly in physically demanding sports; however, misuse without clinical guidance remains a concern.
Effects on Liver
As with most other drugs, the frequency and severity of hepatic adverse effects from AAS arise from several factors including formulation of the drug, route of administration, dosage, duration, and idiosyncratic responses.Citation79 Hepatotoxic effects of testosterone occur due to slower clearance of anabolic steroids like testosterone. These adverse effects are likely to arise from 17-α-alkylation modification, which makes their use desirable for oral intake.Citation66 Moreover, such substitutions facilitate the potency and duration of action of these anabolic steroids. Hepatotoxic events are related to elevated liver transaminases, translating into acute cholestatic syndrome. Testosterone and its derivatives especially have been frequently employed as a causative factor in a specific form of cholestasis called peliosis hepatis, benign and malignant hepatic tumors.Citation80 Other than that, chronic vascular injury, fatty liver diseases associated with toxicants like alcohol, and significant lipoprotein alterations have been observed with the use of AAS. Usually, these modifications are reversible with cessation of steroid use, however, some of them can pose life-threatening conditions.Citation81 Overall, the hepatotoxic mechanisms currently unveiled in AAS-related hepatotoxicity include disturbance of antioxidative factors, upregulation of bile acid synthesis, and induction of hepatocyte hyperplasia.Citation71 The AAS-induced hepatotoxic effects result from various factors, encompassing drug formulation, administration route, dosage, duration, and individual responses. Hepatotoxicity, often linked to elevated liver transaminases and acute cholestatic syndrome, can stem from the 17-α-alkylation modification in AAS, which enhances their oral availability and effectiveness.Citation82 Additionally, AAS usage has been associated with intricate hepatic conditions like peliosis hepatis, benign and malignant tumors, vascular injuries, fatty liver disorders, and disrupted lipoprotein profiles.Citation61 While many alterations are reversible upon discontinuation, certain consequences may escalate into severe, life-threatening scenarios.
Effects on Bones
The mechanism of androgens in the skeletal system is to inhibit bone resorption through osteoclastic activity. This results in the increase of androgen receptor-mediated bone formation.Citation83 The increase in bone formation is associated with longitudinal and periosteal bone growth and an increase in bone mass. In various conditions, including ovariectomized and orchiectomized rats, AAS exhibit anti-catabolic effects by reducing trabecular bone resorption, while also demonstrating the potential to enhance cortical bone strength, providing insights for addressing established osteoporosis related to aging.Citation84,Citation85 In the ovariectomized and ovariectomized rats, AAS have shown decreased trabecular bone resorption which further elaborates their anti-catabolic effects in bone.Citation86 In particular, an increase in the mechanical strength of cortical bone was observed in ovariectomized rats with AAS use, a finding that led to the development of AAS as a potential lead for treating established osteoporosis associated with aging.Citation87 AAS has shown stimulation of endosteal bone formation. These findings have provided recommendations for AAS use particularly ND and nandrolone in estrogen-deficient conditions.Citation88 Administration of ND and nandrolone have revealed their significant effects in increasing bone mass in osteoporotic men and women. The bone gain with AAS use in such patients is almost 3% per annum, however, maximal effects are observed in the initial months of treatment.Citation89 This bone gain is associated with stimulation of bone formation, increase in serum albumin, and fat-free skinfold thickness.Citation90 Research using cellular models like SaOS-2 cells has also shown promising results for AAS use by promoting osteogenic commitment in these osteoblasts.Citation91 Despite their positive effects on bone growth, AAS use has shown a paradoxical effect on tendons and ligaments. Contrary to their anabolic effects on bone formation and bone growth, rupture of biceps and quadriceps tendons has been reported in athletes using AAS.Citation92 Conclusively, despite their deleterious effects on other organs, AAS have desirable effects on bone formation and growth, which calls for more cellular and clinical studies deciphering their effects on bone-related disorders.
Effects on Fat Metabolism
Alteration in fat metabolism due to AA use has shown a decrease in sphingolipids and glycerolipids with palmitic, palmitoleic, stearic, and oleic acids. These lipids serve as a building block in the formation of membrane-bound structures in cells and therefore, play a key role in the wear-and-tear mechanism of cellular growth.Citation93 Lipid profiling in individuals with AAS abuse revealed an increased amount of free fatty acids and glycerophospholipids. This increase is associated with odd-numbered chain fatty acids and/or arachidonic acid.Citation94 Notably, AAS influences HDL-cholesterol dynamics by enhancing hepatic lipase activity, which plays a pivotal role in HDL particle size conversion from HDL2 to HDL3, with even relatively low AAS dosages contributing to significant reductions in HDL-cholesterol level.Citation95 Administration of a low dosage of almost 6 mg per day of AAS compound for 2 weeks has shown a reduction of up to 20% in HDL-cholesterol levels in HL deficiency.Citation61 Reduction in HDL and increase in LDL and triglyceride levels have shown a predisposition to adverse cardiovascular events, thereby reflecting its adverse effects on the cardiovascular system.
Cardiovascular Risks Associated with AAS Abuse
Adverse events associated with AAS use are widely studied for the cardiovascular system. In general, cardiomyopathy, myocardial infarction, and fatal arrhythmias have been reported with AAS use.Citation96 An increase in Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio has been implemented in the pathophysiology of ventricular arrhythmias caused by AAS use, with reports of sudden death.Citation97 Cardiomyopathy in response to AAS use is also contributed by the increase of heart chamber diameters, changes in ventricular relaxation via altered diastolic function, and alterations in left ventricular contractility at the subclinical level.Citation98,Citation99
Neurohumoral responses from AAS include a transient increase in blood pressure, yet, clinically significant hypertension is still to be established. The mechanism postulated to be involved in alteration in neurohumoral response includes an increase in systolic blood pressure with a decrease in plasma MR-proANP levels.Citation100 On the other hand, suppression of testosterone in men has shown an increase in NY-proBNP levels, which were restored by testosterone replacement, thereby formulating the role of testosterone in circulating natriuretic peptide levels.Citation101 Also, there have been reports of a link between AAS abuse and aortic stiffness that can be partly explained by their effects on platelet function and neurohumoral responses together.Citation102
Platelet function in response to AAS abuse involves a pronounced pro-thrombotic state, reflected by the increase in platelet aggregation.Citation103 Parallel coagulatory responses from the humoral regulation lead to a more complex pro-coagulator state characterized by the activation of pro-coagulatory and fibrinolytic pathways. Among these AAS, nandrolone specifically has shown a tendency to accelerate clot development and firmness in Wistar rats.Citation104 This might explain evident alterations in endothelial-dependent or independent vasodilation resulting in clot formation.Citation105
Additionally, the altered lipid metabolism, as discussed above, sets a foundation for the development of atherosclerosis and hypertension,Citation106 alongside an accelerated coagulation cascade, further exacerbating the condition. Persistent use of AAS therefore shows an association with myocardial dysfunction and accelerated coronary atherosclerosis. Such adverse events associated with AAS abuse are wreaking havoc on the current prevalence of cardiovascular events, without the recognition of AAS abuse as a public health concern.Citation107
Effects on Kidney
Effects of AAS on kidneys have been categorized as either direct or indirect effects.Citation108 While indirect effects are linked to cardiovascular or muscular abnormalities discussed above, direct effects refer to focal segmental glomerulosclerosis, acute kidney injury, and predisposition to chronic kidney injury.Citation109 Creatinine is a biochemical marker of kidney functioning and AAS use has shown transient small increases in serum creatinine concentrations (1.05 mg/Dl to 1.11 mg/dL) however, this increase remained clinically insignificant and returned to baseline after discontinuation of AAS use.Citation110 Comparatively, a larger increase in serum creatinine levels was observed in a placebo-controlled trial for a 4-week long duration, with resistance-trained men. These subjects were randomized to either a daily dose of 330 mg of oral prohormone 3β-hydroxy-5α-androst-1-en-17-one (1-androsterone) or placebo,Citation111 thereby reflecting alteration in renal function. Nandrolone decanoate abuse can cause aldosterone and electrolyte imbalance in the body which could be a serious risk factor for cardiovascular-related disorders.Citation112 The impact of AAS on kidneys has been categorized into direct and indirect effects, with indirect effects being linked to the cardiovascular or muscular abnormalities discussed earlier, and direct effects encompassing conditions like focal segmental glomerulosclerosis, acute kidney injury, and susceptibility to chronic kidney injury.Citation5
Effects of AAS Abuse on Sexual Functions
Testosterone, a reproductive hormone in males is known to be involved in metabolism where it enhances basal metabolic rate (BMR) at pharmacological doses.Citation113 As discussed earlier, it also has an impact on fat metabolism, thereby exerting its direct effects on the metabolism of reproductive hormones as well. Supraphysiological doses of AAS downregulate testosterone production.Citation114 Also, the external administration of testosterone in turn suppresses the hypothalamic-pituitary axis. It consequently translates into erectile dysfunction, violent tendencies, and decreased libido. Sexual dysfunction resulting from AAS abuse involves anabolic steroid-induced hypogonadism which includes azoospermia and testicular atrophy.Citation115 In males with ≤ 1 year of AAS abuse, withdrawal of AAS may suffice to normalize testosterone levels, but for abuse exceeding one year, additional therapy with clomiphene or gonadotropin may be needed to restore spermatogenesis.Citation107
Effects on Brain
AAs work by activating dopamine pathways in the brain. Specifically, testosterone acts through the mesolimbic dopamine system, which serves as a common platform in the brain for dependence on drugs of abuse.Citation116 Instead, testosterone resembles other mild reinforcers, such as caffeine, nicotine, or benzodiazepines. The potential for androgen addiction remains to be determined. These pathways are known for reward mechanisms in the brain, making them prone to developing dependence.Citation117 Also, AAS has been linked to modification in some indirect pathways including serotonergic, glutamatergic, and dopaminergic pathways in the lateral-anterior hypothalamus, translating into aggressive behavior.Citation118,Citation119 AAS dependence arises from androgen reinforcement in the brain, which is comparatively lower than that of cocaine or heroin.Citation120 Reinforcements induced by caffeine, nicotine, or benzodiazepines instead are comparable to those induced by AAS.Citation121 Yet the AAS dependence requires further probing due to the adverse events associated with it. AAS dependence in males has exhibited thinning in the brain-wide cortical regions, in particular the pre-frontal cortex which is responsible for inhibitory control and emotional regulation.Citation122 Increased anxiety associated with chronic AAS use involves a direct amygdala-fugal pathway which forms the connection between the central nucleus of the amygdala and brainstem. In normal conditions, this pathway is responsible for cognitive-emotive and homeostatic processes. Among AAS, nalbuphine has shown some degree of dependence predisposing it as a drug of abuse among athletes. Consequently, nalbuphine’s scheduling status has been recommended for re-evaluation.Citation123
Psychological Complications Arising from AAS Abuse
Psychological complications with the use of AAS have brought about an area of concern that needs to be addressed with rising global mental health challenges. AAS use has been shown to cause an increase in aggression and hostility.Citation124,Citation125 Some studies report mood disturbance about the type and dosage of AAS.Citation126 The AAS abuse population at risk of psychopathic traits includes bodybuilders with a prior history of AAS use. Also, sexual and substance use risk-taking behaviors and anger problems, are widely observed in bodybuilders with AAS use history as compared with non-users.Citation127 Since AAS use enhances aesthetic appeal by increasing muscle strength, withdrawal brings about concerns related to the body image of a person, resulting in social physique anxiety, however, this effect has been observed in more severe symptoms of AAS abuse including depression and withdrawal.Citation128 Yet, AAS dependence or withdrawal symptoms like depressed mood have been observed in a small number of AAS users. Also, reverse anorexia syndrome may develop with a dissatisfied body image and low self-esteem in the individuals using AAS, which predisposes such persons to resume the use of AAS.Citation129 So far, only 37.12% of people have shown interest in seeking support from physicians regarding AAS dependence which calls for the need to appreciate seeking help regarding AAS dependence.Citation130 They can lead to early heart attacks, strokes, liver tumors, kidney failure, and psychiatric problems. In addition, stopping use can cause depression, often leading to resumption of use.
Conclusion
Cellular and metabolic complications arising from anabolic steroid misuse have raised a recent public health concern. Health complications arising from anabolic steroid misuse have unveiled an occult cause of metabolic derangements in the younger population. Most studies have deciphered mechanisms where AAS activates common downstream signaling molecules like Akt, explaining unwanted events arising from AA abuse. AAS abuse has shown critical damage to cardiovascular, excretory, reproductive, muscular, hepatic, and nervous systems. Studies on AAS-related adverse effects have also brought about a connection with psychiatric disorders, thereby opening an area of interest for the causative factors of psychiatric disorders. Much is yet to be explored on how overt or occult AAS abuse defines certain behaviors in a normal individual. Can AAS play a role in defining behaviors that are generally attributed to a person’s own choice of behavior or are they influenced by such drugs of abuse needs to be explored to better understand male psychology. Moreover, health complications arising from AAS abuse like gynecomastia, acne, etc. also raise concern over AAS’s potential as a useful drug. While AAS abuse has been linked to many disorders affecting different organs in the body, it is interesting to observe that the effects of AAS have remained largely positive on bone growth. Reports on damage to tendons or ligaments exist, yet the effects of AAS remain largely beneficial for the skeletal system. Further probing into its mechanisms of action on bone growth may be useful for understanding the reason for its limited adverse effects on them. Moreover, clinical studies utilizing AAS for enhancing fracture repair in different ages and different kinds of fractures may provide useful insight into its clinical use. Conclusively, while AAS abuse has proven to be associated with many health complications, its effectiveness in treating skeletal disorders is largely unexplored. Probing its effectiveness for bone-related disorders with careful monitoring may offer a new mode of treatment for accelerated healing from complicated skeletal disorders.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The authors are thankful to Dr Noor Bahadar for reviewing the manuscript and suggesting grammatical corrections.
References
- Christiansen AV, Henning A, Lopez Frias FJ, Hoberman JM. Book symposium: ask vest christiansen’s gym culture, identity and performance-enhancing drugs’. 2021.
- Machek SB, Cardaci TD, Wilburn DT, Willoughby DS. Considerations, possible contraindications, and potential mechanisms for deleterious effect in recreational and athletic use of selective androgen receptor modulators (SARMs) in lieu of anabolic androgenic steroids: a narrative review. Steroids. 2020;164:108753. doi:10.1016/j.steroids.2020.108753
- Bird SR, Goebel C, Burke LM, Greaves RF. Doping in sport and exercise: anabolic, ergogenic, health and clinical issues. Ann Clin Biochem. 2016;53(2):196–221. doi:10.1177/0004563215609952
- Smit DL, Bond P, de Ronde W. Health effects of androgen abuse: a review of the HAARLEM study. Curr Opin Endocrinol Diabetes Obes. 2022;29(6):560–565. doi:10.1097/MED.0000000000000759
- Pope HG, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev. 2014;35(3):341–375. doi:10.1210/er.2013-1058
- Moore E, Morrison J. In defense of medically supervised doping. J Philos Sport. 2022;49(2):159–176. doi:10.1080/00948705.2022.2066538
- Grant B, Minhas S, Jayasena CN. A review of recent evidence on androgen abuse from interviews with users. Curr Opin Endocrinol Diabetes Obes. 2023;30(6):285–290. doi:10.1097/MED.0000000000000834
- Bhasin S, Hatfield DL, Hoffman JR, et al. Anabolic-androgenic steroid use in sports, health, and society. Med Sci Sports Exerc. 2021;53(8):1778–1794. doi:10.1249/MSS.0000000000002670
- Phan J. The Role of Lipin as a Modulator of Adiposity and Energy Homeostasis: Insights from the Fatty Liver Dystrophy Mouse. Los Angeles: University of California; 2004.
- Kornbeck J. EU Antitrust Law and Sport Governance: The Next Frontier? Taylor & Francis; 2022.
- Masteralexis L. Regulating player agents. In: Research Handbook of Employment Relations in Sport. Edward Elgar, Cheltenham; 2016:99–123.
- Cowan DA, Kicman AT. Doping in sport: misuse, analytical tests, and legal aspects. Clin Chem. 1997;43(7):1110–1113. doi:10.1093/clinchem/43.7.1110
- Ljungqvist A. Brief history of anti-doping. In: Acute Topics in Anti-Doping. Vol 62. Karger Publishers; 2017:1–10.
- Graham MR, Davies B, Grace FM, Kicman A, Baker JS. Anabolic steroid use: patterns of use and detection of doping. Sports Med. 2008;38(6):505–525. doi:10.2165/00007256-200838060-00005
- Kornbeck J. Conclusion: EU antitrust law and the future of the sports pyramid and the ‘one federation’principle. In: EU Antitrust Law and Sport Governance. Routledge; 2022:133–157.
- Baker JS, Graham MR, Davies B. Steroid and prescription medicine abuse in the health and fitness community: a regional study. Eur J Intern Med. 2006;17(7):479–484. doi:10.1016/j.ejim.2006.04.010
- Hard M. Caught in the Net: athletes’ rights and the world anti-doping agency. Cal Interdisc LJ. 2009;19:533.
- Gopinath P. Scarecrows of Chivalry: English Masculinities After Empire. University of Virginia Press; 2013.
- Mazzeo F. Anabolic steroid use in sports and in physical activity: overview and analysis. Sport Mont. 2018;16(3):113–118. doi:10.26773/smj.181020
- Brower KJ. Anabolic steroid abuse and dependence. Curr Psychiatry Rep. 2002;4(5):377–387. doi:10.1007/s11920-002-0086-6
- Bonnecaze AK, O’Connor T, Burns CA. Harm reduction in male patients actively using anabolic androgenic steroids (AAS) and performance-enhancing drugs (PEDs): a review. J Gen Intern Med. 2021;36(7):2055–2064. doi:10.1007/s11606-021-06751-3
- Kolliari-Turner A, Oliver B, Lima G, et al. Doping practices in international weightlifting: analysis of sanctioned athletes/support personnel from 2008 to 2019 and retesting of samples from the 2008 and 2012 Olympic Games. Sports Med - Open. 2021;7(1):4. doi:10.1186/s40798-020-00293-4
- Bailey K, Yazdi T, Masharani U, Tyrrell B, Butch A, Schaufele F. Advantages and limitations of androgen receptor-based methods for detecting anabolic androgenic steroid abuse as performance enhancing drugs. PLoS One. 2016;11(3):e0151860. doi:10.1371/journal.pone.0151860
- Chandler M, McVeigh J. Steroids and Image Enhancing Drugs 2013 Survey Results. Liverp LJMU Cent Public Health; 2014:1–26.
- Häggström M and Richfield D. Diagram of the pathways of human steroidogenesis. Wiki J Med. 2014;1(1):1–5. doi:10.15347/wjm/2014.005
- Shah RS, Patel NS, Rami NV, et al. DOPE AS A DOSE OF PHYSIOLOGICAL ENHANCERS. Pharm Global. 2016;7(3):1.
- da Justa Neves DB, Caldas ED. GC–MS quantitative analysis of black market pharmaceutical products containing anabolic androgenic steroids seized by the Brazilian Federal Police. Forensic Sci Int. 2017;275:272–281. doi:10.1016/j.forsciint.2017.03.016
- Magnolini R, Falcato L, Cremonesi A, Schori D, Bruggmann P. Fake anabolic androgenic steroids on the black market–a systematic review and meta-analysis on qualitative and quantitative analytical results found within the literature. BMC Public Health. 2022;22(1):1–15. doi:10.1186/s12889-022-13734-4
- Câmara LC. Anabolic androgenic steroids from underground market: drug quality and implications for research. Asian J Res Med Pharm Sci. 2023;12(3):59–64.
- Heinsvig PJ, Christiansen AV, Ayoubi D, Heisel LS, Lindholst C. Do you get what you see? The illicit doping market in Denmark—An analysis of performance and image enhancing drugs seized by the police over a 1‐year period. Drug Test Anal. 2023;15(6):668–677. doi:10.1002/dta.3472
- Vanhee C, Jacobs B, Mori M, et al. Uncovering the quality deficiencies with potentially harmful effects in substandard and falsified PDE-5 inhibitors seized by Belgian controlling agencies. Forensic Sci. 2023;3(3):426–451. doi:10.3390/forensicsci3030031
- Graham MR, Ryan P, Baker JS, et al. Counterfeiting in performance‐and image‐enhancing drugs. Drug Test Anal. 2009;1(3):135–142. doi:10.1002/dta.30
- Ip EJ, Doroudgar S, Lau B, Barnett MJ. Anabolic steroid users’ misuse of non-traditional prescription drugs. Res Soc Adm Pharm. 2019;15(8):949–952. doi:10.1016/j.sapharm.2018.07.003
- Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154(3):502–521. doi:10.1038/bjp.2008.165
- Kicman AT, Gower DB. Anabolic steroids in sport: biochemical, clinical and analytical perspectives. Ann Clin Biochem. 2003;40(4):321–356. doi:10.1258/000456303766476977
- Parr MK, Flenker U, Schänzer W. Sports-related issues and biochemistry of natural and synthetic anabolic substances. Endocrinol Metab Clin. 2010;39(1):45–57. doi:10.1016/j.ecl.2009.11.004
- Wagner U. Towards the construction of the world anti-doping agency: analyzing the approaches of FIFA and the IAAF to doping in sport. Eur Sport Manag Q. 2011;11(5):445–470. doi:10.1080/16184742.2011.624107
- Challab SM, Anwer ZM, Khalil HH. Knowledge and attitude of using anabolic androgenic steroids among male bodybuilders in al-russafa baghdad province. Ibn AL-Haitham J Pure Appl Sci. 2023;36(2):22–32. doi:10.30526/36.2.3004
- de Zeeuw TI, Brunt TM, Van amsterdam J, van de Ven K, van den Brink W. Anabolic androgenic steroid use patterns and steroid use disorders in a sample of male gym visitors. Eur Addict Res. 2023;29(2):99–108. doi:10.1159/000528256
- Greenway CW, Price C. Muscle dysmorphia and self-esteem in former and current users of anabolic-androgenic steroids. Perform Enhanc Health. 2020;7(3–4):100154. doi:10.1016/j.peh.2019.100154
- Mittra J, Mastroeni M, Haddow G, Wield D, Barlow E. Re-imagining healthcare and medical research systems in post-devolution Scotland. Sociol Res Online. 2019;24(1):55–72. doi:10.1177/1360780418823221
- Denham BE. Anabolic steroid cases in United States district courts (2013–2017): defendant characteristics, geographical dispersion, and substance origins. Contemp Drug Prob. 2019;46(1):41–57. doi:10.1177/0091450918800823
- Gheddar L, Pélissier AL, Desfeux J, Niort F, Raul JS, Kintz P. Testing for trenbolone, an anabolic steroid, in biological fluids and head hair in a postmortem case. J Anal Toxicol. 2022;46(2):e88–e91. doi:10.1093/jat/bkab091
- Harries RL, De Paoli G, Hall S, Nisbet LA. A review of the analytical techniques for the detection of anabolic–androgenic steroids within biological matrices. Wiley Interdiscip Rev Forensic Sci. 2023;e1504. doi:10.1002/wfs2.1504
- Frati P, Busardo F, Cipolloni L, De Dominicis E, Fineschi V. Anabolic androgenic steroid (AAS) related deaths: autoptic, histopathological and toxicological findings. Curr Neuropharmacol. 2015;13(1):146–159. doi:10.2174/1570159X13666141210225414
- Solimini R, Rotolo MC, Mastrobattista L, et al. Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping. Eur Rev Med Pharmacol Sci. 2017;21(1 Suppl):1.
- Wu C, Kovac JR. Novel uses for the anabolic androgenic steroids nandrolone and oxandrolone in the management of male health. Curr Urol Rep. 2016;17(10):72. doi:10.1007/s11934-016-0629-8
- Cohen J, Collins R, Darkes J, Gwartney D. A league of their own: demographics, motivations and patterns of use of 1955 male adult non-medical anabolic steroid users in the United States. J Int Soc Sports Nutr. 2007;4(1):12. doi:10.1186/1550-2783-4-12
- Pope HG, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. The lifetime prevalence of anabolic‐androgenic steroid use and dependence in Americans: current best estimates. Am J Addict. 2014;23(4):371–377. doi:10.1111/j.1521-0391.2013.12118.x
- Adams NN. A triad of physical masculinities: examining multiple ‘hegemonic’bodybuilding identities in Anabolic-Androgenic Steroid (AAS) online discussion groups. Deviant Behav. 2023;3:1–19.
- Brennan DJ, Craig SL, Thompson DEA. Factors associated with a drive for muscularity among gay and bisexual men. Cult Health Sex. 2012;14(1):1–15. doi:10.1080/13691058.2011.619578
- Al-Harbi FF, Gamaleddin I, Alsubaie EG, Al-Surimi KM. Prevalence and risk factors associated with anabolic-androgenic steroid use: a cross-sectional study among gym users in Riyadh, Saudi Arabia. Oman Med J. 2020;35(2):e110. doi:10.5001/omj.2020.28
- Meller L, Wilson K, Huang B, Kalavacherla S, Vitale K. Underlying subclavian artery occlusion initially misdiagnosed in weightlifter using anabolic steroids: a case report and review of literature. Cureus. 2023;18. doi:10.7759/cureus.37763
- Kolliari-Turner A, Lima G, Hamilton B, Pitsiladis Y, Guppy FM. Analysis of anti-doping rule violations that have impacted medal results at the summer Olympic Games 1968–2012. Sports Med. 2021;51(10):2221–2229. doi:10.1007/s40279-021-01463-4
- Salcher‐Konrad M, Naci H, Davis C. Approval of cancer drugs with uncertain therapeutic value: a comparison of regulatory decisions in Europe and the United States. Milbank Q. 2020;98(4):1219–1256. doi:10.1111/1468-0009.12476
- Swaab DF. Eating disorders (Fig. 23A). 2004.
- Burrows PJ, Schrepferman CG, Lipshultz LI. Comprehensive office evaluation in the new millennium. Urol Clin. 2002;29(4):873–894. doi:10.1016/S0094-0143(02)00091-5
- Evangelista M A guide on the classes of performance-enhancing substances. 2023.
- Basaria S, Wahlstrom JT, Dobs AS. Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86(11):5108–5117. doi:10.1210/jcem.86.11.7983
- Piatkowski T, Gibbs N, Dunn M. Beyond the law: exploring the impact of criminalising anabolic–androgenic steroid use on help-seeking and health outcomes in Australia. J Criminol. 2023;26338076231209044. doi:10.1177/26338076231209044
- Bond P, Smit DL, de Ronde W. Anabolic–androgenic steroids: how do they work and what are the risks? Front Endocrinol. 2022;13:1059473. doi:10.3389/fendo.2022.1059473
- Dubois V, Laurent M, Boonen S, Vanderschueren D, Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69(10):1651–1667. doi:10.1007/s00018-011-0883-3
- Santos HO, Haluch CE. Downregulation of androgen receptors upon anabolic-androgenic steroids: a cause or a flawed hypothesis of the muscle-building plateau? Muscles. 2022;1(2):92–101. doi:10.3390/muscles1020010
- Covarrubias AJ, Aksoylar HI, Horng T. Control of macrophage metabolism and activation by mTOR and Akt signaling. In: Seminars in Immunology. Vol. 27. Elsevier; 2015:286–296.
- Patt M, Beck KR, Di Marco T, et al. Profiling of anabolic androgenic steroids and selective androgen receptor modulators for interference with adrenal steroidogenesis. Biochem Pharmacol. 2020;172:113781. doi:10.1016/j.bcp.2019.113781
- Park J, Mcllvain V, Rosenberg J, Donovan L, Desai P, Kim JY. The mechanisms of anabolic steroids, selective androgen receptor modulators and myostatin inhibitors. Korean J Sports Med. 2022;40(2):67–85. doi:10.5763/kjsm.2022.40.2.67
- Gao H, Zhang JY, Zhao LJ, Guo YY. Synthesis and application of clinically approved small-molecule drugs targeting androgen receptor. Bioorg Chem. 2023;1:106998.
- Hall E, Vrolijk MF. Androgen receptor and cardiovascular disease: a potential risk for the abuse of supplements containing selective androgen receptor modulators. Nutrients. 2023;15(15):3330. doi:10.3390/nu15153330
- Gu L, Yu Q, Shen Y, Wang Y, Xu Q, Zhang H. The role of monoaminergic neurons in modulating respiration during sleep and the connection with SUDEP. Biomed Pharmacother. 2022;150:112983. doi:10.1016/j.biopha.2022.112983
- Carbajal-García A, Reyes-García J, Montaño LM, Llanos P. Androgen effects on the adrenergic system of the vascular, airway, and cardiac myocytes and their relevance in pathological processes. Int J Endocrinol. 2020;2020:1–25. doi:10.1155/2020/8849641
- Albano GD, Amico F, Cocimano G, et al. Adverse effects of anabolic-androgenic steroids: a literature review. In: Healthcare. Vol. 9. MDPI; 2021:97.
- Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin–proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17(7):1807–1819. doi:10.1681/ASN.2006010083
- Kraemer WJ, Duncan ND, Volek JS. Resistance training and elite athletes: adaptations and program considerations. J Orthop Sports Phys Ther. 1998;28(2):110–119. doi:10.2519/jospt.1998.28.2.110
- Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–E1181. doi:10.1152/ajpendo.2001.281.6.E1172
- Vorona E, Nieschlag E. Abuse of Anabolic Androgenic Steroids (AAS) for Doping. In: Andrology: Male Reproductive Health and Dysfunction. Springer; 2023:585–597.
- Yu JG, Bonnerud P, Eriksson A, Stål PS, Tegner Y, Malm C. Effects of long term supplementation of anabolic androgen steroids on human skeletal muscle. PLoS One. 2014;9:9.
- Quattrocelli M, Zelikovich AS, Salamone IM, Fischer JA, McNally EM. Mechanisms and clinical applications of glucocorticoid steroids in muscular dystrophy. J Neuromuscul Dis. 2021;8(1):39–52. doi:10.3233/JND-200556
- Kolliari-Turner A. Anabolic Androgenic Steroid Doping in Weightlifting and the Summer Olympic Games Alongside Their Impact on Muscle Memory and the Human Transcriptome. University of Brighton; 2023.
- Zheng E, Sandhu N, Navarro V. Drug-induced liver injury secondary to herbal and dietary supplements. Clin Liver Dis. 2020;24(1):141–155. doi:10.1016/j.cld.2019.09.009
- Wu S, Daston G, Rose J, et al. Identifying chemicals based on receptor binding/bioactivation/mechanistic explanation associated with potential to elicit hepatotoxicity and to support structure activity relationship-based read-across. Curr Res Toxicol. 2023;5:100108. doi:10.1016/j.crtox.2023.100108
- Kafrouni MI, Anders RA, Verma S. Hepatotoxicity associated with dietary supplements containing anabolic steroids. Clin Gastroenterol Hepatol. 2007;5(7):809–812. doi:10.1016/j.cgh.2007.02.036
- Beyoğlu D, Idle JR. Metabolomic and lipidomic biomarkers for premalignant liver disease diagnosis and therapy. Metabolites. 2020;10(2):50. doi:10.3390/metabo10020050
- Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328(3):688–696. doi:10.1016/j.bbrc.2004.11.097
- Sun J, Tan Y, Su J, et al. Role and molecular mechanism of ghrelin in degenerative musculoskeletal disorders. J Cell Mol Med. 2023;27(23):3681–3691. doi:10.1111/jcmm.17944
- Weber AE, Gallo MC, Bolia IK, Cleary EJ, Schroeder TE, Rick Hatch GF. Anabolic androgenic steroids in orthopaedic surgery: current concepts and clinical applications. JAAOS Glob Res Rev. 2022;6(1):e21.00156. doi:10.5435/JAAOSGlobal-D-21-00156
- Brodziak-Dopierała B. Lead–factors affecting its content in bone tissue. Pomeranian J Life Sci. 2020;66(4):23–29. doi:10.21164/pomjlifesci.745
- Shipov A, Zaslansky P, Riesemeier H, et al. The influence of estrogen deficiency on the structural and mechanical properties of rat cortical bone. PeerJ. 2021;9:e10213.
- Vieira RP, Franca RF, Damaceno-Rodrigues NR, et al. Dose-dependent hepatic response to subchronic administration of nandrolone decanoate. Med Sci Sports Exerc. 2008;40(5):842. doi:10.1249/MSS.0b013e3181666f1c
- Patanè FG, Liberto A, Maria Maglitto AN, et al. Nandrolone decanoate: use, abuse and side effects. Medicina. 2020;56(11):606. doi:10.3390/medicina56110606
- Hutter AM, Kayhoe DE. Adrenal cortical carcinoma: results of treatment with o, p’DDD in 138 patients. Am J Med. 1966;41(4):581–592. doi:10.1016/0002-9343(66)90220-8
- Ghiacci G, Lumetti S, Manfredi E, Mori D, Macaluso GM, Sala R. Stanozolol promotes osteogenic gene expression and apposition of bone mineral in vitro. J Appl Oral Sci. 2018;4:27.
- Seynnes OR, Kamandulis S, Kairaitis R, et al. Effect of androgenic-anabolic steroids and heavy strength training on patellar tendon morphological and mechanical properties. J Appl Physiol. 2013;115(1):84–89. doi:10.1152/japplphysiol.01417.2012
- Pálinkás Z, Békési D, Utczás M. Quantitation of ecdysterone and targeted analysis of WADA-prohibited anabolic androgen steroids, hormones, and metabolic modulators in ecdysterone-containing dietary supplements. Separations. 2023;10(4):242. doi:10.3390/separations10040242
- Balgoma D, Zelleroth S, Grönbladh A, Hallberg M, Pettersson C, Hedeland M. Anabolic androgenic steroids exert a selective remodeling of the plasma lipidome that mirrors the decrease of the de novo lipogenesis in the liver. Metabolomics. 2020;16(1):1–13. doi:10.1007/s11306-019-1632-0
- Ooi EMM. Regulation of Lipoprotein Transport in the Metabolic Syndrome: Impact of Statin Therapy. University of Western Australia; 2007.
- Kennedy MC, Lawrence C. Anabolic steroid abuse and cardiac death. Med J Aust. 1993;158(5):346–348. doi:10.5694/j.1326-5377.1993.tb121797.x
- Elitok A, Öz F, Panc C, et al. Acute effects of Red Bull energy drink on ventricular repolarization in healthy young volunteers: a prospective study. Anatol J Cardiol. 2015;15(11):919. doi:10.5152/akd.2015.5791
- Fadah K, Gopi G, Lingireddy A, Blumer V, Dewald T, Mentz RJ. Anabolic androgenic steroids and cardiomyopathy: an update. Front Cardiovasc Med. 2023;2023:10.
- Torrisi M, Pennisi G, Russo I, et al. Sudden cardiac death in anabolic-androgenic steroid users: a literature review. Medicina. 2020;56(11):587. doi:10.3390/medicina56110587
- Martins T, Vitorino R, Moreira-Gonçalves D, Amado F, Duarte JA, Ferreira R. Recent insights on the molecular mechanisms and therapeutic approaches for cardiac cachexia. Clin Biochem. 2014;47(1–2):8–15. doi:10.1016/j.clinbiochem.2013.10.025
- Bachmann KN, Huang S, Lee H, et al. Effect of testosterone on natriuretic peptide levels. J Am Coll Cardiol. 2019;73(11):1288–1296. doi:10.1016/j.jacc.2018.12.062
- Rasmussen JJ, Schou M, Madsen PL, et al. Increased blood pressure and aortic stiffness among abusers of anabolic androgenic steroids: potential effect of suppressed natriuretic peptides in plasma? J Hypertens. 2018;36(2):277–285. doi:10.1097/HJH.0000000000001546
- Vanberg P, Atar D. Androgenic anabolic steroid abuse and the cardiovascular system. Doping Sport. 2010;2:411–457.
- Melchert RB, Belcher SM, Kennedy RH. Cardiovascular effects of steroidal agents. In: Cardiovascular Toxicology. CRC Press; 2008:379–440.
- Carden DL, Granger DN. Pathophysiology of ischaemia–reperfusion injury. J Pathol. 2000;190(3):255–266. doi:10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6
- Novák J, Bienertová-Vašků J, Kára T, Novák M. MicroRNAs involved in the lipid metabolism and their possible implications for atherosclerosis development and treatment. Mediators Inflamm. 2014;2014:1–14. doi:10.1155/2014/275867
- Mullen C, Whalley BJ, Schifano F, Baker JS. Anabolic androgenic steroid abuse in the United Kingdom: an update. Br J Pharmacol. 2020;177(10):2180–2198. doi:10.1111/bph.14995
- Turillazzi E, Perilli G, Di Paolo M, Neri M, Riezzo I, Fineschi V. Side effects of AAS abuse: an overview. Mini Rev Med Chem. 2011;11(5):374–389. doi:10.2174/138955711795445925
- Lee VWS, Harris DCH. Adriamycin nephropathy: a model of focal segmental glomerulosclerosis. Nephrology. 2011;16(1):30–38. doi:10.1111/j.1440-1797.2010.01383.x
- Banfi G, Colombini A, Lombardi G, Lubkowska A. Metabolic markers in sports medicine. Adv Clin Chem. 2012;56(Suppl 3):1–54.
- Granados J, Gillum TL, Christmas KM, Kuennen MR. Prohormone supplement 3β-hydroxy-5α-androst-1-en-17-one enhances resistance training gains but impairs user health. J Appl Physiol. 2014;116(5):560–569. doi:10.1152/japplphysiol.00616.2013
- Chowdhury P, Mahanta R. Effect of administration of nandrolone decanoate upon aldosterone concentration and serum na+/k+ levels in albino mice. Cardiovasc Hematol Agents Med Chem. 2016;14(3):160–166.
- Buttemer WA, Astheimer LB. Testosterone does not affect basal metabolic rate or blood parasite load in captive male white‐plumed honeyeaters Lichenostomus penicillatus. J Avian Biol. 2000;31(4):479–488. doi:10.1034/j.1600-048X.2000.310407.x
- Skogastierna C, Hotzen M, Rane A, Ekström L. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur J Prev Cardiol. 2014;21(8):1049–1054. doi:10.1177/2047487313481755
- Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. Anabolic steroid–induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014;101(5):1271–1279. doi:doi:10.1016/j.fertnstert.2014.02.002
- Wood RI, Serpa RO. Anabolic-androgenic steroid abuse and cognitive impairment: testosterone IMPAIRS biconditional task performance in male rats. Behav Brain Res. 2020;379:112339. doi:10.1016/j.bbr.2019.112339
- Wood RI, Wallin-Miller KG. Neurobiology of Anabolic-Androgenic Steroid Abuse. Oxford Research Encyclopedia of Neuroscience; 2019.
- Donovan A, Wood RI. Effort-based decision making in response to high-dose androgens: role of dopamine receptors. Behav Pharmacol. 2022;33(7):435–441. doi:10.1097/FBP.0000000000000687
- Mhillaj E, Morgese MG, Tucci P, Bove M, Schiavone S, Trabace L. Effects of anabolic-androgens on brain reward function. Front Neurosci. 2015;9. doi:10.3389/fnins.2015.00295
- Wood RI. Anabolic–androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol. 2008;29(4):490–506. doi:doi:10.1016/j.yfrne.2007.12.002
- Wood RI. Reinforcing aspects of androgens. Physiol Behav. 2004;83(2):279–289. doi:doi:10.1016/j.physbeh.2004.08.012
- Hauger LE, Westlye LT, Fjell AM, Walhovd KB, Bjørnebekk A. Structural brain characteristics of anabolic-androgenic steroid dependence in men . Addiction. 2019;114(8):1405–1415. doi:10.1111/add.14629
- Kanayama G, Cohane GH, Weiss RD, Pope HG. Past anabolic-androgenic steroid use among men admitted for substance abuse treatment: an underrecognized problem? J Clin Psychiatry. 2003;64(2):156–160. doi:10.4088/JCP.v64n0208
- Chegeni R, Notelaers G, Pallesen S, Sagoe D. Aggression and psychological distress in male and female anabolic-androgenic steroid users: a multigroup latent class analysis. Front Psychiatry. 2021;12:629428. doi:10.3389/fpsyt.2021.629428
- Nelson BS, Hildebrandt T, Wallisch P. Anabolic–androgenic steroid use is associated with psychopathy, risk-taking, anger, and physical problems. Sci Rep. 2022;12(1):9133. doi:10.1038/s41598-022-13048-w
- Hauger LE, Westlye LT, Fjell AM, Walhovd KB, Bjørnebekk A. Structural brain characteristics of anabolic–androgenic steroid dependence in men. Addiction. 2019;114(8):1405–1415. doi:10.1111/add.14629
- Hauger LE, Havnes IA, Jørstad ML, Bjørnebekk A. Anabolic androgenic steroids, antisocial personality traits, aggression and violence. Drug Alcohol Depend. 2021;221:108604. doi:10.1016/j.drugalcdep.2021.108604
- Griffiths S, Jacka B, Degenhardt L, Murray SB, Larance B. Physical appearance concerns are uniquely associated with the severity of steroid dependence and depression in anabolic–androgenic steroid users. Drug Alcohol Rev. 2018;37(5):664–670. doi:10.1111/dar.12688
- DiFonzo N, Bordia P. Reproduced with permission of the copyright owner. Further reproduction prohibited without. J Allergy Clin Immunol. 1998;130(2):556.
- Amaral JMX, Kimergård A, Deluca P. Prevalence of anabolic steroid users seeking support from physicians: a systematic review and meta-analysis. BMJ Open. 2022;12(7):e056445. doi:10.1136/bmjopen-2021-056445
