Abstract
Background
The purpose of this study was to draw conclusions from patient-reported experiences in two national surveys from Scandinavia with the intention of comparing treatment strategies and increasing our knowledge of factors that affect the experiences of patients with Parkinson’s disease (PD).
Methods
A total of 2000 individuals in Sweden and 1300 in Norway were invited to complete postal surveys covering PD-related issues. Patient experiences of diagnostic procedures, symptom control, and follow-up in PD and the effects on symptom-related quality of life were collected. Pharmaceutical prescription data on anti-PD drugs and administrative data were collected from national registries.
Results
The surveys were completed by 1553 (78%) of the Swedish cohort and 1244 (96%) of the Norwegian cohort. Only small differences were seen in disease duration and age distribution. Statistically as well as clinically significant differences in symptom control, diagnostic, and follow-up procedures, as well as in pharmacological treatment and impact on quality of life, were found between the national cohorts independent of disease duration.
Conclusion
Information from separate national surveys has the potential to increase our knowledge of patient experiences in PD and can be used to compare, evaluate, educate, and guide health care staff and administrators in optimizing health care for patients with the disease.
Introduction
Parkinson’s disease (PD) is a geographically widespread, progressive, chronic neurodegenerative disorder resulting in motor and nonmotor symptoms.Citation1 PD is not uncommon, and has a prevalence ranging from 0.3% to 1%–2% among persons aged 65 years and older.Citation2 In two Scandinavian studies from 2008 and 2009, the estimated annual incidence in Norway was 12.6 per 100,000 inhabitants (95% confidence interval 11.1–14.2), while the age-adjusted annual incidence rate in a Swedish study was estimated to be 21.5 per 100,000 (95% confidence interval 17.4–25.6).Citation3,Citation4 The prevalence of PD in the Scandinavian countries in the northern part of Europe is estimated to be 0.175% of the total population.Citation5 At the time of the surveys used in this study (2005 and 2006), Norway had a population of 4.7 million inhabitants,Citation6 with an estimate of just over 8000 patients with PD. The corresponding figures for Sweden were 9.1 million and just less than 16,000 estimated patients with PD.
The pharmacological approach to treating PD has advanced in recent decades. From levodopa as the only effective pharmacological treatment of the disease in the early 1960s,Citation7 other compounds such as enzyme inhibitors and dopamine agonists have evolved. Pharmacoepidemiological data also show that the traditional patterns of prescribing differ between countries.Citation8
National health care systems in different countries face different challenges, largely due to their varying economic and political conditions, but also because of strict demographic and geographical factors. A predicted increase in life expectancyCitation9 will most probably result in a growing number of elderly patients with PD and accompanying health care costs.Citation10 As the prevalence of chronic disorders such as PD rises, the burden on relatives of sufferers also increases.Citation11,Citation12 Therefore, providers of managed care and health care planners need a broad understanding of PD and its management, as well as good insights into prescribing habits and cost-benefit analyses.Citation13
All these facts stress the need for a systematic evaluation of the therapies that are implemented today. However, our knowledge about how symptom control is influenced by additional, often costly, pharmacological prescriptions is insufficient. Patient adherence with the medication regimen is also critical to treatment outcome.Citation14
Further, we also lack an adequate understanding of how different organizational approaches influence patient experiences with treatment of their PD over time. The lifelong burden of a disease is influenced by many factors, only a few of which are possible to capture on general levels. In a new era of rapid and often digitalized communication, the opportunities to collect data from large anonymous cohorts of patients have increased. Therefore, we decided to compile data from two national surveys in an attempt to broaden our understanding of this field and discover new perspectives.
The primary aim of this study was to consider the general usefulness of national surveys by comparing patients’ self-reported answers from two examples. The secondary aim was to compare the effects of differences in diagnostic procedures and anti-Parkinson pharmaceutical prescribing patterns on self-reported patient experiences of symptom control and symptom-related quality of life in Sweden and Norway.
Patients and methods
A total of 2000 Swedish patients with PD were asked to answer a questionnaire attached to the Journal of the Swedish Parkinson’s Disease Association. In the same way, 1300 Norwegian patients with PD were asked to answer a corresponding questionnaire in the Journal of the Norwegian Parkinson’s Disease Association. Patients were randomly chosen and account for one third of subscribers to the journal. The figures also correspond to 9.8% and 16.1% of the estimated PD populations of Sweden and Norway, respectively.
Both surveys contained questions on demographics as well as individual disease data with regard to diagnosis, treatment, and follow-up. The impact on quality of life due to symptoms of PD was assessed using a global measure on a five-point Likert scale ranging from little impact to high impact (“Are the symptoms from your Parkinson disease affecting your quality of life in a negative way?”).Citation15
The pharmacological profile of anti-PD medication in Sweden for the actual year was collected from the national pharmaceutical database.Citation16 Data concerning the corresponding pharmacological profile in Norway were extracted from the prescription database at the Norwegian Institute of Public Health.Citation17
Before commencing the study, approval was secured from the ethics committee at the University of Linköping, Sweden (2010/62–31). In a covering letter to respondents, it was stated that all results would be processed anonymously and presented in a nonidentifiable way. No identification data were used in the database. Because most data were in ordinal scales, nonparametric statistics were used. The Chi-square test, Mantel-Haenszel Chi-square, and Fisher’s Exact test were used as appropriate.
Results
Demographic data
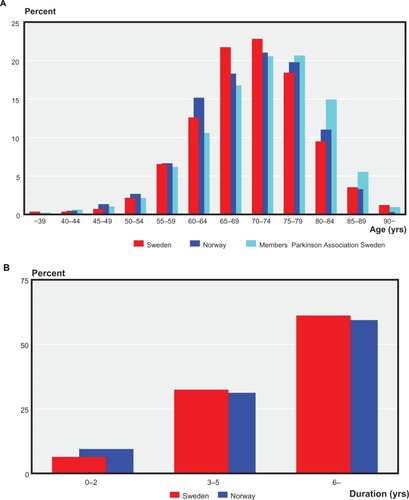
show the age distribution of members of the Swedish and Norwegian Parkinson’s Disease Associations. Median age was 71 (25th/75th percentiles) (67/77) years of the respondents. The oldest members of the association (>80 years) were less likely to participate in the survey. Median (25th/75th percentile) age at onset of disease was 61 (55/69) years in the Swedish cohort and 62 (54/68) years in the Norwegian cohort. Corresponding values for duration of disease in the Swedish and Norwegian cohorts were eight (5/13) and eight (4/12) years.
Figure 1 (A) Age distribution of respondents in Sweden and Norway versus the age distribution of all members of the Swedish Parkinson’s Disease Association (data from the Norwegian Movement Disorders Association not available). (B) Duration of Parkinson’s disease among respondents in the Swedish and Norwegian cohorts.
Diagnosis and follow-up
In both countries, the majority of patients were diagnosed by neurologists. Of the remaining patients, significantly more were diagnosed by geriatricians in the Swedish cohort (4%) than in the Norwegian cohort (0.6%). General practitioners also diagnosed more patients with PD in Sweden than in Norway ().
Table 1 Specialists diagnosing Parkinson’s disease in Sweden and Norway
In Norway, follow-up of patients with PD was more often performed by general practitioners (17% in the Norwegian cohort and 7% in the Swedish cohort). The opposite was found for geriatricians (0.2% in the Norwegian cohort and 8% in the Swedish cohort), but according to the respondents, the vast majority of patients in both countries were followed up by neurologists (91% in the Norwegian cohort and 80% in the Swedish cohort). In addition, more than one specialist was often in contact with a patient, probably to treat concomitant illnesses. Contact between other caregivers and patients also differed between respondents from the different countries. Significantly more patients in the Swedish cohort (P < 0.0001) made their own contacts and appointments with caregivers than in the Norwegian cohort.
Nurses had a more active role in the Swedish cohort, with 3% (51/1501) of respondents reporting medication adjustments through contact with a PD nurse. Frequencies of contacts between caregiver and patient and initiatives to the contacts are visualized in . This was significantly different (P = 0.002) from the Norwegian cohort, where only 1.5% (19/1275) reported medication adjustments without contact with their doctor. Neurologists, geriatricians, general practitioners, and nurses cooperated more often in dose adjustments in the Swedish cohort, 4% (n = 54) versus 1% (n = 13) in the Norwegian cohort. However, in the Norwegian cohort, cooperation between neurologists and general practitioners was common, with 12% (n = 159) of respondents reporting this.
Table 2 Contacts patterns between patients and doctors/PD nurses in Sweden and Norway
Pharmacological therapy
The market shares of anti-PD drugs in Sweden and Norway in defined daily doses for the years surveyed are shown in . Use of different groups of anti-PD drugs among respondents to the survey is shown in . Levodopa-benserazide or levodopa-carbidopa were most commonly used as single therapy in both countries (27% in the Swedish cohort and 20% in the Norwegian cohort). Levodopa-carbidopa represented a higher proportion in the Norwegian cohort, than in the Swedish, and 38% of responders in Sweden reported using selegiline, a monoamine oxi-dase B inhibitor. This contrasts strongly with the Swedish cohort, where only 11% reported using selegiline. Even when taken in combination with other antiparkinsonian medications, taking levodopa-benserazide or levodopa-carbidopa was slightly more common in the Swedish cohort. Total use was 72% in the Swedish cohort compared with 71% in the Norwegian cohort.
Figure 2 Market shares of antiparkinsonian drugs in Sweden and Norway in defined daily doses for 2008. *Includes carbidopa-levodopa delivered via intraintestinal pump, rasagiline, amantadine, and others.
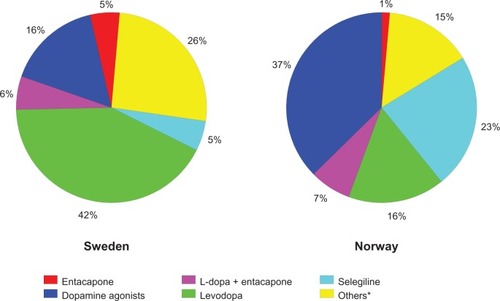
Figure 3 Antiparkinsonian medication among respondents according to pharmacological group. Note that one patient might have a combination of several drugs.
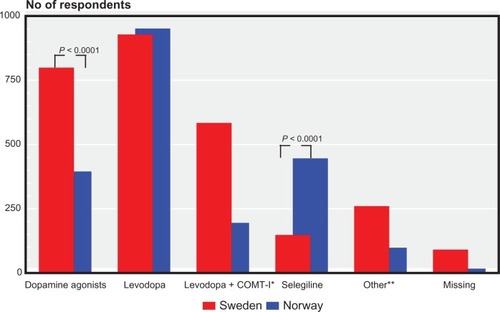
More than one drug was used by 68% of the Swedish respondents and 72% of the Norwegian respondents. The most common combination therapy among Swedish respondents was levodopa-benserazide or levodopa-carbidopa and pramipexole, which were used by 14% of respondents. In the Norwegian cohort, the corresponding proportion was 7%, and combination therapies of selegiline and levodopa-carbidopa or levodopa-benserazide were most common (reported by 10% of respondents). This combination therapy was only reported by 2.5% of respondents in the Swedish cohort. These differences were highly significant (P < 0.0001, Fisher’s Exact test).
Motor complications, disease duration, and satisfaction with treatment
Wearing off more than once a day was experienced by 38% in the Norwegian cohort compared with 18% in the Swedish cohort. No wearing off or less than one episode per day was reported by 243 patients (19%) in the Norwegian cohort and 453 (31%) in the Swedish cohort. Once again, these differences were significant (P < 0.0001, Fisher’s Exact test).
Despite the reported differences in the frequency of motor complications, significantly more patients in the Norwegian cohort (63%) were satisfied with their medication than those in the Swedish cohort (52%, P < 0.0001, Fisher’s Exact test). Data were missing for 7% of the Swedish cohort and for 4% of the Norwegian cohort.
About one third of respondents reporting satisfaction with medication were prescribed one single drug (31% in the Swedish cohort and 36% in the Norwegian cohort). However, most patients reporting satisfaction with their pharmacological treatment (38% in the Swedish cohort and 46% in the Norwegian cohort) were prescribed a combination with two antiparkinsonian drugs. Corresponding answers for respondents prescribed more than four different drugs were 13% for the Swedish cohort and 2% for the Norwegian cohort.
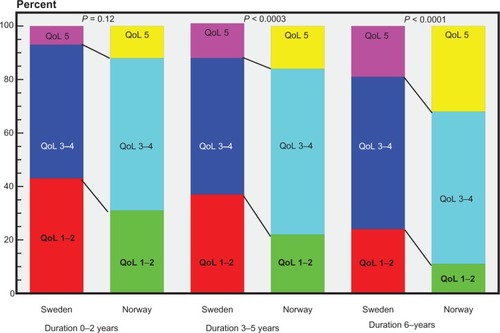
When comparing satisfaction with medication (“Are you satisfied with your present Parkinson medication?”), experiencing symptoms of wearing off, and duration of disease in the Norwegian cohort, the following was found: of those with a disease duration less than two years, 61% were satisfied with their medication (P = 0.02, Fisher’s Exact test); of those with a disease duration of 2–6 years, 58% were satisfied (P < 0.0001, Fisher’s Exact test); and of those with a duration of more than six years, 50% were satisfied. There was also a statistically significant association between a long disease duration and low satisfaction with medication (P < 0.0001, Cochran-Mantel-Haenszel test). The degree of satisfaction with medication changes during disease progression. Wearing-off as a motor complication increases with duration of disease. The differences in satisfaction with medication among patients with and without wearing-off is illustrated in .
Table 3 Relation between disease duration, motor complications (wearing-off) and satisfaction with medication among PD patients’ in Sweden and Norway
For the Swedish cohort, the question was formulated as “How satisfied are you with your levodopa medication?” Among those experiencing symptoms of wearing off with a disease duration of less than two years, 57% were satisfied with their medication (P = 0.04, Fisher’s Exact test), among those with a disease duration of 2–6 years, 33% were satisfied (P < 0.0001, Fisher’s Exact test), and among those with a disease duration of more than six years, 37% were satisfied. There was also a statistically significant association between a long disease duration and low satisfaction with medication (P < 0.0001, Cochran-Mantel-Haenszel test).
In the Norwegian respondents, 36% (332/931) reported selegiline as one of their prescribed drugs. As shown above, this was the most common drug combined with one other compound. When combining the Norwegian and Swedish cohort data and analyzing respondents with a disease duration of 2–6 years, selegiline in combination with one other drug was used by 63 responders. Wearing off more than once daily was experienced by 52% (n = 33) in this group, and a significantly larger proportion of responders reported dissatisfaction with their medication (80% versus 44%, P = 0.02). For a disease duration of more than six years, 116 responders reported combination therapy of selegiline and one other compound. Of the patients in this longer duration group, 69% (80/116) reported wearing off. Significantly more patients (85%, 34/40) were dissatisfied with their medication compared with those who were satisfied in this longer disease duration group (P = 0.007). depicts the answers to the quality of life question of “How do the symptoms affect your quality of life” divided by duration of disease/country.
Discussion
In this study, we found both clinically and statistically significant differences between two neighboring national cohorts (Sweden and Norway) regarding patient assessment of their symptom-related quality of life; patients with PD in the Swedish cohort rated their quality of life higher than those in the Norwegian cohort. These differences were significant in patients with a disease duration longer than two years. This finding could simply be due to demographic differences in the two PD cohorts rather than a true difference. However, only small differences were seen in terms of age and disease duration between the two cohorts. In addition, PD is a well defined clinical diagnosisCitation18 and there is no reason to believe that it should differ between the two countries in terms of prevalence, incidence, or spread of disease severity. Moreover, Sweden and Norway are two countries with similar socioeconomic profiles.Citation19
When health-related quality of life in patients with PD was reviewed by Dowding et al,Citation20 the most effective drug treatment was mentioned as one of the specific areas that needed to be addressed further. In our study, comparison between the two countries revealed substantial differences in pharmaceutical profiles, not only in terms of type of PD medication, follow-up procedures, dose adjustment, and patient satisfaction, but also in symptom-related quality of life in the first two years after diagnosis. This study also highlights other differences between the two neighboring countries that might have an impact on the quality of life experienced.
Diagnostics and follow-up
The diagnostic procedure in terms of the professional categories involved differed in some ways, as did the follow-up procedures. Neurologists most often diagnosed PD in both countries. However, in the Swedish cohort, geriatricians and general practitioners played a more dominant role in the diagnostic process than in the Norwegian cohort. One in five patients in the Swedish cohort was diagnosed by these specialists compared with one in ten in the Norwegian cohort. These figures should be viewed in the light of the fact that about 150 specialists in geriatrics, 3000 specialists in general medicine, and 450 specialists in neurology were working in Norway at the time of the study, corresponding to 0.8%, 16%, and 2.5%, respectively, of the total number of specialists.Citation21 This contrasts with the approximately 500 geriatricians, 6000 specialists in general medicine, and 300 neurologists active in Sweden, corresponding to 2%, 25%, and 1%, respectively, of all specialists.Citation22 Hence, the Swedish population of about nine million inhabitants has a greater proportion of specialists in geriatrics available but less access to neurologists compared with Norway and its 4.7 million inhabitants.
More patients in the Swedish cohort made their own appointments with their doctor and had independent contact with their PD nurse than in the Norwegian cohort. This indicates that the former cohort includes more empowered patients and caregivers who actively try to influence and improve their situation and quality of life. To a small extent, Swedish nurses are also able to adjust pharmacological therapy, which was not the case in Norway. These findings could also be related to the greater emphasis on team education, self-care, and lifestyle programs stimulated by the Swedish health care authorities during recent decades. The implications of the differences in the diagnostic and follow-up procedures, with a clearer focus on the Swedish respondents’ own initiatives and self-responsibility, might contribute to a feeling of safety and empowerment. This feeling, combined with access to health professionals, is probably important for their perception of quality of life.Citation20
Pharmacological treatments and motor complications
Anti-PD medication has been shown to improve the severity of motor symptoms and motor complications in advanced PD. From a patient’s point of view, there is evidence that this can be translated into improved quality of life.Citation23,Citation24 However, quality of life is also influenced by a variety of nonmotor symptoms, such as depression and sleep disturbance, as well as by social interaction and feelings of safety.Citation25
Not surprisingly, comparing patients with short (less than or up to two years), medium to long (2–6 years), and long (more than six years) duration of PD reveals significant differences in the proportion of wearing off episodes. When the rates of wearing off symptoms between the two cohorts were compared, only limited differences that could explain the differences in satisfaction were found.
The national pharmacological databases of each countryCitation17,Citation26 showed significant differences in the pharmacological approaches to PD treatment between the cohorts as well as between the countries as a whole. For example, significantly more use of selegiline was made in the Norwegian cohort. Selegiline, as a compound for reducing off-time, has been somewhat controversial.Citation27,Citation28
In this study, we had a special focus on combination therapy with selegiline because this was the most common therapy in the Norwegian cohort. Combining data from the Swedish and Norwegian cohorts for this aspect of treatment showed that significantly more responders with a disease duration longer than two years reported dissatisfaction with their medication, when wearing off was experienced more than once daily, compared with the group that was still satisfied despite experiencing wearing off episodes. When initiating polypharmacy in PD, which is very common even after a short disease duration, consideration must be given to its purpose in terms of patient experience. The frequent use of selegiline in the Norwegian cohort might be due to results of Norwegian research conducted on selegiline and published by Larsen et al in the late 1990s.Citation29 This introduced another hypothesis, ie, that selegiline might be able to modify the progression of early PD.
For a number of newer anti-PD agents, there is evidence that improvement of objective measures often translates into improvements in quality of life. For instance, entacapone resulted in improvement of quality of life in non-fluctuating patients, but not significantly so in those with motor fluctuations.Citation30 Similarly, rasagiline improved quality of life as monotherapy in early PD,Citation31 but not significantly in its later stages. Taken together, these findings should be forwarded to politicians and health care staff through seminars, lectures, Internet, and personal contacts in order to increase awareness of these issues.
Limitations
Only members of the patient associations of the respective countries were eligible for the questionnaire because it was distributed together with the journals of the association. Patients who join their association may well be more active than those who do not. Further, the responders to these surveys possibly represent a more active cohort of patients and relatives, which may contribute to bias in the study. We do not suggest that our findings are valid for the whole PD population of the respective countries. Because no national PD registries are available, our method was a way to catch a high number of patients without contravening the laws concerning personal data security or individual integrity. As a limitation it is important to mediate that there was no general reminder to the questionnaire. Despite this the response rate was high. In the Swedish survey, a technical error resulted in no data being available on gender. Neither were corresponding data for the national association members available in the Norwegian survey. However, there is no reason to believe that there should be any difference in gender composition or age distribution of association members between the two countries.
Conclusion
This study demonstrates differences between countries in diagnosing, follow-up, treatment regimens, experience of symptoms, and quality of life in patients with PD. Information of this kind can be used to compare, evaluate, educate, and guide health care staff and administrators to optimize health care for patients with PD. The development and implementation of nationally-based quality registers has been a feature of the health care systems of Sweden and Norway for more than a decade, and they have now been launched in Sweden through the Swedish Movement Disorder Association. Future studies should collect longitudinal data from the same patients at appropriate time intervals, given the well known risk of an increasing dropout rate. This might be easier and more economically performed by web-based procedures, but will probably encounter difficulties in obtaining answers from older patients because of their inexperience with the Internet. Tools for further refining and promoting these processes will probably be a core issue in coming decades.
Acknowledgments
We are grateful for the financial support of the Parkinson Fund and the committees of the Swedish and Norwegian patient associations for Parkinson’s disease for permission to evaluate the questionnaires, and to Futurum (Academy for Healthcare, Jonkoping County Hospital, Sweden).
Disclosure
The authors report no conflicts of interest in this work.
References
- MaetzlerWLiepeltIBergDProgression of Parkinson’s disease in the clinical phase: potential markersLancet Neurol20098121158117119909914
- von CampenhausenSBornscheinBWickRPrevalence and incidence of Parkinson’s disease in EuropeEur Neuropsychopharmacol200515447349015963700
- AlvesGMullerBHerlofsonKIncidence of Parkinson’s disease in Norway: the Norwegian ParkWest studyJ Neurol Neurosurg Psychiatry200980885185719246476
- LinderJStenlundHForsgrenLIncidence of Parkinson’s disease and parkinsonism in northern Sweden: a population-based studyMov Disord201025334134820108376
- FallPAAxelsonOFredrikssonMAge-standardized incidence and prevalence of Parkinson’s disease in a Swedish communityJ Clin Epidemiol19964966376418656224
- Nordic Council of MinistersNordic Statistical YearbookCopenhagen, DenmarkNordic Council of Ministers2006
- AndenNECarlssonAKerstellJOral L-dopa treatment of parkinsonismActa Med Scand197018742472554911257
- RosaMMFerreiraJJCoelhoMFreireRSampaioCPrescribing patterns of antiparkinsonian agents in EuropeMov Disord20102581053106020222132
- CohenJEHuman population: the next half centuryScience200330256481172117514615528
- WinterYBalzer-GeldsetzerMvon CampenhausenSTrends in resource utilization for Parkinson’s disease in GermanyJ Neurol Sci20102941–2182220493500
- LokkJCaregiver strain in Parkinson’s disease and the impact of disease durationEur J Phys Rehabil Med2008441394518385627
- BaandersANHeijmansMJThe impact of chronic diseases: the partner’s perspectiveFam Community Health200730430531717873637
- VossiusCGjerstadMBaasHLarsenJPDrug costs for patients with Parkinson’s disease in two different European countriesActa Neurol Scand2006113422823216542161
- BainbridgeJLRuscinJMChallenges of treatment adherence in older patients with Parkinson’s diseaseDrugs Aging200926214515519220071
- SvenssonEConstruction of a single global scale for multi-item assessments of the same variableStatist Med2001203831384616
- SLD, Swedish pharmaceutical data, a part of IMS Health Available from: http://www.sld.fiAccessed January, 2011
- FolkhelseinstitutetRapport 2008: Reseptregisteret 2004–2007/Norwegian Prescription Database 2004–2007 Available from: http://www.norpd.no/Accessed January, 2011
- CalneDBSnowBJLeeCCriteria for diagnosing Parkinson’s diseaseAnn Neurol199232SupplS125S1271510370
- RoosEKivelaKLahelmaE[Small changes in health differences in the Nordic countries during the 1980s and 1990s.]Lakartidningen2001982125762577 Swedish11433993
- DowdingCHShentonCLSalekSSA review of the health-related quality of life and economic impact of Parkinson’s diseaseDrugs Aging200623969372117020395
- Norwegian Medical Association2011 Available from: http://www.legeforeningen.no/id/171362Accessed January, 2011 [Norwegian]
- Access to specialist doctors in Sweden. Welfare2007 Available from: http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/18153/2010-2011-1.pdfAccessed January, 2011 [Swedish]
- PechevisMClarkeCEViereggePEffects of dyskinesias in Parkinson’s disease on quality of life and health-related costs: a prospective European studyEur J Neurol2005121295696316324089
- DodelRCEggertKMSingerMSEichhornTEPogarellOOertelWHCosts of drug treatment in Parkinson’s diseaseMov Disord19981322492549539337
- PandyaMKubuCSGirouxMLParkinson disease: not just a movement disorderCleve Clin J Med2008751285686419088004
- IMS Health Available from: http://www.imshealth.com/portal/site/imshealthAccessed January, 2011
- WatersCHSethiKDHauserRAMolhoEBertoniJMZydis selegiline reduces off time in Parkinson’s disease patients with motor fluctuations: a 3-month, randomized, placebo-controlled studyMov Disord200419442643215077240
- OndoWGSethiKDKricorianGSelegiline orally disintegrating tablets in patients with Parkinson disease and “wearing off” symptomsClin Neuropharmacol200730529530017909308
- LarsenJPBoasJErdalJEDoes selegiline modify the progression of early Parkinson’s disease? Results from a five-year study. The Norwegian-Danish Study GroupEur J Neurol19996553954710457386
- GallagherDASchragAImpact of newer pharmacological treatments on quality of life in patients with Parkinson’s diseaseCNS Drugs200822756358618547126
- BiglanKMSchwidSEberlySRasagiline improves quality of life in patients with early Parkinson’s diseaseMov Disord200621561662316450340