Abstract
Purpose
To analyze leptin levels in placental tissue and premature infants undergoing phototherapy and to evaluate the potential for prescribing passive exercise after phototherapy in this population.
Patients and Methods
This analytical, longitudinal, prospective cohort study included 108 parturients and their respective premature infants. Variables examined included weight, gestational age, body mass index, sex, serum leptin levels in placental tissue, serum bilirubin levels, and reticulocyte count.
Results
When comparing each group to a leptin threshold, statistically significant differences were observed at all evaluated time points for placental leptin levels (p < 0.001). Additionally, reticulocyte count decreased in relation to rebound time (p < 0.004). No correlations were found between leptin/bilirubin levels, leptin/reticulocytes, onset of nutrition, and BMI/leptin levels.
Conclusion
The findings regarding leptin levels suggest that prescribing passive exercises to premature infants undergoing phototherapy may be feasible because this intervention did not increase leptin levels.
Introduction
According to the World Health Organization (WHO), the prevalence of preterm births is 5–18%, with a higher incidence in low-income populations.Citation1 Prematurity is a considerable perinatal challenge associated with morbidity and mortality in early life as well as clinical and economic implications.Citation2–4 In Brazil, where nearly three million births occur annually, approximately 345,000 children are born prematurely, with 54,000 born before 32 weeks of gestation.Citation5,Citation6 Data from 2020 indicate that out of 2,726,025 births in Brazil, 307,820 were premature (<37 weeks), including 41,308 between 22 and 31 weeks and 38,579 with birth weights <1500 g.Citation7 Prematurity is the leading cause of infant mortality in Brazil’s first year of life, with its etiology associated with maternal, fetal, and environmental factors. Various determinants, including preconceptional, gestational, placental, and fetal conditions, can impact the neonatal period and the child’s long-term health.Citation2,Citation5
Various health issues are linked to prematurity, encompassing metabolic disorders, changes in neurodevelopment, cognitive and behavioral changes, and chronic conditions extending into childhood and adulthood, such as hypertension, diabetes, dyslipidemia, and obesity.Citation4,Citation6,Citation8 The incidence and severity of prematurity complications tend to increase with decreasing gestational age. Premature neonates commonly face complications associated with their immaturity, such as necrotizing enterocolitis, retinopathy of prematurity, bronchopulmonary dysplasia, and intraventricular hemorrhage, which are less prevalent in late preterm infants. Additionally, challenges arising from the extrauterine environment, including nutritional factors and side effects of medications, can result in metabolic changes such as hypoglycemia and hyperbilirubinemia.Citation9
Among neonatal period complications, jaundice commonly manifests as the clinical expression of hyperbilirubinemia. Therapies aimed at controlling serum indirect bilirubin levels during this period include phototherapy and exchange transfusion. Phototherapy, while effective, is a sensory stressor in the Neonatal Intensive Care Unit (NICU) and can induce physiological changes in premature infants.Citation8,Citation10 Consequently, there has been considerable debate surrounding the physiological changes occurring during or after phototherapy application, given the potential risks of photooxidative damage that may impact serum leptin levels.Citation11,Citation12 Notably, phototherapy can induce oxidative damage to cell membranes and DNA, particularly in low birth weight newborns, owing to greater light penetration through their thin, gelatinous skin. Therefore, careful monitoring during phototherapy is essential, encompassing assessment of adverse reactions, radiance dosage, body temperature, weight, diuresis, newborn hydration, exposure duration, and serum total bilirubin levels.Citation13,Citation14
Leptin, a protein encoded by the obese gene primarily secreted by adipocytes, is not only linked to metabolic bone disease and jaundice but also plays a crucial role in maintaining bone mass in jaundiced patients,Citation11 suggesting its broader physiological involvement in appetite regulation, energy expenditure, fat metabolism, and body weight control.Citation15 Conversely, passive exercise can potentially increase leptin levels in neonates by enhancing bone mineralization, consequently promoting weight gain, which is pivotal for the prognosis of this populationCitation11 and may contribute to childhood obesity.Citation15 In the neonatal population, weight is a growth indicator and a criterion for hospital discharge, with associations observed between weight gain and improved cognitive outcomes in premature infants.Citation4
Some studies have shown that daily movement interventions correlate with weight gain and increased leptin levels in premature infants. Considering the implications of passive exercise on circulating leptin levels, it may be a potential factor contributing to childhood obesity.Citation15 To ascertain the safety and efficacy of passive exercise and its precise indication in this population, we analyzed placental leptin levels alongside those in premature infants exposed to phototherapy. This assessment aimed to evaluate the viability of prescribing passive exercise after phototherapy intervention in this population.
Materials and Methods
This study adopts an analytical, longitudinal, prospective cohort design, with data collection performed from May to December 2016 in the Neonatal Intensive Care Unit (NICU) at the Hospital Universitário Evangélico Mackenzie (HUEM), Curitiba, PR, Brazil. Ethical approval for this research was granted by the Ethics Committee for Research Involving Human Subjects at Faculdade Evangélica Mackenzie do Paraná, Curitiba, PR, Brazil, under Approval Letter number 1.489.254 on April 12th, 2016. The conduct of this study complies with the Declaration of Helsinki.
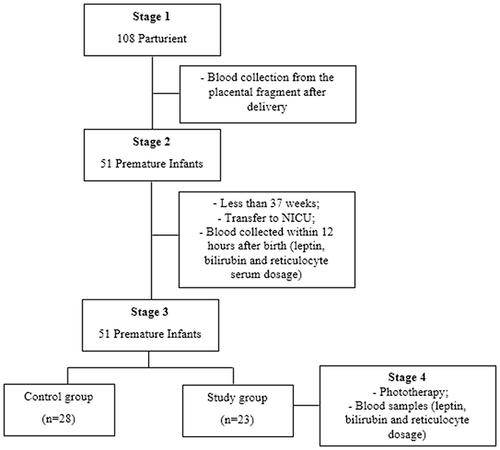
The study comprised four consecutive stages and involved a non-probabilistic sample of 108 parturients and their respective premature infants. As part of the high-risk pregnancy program at HUEM, all parturients received detailed information regarding the institution’s care protocol during the pre-, intra-, and postpartum periods, including the collection of placental blood fragments for newborn diagnosis and clinical management. Before delivery, parturients provided approval for the collection of maternal and neonatal data for the study by signing an informed consent form.
The first stage involved collecting blood from placental fragments after delivery and routine tests to determine serum leptin levels.
In the second stage, preselection of premature infants transferred to the NICU was performed, comprising 51 infants born at less than 37 weeks and 0 days gestation. In the NICU, the intensivist physician collected venous blood samples within 12 h of birth, with 3 mL of blood utilized for serum leptin, bilirubin, and reticulocyte analysis.
In the third stage, the population of premature infants was divided into two groups: control (n = 28) and study (n = 23).
The control group adhered to the following inclusion criteria:
a) Blood collection from placental fragments and within 12 h of life for serum leptin assessment.
b) Absence of major risk factors for hyperbilirubinemia (maternal history, delivery conditions) or typical neonatal phase factors for hyperbilirubinemia.
c) Dermal jaundice involvement (Kramer’s zones) of ≤2, as verified by Bilicheck® bilirubinometer readings, indicating no requirement for phototherapy.
d) Small for gestational age, falling below the 10th percentile, or appropriate for gestational age, between the 10th and 90th percentiles on the Alexander curve 10.Citation16
The study group adhered to the following inclusion criteria:
a) Blood collection from placental fragments and 12 h of life for serum leptin assessment.
b) Presence of major risk factors for hyperbilirubinemia (maternal history, delivery conditions) or typical neonatal phase factors for hyperbilirubinemia.
c) Dermal jaundice involvement (Kramer’s zones) of ≤2, as verified by Bilicheck® bilirubinometer readings, indicating no requirement for phototherapy.
d) Premature infants with serum bilirubin and reticulocyte levels (via venipuncture) indicative of phototherapy.
e) Small for gestational age, falling below the 10th percentile, or appropriate for gestational age, between the 10th and 90th percentiles on the Alexander curve 10.Citation16
In the fourth stage, we continued to monitor the premature infants from the study group identified for phototherapy until its cessation (24 h). Serial blood samples were collected for serum leptin, bilirubin, and reticulocyte analysis throughout the follow-up period. provides detailed information on patient flow during the study period.
The following exclusion criteria were adopted: patients categorized as a newborn term at >38 weeks; early term newborns (37–38 weeks); infants with congenital anomalies and genetic syndromes; those transferred from other wards; infants with congenital infections; and infants requiring exchange transfusion after 12 h of life, as determined by the criteria established by Bhutani et al.Citation17
Clinical Descriptive Variables
This study’s inclusion criteria encompassed all premature infants with a gestational age of <37 weeks who were transferred to the NICU. Data collection involved gathering information on gestational and obstetric history from the parturient, birth conditions, demographic data, neonatal history, clinical progression, and nutritional intake, with particular emphasis on the initiation of feeding within hours of birth among premature infants.
Anthropometric Variables
The following anthropometric factors were assessed:
a) Weight: Measured with a scale adjusted to an accuracy of 0.1 kg and categorized based on weight-for-gestational-age reference curves 10.Citation16
b) Height: Measured using an anthropometer graduated in centimeters, comprising a fixed rod positioned on top of the head and a mobile rod near the calcaneus.
c) Head circumference.
d) Body mass index (BMI): Calculated by dividing mass by the square of height (kg/m2). For children aged 0–5 years, BMI categories were defined as follows: low for age (<3rd percentile), adequate or eutrophic (≥3rd percentile and <85th percentile), overweight (≥85th percentile and <97th percentile), and obesity (≥97th percentile).
Laboratorial Variables
Leptin
Leptin concentration was measured using the ELISA-AIDTM assay, which lasted five days. The analysis was performed using the DIAsource leptin-EASIA antibody kit, following the manufacturer’s protocol, and the results were expressed in ng/mL. Both placental and premature infants’ leptin levels were assessed. Blood samples were obtained from the placental fragment immediately after delivery using venipuncture with a 40×12 needle and a 5 mL syringe. The procedure was identical at all times.Citation18
Serum Bilirubin and Reticulocyte Count
In the second stage, serum bilirubin was collected from all premature infants presenting risk factors (maternal history, delivery conditions) or typical neonatal factors for hyperbilirubinemia. If the Bilicheck® bilirubin meter reading within the first 24 h of life exceeded 5, the premature infant underwent superficial venous puncture. Concurrently, in this stage, the initial reticulocyte count was obtained from the same blood sample. In the third and fourth stages, the collection procedure remained consistent.
The final reticulocyte count was measured only after 24 h or more following the cessation of phototherapy, guided by well-defined clinical criteria and subsequent quantification of rebound bilirubin. Therefore, it was assessed solely to determine the necessity for reinitiating phototherapy.
Bilirubin Total
The collection was conducted via superficial venous puncture, with 0.4 mL/dl of blood being obtained. These samples were protected from light during the process, and a colorimetric test was employed using the coupling reaction with 2, 4-dichloroanilinediazotate. The color intensity directly correlated with the total bilirubin concentration in the sample, with results expressed in mg/dl. The technique utilized BioCal, BioControl N, BioControl P, and Bioclin reagent kits. Reference values were determined in accordance with Bhutani et al.Citation17
Reticulocytes
We performed both relative and absolute quantification of Reticulocytes in the whole blood sample. For elevated values, we adjusted the threshold for phototherapy initiation to be two points lower than initially proposed, thus enabling earlier initiation for premature infants exhibiting such levels. The employed method was the brilliant cresyl blue staining technique, with results recorded using the BC 5380 Hematology Automated Analyzer.
Statistical Analysis
Quantitative variables were described using mean, median, minimum, and maximum values, 1st and 3rd quartiles, and standard deviation. Qualitative variables were summarized through frequencies and percentages. Group comparisons for quantitative variables were conducted using Student’s t-test for independent samples or the non-parametric Mann–Whitney test. Within-group comparisons were performed using Student’s t-test for paired samples. Assessment of leptin results across different time points within each group was performed using Friedman and Wilcoxon non-parametric tests. When comparing evaluation time points pairwise within the study group, adjustments were made using Bonferroni multiple comparisons based on the number of comparisons (p = 0.05/n, where n is the number of comparisons). Statistical significance was defined as p < 0.05. The statistical analysis was carried out using Stata/SE v.14.1 by StataCorpLP, USA.
Results
During the study period, 108 parturient women and their respective premature infants were evaluated. Among them, 51 premature infants requiring transfer to the NICU were analyzed and subsequently divided into control and study groups. The characteristics of the subjects are outlined in . Notably, the groups demonstrated homogeneity, with no statistically significant differences observed in their characteristics.
Table 1 Demographic Characteristics of Premature Infants in the Study and Control Groups
No statistically significant differences were observed in the leptin concentration between the placenta and premature infants within 12 h after birth. However, the leptin concentrations at these identical time points (placenta and premature infants up to 12 h after birth) exhibited similarities (p = 0.8 and p = 0.12). Notably, the leptin concentration at the placental time point in the control group measured 0.6, which was double the value observed in the study group (0.3).
Significant differences were noted across all assessment time points within the study group regarding the leptin concentrations at various assessment time points. The most notable distinction was observed between the placental and subsequent time points (p = 0.001). Additionally, a trend was observed between the 24-h mark, phototherapy, and rebound time points (). These findings suggest a progressive decrease in leptin levels in premature infants over the hours following birth, eventually falling below the levels detected in the placenta.
Table 2 Comparison Between the Moments of Evaluation 2 to 2 of the Study Group, Regarding Leptin Concentration (n=23)
Both groups exhibited similar Results regarding leptin concentration in the placenta and within the first 12 h of life. However, in the control group, a statistically significant difference was observed in leptin concentration between the placenta and within the first 12 h of life (p = 0.002).
Leptin was dichotomized into categories “less than 0.1” and “greater than or equal to 0.1” for analysis. When comparing the distribution of leptin proportions at two time points with levels lower than 0.1, only the placenta and within the first 12 h of life showed a statistically significant difference in the study group (p = 0.001). These findings suggest that leptin levels remained relatively constant in preterm infants after birth.
In the descriptive analysis of bilirubin levels at each time point within the study group, we observed no statistically significant difference (p = 0.158), indicating a gradual decrease in bilirubin over time.
Among the 23 premature infants, 12 required reticulocyte collection for differential diagnosis purposes, particularly in cases where jaundice persisted after treatment. Reticulocyte collection was deemed necessary to ascertain the underlying cause of hyperbilirubinemia and guide further interventions (). A notable trend was observed, indicating a decrease in reticulocyte count in relation to the onset/rebound period (p = 0.004), suggesting improvement in hyperbilirubinemia among premature infants. “Rebound” refers to the blood sample obtained 24 h after phototherapy.
Table 3 Descriptive Analysis of Reticulocyte Values at Various Time Points After Phototherapy (n = 12)
No association was found between bilirubin/leptin and reticulocyte/leptin levels before and after phototherapy. Similarly, no correlation was observed when comparing the leptin results at the time of nutrition initiation with leptin levels at each assessment time point. These findings suggest that phototherapy does not influence leptin concentrations ().
Table 4 Evaluation of the Association Between Bilirubin and Leptin, and Nutrition and Leptin (n = 23)
When analyzing the association between BMI and leptin in each group, we observed no correlation between the groups during the first 12 h of life. This lack of correlation also persisted across other time points within the study group ().
Table 5 Association Between BMI and Leptin
Discussion
In this study, we examined serum leptin levels in premature infants exposed to phototherapy, correlating them with serum bilirubin levels that could affect leptin levels, aiming to establish safe recommendations for passive exercise in this population. Passive exercise may increase serum leptin levels,Citation15 a factor linked to childhood obesity. Our findings indicate a decrease in leptin levels in premature infants after birth, remaining lower than placental levels. Thus, passive exercises could be recommended after phototherapy and feeding initiation.
Leptin levels appear independent of placental production and can be viewed as a marker of fetal adiposity.Citation18,Citation19 Leptin produced from fat deposits affects cells associated with bone diseases and blood substances (hyperbilirubinemia). This influence on osteoblast proliferative capacity suggests a physiological role for leptin in maintaining bone mass in jaundiced patients.Citation6,Citation11
The primary function of leptin is to communicate energy reserve information to hypothalamic structures, regulating energy balance by inducing an anorectic effect and increasing energy expenditure. However, during early childhood, leptin appears to lack this anorexigenic effect.Citation20 Evidence suggests that serum leptin concentrations mirror body fat mass throughout fetal life, childhood, and adulthood. Leptin has emerged as a significant factor in fetal growth and neonatal development, with a positive correlation between leptin levels in umbilical cord blood and birth weight. Some researchers propose that fetal adipose tissue is the primary source of circulating leptin.Citation20 Thus, leptin may exert local and endocrine effects on the fetus, which is crucial for normal prebirth growth and development. It acts as a signal of adipose tissue status during pregnancy and infancy. Our results indicate that premature infants exhibit a decrease in leptin levels after birth, which eventually fall below placental levels and stabilize thereafter.
Phototherapy is the most prevalent intervention for treating hyperbilirubinemia, widely acknowledged for its safety.Citation21–23 Despite debates surrounding its potential adverse effects stemming from photooxidative stress and lipid peroxidation induction, bilirubin is a crucial laboratory marker assessed at various intervals in this study group. Our findings reveal no association between bilirubin/leptin and reticulocyte/leptin levels before and after phototherapy or when comparing results at the initiation of nutrition with leptin levels at each assessment time point. These results suggest that phototherapy does not affect leptin concentrations.
Another descriptive variable examined in this study was sex differences, with balanced samples. Our analysis indicates no correlation between leptin concentrations and serum estradiol and testosterone levels. This suggests that genetic differences may play a more significant role than fat distribution or hormonal status because lower leptin levels were observed among males and females.Citation24 In a study by Shekhawat et al, no sex differences were found in cord blood leptin levels, indicating that maternal obesity did not affect cord leptin. In contrast, exogenous maternal steroids increased neonatal leptin concentrations.Citation25
Leptin concentrations exhibit differences between the sexes, increasing in women starting from puberty and persisting after sexual maturation. This difference is attributed to the greater percentage of body fat in women and the inhibitory effect of testosterone on leptin secretion in men.Citation26 Rump et al demonstrated an increase in leptin concentration in pregnant women during childbirth, with levels even doubling, followed by a reduction postpartum. This decline is concurrent with a desensitization process of hypothalamic receptors, potentially contributing to postpartum weight retention.Citation27
Regarding birth weight, BMI, and sex differences between the groups, our findings align with existing literature.Citation28,Citation29 Prematurity is a multifaceted process that begins before gestation and is influenced by a complex interaction between biological factors.Citation28 Predicting ideal growth for premature infants is challenging given the continuous and intricate nature of growth, influenced by genetic, environmental, nutritional, and hormonal factors.Citation30,Citation31
The stage of body maturation correlates directly with adipocytokines such as leptin and adiponectin, which play roles in regulating fetal growth. Consequently, their serum concentrations exhibit mutual relationships and correlations with anthropometric data, indicating fetal growth maturity in full-term and preterm neonates.Citation32,Citation33 In this study, the BMI served as a descriptive variable, with a mean value of 11.9, indicating that the studied population fell below the 3rd percentile. While many studies have included BMI in the assessment of premature newborns, establishing predictive values defining a reference for this population to estimate body fat remains challenging.Citation34
The association with the onset of nutrition has been shown to induce changes in serum leptin levels in neonates. Leptin concentrations in breast milk positively correlate with circulating levels in newborns, BMI, and adiposity.Citation20 Moreover, it has been observed that leptin levels decrease after the Introduction of solid foods at 6 months, resulting in breastfed infants having significantly higher serum leptin levels than formula-fed infants during the first 6 months of life. However, this difference disappears later in life. In this study, we proposed that breastfed newborns received leptin produced by their adipocytes and that found in human milk. However, we found no association between the onset of feeding and leptin levels in the study group. Thus, further investigation into the nutritional factors influencing this population is warranted.
Postnatal provision of bone nutrients enhances mineralization, albeit not to the extent achieved during intrauterine development. Increased dietary intake of bone mineral substrates such as calcium, phosphorus, magnesium, and protein is only one aspect of fostering healthy bone growth and development. Other factors influencing bone growth and density include genetics, endocrine function, and physical activity.Citation17 Leptin levels derived from adipose tissue influence cells involved in bone diseases and substances present in the bloodstream (hyperbilirubinemia), which may impede osteoblast proliferative capacity. Therefore, it is proposed that leptin plays a physiological role in maintaining bone mass, particularly in patients with jaundice.Citation11 Leptin stimulates linear growth, regulates the body’s energy balance, and promotes the production and secretion of growth hormone from the hypothalamus. Additionally, it participates in bone remodeling and directly affects chondrocytes in the growth plate.Citation19
Several changes in leptin levels occur during intrauterine life, childhood, and adulthood.Citation20 Unfortunately, a database providing normal leptin reference values across different age groups is not available in the literature, limiting our study. Further research is needed to establish reference values for leptin levels across various age groups.
Conclusion
To establish the safety of implementing passive exercise techniques in premature infants, we examined serum levels of leptin and bilirubin during phototherapy exposure. Our findings indicate that phototherapy does not increase serum leptin levels in premature infants, confirming passive exercise’s safety in this population.
Although passive exercises have been associated with potential increases in leptin levels, our results suggest that such exercises can be safely conducted in premature infants because premature infants do not exhibit increased leptin levels after birth.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Falcon Scientific Editing and the Hospital Universitário Evangélico Mackenzie for allowing us to conduct this study at the NICU’s facilities.
References
- Dbstet A. WHO: recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14. Acta Obstet Gynecol Scand. 1977;56(3):247–253. doi:10.3109/00016347709162009
- De Jesus RLR, Dos Santos GM, Barreto MTS, Monteiro MJS, Silva RVS, da Silva HJN. Caracterização dos recém-nascidos pré-termo nascidos no estado do Piauí entre 2011 a 2015 [Characterization of preterm newborns born in the state of Piauí between 2011 and 2015]. Arch of Health Investig. 2019;8(4):217–223. doi:10.21270/archi.v8i4.3193
- França EB, Lansky S, Rego MAS, et al. Leading causes of child mortality in Brazil, in 1990 and 2015: estimates from the Global Burden of Disease study. Rev Bras Epidemiol. 2017;20(1):46–60. doi:10.1590/1980-5497201700050005
- Ou-Yang MC, Liebowitz M, Chen CC, et al. Accelerated weight gain, prematurity, and the risk of childhood obesity: a meta-analysis and systematic review. PLoS One. 2020;15(5):e0232238. doi:10.1371/journal.pone.0232238
- Barros FC, Papageorhiou AT, Victoria CG, et al. The distribution of clinical phenotypes of preterm birth syndrome implications for prevention. JAMA Pediatrics. 2015;169(3):220–229. doi:10.1001/jamapediatrics.2014.3040
- Leal MD, Esteves-Pereira AP, Nakamura-Pereira M, et al. Prevalence and risk factors related to preterm birth in Brazil. Reprod Health. 2016;13(Suppl 3):127. doi:10.1186/s12978-016-0230-0
- Guinsburg R, Fernanda M, Almeida de B. Reanimação do recém-nascido <34 semanas em sala de parto: diretrizes 2022 da Sociedade Brasileira de Pediatria [Resuscitation of newborns <34 weeks in the delivery room: 2022 guidelines from the Brazilian Society of Pediatrics]. Soc Bras Pediatr. 2022;2022:1–42.
- Lançoni SS, Souto LRT, Albuquerque JP. Inspirando Fisioterapia em Pediatria e Neonatologia [Inspiring Physiotherapy in Pediatrics and Neonatology]. Curitiba: Livraria e Editora Andreoli; 2018.
- Ribeiro AM, Lima MC, Lira PIC, Silva GAP. Baixo peso ao nascer e obesidade: associação causal ou casual? [Low birth weight and obesity: causal or casual casual association?] Rev Paul Pediatr. 2015;33(3). doi:10.1016/j.rpped.2014.09.007
- Okwundu CI, Okoromah CAN, Shah PS. Cochrane review: prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants. Cochrane Database Syst Rev Evid Based Child Health. 2012. doi:10.1002/14651858.CD007966.pub2
- Hosanwek S, Chaiwatanarat T, Chongsrisawat V, Thawornsuk N, Vejchapipat P, Poovorawan Y. Circulating leptin levels and bone mineral density in children with biliary atresia. Acta Paediatrica. 2008;97(2):206–211. doi:10.1111/j.1651-2227.2007.00596.x
- Soyer T, Ayva S, Aliefendioglu D, et al. Effect of phototherapy on growth factor levels in neonatal rat skin. J of Pediatric Sur. 2011;46(11):2128–2131. doi:10.1016/j.jpedsurg.2011.06.012
- Burke B, Robbins J, Hobbs C. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi:10.1542/peds.114.1.297
- Taksande A, Selvam S. Side effects of phototherapy in neonatal hyperbilirubinemia. Acta Sci Paediatr. 2018;1:24–30.
- Eliakim A, Dolfin T, Weiss E, et al. The effects of exercise on body weight and circulating leptin in premature infants. J Perinatol. 2022;22:550–554. doi:10.1038/sj.jp.7210788
- Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–168. doi:10.1016/0029-7844(95)00386-X
- Bhutani VK, Johnson LH, Shapiro SM. Kernicterus in sick and preterm infants (1999–2002): a need for an effective preventive approach. Semin Perinatol. 2004;28(5):319–325. doi:10.1053/j.semperi.2004.09.006.
- Veselá PK, Kaniok R, Bayer M. Markers of bone metabolism, serum leptin levels and bone mineral density in preterm babies. J Pediatr Endocrinol Metab. 2016;29(1):27–32. doi:10.1515/jpem-2013-0474
- Bajoria R, Sooranna SR, Ward BS, Chatterjee R. Prospective function of placental leptin at maternal-fetal interface. Placenta. 2002;23(2–3):103–115. doi:10.1053/plac.2001.0769
- Savino F, Rossi L, Benetti S, Petrucci E, Sorrenti M, Silvestro L. Serum reference values for leptin in healthy infants. PLoS One. 2014;9(11):e113024. doi:10.1371/journal.pone.0113024
- Singer S, Berneburg M. Phototherapy. J of German Soc of Dermatol. 2018;16(9):1120–1131. doi:10.1111/ddg.13646
- Mitra S, Rennie J. Neonatal jaundice: aetiology, diagnosis and treatment. Br J Hosp Med. 2017;78(12):699–704. doi:10.12968/hmed.2017.78.12.699
- Stevenson DK, Wong RJ. The biology of bilirubin production: overview of detection and inhibition. Pediatr Med. 2021;4:16. doi:10.21037/pm-21-8
- Shroff MR, Holzman C, Tian Y, Evans RW, Sikorskii A. Mid-pregnancy maternal leptin levels, birthweight for gestational age and preterm delivery. Clin Endocrinol. 2013;78(4):607–613. doi:10.1111/cen.12029
- Shekhawat P, Garland J, Shivpuri C, et al. Neonatal cord blood leptin: its relationship to birth weight, body mass index, maternal diabetes, and steroids. Pediatr Res. 1998;43:338–343. doi:10.1203/00006450-199803000-00005
- Goumenou AG, Matalliotakis IM, Koumantakis GE, Panidis DK. The role of leptin in fertility. Eur J Obstetr Gynecol Reprod Biol. 2003;106(2):118–124. doi:10.1016/s0301-2115(02)00359-7
- Rump P, Otto SJ, Hornstra G. Leptin and phospholipid-esterified docosahexaenoic acid concentrations in plasma of women: observations during pregnancy and lactation. Eur J Clin Nutr. 2001;55(4):244–251. doi:10.1038/sj.ejcn.1601151
- Laivuori H, Gallaher M, Collura L, et al. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol Hum Reprod. 2006;12:551–556. doi:10.1093/molehr/gal064
- Knegt VE, Hedley PL, Kanters JK, et al. The role of leptin in fetal growth during pre-eclampsia. Int J Mol Sci. 2021;22(9):4569. doi:10.3390/ijms22094569
- Fonseca V, Sichieri R, Moreira M, et al. Early postnatal growth in preterm infants and cord blood leptin. J Perinatol. 2004;24:751–756. doi:10.1038/sj.jp.7211188
- Litmanovitz I, Eliakim A. Exercise, nutrition, and anthropometry of bone development in term and preterm infants. In: Preedy V, editor. Handbook of Anthropometry. New York, NY: Springer; 2012. doi:10.1007/978-1-4419-1788-1_108.
- Palchevska S, Krstevska M, Shukarova E, et al. Comparing preterm and term newborns serum adiponectin and leptin concentrations and their correlations with anthropometric parameters. Maced J Med Sci. 2012;5(3):317–323. doi:10.3889/MJMS.1857-5773.2012.0264
- Plowden TC, Zarek SM, Rafique S, et al. Preconception leptin levels and pregnancy outcomes: a prospective cohort study. Obes Sci and Pract. 2020;6(2):181–188. doi:10.1002/osp4.399
- Cardoso LEB, Falcão MC. Importância da avaliação nutricional de recém- nascidos pré-termo por meio de relações antropométricas [The importance of the nutritional assessment of premature newborn infants by anthropometric relationships]. Rev Paulista Pediatria. 2007;25(2):135–141. doi:10.1590/S0103-05822007000200007