Abstract
Background
To investigate the association between the NLR and the risk of all-cause and cardiovascular mortality in US adults with diabetic kidney disease (DKD).
Methods
The data utilized for this analysis were sourced from ten National Health and Nutrition Examination Survey cycles (1999–2018) with mortality data (up to 31 December 2019) via linkage to the National Death Index. The optimum NLR threshold for predicting survival outcomes was determined through the maximally selected rank statistics. Restricted cubic spline (RCS), weighted Cox proportional hazard regression, stratified analyses, and time-dependent receiver-operating characteristic curve (ROC) were employed to delineate the prospective correlations of the NLR with both all-cause and cardiovascular mortality.
Results
In this investigation, a cohort comprising 2581 patients diagnosed with DKD was examined, encompassing 624 individuals with a higher NLR (≥3.07) and 1957 subjects with a lower NLR (<3.07). Over a median follow-up of 79 months (interquartile range, 44–128 months), 1103 deaths occurred, including 397 from cardiovascular causes and 706 from non-cardiovascular causes. The RCS analysis elucidated the positive linear correlation (both nonlinear P > 0.05). In the multivariable analyses, each one-unit increase in the NLR value was correlated with a 51% increased risk of all-cause mortality (1.51(1.28, 1.77)) and a 71% increased risk of cardiovascular mortality (1.71(1.32, 2.21)). The results were largely consistent across stratified analyses encompassing variables such as age, sex, race/ethnicity, marital status, family income, education levels, BMI, drinking status, smoking status, hypertension, CVD, and anti-infective drugs (P for interaction >0.05 for all). Time-dependent ROC analyses underscored the NLR’s credible predictive efficacy for both short-term and extended durations in forecasting both all-cause and cardiovascular mortality.
Conclusion
The findings emphasize the promising use of the NLR in stratifying and prognosticating the risk of mortality in DKD in clinical practice.
Introduction
Diabetic kidney disease (DKD) is increasingly recognized as the foremost cause of end-stage kidney disease (ESKD) on a global scale.Citation1–3 This is primarily attributable to the escalating incidence of diabetes, which is fueled by the unprecedented increase in the obesity epidemic. Notably, approximately 30–50% of subjects with diabetes ultimately develop DKD.Citation1 Current evidence indicates that subjects with both diabetes and chronic kidney disease (CKD) were found to be related with higher rates of myocardial infarction and 10-year cumulative mortality compared to those with diabetes alone.Citation4,Citation5 Thus, it is imperative to promptly identify more risk factors to mitigate the progression of DKD and DKD-related mortality.
There is increasing evidence delineating the pivotal role of inflammation in both the initiation and progression of DKD.Citation6–8 Several immune infiltrating cells are apparent in glomerular and tubular cells and their activation is closely correlated with sustained kidney inflammation.Citation6,Citation9 The neutrophil–lymphocyte ratio (NLR), a cheap and readily accessible measure of systemic inflammation and immune system activation in clinical use and comprehensive reflection of both innate (neutrophils) and adaptive (lymphocytes) immunological responses,Citation10 is regarded as a factor associated with adverse outcomes across a spectrum of diseases.Citation9–13 Association of the NLR and kidney impairment in diabetes is well established.Citation14,Citation15
Inflammation is also recognized as playing a pivotal role associated with the etiology of cardiometabolic diseases.Citation16,Citation17 Recent researchers have identified the NLR as the strongest predictor of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke in two randomized clinical trials, which comprised 6480 participants with diabetes.Citation18 The NLR also has been found to be correlated with all-cause and cardiovascular mortality in diabetic individuals.Citation19 However, data are scarce on the linkage of the NLR with all-cause and cardiovascular mortality in DKD.
Given the high prevalence of DKD, understanding whether the NLR has a prognostic value for the risk of mortality in DKD is an important research area. To fill these knowledge gaps, this study aimed to prospectively examine the relationship of the NLR with all-cause and cardiovascular mortality in a nationally representative sample of US adults with DKD.
Materials and Methods
Study Population
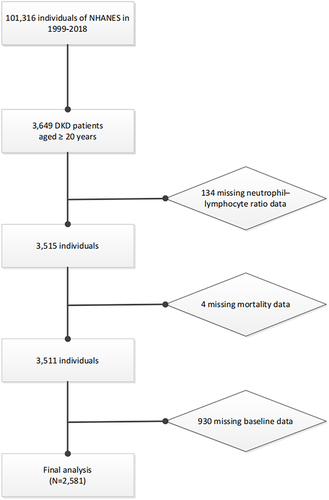
This study is a prospective cohort study utilizing data derived from the National Health and Nutrition Examination Survey (NHANES), which is a continuing, nationally representative, repeated populated-based study conducted by the Centers for Disease Control (CDC) and the National Center for Health Statistics (NCHS). The study’s design and methodologies have been comprehensively elucidated in previous literature.Citation20,Citation21 We subsumed ten cycles from 1999 to 2000 through 2017 to 2018. There was a total of 3649 adults aged 20 years or older with DKD. Of this group, we excluded individuals with missing data on NLR (n = 134) or mortality status (n = 4). A whole number of 930 individuals with missing data on marital status, family income, education levels, body mass index (BMI), glycohemoglobin (HbA1c), serum creatinine (Scr), blood urea nitrogen (BUN), total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), urinary albumin creatinine ratio (UACR), estimated glomerular filtration rate (eGFR), drinking status, smoking status, hypertension, CVD, and use of anti-infective drugs were excluded. Subsequent to these exclusions, the final analytic sample size for this investigation comprised 2581 participants (). All study procedures were authorized by the National Center for Health Statistics Ethics Review Board prior to data collection, and each subject furnished written informed consent.
DKD Definition and Blood Cell Counts Measurement
Diabetes was diagnosed according to the criteria of (1) fasting plasma glucose ≥126mg/dL, or (2) 2-hour oral glucose tolerance ≥200mg/dL, or (3) HbA1c ≥6.5%, or (4) administering antidiabetic medications, or (5) self-reported diabetes. The urine albumin/creatinine ratio was utilized to evaluate the ACR. The Chronic Kidney Disease Epidemiology Collaboration equation was utilized to evaluate eGFR. DKD in participants with diabetes was diagnosed according to the criteria of eGFR <60 mL/min/1.73 m2 or UACR ≥30 mg/g.Citation22,Citation23 The Beckman Coulter methodology was employed to conduct the complete blood count. The NLR was evaluated by the ratio of the absolute neutrophil count to the absolute lymphocyte count from an identical automated complete blood count specimen.
Ascertainment of All-Cause and Cardiovascular Mortality
Mortality status and causes of death were ascertained through linkage with the National Death Index (NDI) database by the NCHS based on a probability matching algorithm. The duration of follow-up (in months) spanned from the baseline examination date to the recorded date of death or until 31 December 2019 (the latest available date of the NDI database). The International Classification of Diseases (ICD), 10th Edition, was utilized for ascertaining the primary cause of mortality. Specifically, cardiovascular mortality encompassed fatalities attributed to heart diseases (codes I00 to I09, I11, I13, I20 to I51) and cerebrovascular ailments (codes I60-I69).
Covariables
Covariate selection was informed by comprehensive evidence from literature.Citation19,Citation22 The chosen covariates encompassed age, sex, race/ethnicity, marital status, family income, education levels, BMI, HbA1c, Scr, BUN, TC, HDLC, UACR, eGFR, drinking status, smoking status, hypertension, CVD, and use of anti-infective drugs. Race/ethnicity categorization included White, Black, Mexican, and Other. Marital classifications comprised individuals who were married or living with partner, those who had never married, and those who were widowed, divorced, or separated. Height and weight were utilized to evaluate BMI in kg/m2. Smoking habits were delineated as never smoker, former smoker, or current smoker. Education levels were stratified into categories: less than high school, high school or equivalent, and college or above. Family income was categorized into <1 (poor), 1–3 (near poor), and ≥3 (not poor).Citation21 Drinking status was characterized as follows: never drinker, former drinker, mild drinker (≤1 drink for females or ≤2 drinks for males daily on average over the past year), moderate drinker (1–3 drinks for females or 2–4 drinks for males daily on average over the past year), and heavy drinker (≥4 drinks for females or ≥5 drinks for males daily on average over the past year).Citation21 The average of the first three blood pressure readings was utilized to assess systolic and diastolic blood pressure. Hypertension was delineated as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg. The history of CVD, encompassing conditions such as coronary heart disease, congestive heart failure, heart attack, stroke, and angina, and use of anti-infective drugs were obtained from the standardized questionnaires. Blood samples were procured and sent to central laboratories to measure HbA1c, Scr, BUN, TC, and HDLC. Standard protocols were followed to assess the aforementioned measurements.
Statistical Analysis
We accounted for the complex sampling design and incorporated mobile examination center sample weights into all statistical evaluations across the study to ensure nationally representative estimates as stipulated by the NHANES analytic and reporting guidelines.Citation24 Continuous variables were delineated as mean (standard deviation [SD]), while categorical variables were presented as frequency (percentages). To discern disparities between two cohorts concerning continuous variables, we employed either the weighted t-test or the Mann–Whitney U-test. Concurrently, the weighted chi-square test was engaged for dichotomous variables.
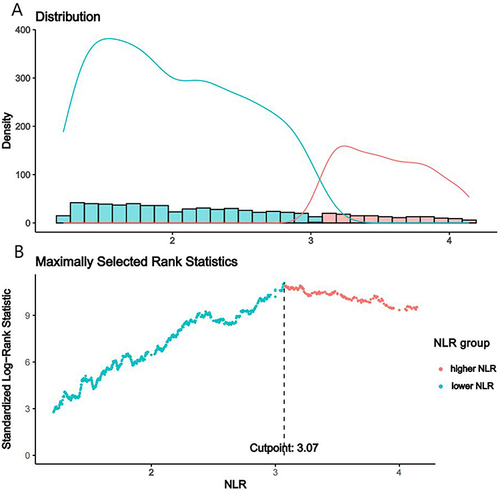
The optimum NLR threshold for predicting survival outcomes was determined through the utilization of the maximally selected rank statistics.Citation19 Subsequently, participants were stratified into cohorts delineated by low and high NLR values based on this optimal threshold. We also modeled the NLR utilizing restricted cubic spline (RCS) (3 knots at the 5th, 50th, and 95th percentiles) to flexibly explore any potentially nonlinear correlation between the NLR and all-cause mortality and cardiovascular mortality in DKD participants. We utilized weighted Cox proportional hazard regression to model the prospective correlations of the NLR with all-cause mortality and cardiovascular mortality in DKD participants. Four models were applied: a crude model; Model 1 included age, sex, race, marital status, family income, and education levels; Model 2 included all covariables in Model 1 plus drinking status and smoking status; Model 3 included all covariables in Model 2 plus BMI, HbA1c, Scr, BUN, TC, HDLC, UACR, eGFR, hypertension, CVD, and anti-infective drugs. The cumulative Kaplan–Meier (KM) method for the probabilities survival outcomes during follow-up was plotted. Age, sex, race/ethnicity, marital status, family income, education levels, BMI, drinking status, smoking status, hypertension, CVD, and anti-infective drugs were all taken into account in stratified analyses, and P for interaction was explored. To ascertain the precision of the NLR at various temporal intervals for predicting survival outcomes, we instituted a time-dependent receiver-operating characteristic curve (ROC) leveraging the “timeROC” package.Citation25 P < 0.05 denoted statistical significance. Statistical analyses were performed using R version 4.0.4 (http://www.R-project.org, The R Foundation).
Results
Subject Characteristics
The final analysis utilized data from 2581 subjects within the NHANES cohort, representing 7,514,268 subjects with DKD of the USA (). The cohort study encompassed 624 individuals with a higher NLR (≥3.07) and 1957 subjects with a lower NLR (<3.07), through the utilization of the optimum NLR threshold for predicting survival outcomes (). A comprehensive characterization of the cohort is elucidated in . The mean age of 2581 individuals was 63.80 years, more than half of the subjects were male, and 65.07% were White. The subjects in the higher NLR group were more likely to be correlated with CVD, be older, male, White, former smokers, and married or living with partner, be without the use of anti-infective drugs, to have a higher white blood cell count, BUN, UACR, and HDLC and lower eGFR, HbA1c, and TC, relative to those in the lower NLR group (P < 0.05). BMI, drinking status, education levels, family income, platelet count, and hypertension did not show statistical significance (P > 0.05).
Table 1 Characteristic of Participants
NLR and All-Cause Mortality
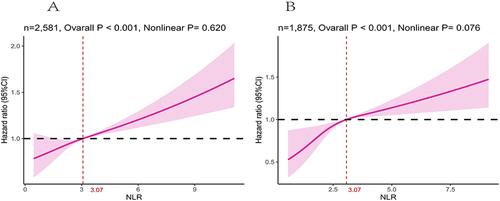
Over a median follow-up of 79 months (interquartile range (IQR), 44–128 months), there were 1103 (42.74%) deaths, including 397 (15.38%) from cardiovascular causes and 706 (27.35%) from non-cardiovascular causes. The NLR exhibited a positive linear association with mortality in DKD subjects according to the RCS analysis (nonlinear P = 0.62) (). With respect to the higher-NLR group (), the NLR led to a 2-fold increased all-cause mortality risk in the crude model (hazard ratio (HR) = 2.10, 95% CI = 1.77, 2.49, P < 0.001). In multivariable models, the NLR led to an approximate doubling of the HRs in Model 1 (HR = 1.74, 95% CI = 1.48, 2.05, P < 0.001) and Model 2 (HR = 1.72, 95% CI = 1.47, 2.02, P < 0.001). In Model 3, participants with higher NLR have an up to 1.51-fold increased relative risk for all-cause mortality (HR = 1.51, 95% CI = 1.28, 1.77, P < 0.001), respectively.
Table 2 The Relationships Between NLR and Mortality in DKD
Figure 3 Association between NLR and all-cause (A) and cardiovascular mortality (B) among DKD visualized by restricted cubic spline (NLR breakpoint: 3.07). Adjusted for age, sex, race, marital status, family income, education levels, BMI, HbA1c, Scr, BUN, TC, HDLC, UACR, eGFR, drinking status, smoking status, hypertension, CVD and anti-infective drugs. Both P value for nonlinearity > 0.05.
NLR and Cardiovascular Mortality
A total of 1875 DKD patients, incorporating 390 having higher NLR and 1485 having lower NLR, were taken to evaluate the correlation of the NLR with cardiovascular mortality apart from 706 non-cardiovascular deaths. A non-linear correlation was identified between the NLR and the HR of cardiovascular mortality after adjusted for age, sex, race, marital status, family income, education levels, BMI, HbA1c, Scr, BUN, TC, HDLC, UACR, eGFR, drinking status, smoking status, hypertension, CVD, and anti-infective drugs (). The Cox proportional hazard regression model indicated that DKD patients with higher NLR had an increased risk of cardiovascular mortality (HR = 2.34, 95% CI = 1.79, 3.06, P < 0.001). The risk of cardiovascular mortality remained significant after adjusting for full adjustment (Model 3, HR = 1.71, 95% CI = 1.32, 2.21, P < 0.001), manifesting that each one-unit increase in the NLR value was correlated with a 71% increased risk of cardiovascular mortality ().
Survival Analysis
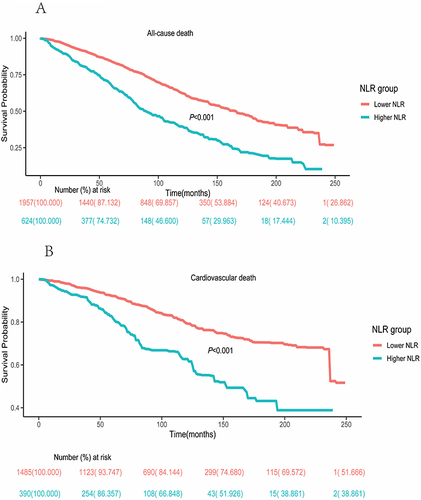
The KM analysis for all-cause mortality underscored a marked disparity between the higher- and lower-NLR groups (P < 0.001) (). The results of KM survival rates for cardiovascular mortality indicated that the survival rate was diminished in DKD patients with the higher NLR (P < 0.001) ().
Stratification Analysis and Ascertainment of the Prognostic Ability
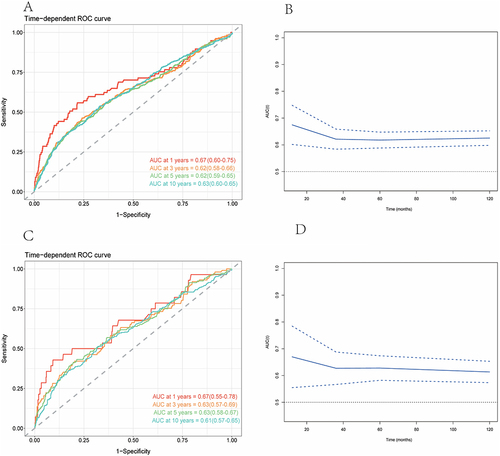
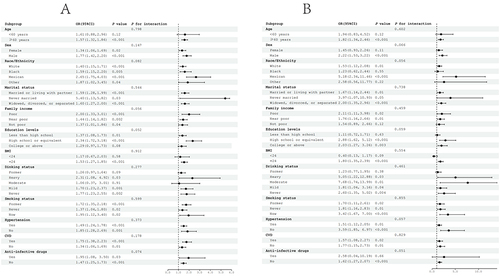
Concurrently, sensitivity analyses were meticulously stratified across a spectrum of variables, including age, sex, race/ethnicity, marital status, family income, education levels, BMI, drinking status, smoking status, hypertension, CVD, and anti-infective drugs, to discern the correlation of the NLR with all-cause () and cardiovascular mortality (). We investigated the heterogeneity within each subgroup using interaction terms and no significant difference was uncovered (P for interaction >0.05 for all), revealing that the results were largely consistent when the individuals were divided into different subgroups. In assessing the predictive performance of the NLR for all-cause and cardiovascular mortality in DKD using time-dependent ROC analysis, it was determined that the NLR exhibited superior predictive value for both all-cause and cardiovascular mortality across short-term and extended durations (1-year, 3-year, 5-year, and 10-year periods) ().
Figure 5 Subgroup analysis of the associations between NLR and mortality among DKD. Adjusted for age, sex, race, marital status, family income, education levels, BMI, HbA1c, Scr, BUN, TC, HDLC, UACR, eGFR, drinking status, smoking status, hypertension, CVD, and anti-infective drugs. (A) All-cause mortality. (B) Cardiovascular mortality.
Discussion
In this large-sample nationally representative cohort investigation, we explored the correlation of the NLR with survival outcomes among adults with DKD utilizing the NHANES data collected from 1999 to 2000 through 2017 to 2018. To the best of our knowledge, this study is the first to prospectively examine the relationship of the NLR with mortality in DKD. Our findings underscore that higher NLR levels corresponded with augmented incidences of both all-cause and cardiovascular mortality in a medium follow-up time of 79 months. After adjusting for confounders, the NLR was still significantly correlated to increasing all-cause (HR = 1.51, 95% CI: 1.28, 1.77) and cardiovascular mortality (HR = 1.71, 95% CI: 1.32, 2.21). A variety of stratified analyses and sensitivity analyses demonstrated the robustness of our findings. These results emphasize the promising use of the NLR in stratifying and prognosticating the risk of mortality in DKD in clinical practice.
Emerging scholarly consensus postulates that inflammation significantly mediates the onset and progression of DKD.Citation6–8 The NLR affords insights into the individual’s systemic inflammation and immune system activation. A high neutrophil count signifies persistent inflammatory cascades, while a low lymphocyte count signifies an insufficiency in the regulatory and dormant immune pathways. Preclinical evidence supports that a recruitment of immune infiltrating cells into the kidney, including neutrophils and lymphocytes, releases inflammatory mediators, resulting in formulating a feedback inflammatory loop that enhances chronic inflammation and involves the progression of DKD.Citation6,Citation26,Citation27 In clinical investigations, the NLR has been widely established as a harbinger for the progression of DKD.Citation14,Citation28–31 In a longitudinal cohort investigation spanning 3 years, Azab et al reported that increased NLR was correlated with an increased risk of worsening renal function in diabetes.Citation14 In cross-sectional studies, the NLR has proven to be a prognostic marker for DKD among Indian,Citation29 Syrian,Citation28 Chinese,Citation30 and Turkish populations.Citation31 With respect to CVD, a systematic review and meta-analysis comprising 76,002 participants reported that persons with higher NLR had a higher risk for CVD,Citation32 and a prospective cohort study comprising 338 diabetic patients in the USA found that participants who had higher NLR had a higher risk for major adverse cardiac events.Citation33 These results validate our findings of the NLR being an independent factor of adverse outcomes in DKD patients.
Prior investigations have delineated correlations between higher NLR levels and mortality risks across a spectrum of diseases.Citation19,Citation34–36 In diabetes, after a median follow-up 91 months, Dong and colleagues showed that persons with higher NLR had a 2.03-fold higher risk for all-cause mortality and 2.76-fold higher risk for cardiovascular mortality.Citation19 In amputated diabetic foot, higher NLR carried a 1.1 times elevated risk of 1-year mortality.Citation34 In ESKD patients undergoing peritoneal dialysis, the NLR was an independent predictor of all-cause and cardiovascular mortality.Citation35 In ESKD patients undergoing hemodialysis, higher pre-dialysis NLR was linked with higher risk of short-term all-cause mortalityCitation36 Nevertheless, the available evidence is scarce on the linkage of the NLR with all-cause and cardiovascular mortality in DKD. Our investigation effectively bridges this lacuna, furnishing novel evidence accentuating the NLR’s pivotal role as a standalone predictor of all-cause and cardiovascular mortality in DKD.
Metabolic inflammation is recognized as playing a pivotal role in the mechanisms linking DKD and CVD risk.Citation37 An increase in neutrophils can exacerbate chronic inflammation,Citation38 while decreased lymphocyte counts contribute to a weakening of immune defenses, resulting in DKD patients with higher NLR being at increased risk of developing CVD. With respect to the mechanisms, firstly, increased metabolic endotoxemia leads to metabolic inflammation. In the Chronic Renal Insufficiency Cohort (CRIC) study, the accumulation of several uremic solutes in DKD, such as asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), and trimethylamine N-oxide (TMAO), resulted in a 1-fold or 2-fold increased risk of cardiovascular mortality.Citation39 Secondly, lipid accumulation likely serves as a core element in metabolic inflammation. Emerging evidence suggests that lipid metabolism disorders in patients with DKD were linked to inflammation and cardiovascular disease.Citation40,Citation41 Of note, both innate and adaptive immune responses play a crucial role in initiating metabolic inflammation linked to DKD and cardiovascular disease. Neutrophils representing the innate immune response contribute to inflammatory reaction by releasing inflammatory mediators that lead to dysfunction of the vascular wall, while lymphocytes (representing the adaptive immune response) can either enhance or dampen inflammatory responses.Citation37 In our study, our findings revealed that a higher NLR was correlated with increased all-cause and cardiovascular mortality in a population of patients with DKD, which holds important suggestions for clinical practice and patient care.
The present study has several strengths. First, this study included a large sample-scale population-based sample with extended follow-up periods (a median follow-up of 79 months) and a variety of stratified analyses and sensitivity analyses, thus providing more robust evidence. Second, this is the first study to explore the role of the NLR in the all-cause and cardiovascular mortality in DKD. Several limitations cannot be ignored. First, we cannot exclude the potentiality that the NLR is influenced by latent confounding variables. Second, the data included in this study were only accessible at baseline, which means that it is uncertain whether changes in NLR levels during the follow-up period still predict all-cause and cardiovascular mortality in DKD. Third, this study was conducted among individuals with DKD in the United States. Therefore, the generalizability of the conclusions to other populations warrants further exploration.
Conclusions
In sum, our expansive, nationally representative cohort study indicates the NLR is a predictor of all-cause and cardiovascular mortality in DKD. The findings emphasize the promising use of the NLR in stratifying and prognosticating the risk of mortality in DKD in clinical practice.
Ethics Approval and Informed Consent
The NHANES 1999–2018 was approved by the NCHS Research Ethics Review Board (Continuation of Protocol #1999–2018), and each participant signed the written informed consent.
Disclosure
The authors declare that they have no competing interests in this work.
Acknowledgments
We thank the NHANES participants and staff for their contributions. We also thank Zhang Jing, Second Department of Infectious Disease, Shanghai Fifth People’s Hospital, Fudan University, for his work on the NHANES database. His outstanding work, nhanesR package, and webpage make it easier for us to explore the NHANES database.
Data Sharing Statement
The National Health and Nutrition Examination Survey dataset is publicly available at the National Center for Health Statistics of the Centers for Disease Control and Prevention (https://www.cdc.gov/nchs/nhanes/index.htm).
References
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi:10.2215/cjn.11491116
- White S, Chadban S. Diabetic kidney disease in Australia: current burden and future projections. Nephrology. 2014;19(8):450–458. doi:10.1111/nep.12281
- Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864–2883. doi:10.2337/dc14-1296
- Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–814. doi:10.1016/s0140-6736(12)60572-8
- Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308. doi:10.1681/asn.2012070718
- Rayego-Mateos S, Morgado-Pascual JL, Opazo-Ríos L, et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int J Mol Sci. 2020;21(11):3798. doi:10.3390/ijms21113798
- Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, Egido J. Therapeutic approaches to diabetic nephropathy--beyond the RAS. Nat Rev Nephrol. 2014;10(6):325–346. doi:10.1038/nrneph.2014.74
- Perez-Gomez MV, Sanchez-Niño MD, Sanz AB, et al. Targeting inflammation in diabetic kidney disease: early clinical trials. Expert Opin Investig Drugs. 2016;25(9):1045–1058. doi:10.1080/13543784.2016.1196184
- Cai A, Shen J, Yang X, et al. Dapagliflozin alleviates renal inflammation and protects against diabetic kidney diseases, both dependent and independent of blood glucose levels. Front Immunol. 2023;14:1205834. doi:10.3389/fimmu.2023.1205834
- Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11(1):55–59. doi:10.1586/erc.12.159
- Williams KA, Labidi-Galy SI, Terry KL, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014;132(3):542–550. doi:10.1016/j.ygyno.2014.01.026
- Santoni M, De Giorgi U, Iacovelli R, et al. Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Br J Cancer. 2013;109(7):1755–1759. doi:10.1038/bjc.2013.522
- Zhou E, Wu J, Zhou X, Yin Y. The neutrophil-lymphocyte ratio predicts all-cause and cardiovascular mortality among U.S. adults with rheumatoid arthritis: results from NHANES 1999-2020. Front Immunol. 2023;14:1309835. doi:10.3389/fimmu.2023.1309835
- Azab B, Daoud J, Naeem FB, et al. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study). Ren Fail. 2012;34(5):571–576. doi:10.3109/0886022x.2012.668741
- Moh MC, Low S, Shao YM, Subramaniam T, Sum CF, Lim SC. Association between neutrophil/lymphocyte ratio and kidney impairment in type 2 diabetes mellitus: a role of extracellular water/total body water ratio. Diabet Res Clin Pract. 2023;199:110634. doi:10.1016/j.diabres.2023.110634
- Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 2021;117(13):2525–2536. doi:10.1093/cvr/cvab303
- Angkananard T, Anothaisintawee T, Ingsathit A, et al. Mediation effect of neutrophil lymphocyte ratio on cardiometabolic risk factors and cardiovascular events. Sci Rep. 2019;9(1):2618. doi:10.1038/s41598-019-39004-9
- Verma S, Husain M, Madsen CM, et al. Neutrophil to lymphocyte ratio predicts cardiovascular events in people with type 2 diabetes: post hoc analysis of the SUSTAIN 6 and PIONEER 6 cardiovascular outcomes trials. Diabetes Obes Metab. 2023;25(8):2398–2401. doi:10.1111/dom.15088
- Dong G, Gan M, Xu S, Xie Y, Zhou M, Wu L. The neutrophil-lymphocyte ratio as a risk factor for all-cause and cardiovascular mortality among individuals with diabetes: evidence from the NHANES 2003-2016. Cardiovasc Diabetol. 2023;22(1):267. doi:10.1186/s12933-023-01998-y
- Zeng G, Chen X, Jiang Z, Lin J, Wu Y, Wei J. Relationship between diet-related inflammation and bone health under different levels of body mass index. J Orthop Surg Res. 2023;18(1):1. doi:10.1186/s13018-022-03481-y
- Zeng G, You D, Ye L, et al. n-3 PUFA poor seafood consumption is associated with higher risk of gout, whereas n-3 PUFA rich seafood is not: NHANES 2007-2016. Front Nutr. 2023;10:1075877. doi:10.3389/fnut.2023.1075877
- Guo W, Song Y, Sun Y, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: evidence from NHANES 2011-2018. Front Endocrinol. 2022;13:1071465. doi:10.3389/fendo.2022.1071465
- de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi:10.1001/jama.2011.861
- Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. 2013;161:1–24.
- Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17(1):53. doi:10.1186/s12874-017-0332-6
- Seok SJ, Lee ES, Kim GT, et al. Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol Dial Transplant. 2013;28(7):1700–1710. doi:10.1093/ndt/gfs555
- Rayego-Mateos S, Rodrigues-Diez RR, Fernandez-Fernandez B, et al. Targeting inflammation to treat diabetic kidney disease: the road to 2030. Kidney Int. 2023;103(2):282–296. doi:10.1016/j.kint.2022.10.030
- Jaaban M, Zetoune AB, Hesenow S, Hessenow R. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as novel risk markers for diabetic nephropathy in patients with type 2 diabetes. Heliyon. 2021;7(7):e07564. doi:10.1016/j.heliyon.2021.e07564
- Subramani M, Anbarasan M, Shanmugam D, Muthumani LN, Vasudevan P. Role of neutrophil-lymphocyte ratio as a prognostic marker for type 2 diabetic nephropathy among Indians. Bioinformation. 2023;19(4):375–379. doi:10.6026/97320630019375
- Zhang J, Zhang R, Wang Y, et al. Effects of neutrophil-lymphocyte ratio on renal function and histologic lesions in patients with diabetic nephropathy. Nephrology. 2019;24(11):1115–1121. doi:10.1111/nep.13517
- Kahraman C, Kahraman NK, Aras B, Coşgun S, Gülcan E. The relationship between neutrophil-to-lymphocyte ratio and albuminuria in type 2 diabetic patients: a pilot study. Arch Med Sci. 2016;12(3):571–575. doi:10.5114/aoms.2016.59931
- Angkananard T, Anothaisintawee T, McEvoy M, Attia J, Thakkinstian A. Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:2703518. doi:10.1155/2018/2703518
- Azab B, Chainani V, Shah N, McGinn JT. Neutrophil-lymphocyte ratio as a predictor of major adverse cardiac events among diabetic population: a 4-year follow-up study. Angiology. 2013;64(6):456–465. doi:10.1177/0003319712455216
- Yüce A, Yerli M, Erkurt N, Çakar M. The preoperative neutrophil-lymphocyte ratio is an independent predictive factor in predicting 1-year mortality in amputated diabetic foot patients. J Foot Ankle Surg. 2023;62(5):816–819. doi:10.1053/j.jfas.2023.04.007
- Lau LFS, Ng JK, Fung WWS, et al. Relationship between serial serum neutrophil-lymphocyte ratio, cardiovascular mortality, and all-cause mortality in Chinese peritoneal dialysis patients. Kidney Blood Press Res. 2023;48(1):414–423. doi:10.1159/000530554
- Sato H, Takeuchi Y, Matsuda K, et al. Pre-Dialysis Neutrophil-Lymphocyte Ratio, a Novel and Strong Short-Term Predictor of All-Cause Mortality in Patients With Diabetic Nephropathy: results From a Single-Center Study. Ther Apher Dial. 2017;21(4):370–377. doi:10.1111/1744-9987.12533
- d’Aiello A, Bonanni A, Vinci R, et al. Meta-inflammation and new anti-diabetic drugs: a new chance to knock down residual cardiovascular risk. Int J Mol Sci. 2023;24(10). doi:10.3390/ijms24108643
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi:10.1038/nri3024
- Schrauben SJ, Sapa H, Xie D, et al. Association of urine and plasma ADMA with atherosclerotic risk in DKD cardiovascular disease risk in diabetic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Nephrol Dial Transplant. 2023;38(12):2809–2815. doi:10.1093/ndt/gfad103
- Thakur M, Tupe RS. Lipoxin and glycation in SREBP signaling: insight into diabetic cardiomyopathy and associated lipotoxicity. Prostaglandins Other Lipid Mediat. 2023;164:106698. doi:10.1016/j.prostaglandins.2022.106698
- Zhang W, Lu J, Wang Y, et al. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway. Int J Mol Sci. 2023;24(1). doi:10.3390/ijms24010858