Abstract
Purpose
Patent foramen ovale (PFO) has been implicated as a risk factor for cryptogenic ischemic stroke (CS). However, there is still a lack of widely accepted, undisputed indications for PFO closure. The present study describes the concept of the multidisciplinary PFO conference and a decision making process for closure versus no closure that was developed into a formalized clinical algorithm, and presents the results of implementing these, in terms of number and proportion of PFO closures as well as repeat referrals.
Design
Five specialists in neurology, cardiology, internal medicine, thromboembolism, and echocardiography evaluated the clinical data of 311 patients at PFO conferences during 2006 to 2009. The main criteria for closure were patients with first-ever CS with PFO and atrial septal aneurysm, or patients with recurrent CS and PFO without atrial septal aneurysm.
Results
A total of 143 patients (46%) were accepted for closure and 167 patients were rejected. Patients accepted for closure were younger (mean 50 years versus 58 years) (P < 0.001). The acceptance rate for PFO closure was similar throughout these years, with an average of 45%. Three of 167 patients (1.8%) initially rejected for PFO closure were re-referred due to recurrent stroke, and the PFO closure was subsequently performed.
Conclusion
The acceptance rate of less than 50% in the present study underscores the complex relationship between CS and PFO. Whatever the criteria used for PFO closure, any unit caring for these patients needs to have a rigorous process to avoid overtreatment as well as undertreatment and to ensure that personal preferences and economic incentives do not steer the selection process. Our algorithm provides a stable acceptance rate and a low rate of repeat referrals.
Introduction
Cryptogenic ischemic stroke (CS) is defined as a stroke that cannot be attributed to any specific cause after an extensive search for the most common ones, such as cardiac emboli, or large or small vessel disease. It is present in about 25% of ischemic stroke patients below 70 years.Citation1
Patent foramen ovale (PFO) has been implicated as a risk factor for CS, due to paradoxical embolism. A few studies have reported a significantly higher prevalence of PFO in patients with CS than in normal controls (44%–66% versus 0%–27%).Citation2–Citation5 The reported annual recurrence rate in patients with PFO and CS ranges from 3.8% to 16%, indicating the need for prevention.Citation6–Citation12 During the last decades, the therapeutic measures that have been introduced for prevention of recurrent cerebrovascular events in patients with PFO and CS are long-term anticoagulation or antiplatelet medication, surgical closure, and transcatheter closure. The observational data examining outcomes have been very promising. When studying the five largest observational trials, we found a pooled effect indicating that PFO closure by device lowers the relative risk for recurrent cerebrovascular events by almost 80%.Citation13–Citation17 Many randomized trials in this field have been started,Citation18 and to date, three randomized trials have been published.Citation19–Citation21 Yet, widely accepted indications are lacking, and in the clinical setting, there are difficulties in defining whether CS is present or not. Given these ambiguities, which are compounded by the increasing number of cases and the complexity of CS, there is a real risk of variation in clinical decision making between doctors. Consequently, to address this, we have formalized a multidisciplinary approach using a dedicated PFO conference involving experts in interventional cardiology, neurology, internal medicine, cardiac imaging, thromboembolism, and cardiology. Since 1997, the Gothenburg Center for Grown-Up Congenital Heart Disease (GUCH) has performed percutaneous closure of PFO, in order to reduce the risk of recurrent stroke in selected patients.
The present study describes the concept of the multidisciplinary PFO conference and the process for making closure versus no closure decisions that was developed into a formalized clinical algorithm, and presents the results of implementing these, in terms of number and proportion of PFO closures, as well as repeat referrals.
Material and methods
Patient selection
The present study included 311 consecutive patients with cerebrovascular events (including transient ischemic attack [TIA] and ischemic strokes) and a PFO, referred to our GUCH unit, Sahlgrenska University Hospital/Östra, during the period between January 2006 and December 2009. Patients who were referred because of decompression illness, platypnea-orthodeoxia, or migraine headache were excluded. The patients were referred from hospitals all over Sweden but mainly from the western and central parts of the country. The GUCH unit at our hospital is a tertiary center, serving a population of approximately three million. The present study was approved by the regional ethics review board in Gothenburg. Patients were informed by referring physicians and agreed to participate in this study.
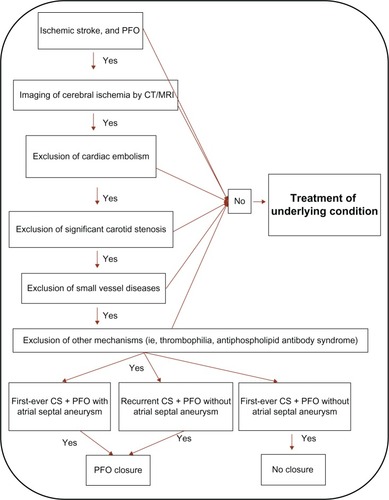
The main criteria for closure were patients with a first-ever CS with high-risk morphology (PFO with atrial septal aneurysm) or recurrent CS and a PFO with or without atrial septal aneurysm (high- or low-risk morphology). Recurrent stroke was defined as either more than one clinical event of ischemic stroke, or at least one clinical event of stroke and multiple ischemic brain lesions, of different ages, on a computed tomography (CT) scan or magnetic resonance imaging (MRI) scan of the brain. All patients who were considered for PFO closure had a transesophageal echocardiography (TEE)-verified right-to-left shunting at rest or under Valsalva maneuver.
The diagnostic workup to verify ischemic stroke or TIA in these patients occurred in two stages
In the first stage, the diagnosis of ischemic stroke or TIA was made by the stroke team at the neurological or stroke medicine unit of the hospital where the patient had been admitted for an index event. The stroke team consisted of neurologists or internal medicine specialists working with stroke medicine, specialist stroke nurses, physiotherapists, occupational therapists, psychologists, and other members of the multidisciplinary team. All patients had a clinical diagnosis of CS (that is, an identifiable cause of an ischemic cerebral stroke was not found) before they were referred to our GUCH unit. In order not to miss any information about patients at the time of conference, we asked the referring medical staff to fill in the PFO questionnaire, which gathers factual information about patients’ demographic data, the investigation process for the diagnosis of CS, such as CT scan and MRI of the brain and vertebral circulation, carotid Doppler, serum lipids, presence of thrombophilia, medical treatment, and other concomitant diseases.
The PFO questionnaire is in Swedish and is available at our website, http://www.guch.nu/guch%20hemsida/Gbg/information_lakare/PFO_konferensunderlag.pdf.
In the second stage, PFO conferences were held twice a month at our GUCH unit, in which five specialists in neurology, cardiology, internal medicine, thromboembolism, and echocardiography met to discuss patients’ data, including medical records, TEE, CT scans, and MRI of the brain. The stroke etiology and morphological risk were evaluated for each patient. Decisions were made by consensus ().
Implementation of treatment
Closure of the PFO, guided by a perioperative TEE, was performed under general anesthesia. All patients were taking warfarin or antiplatelet therapy before closure and received intravenous prophylactic antibiotics during the procedure; they were given a loading dose of 160 mg aspirin or 300 mg clopidogrel and low-molecular-weight heparin. The PFO was visualized and its size was measured both by balloon sizing and TEE. The device was chosen according to the balloon size of the PFO.
The vast majority of closures used an Amplatzer® PFO Occluder device (AGA Medical Corp, Plymouth, MN, USA). If the PFO size was more than 15 mm, an Amplatzer Septal Occluder (atrial septal defect [ASD] closure device) or an Amplatzer Multi-Fenestrated Septal Occluder “Cribriform” (multi-fenestrated ASD closure device) could be used; if it was less than 7 mm, a BioSTAR® (NMT Medical, Inc., Boston, MA, USA), a Solysafe® Septal Occluder (Swissimplant AG, Solothurn, Switzerland), or a GORE® HELEX® Septal Occluder (WL Gore and Assoc, Inc, Newark, DE, USA) device could be considered, at the operator’s discretion. Patients were monitored by telemetry (continuous 12-lead electrocardiogram [ECG]) during the next 24 hours after closure. The day after the closure, a transthoracic echocardiogram was repeated to confirm proper position of the device and exclude leakage, whereupon the patient was discharged.
Transesophageal echocardiography
TEE is considered the method of choice for PFO detection.Citation22 All patients were investigated with TEE before they were referred to us. PFO was diagnosed if contrast bubbles entered the left atrium through the oval structure or if color Doppler detected right-to-left flow between the two septa. Agitated NaCl solution, mixed with 5%–10% air by repeated and forceful injection from one syringe to another through a three-way stopcock, was used as a contrast medium. A PFO was defined as the appearance of microbubbles in the left atrium within three heartbeats from when the contrast filled the right atrium, in the absence of a tissue defect.Citation23 It is important to mention that a PFO is functionally closed most of the time, due to higher left than right atrial pressure. A provocation, such as the Valsalva maneuver, may be used in order to invert the interatrial pressure gradient and thus open the PFO. Right-to-left shunting, at rest or during the Valsalva maneuver, was detected in all patients by TEE before the PFO conferences.
Statistical analyses were performed using PASW Statistics for Windows, Version 18.0 software (IBM Corporation, Armonk, NY, USA). Variables were compared using Pearson chi-square test, and P < 0.05 was considered to be a significant difference between groups.
Results
In total, 311 patients were evaluated at the PFO conferences. We accepted 144 patients for PFO closure (99 men and 45 women), whereas 167 patients were rejected (93 men and 74 women). Our acceptance rate for closure was similar throughout these years, with an average of 45% (43% in 2006, 42% in 2007, 52% in 2008, and 42% in 2009). Patients accepted for closure were younger (mean 50 years versus 58 years) (P < 0.001). The mean age for men was 51 years and for women was 47 years in the closure group versus 57 years for men and 59 years for women in the group that was rejected for PFO closure. Of the patients in the closure group, 84% were under 60 years and 94% were under 65 years. As shown in , the presence of risk factors, such as smoking, diabetes, hyperlipidemia, and hypertension, was slightly higher in the rejected group. One patient with atrial fibrillation was accepted for closure due to epilepsy and contraindication to warfarin. This patient had already had several recurrent strokes when referred to us ().
Table 1 Baseline characteristics of patients referred to the PFO conference
Inclusion criteria for closure
The most common criterion for closure was a first-ever stroke with high-risk morphology (PFO with atrial septal aneurysm) or a recurrent CS with a low-risk morphology (PFO without atrial septal aneurysm). Our criteria for PFO closure also included patients with one CS and another thromboembolic risk factor, in this case activated protein C (APC) resistance, and patients with CS with a low-risk but very large PFO, indicated by massive right-to-left passage of more than 20 bubbles, without atrial septal aneurysm. Two patients with only one CS and low risk for PFO were accepted for device closure for other reasons.
The following types of devices were used for PFO closure in the 144 patients: Amplatzer Septal Occluder device in 98 patients, BioSTAR® (NMT Medical, Inc.) in 30 patients, the GORE HELEX Septal Occluder in two patients, the Solysafe Septal Occluder in four patients, the Amplatzer ASD Cribriform Occluder in ten patients. The mean balloon size of the PFO, as measured by TEE, was 9.25 millimeters (ranging 2 mm–24 mm, with a standard deviation of 4.58 mm).
Patients rejected for PFO device closure
In 167 of the referred patients, we did not find an indication to perform PFO closure, when adopting the criteria described above. The most common reason to refrain from closure was that the multidisciplinary panel did not regard the stroke as cryptogenic, and the second most common reason was the occurrence of only one stroke in patients with PFO with low-risk morphology. Three patients were initially rejected for PFO closure and given medical treatment instead but were referred again after a second cerebrovascular event and were then accepted for closure. The criteria for acceptance and rejection for PFO closure are shown in .
Table 2 Outcome of PFO conferences, criteria for closure or rejection
Discussion
The relationship between PFO and stroke is not straightforward. PFO is not a risk factor for stroke per se, but for CS in particular. This makes it pivotal that only CS patients are treated with closure of their PFO, as the other patients have a known etiology for their stroke, and their risk of stroke recurrence is thus unaffected by PFO closure. CS is a diagnosis of exclusion, which is dependent on the thoroughness of the clinical investigation. In 44% to 66% of patients with CS, diagnostic workup reveals a PFO,Citation4,Citation24,Citation25 and an association between PFO and CS has been described in observational clinical trials.Citation26,Citation27 Patients with documented PFO and previous embolic events are at an increased risk, of up to 4.2% per year, for recurrent stroke, even in the context of therapeutic anticoagulation.Citation9,Citation28,Citation29 Patients with PFO and atrial septal aneurysm are at higher risk of recurrent stroke than those with PFO alone.Citation11 Transcatheter treatment of atrial septal aneurysm and PFO is reported to be safe and effective in patients with paradoxical embolism.Citation30
We established PFO conferences to create a balanced view of decision making by involving experts from different relevant fields and to avoid leaving the decision to the interventionist or neurologist alone. We have developed an algorithm to facilitate the diagnostic workup (). This algorithm is based on evidence from the literature and from clinical decision-making on our patients since 1997. At the PFO conference, the specialists in internal medicine and neurology assess whether the neurological symptoms were related to a stroke and whether other etiologies can be ruled out and thus, whether the stroke can be diagnosed as cryptogenic. The interventional cardiologist and specialist in echocardiography assess whether the PFO is suitable for closure and whether the TEE provides enough information to distinguish high risk from low risk PFO. Other concomitant disease that may influence the treatment of the individual patient is also taken into consideration.
The patients who were accepted for PFO closure were younger than those who were recommended medical treatment only. Since other etiologies, namely large-vessel atherosclerosis, small-artery disease, and cardiac embolism, are more frequent in the elderly, the diagnosis of CS is less frequent than in the young. In addition, the association of PFO with CS has consistently been reported in patients younger than 55 years, whereas the association in those older than 55 years remains uncertain.Citation27 Among our patients in the closure group, 72% were younger than 55 years. Of those older than 55 years, 28% were judged to have PFO and CS and therefore were considered for closure.Citation31
Regarding sex, we accepted fewer women than men (31% versus 69%), and moreover, fewer women were referred. Women were older, but did not differ in the proportion of risk factors (data not shown). A trend of less prevalent previous cerebrovascular disease among women, as compared with men, was also observed (chi-square P = 0.052). The observed difference in this retrospective analysis could have been caused by selection bias, but further study, to investigate this putative sex difference, is warranted.
Although device closure of PFO has increasingly been performed since the early 1990s, there is still not sufficient evidence to establish that device closure is more efficient than medical treatment. On the one hand, the first randomized trial to be published concluded that percutaneous closure of PFO with the STARFlex device (NMT Medical, Inc.) plus medical therapy did not offer any significant benefit over medical therapy alone for the prevention of recurrent stroke or TIA, in patients younger than 60 years presenting with CS or TIA and a PFO.Citation19
On the other hand, by pooling the results of the five largest observational trials, we found a relatively large effect indicating that PFO closure by device lowers the relative risk for recurrent cerebrovascular events.Citation13–Citation17
One previous study reported the use of multidisciplinary management of PFO and CS, but it included fewer patients and was conducted under a shorter time period.Citation32 In the setting of multiple treatment options, unclear evidence, the complexity of CS, as well as the difficulty, in the clinical setting, of defining whether CS is present or not, a multidisciplinary approach may enhance the stability of clinical decision making and improve quality of care. It maintains transparency and clarity of medical decision making, which is important for both patients and payers of health care. The regular PFO conferences may also promote adherence to the decision algorithm for how to exclude and include PFO patients with stroke or TIA for closure. We performed approximately the same proportion of closures over all years monitored, and we have a low rate of repeat referrals.
The results of this study should be considered in the light of the following limitations. Firstly, the diagnosis of paradoxical embolism remains presumptive and cannot be considered synonymous with CS or TIA. Secondly, the patient population in this study was a selected group referred to our hospital in a nonrandomized, retrospective, consecutive order, which may not be the case in other published studies.
In conclusion, less than 50% of referred cases with PFO and suspected CS fulfilled our acceptance criteria for closure. The acceptance rates were constant through this period. Three patients were initially rejected for closure and given medical treatment instead but were referred again after a second cerebrovascular event and were then accepted for closure. Our currently used algorithm seems to have given a low rate of misclassification, of below 1.8%. In view of the 1%–2% rate of severe periprocedural complications in most published series, this misclassification rate does not seem unreasonable. We believe that a standardized multidisciplinary approach and a clinical algorithm, as described in this paper, are important for the proper assessment of PFO as a risk factor for CS.
Acknowledgments
The present study was supported by the Swedish Society of Medicine, and by grants from the Swedish state (under the LUA/ALF agreement) and from the Region Västra Götaland.
The authors thank research assistants Görel Hultsberg-Olsson and Helena Svensson for excellent assistance throughout the study. We also acknowledge the secretarial assistance from Eva Thydén.
Disclosure
The authors report no conflicts of interest in this work.
References
- JoodKLadenvallCRosengrenABlomstrandCJernCFamily history in ischemic stroke before 70 years of age: the Sahlgrenska Academy Study on Ischemic StrokeStroke20053671383138715933254
- HagenPTScholzDGEdwardsWDIncidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal heartsMayo Clin Proc198459117206694427
- LechatPMasJLLascaultGPrevalence of patent foramen ovale in patients with strokeN Engl J Med198831818114811523362165
- WebsterMWChancellorAMSmithHJPatent foramen ovale in young stroke patientsLancet19882860111122898621
- WuLAMaloufJFDearaniJAPatent foramen ovale in cryptogenic stroke: current understanding and management optionsArch Intern Med2004164995095615136302
- BogousslavskyJGaraziSJeanrenaudXAebischerNVan MelleGStroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study GroupNeurology1996465130113058628471
- ComessKADeRookFABeachKWLytleNJGolbyAJAlbersGWTransesophageal echocardiography and carotid ultrasound in patients with cerebral ischemia: prevalence of findings and recurrent stroke riskJ Am Coll Cardiol1994237159816038195520
- CujecBMainraRJohnsonDHPrevention of recurrent cerebral ischemic events in patients with patent foramen ovale and cryptogenic strokes or transient ischemic attacksCan J Cardiol1999151576410024860
- HannaJPSunJPFurlanAJStewartWJSilaCATanMPatent foramen ovale and brain infarct. Echocardiographic predictors, recurrence, and preventionStroke19942547827868160221
- HommaSSaccoRLDi TullioMRSciaccaRRMohrJPPFO in Cryptogenic Stroke Study (PICSS) InvestigatorsEffect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke StudyCirculation2002105222625263112045168
- MasJLArquizanCLamyCPatent Foramen Ovale and Atrial Septal Aneurysm Study GroupRecurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or bothN Engl J Med2001345241740174611742048
- PaciaroniMAgnelliGBertoliniAFORI (Foramen Ovale Registro Italiano) InvestigatorsRisk of recurrent cerebrovascular events in patients with cryptogenic stroke or transient ischemic attack and patent foramen ovale: the FORI (Foramen Ovale Registro Italiano) studyCerebrovasc Dis201131210911621088390
- HarrerJUWesselsTFrankeALucasSBerlitPKlötzschCStroke recurrence and its prevention in patients with patent foramen ovaleCan J Neurol Sci2006331394716583720
- SchuchlenzHWWeihsWBergholdALechnerASchmidtRSecondary prevention after cryptogenic cerebrovascular events in patients with patent foramen ovaleInt J Cardiol20051011778215860387
- ThanopoulosBVDardasPDKaranasiosEMezilisNTranscatheter closure versus medical therapy of patent foramen ovale and cryptogenic strokeCatheter Cardiovasc Interv200668574174617039525
- WeimarCHolleDNBenemannJGerman Stroke Study CollaborationCurrent management and risk of recurrent stroke in cerebrovascular patients with right-to-left cardiac shuntCerebrovasc Dis200928434935619628936
- WindeckerSWahlANedeltchevKComparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic strokeJ Am Coll Cardiol200444475075815312853
- O’GaraPTMesseSRTuzcuEMCathaGRingJCAmerican Heart AssociationAmerican Stroke AssociationAmerican College of Cardiology FoundationPercutaneous device closure of patent foramen ovale for secondary stroke prevention: a call for completion of randomized clinical trials: a science advisory from the American Heart Association/American Stroke Association and the American College of Cardiology FoundationCirculation2009119202743274719433754
- FurlanAJReismanMMassaroJClosure or medical therapy for cryptogenic stroke with patent foramen ovaleNew Engl J Med20123661199199922417252
- MeierBKalesanBMattleHPPC Trial InvestigatorsPercutaneous closure of patent foramen ovale in cryptogenic embolismN Engl J Med2013368121083109123514285
- CarrollJDSaverJLThalerDERESPECT InvestigatorsClosure of patent foramen ovale versus medical therapy after cryptogenic strokeN Engl J Med2013368121092110023514286
- SiostrzonekPLangWZangenehMSignificance of left-sided heart disease for the detection of patent foramen ovale by transesophageal contrast echocardiographyJ Am Coll Cardiol1992196119211961564219
- JohanssonMCErikssonPPekerYHednerJRåstamLLindbladUThe influence of patent foramen ovale on oxygen desaturation in obstructive sleep apnoeaEur Respir J200729114915517005584
- JobFPRingelsteinEBGrafenYComparison of transcranial contrast Doppler sonography and transesophageal contrast echocardiography for the detection of patent foramen ovale in young stroke patientsAm J Cardiol19947443813847914717
- SteinerMMDi TullioMRRundekTPatent foramen ovale size and embolic brain imaging findings among patients with ischemic strokeStroke19982959449489596240
- Di TullioMSaccoRLGopalAMohrJPHommaSPatent foramen ovale as a risk factor for cryptogenic strokeAnn Intern Med199211764614651503349
- OverellJRBoneILeesKRInteratrial septal abnormalities and stroke: a meta-analysis of case-control studiesNeurology20005581172117911071496
- ChatterjeeTPetzschMInceHInterventional closure with Amplatzer PFO occluder of patent foramen ovale in patients with paradoxical cerebral embolismJ Interv Cardiol200518317317915966921
- De CastroSCartoniDFiorelliMMorphological and functional characteristics of patent foramen ovale and their embolic implicationsStroke200031102407241311022072
- WahlAKrumsdorfUMeierBTranscatheter treatment of atrial septal aneurysm associated with patent foramen ovale for prevention of recurrent paradoxical embolism in high-risk patientsJ Am Coll Cardiol200545337738015680715
- HandkeMHarloffAOlschewskiMHetzelAGeibelAPatent foramen ovale and cryptogenic stroke in older patientsNew Engl J Med2007357222262226818046029
- RigatelliGBraggionGChinagliaMSetting up a multidisciplinary program for management of patent foramen ovale-mediated syndromesJ Interv Cardiol200619326426816724970