Abstract
Purpose
The objective of this study was to investigate latent classes of oral health and the factors associated with them in acutely admitted elderly inpatients.
Patients and Methods
A cross-sectional study was conducted using purposive sampling to survey Chinese older-adult acutely inpatients. Data was collected utilizing several instruments, including a general information questionnaire, Brief Oral Health Status Examination (BOHSE), Oral Frailty Index-8 (OFI-8), Mini-Nutritional Assessment Short Form (MNA-SF), and Frailty Screening Questionnaire (FSQ). Latent class analysis was applied to identify distinct categories of oral health among elderly inpatients, and multinomial logistic regression was employed to analyze the factors associated with different oral health categories.
Results
In this study, a total of 504 elderly patients were ultimately included, leading to the identification of three latent classes of oral health: “oral health-low level group (41.27%)”, “oral health-moderate level group (25.4%)”, and “oral health-high level group (33.33%)”. The findings revealed that patients with advanced age, elevated neutrophil percentage, and higher C-reactive protein (CRP) values were more likely to be classified in the low oral health group. Additionally, individuals experiencing malnutrition and frailty had a higher risk of falling into the low oral health category. Those with comorbidities and oral frailty were more prevalent in the moderate oral health group. At the same time, elderly patients with higher BMI (22.95±3.043) ratios were more likely to be categorized in the high oral health group.
Conclusion
This study sheds light on three distinct latent classes of oral health among acutely admitted elderly inpatients. These findings underscore the importance of healthcare professionals focusing on the assessment and health education of elderly patients’ oral health. Furthermore, personalized interventions should be developed to promote healthy aging, with particularly attention to enhancing oral health outcomes in this population.
Introduction
Statistics from the World Health Organization (WHO) project a notable increase in the global population aged 60 years and over, expected to rise from 12% to 22% by the year 2050.Citation1 In China, the latest demographic data reveals that around 13.5% of the total population is aged 65 years and older, and this figure has reached 18.7% by the year 2020.Citation2 The acceleration of the aging process is contributing to a surge in emergency department visits by older adults. In the United States, elderly patients accounted for over 20 million emergency department visits, representing more than 15% of all visits. Similarly, in France, there were over 2.7 million emergency department visits by the elderly in 2019. In China, older adults constituted 18% of emergency department visits.Citation3,Citation4 Highlighting the significance of oral health in the older population, the State of Global Oral Health Report underscores that oral health is a crucial indicator of overall health in older individuals. Furthermore, oral diseases can impose a substantial burden on an individual’s health.Citation5 Notably, studies, such as the one conducted by Ní Chróinín et al, reveal that oral health is unfavorable in older acutely hospitalized patients.Citation6 These findings emphasize the pressing need for urgent attention to the oral health of acutely admitted elderly patients, especially as the aging demographic grows globally.
The WHO’s comprehensive definition of oral health extends beyond the physical state, incorporating social functioning and mental health considerations.Citation5 Globally, common oral diseases, such as dental caries, periodontal disease, and tooth loss, impact approximately 45% of the population.Citation7,Citation8 Alarmingly, around 2.5 billion people suffer from untreated oral diseases, with a striking 96% of individuals aged 65 years and older grappling with oral health issues like dental caries.Citation7,Citation8 Despite its significance, oral health tends to be neglected, particularly among older adults. Oral diseases can induce pain, discomfort, and increase the risk of malnutrition due to difficulties in chewing or swallowing.Citation9 Furthermore, the aesthetic aspects of poor oral health, including dental appearance, defects, and oral odor, can diminish self-esteem and prompt older adults to avoid social activities and social isolation, impacting their psychosocial health.Citation10 Research has unveiled that compromised oral health and swallowing difficulty in elderly patients heightens the risk of inhaling oral bacteria into the lungs, elevating the likelihood of infectious diseases like hospital-acquired pneumonia.Citation10,Citation11 Additionally, it influences hospital mortality, length of stay, and healthcare costs for elderly patients.Citation11 The burden of oral health extends beyond physiological implications, permeating into the social and psychological realms, significantly affecting the overall quality of life for older adults. Given that elderly patients often grapple with a combination of acute and underlying illnesses with more medically complex and typically take a higher number of medications compared to their younger counterparts in the emergency department, they are at a heightened risk for adverse health-related outcomes. An observational study conducted by Maeda et al revealed that poor oral health upon admission served as an independent predictor of death during hospitalization in elderly patients.Citation12 The assessment of oral health in acutely admitted elderly inpatients becomes crucial not only for diagnosing oral diseases but also for evaluating the risk of systemic diseases. Consequently, integrating comprehensive oral health assessments into the care of elderly inpatients is paramount to addressing both oral and overall health outcomes in this vulnerable population.
Various perspectives exist regarding the factors contributing to poor oral health in older adults. Sheiham and Watt proposed that common risk factors for oral health encompass smoking, alcohol consumption, unhealthy diet, stress, and lack of exercise.Citation13 They suggested that the cumulative impact of these risk factors significantly influences oral health.Citation13–15 Additionally, research indicates that poor oral health in older adults correlates with conditions such as sarcopenia, cognitive impairment, comorbidities, and frailty, revealing a complex interplay of multiple factors severely affecting the quality of life for older individuals.Citation16–18 The oral health of older adults exhibits complexity, variability, and heterogeneity across different settings, with the oral health of acutely admitted elderly inpatients often overlooked. Prior studies assessing the oral health of elderly patients utilized aggregate scores on scales and a single assessment dimension, neglecting the heterogeneity among groups with distinct characteristics.Citation19 Latent class analysis is a labelling of a form of finite mixed model in which the observed indicators are categorical.Citation20 Using latent category analysis, an analytical method that identifies latent characteristics based on individuals’ responses and groups those with similar characteristics together.Citation20 This study aimed to (1) identify latent classes of oral health in acutely admitted elderly inpatients and (2) explore factors associated with categorizing individuals into these latent classes. This research seeks to establish a theoretical foundation for promoting oral health and healthy aging among elderly inpatients.
Material and Methods
Study Design and Setting
This cross-sectional study aimed at analyzing the oral health status and associated factors in acutely admitted elderly inpatients using a latent category approach. While subgroup analysis from a traditional perspective seeks patient characteristics associated with disease outcomes, these characteristics may manifest differently in individual patients. To comprehend the nuanced differences between patients, a person-centered approach focuses on similarities or relationships between individuals. Latent category analysis, identifying individuals with common characteristics, emerges as the preferred method for health behavior research.Citation21 The emergency department witnesses an annual average of over 3000 cases of acute admissions of elderly patients, ensuring an ample and diverse sample size for this study.
Study Participants
This study employed a purposive sampling method to select elderly patients attending the emergency department of the Fourth Affiliated Hospital, Zhejiang University School of Medicine, between October 20 and December 31, 2023. Inclusion criteria encompassed individuals aged 65 years and above, experiencing an acute onset of illness leading to hospitalization, and providing informed consent to participate in the study voluntarily. Exclusion criteria comprised patients with cardiac arrest, severe trauma, snakebites, poisonings, visual and hearing impairments, as well as those with automatic discharges. Nylund’s simulation modeling demonstrated that the information criterion and likelihood test reliably identify the correct model when the sample size ranges between 500–1000 models.Citation22 Therefore, this study aimed to recruit at least 500 study participants.
Measuring Instrument
The study gathered demographic: including age, gender, education level, smoking history (defined as a duration of previous smoking ≥1 year), drinking history (defined as a duration of previous drinking ≥1 year), place of residence (categorized as urban or suburban). We also collected clinical information: Body Mass Index (BMI, calculated as weight in kilograms divided by the square of height in meters), white blood cell count, percentage of neutrophils, percentage of lymphocytes, C-reactive protein (CRP) levels, multimorbidity (defined as the presence of two or more chronic diseases simultaneously), polypharmacy (defined as the use of five or more medications, following China’s catalog of inappropriate medicines for the elderly),Citation23 nutritional status, general frailty, and oral frailty.
Brief Oral Health Status Examination (BOHSE)
BOHSE developed by Kayser-Jones in 1995, comprises ten items assessing various oral components, including lymph nodes, lips, tongue, mucosal tissues, gingiva, salivary secretion, natural teeth, denture, teeth bite during chewing and oral hygiene. Each item is scored on a 3-point Likert scale, with a score of 0 indicating “healthy”, 1 indicating “healthy change”, and 2 indicating “unhealthy”. The Likert-3 scale was employed, with 0 denoting “healthy”, 1 indicating “altered health”, and 2 indicating “unhealthy”, where a higher score corresponds to a poorer oral condition.Citation24 The Chinese version of the BOHSE demonstrates a Cronbach’s coefficient of 0.873 and a re-test reliability of 0.775, making it suitable for use by non-dental professionals in assessing the oral health status of elderly inpatients.Citation25
Oral Frailty Index-8 (OFI-8)
This instrument was employed to evaluate oral frailty in the elderly, encompassing aspects such as denture use, swallowing ability, chewing ability, oral health-related behaviors, and social participation. This tool consists of five dimensions with eight entries, resulting in a total score ranging from 0 to 11, where a score of ≥4 indicates oral frailty. The overall Cronbach’s α coefficient for the scale was 0.692.Citation26 Additionally, the Chinese version of the oral weakness screening scale for the elderly demonstrated a Cronbach’s α coefficient of 0.949 and a re-test reliability coefficient of 0.786.Citation27 This Chinese version exhibits robust reliability and validity, positioning it as a valuable tool for assessing oral weakness in the elderly.Citation27
Mini-Nutritional Assessment Short Form (MNA-SF)
The MNA-SF, developed by Rubenstein et al, serves as a tool for assessing nutritional status in older adults.Citation28 Comprising six entries covering Body Mass Index (BMI), recent weight changes, acute illness or major psychological changes, mobility, neuropsychiatric disorders, and food intake, the scale yields a total score ranging from 0 to 14. A score of ≥11 is indicative of normal nutrition, while a score of <11 suggests the presence of malnutrition. Zhang et al demonstrated that the scale’s Cronbach’s α coefficient was 0.711, indicating suitability for nutritional assessment in elderly inpatients.Citation29
Frailty Screening Questionnaire (FSQ)
FSQ is a tool designed by Ma et al specifically for screening frailty in community-dwelling older adults in China. This questionnaire is rooted in the frailty phenotype proposed by Fried et al and is structured into five self-reported sections.Citation30 The sections cover various aspects: slow walking, defined as the inability to walk 250 meters independently or with an assistive device other than a wheelchair, regardless of speed; frailty, defined as difficulty in lifting or carrying 5 kg; exhaustion, indicated by responses such as “I struggle with everything I do” or “I cannot do it”. inactivity, defined as spending less than 3 hours per week on leisure activities; and weight loss, defined as unintentional weight loss of at least 4.5 kg in the past year. Liu et al demonstrated the feasibility of the FSQ for routine frailty screening in elderly patients admitted to the hospital in an emergency setting.Citation31
Data Collection
The data collection process was carried out by the study team members within two hours of the elderly patient’s admission to the hospital. We use the Chinese version of the questionnaire for data collection. For elderly patients with multiple emergency visits during the survey period, the team recorded information from their first visit. The study team underwent five 1-hour professional training sessions, overseen by a dental professional, to instruct team members on examining and assessing the oral health status of the elderly. The training encompassed topics such as oral anatomy, common oral diseases, and the use of examination instruments. Each team member successfully passed an examination before completing the training. All examinations of elderly patients were conducted while they were in bed and took approximately 8–25 minutes to complete, with an average examination time of 11.6 minutes. Each study team member had an examination kit comprising a tongue depressor, gauze, hand-held light, and disposable gloves. The assessments were consistently performed in the same order, initiating with the observation and palpation of enlarged lymph nodes and concluding with the documentation of oral cleanliness status.
Statistical Methods
Data analysis was performed using SPSS 20.0 software. GraphPad Prism 9.5.1 was used to plot line graphs. Descriptive statistics were employed, expressing measurement data as mean and standard deviation, and count data as frequency and percentage. Univariate analysis of latent classes of oral health in elderly patients involved using the chi-square test or Fisher’s exact probability method for categorical information, ANOVA for measurement information, and non-parametric tests in cases of heterogeneous variance. Missing variables were substituted with the proximal point median. Multinomial logistic regression analyses were conducted to explore the factors influencing the latent classes of oral health in elderly inpatients. Statistical significance was determined at P < 0.05.
Mplus software (version 8.3) was utilized for latent class analysis. The data derived from the Brief Oral Health Checklist were initially transformed into binary form (0 and 1). A raw entry score of ≥1 was designated a value of 0, representing unhealthy oral health, while a raw entry score of 0 was assigned a value of 1, indicating healthy oral health. Starting with a single-category model, the number of categories in the model was incrementally increased until the model fitting indicators reached the optimal values. Model fit indexes comprised: (1) log-likelihood ratio, Akaike information criterion (AIC), Bayesian information criterion (BIC), and adjusted BIC (aBIC), where smaller values of AIC, BIC, and aBIC indicated better model fit; (2) information entropy, with values ranging from 0 to 1, where a closer value to 1 suggested more accurate classification (values <0.6 indicated over 20% classification errors, while values >0.8 suggested a model classification accuracy of 90%); and (3) Lo Mendell-Rubin (LMR) and Bootstrapped likelihood ratio test (BLRT), with P < 0.05 signifying that the kth model performed better than the k-1th model.Citation22,Citation32,Citation33 In this study, to improve the reliability of the assessment, each oral health assessment was conducted by two members at the same time, and when there was a disagreement, it was assessed by a dentist specializing in dentistry.
Ethical Considerations
The original study received approval from the Human Research Ethics Committee of the Fourth Affiliated Hospital Zhejiang University School of Medicine (Approval NO.: K2023153). Before the commencement of the study, written informed consent was obtained from all participants or their caregivers. Participants retained the right to withdraw from the study at any point, and all data collected were exclusively used for this study. The conduct of this study complies with the Declaration of Helsinki.
Results
In this study, a total of 522 elderly patients were initially recruited, including 18 with repeat admissions, ultimately resulting in the inclusion of 504 elderly patients in the research. The average age of the survey respondents was 77.27 years, ranging from a minimum age of 65 years to a maximum of 99 years. All elderly patients included in the study were married. The descriptive statistics for height were (163.15±6.862) cm, for weight (58.68±8.98) kg, and detailed Results for all variables are presented in .
Table 1 Descriptive Statistics and Analysis of Variance for All Variables (n=504)
Latent class analysis was applied to the oral health assessment results of elderly inpatients, and a total of four latent class models were fitted in this study. The fitting metrics for each model are presented in . Model 4 exhibited entropy values less than 0.8, and LMR was not statistically significant, leading to its exclusion from further consideration. The single-category models displayed the largest AIC, BIC, and ABIC values. Moreover, LR tests for models 2 and 3 were statistically significant (LMR < 0.0001 and BLRT < 0.0001), indicating population heterogeneity. When comparing Models 2 and 3, Model 3 demonstrated the smallest AIC and BIC values, the highest entropy (0.874), and statistically significant p-values for LMR and BLRT. Although the p-values of LMR and BLRT were also statistically significant in Model 2, Model 3 presented the lowest AIC and BIC values, along with the highest entropy value, suggesting it as the best fit for this study. Consequently, Model 3 was selected for latent class classification.
Table 2 Model Fit Indices Derived from Latent Class Analysis on Models with 1–4 Classes
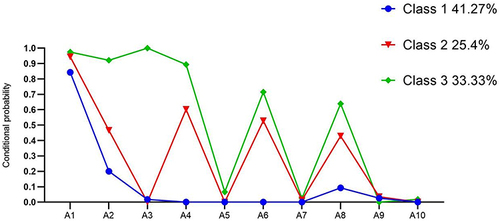
illustrates the 3-category model, displaying the probability of occurrence for ten oral health entries in each category. Latent class 1 was designated as the “Oral Health-Low Level Group (C1)“ and comprised 41.27% of subjects (n = 208). C1 exhibited a low overall oral health level, with the probability of a lymph node response at 0.843, albeit lower thanC2 and C3. Latent class 2 was named the “Oral Health-Moderate Level Group (C2)” and encompassed 25.4% of subjects (n = 128). C2 demonstrated a moderate overall oral health level, with response probabilities falling between C1 and C3, except for the peri-dental and oral hygiene entries, which had a probability of zero. Latent class 3 was denoted as the ”Oral Health-High Level Group (C3)”, incorporating 33.33% of subjects (n = 168). C3 showcased a high overall oral health level, with response probabilities for periapical, chewing, natural teeth, and oral hygiene surpassing those for C1 and C3, although the probabilities of responding to the entries for periapical, chewing, natural teeth, and oral hygiene were all less than 0.1.
Figure 1 The three-class model and probability of 10 oral health symptoms within each class (n = 504).
The analysis of variance for all variables revealed that age, BMI, white blood cell count, percentage of neutrophils, CRP, co-morbidities, nutritional status, frailty, and oral frailty exhibited statistically significant differences (P < 0.05) when compared among the three latent classes of elderly patients. In contrast, the differences in the remaining variables were not statistically significant (P > 0.05), as detailed in .
To explore the factors associated with the latent classes of oral health in elderly inpatients (C1, C2, and C3), multinomial logistic regression was employed, see . Nine variables (age, BMI, leukocyte count, percentage of neutrophils, CRP, multimorbidity, nutritional status, frailty, and oral frailty) were included in the regression equations for the latent classes, and no significant covariance was observed between the variables. The results of the regression equations were presented in the C1, C2, and C3 regression models. When comparing C3 to C1 (with C1 as the reference category), the findings indicated that older patients were more likely to be categorized as C1 (OR =1.149, 95% CI: 1.102–1.198, P<0.001); older patients with a higher BMI were more likely to be classified as C3 (OR =0.86, 95% CI: 0.786–0.941, P=0.001); higher neutrophil percentage and higher CRP were more likely to be categorized as C1 (OR = 1.024, 95% CI: 1.002–1.046, P=0.03; OR = 1.008, 95% CI: 1.003–1.014, P=0.003); and elderly patients with normal nutritional status were more likely to be categorized as C3 compared to malnourished elderly patients (OR =0.546, 95% CI: 0.301–0.991, P=0.047). Additionally, elderly patients without frailty had a higher risk of being categorized as C3 compared to debilitated elderly patients (OR =0.52, 95% CI: 0.031–0.898, P=0.019). In the comparison of C2 to C1 (with C1 as the reference category), the results showed that the older the elderly patient, the more likely they were to be categorized as C1 (OR = 0.945, 95% CI: 0.913–0.978, P=0.001); higher percentages of neutrophils and CRP were more likely to be classified as C1 (OR = 0.975, 95% CI: 0.956–0.995, P=0.016; OR = 0.993, 95% CI: 0.988–0.998, P=0.01); and compared to frail older patients, older patients without frailty had a higher risk of being categorized as C2 (OR = 1.827, 95% CI: 1.083–3.082, P=0.024). When C2 was compared to C3 (with C3 as the reference category), the findings revealed that older patients were more likely to be categorized as C2 (OR = 1.085, 95% CI: 1.04–1.132, P<0.001); older patients with greater BMI were more likely to be categorized as C3 (OR = 0.89, 95% CI: 0.82–0.976, P=0.012); older patients without comorbidities had a higher risk of being categorized as C3 compared to older patients with comorbidities (OR = 0.549, 95% CI: 0.319–0.947, P=0.031); and older patients with oral frailty were at a higher risk of being categorized as C2 (OR =0.496, 95% CI: 0.255–0.966, P=0.039).
Table 3 Multiple Multinomial Logistic Regression for Latent Classes
Discussion
In this study, oral health and its associated factors in acutely admitted elderly inpatients were explored through latent class analysis, marking an attempt to identify latent classes of oral health in this population. The primary findings can be summarized as follows: delineation of three distinct subtypes of oral health, namely, the “oral health-low group”, “oral health-moderate group”, and “oral health-high group”. The analysis revealed that as age increased, the level of oral health also decreased. Specifically, elderly patients with higher age, neutrophil percentage, and CRP values were more likely to be categorized as the low oral health group. Additionally, malnourished and debilitated elderly patients faced a higher risk of being classified into the low oral health group. Co-morbid elderly patients and those with debilitation were more likely to be categorized as the medium oral health group. Conversely, elderly patients with higher BMI ratios were more likely to be classified into the high oral health group. These findings contribute valuable insights into the nuanced relationship between age, health indicators, and oral health status among acutely admitted elderly inpatients.
The findings of this study revealed that older inpatients with advanced age faced an elevated risk of experiencing low levels of oral health—a correlation supported by prior research emphasizing age as a crucial predictor of oral health.Citation34 As individuals age, the various bodily systems undergo a gradual decline in function, attributable to the accumulation of damaged tissues and substances resulting from intrinsic or extrinsic mechanisms. Consequently, oral challenges such as tooth loss and difficulties in chewing become more prevalent.Citation35 Elderly patients commonly exhibit weak oral health behaviors, characterized by a lack of awareness regarding oral health care. They frequently neglect oral hygiene, engage in infrequent or completely absent daily tooth brushing, and are less inclined to seek professional assistance for oral issues. The coexistence of aging and weak oral health behaviors places elderly individuals at an increased susceptibility to poor oral health. Recognizing this, the Global Strategy for Oral Health emphasizes that the adverse consequences of oral health problems accumulate over time, exerting a negative impact on later life.Citation36 Given the cumulative nature of these oral health challenges, it is imperative to prioritize the oral health of elderly patients with advanced age. This issue necessitates a concerted effort to enhance health education initiatives targeted specifically at older adults, aiming to bolster awareness and improve oral health behaviors among this demographic.
The findings of this study also indicated that elderly individuals with higher BMI (22.95±3.043) values were more likely to be classified into the high oral health group compared to the low (21.35±2.976) and intermediate (21.94±2.855) oral health groups. While Han et alCitation37 found a correlation between lower BMI and poorer oral health in adults aged ≥65 years, a cohort study by Chang et alCitation38 suggested an association between poor oral health and higher BMI, presenting a contrast with the current study’s findings. Discrepancies in results were attributed to differences in age groups included in the cohort study, as well as variations in the baseline oral health levels of older adults across different settings and countries. BMI, an anthropometric measure of body fat relative to a person’s overall mass, is also recognized as a straightforward method for assessing nutritional status.Citation39 Malnutrition, often evidenced by reduced body weight and BMI, is primarily a consequence of insufficient protein or energy intake. This study’s results further indicated that malnourished elderly inpatients faced a heightened risk of developing low oral health levels, exhibiting a 0.546 times higher risk than those with high oral health levels, aligning with findings from Toniazzo et al’s study.Citation40 Poor oral health can hinder the consumption of hard foods, negatively impacting nutrient intake and elevating the risk of malnutrition. Inadequate intake of various nutrients, in turn, heightens the risk of oral health problems such as gum disease, dental caries, and insufficient salivation.Citation41 Physiological changes resulting from aging, chronic diseases, and multiple medications may contribute to insufficient salivary secretion, causing challenges in chewing and swallowing and amplifying the risk of malnutrition.Citation42,Citation43 Given the reciprocal causation between oral health and malnutrition, where malnutrition adversely impacts oral health, and poor oral health influences dietary intake, potentially leading to malnutrition, healthcare professionals should intensify assessments of the oral health and nutritional status of elderly patients. Additionally, multidisciplinary and interdisciplinary teams should collaborate to ensure patients maintain optimal nutritional and oral health statuses.
The study findings revealed that higher neutrophil percentage and CRP values increased the risk of developing low oral health levels compared to moderate and high levels. Neutrophil percentage, commonly employed in clinical practice as an indicator of pathogen prediction, serves as the primary response cell during body inflammation. CRP, a non-specific inflammatory marker and acute phase response protein, also signals inflammatory response. Elevated neutrophil percentage and CRP levels both indicate inflammation in the body. While Khoury et al demonstrated that neutrophils could function as early prediction and diagnosis biomarkers for oral health, the present study relied on serum neutrophil percentage as a potential indicator associated with poor oral health, necessitating further validation through expanded sample sizes.Citation44 Notably, Kotronia et al’s cross-sectional survey of community-dwelling older adults linked poor oral health with elevated CRP levels.Citation45 Other studies have established that conditions like periodontitis, constituting poor oral health, act as risk factors for exacerbating neuroinflammation, potentially leading to a heightened chronic inflammatory state.Citation46,Citation47 Oral pathogens may impact systemic health by invading the airways or bloodstream. Dental issues such as tooth loss, associated with poor oral health, can result in chewing difficulties, affecting nutrient intake and causing deficiencies in antioxidants and vitamins, consequently influencing systemic inflammation levels.Citation45 The observed association between poor oral health, increased neutrophil percentage, and elevated CRP underscores the need for attention to inflammation levels in the medical management of elderly patients.
The findings of this study indicated that elderly patients with multimorbidity faced an elevated risk of developing moderate oral health as opposed to achieving high levels of oral health. Multimorbidity, defined as the coexistence of two or more chronic diseases within an individual,Citation48 has seen an increased prevalence, with chronic diseases contributing to 88.5% of total deaths in China in 2019.Citation49 As the population ages, the confluence of multimorbidity and oral diseases becomes more apparent, with poor oral health, including tooth loss and oral inflammation, potentially leading to malnutrition, pain, and other adverse impacts on both quality of life and overall health. These factors collectively contribute to unhealthy aging and exacerbate chronic diseases in the elderly. Notably, periodontal inflammation emerges as a risk factor for systemic inflammation, ultimately culminating in cardiovascular disease.Citation50,Citation51 The strong association between poor oral health in older adults and chronic diseases, encompassing cardiovascular disease, diabetes, and Alzheimer’s disease, underscores the need to address oral health within the broader context of overall health management.Citation50,Citation52 Emerging theories propose that, alongside microvascular and macrovascular damage, endothelial dysfunction triggers local and systemic inflammatory responses, shaping the intricate relationship between chronic diseases and oral health.Citation53,Citation54 In light of these associations between oral health and diverse chronic diseases, it becomes imperative to enhance oral health behaviors among elderly patients and provide personalized health education.
The study findings indicated that debilitated elderly patients were at a higher risk of being categorized with a low level of health. Furthermore, elderly patients experiencing oral debilitation are more prone to be classified with a moderate level of oral health as opposed to a high level, as evidenced by Chew et al’s findings, which demonstrate a significant association between poor oral health and frailty in elderly inpatients.Citation55 Oral health emerges as a predictive factor for frailty in the elderly, operating through various pathways, including functional and psychosomatic routes. Poor oral health leads to reduced protein and micronutrient intake, contributing to the onset of frailty in the elderly.Citation18 Additionally, the impact of poor oral health extends to self-esteem and social interactions, exacerbating the development of senile debility. Oral frailty is a series of phenomena and processes resulting in changes in various oral conditions (number of teeth, oral function, oral hygiene, etc). with age. It involves a diminished interest in oral health, a decrease in physical and mental reserve capacity, and dysfunction in eating, collectively leading to a deterioration in physical and psychological functioning.Citation26 Importantly, this definition emphasizes that oral frailty is not solely an anatomical problem but also encompasses effects on the entire organism and psychological aspects. With a prevalence of 8.4%-22.7% in the elderly, oral frailty is closely linked to frailty, representing one of its types in the elderly.Citation56 Oral frailty predicts the onset of debilitation, contributing to its development through malnutrition, systemic inflammatory response, and reduced quality of life.Citation18 The interaction between frailty and oral frailty increases the risk of poor oral health in elderly inpatients. The study recommends that multidisciplinary teams, comprising professionals from dentistry, geriatrics, and nursing, pay close attention to oral frailty and debilitation. Early screening and assessment for debilitation, the establishment of early warning and predictive diagnostic models, and timely interventions and health education are crucial in improving the quality of life for elderly inpatients.
This study’s strength lies in its emphasis on the heterogeneity of characteristics among various groups, particularly in analyzing oral health and related factors in acutely admitted elderly inpatients. The exploration of factors such as age, CRP, frailty, and nutritional status provides valuable insights into their impact on the oral health of elderly inpatients. The comprehensive data collection, encompassing general demographic information, social characteristics, biochemical indicators, and other clinical details, facilitates a detailed analysis of the influencing factors. Consequently, the conclusions derived from this study are considered representative and contribute significantly to the understanding of the complex interplay between these factors and oral health in elderly inpatients.
The cross-sectional design of this study limited the researcher’s ability to establish causal relationships between identified risk factors and latent class memberships. Therefore, future research should employ a more comprehensive approach, incorporating various study designs, such as longitudinal studies, to delve into the intricacies of oral health among acutely admitted elderly inpatients. Given the diverse social environments and lifestyle habits of elderly patients across different geographic regions, subsequent studies in China should adopt a multicenter, large-sample approach to comprehensively analyze the factors associated with oral health among this population. This study faced limitations related to the survey instrument. Specifically, subgroups based on factors like periodontitis, or the number of teeth were not utilized to analyze poor oral health. Future investigations may benefit from a more nuanced exploration of subgroup analysis to enhance the understanding of the factors influencing oral health outcomes in elderly inpatients.
Conclusion
Through latent category analysis, three distinct latent classes of oral health were identified among acutely admitted elderly inpatients. Age, BMI, white blood cell count, neutrophil percentage, CRP, multimorbidity, nutritional status, frailty, and oral frailty emerged as significant factors influencing the oral health of older inpatients. To foster healthy aging among the elderly, both community and healthcare facilities must enhance oral health screening and assessment protocols tailored specifically for older adults. Additionally, collaborative efforts involving multidisciplinary teams comprising professionals from dentistry, nutrition, geriatrics, and nursing should be implemented to effectively manage the oral health needs of elderly patients. Moreover, adopting multidisciplinary approaches to deliver comprehensive oral health education and promote positive oral health behaviors among the elderly is paramount.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Additional information
Funding
References
- World Health Organization. Ageing and health. Available from: http://www.who.int/zh/health-topics/ageing#tab=tab_1. Accessed September 11, 2023.
- Ministry of Civil Affairs. 2021 statistical bulletin on development of civil affairs. Available from: https://www.mca.gov.cn/images3/www2017/file/202208/2021mzsyfztjgb.pdf. Accessed December 3, 2023.
- Shenvi CL, Platts-Mills TF. Managing the Elderly Emergency Department Patient. Ann Emerg Med. 2019;73(3):302–307. doi:10.1016/j.annemergmed.2018.08.426
- Naouri D, Yordanov Y, Lapidus N, Pelletier-Fleury N. Cost-effectiveness analysis of direct admission to acute geriatric unit versus admission after an emergency department visit for elderly patients. BMC Geriatr. 2023;23(1):283. doi:10.1186/s12877-023-03985-0
- World Health Organization. Global oral health status report: towards universal health coverage for oral health by 2030. Available from: https://www.who.int/publications/i/item/9789240061484. Accessed December 5, 2023.
- Chróinín D N, Montalto A, Jahromi S, Ingham N, Beveridge A, Foltyn P. Oral health status is associated with common medical comorbidities in older hospital inpatients. J Am Geriatr Soc. 2016;64(8):1696–1700. doi:10.1111/jgs.14247
- Benzian H, Watt R, Makino Y, Stauf N, Varenne B. WHO calls to end the global crisis of oral health. Lancet. 2022;400(10367):1909–1910. doi:10.1016/S0140-6736(22)02322-4
- Centers for Disease Control and Prevention. Oral Health Surveillance Report: trends in Dental Caries and Sealants, Tooth Retention, and Edentulism, United States, 1999–2004 to 2011–2016. Available from: https://www.cdc.gov/oralhealth/publications/OHSR-2019-index.html. Accessed December 11, 2023.
- Poisson P, Laffond T, Campos S, Dupuis V, Bourdel-Marchasson I. Relationships between oral health, dysphagia and undernutrition in hospitalised elderly patients. Gerodontology. 2016;33(2):161–168. doi:10.1111/ger.12123
- Ngwu CC, Fadare AS. Oral Health Condition of Geriatrics. Orapuh Lit Rev. 2023;3(1):ORO11.
- Gibney JM, Wright FA, D’Souza M, Naganathan V. Improving the oral health of older people in hospital. Australas. J Ageing. 2019;38(1):33–38. doi:10.1111/ajag.12588
- Maeda K, Mori N. Poor oral health and mortality in geriatric patients admitted to an acute hospital: an observational study.. BMC Geriatr. 2020;20(1):26. doi:10.1186/s12877-020-1429-z
- Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000;28(6):399–406. doi:10.1034/j.1600-0528.2000.028006399.x
- World Health Organization. Constitution of WHO: Principles. World Health Organization. Available from: http://www.who.int/about/mission/en/. Accessed December 11, 2023.
- Williams DM, Mossey PA, Mathur MR. Leadership in global oral health. J. Dent. 2019;87:49–54. doi:10.1016/j.jdent.2019.05.008
- Gil-Montoya JA, Sánchez-Lara I, Carnero-Pardo C, et al. Oral Hygiene in the Elderly with Different Degrees of Cognitive Impairment and Dementia. J. Am Geriatr Soc. 2017;65(3):642–647. doi:10.1111/jgs.14697
- Ahmadi B, Alimohammadian M, Yaseri M, et al. Multimorbidity: Epidemiology and Risk Factors in the Golestan Cohort Study, Iran: a Cross-Sectional Analysis. Medicine. 2016;957:e2756. doi:10.1097/MD.0000000000002756
- Hakeem FF, Bernabé E, Sabbah W. Association between oral health and frailty: a systematic review of longitudinal studies. Gerodontology. 2019;36(3):205–215. doi:10.1111/ger.12406
- Yang H, Xiao J, Cui S, Zhang L, Chen L. Oral Health Assessment Tools for Elderly Adults: a Scoping Review. J Multidiscip Healthc. 2023;16:4181–4192. doi:10.2147/JMDH.S442439
- Lanza ST, Rhoades BL. Latent class analysis: an alternative perspective on subgroup analysis in prevention and treatment. Prev Sci. 2013;14(157):68. doi:10.1007/s11121-011-0201-1
- Kongsted A, Nielsen AM. Latent Class Analysis in health research. J Physiother. 2017;63(1):55–58. doi:10.1016/j.jphys.2016.05.018
- Aflaki K, Vigod S, Ray JG. Part II: a step-by-step guide to latent class analysis [retracted in. J Clin Epidemiol. 2023;159:348–351. doi:10.1016/j.jclinepi.2022.05.009
- Du LN. Study on the relationship between Potential InappropriateMedication and frailty in elderly diabetic patients. Xian: XIAN Medical University. 2022. doi:10.27909/d.cnki.gxaxy.2021.000039
- Kayser-Jones J, Bird WF, Paul SM, Long L, Schell ES. An instrument to assess the oral health status of nursing home residents. Gerontologist. 1995;35(6):814–824. doi:10.1093/geront/35.6.814
- Zhao CJ, Ding F. Translation and psychometric evaluation of the Kayser-Jones Brief Oral Health Status Examination. Chin Nur Mana. 2016;16(01):38–41. doi:10.3969/j.issn.1672-1756.2016.01.011
- Tanaka T, Hirano H, Ohara Y, Nishimoto M, Iijima K. Oral Frailty Index-8 in the risk assessment of new-onset oral frailty and functional disability among community-dwelling older adults [published correction appears in Arch Gerontol Geriatr. 2021 Sep-Oct;96:104466]. Arch Gerontol Geriatr. 2021;94:104340. doi:10.1016/j.archger.2021.104340
- Chen ZM, Tan Y, Liang YJ, Zhang HH, Jiang Y. Shi GF.Chinesization of the Oral Frailty Index-8 and its reliability and validity test. Chin Nur Res. 2023;37(21):3808–3812. doi:10.12102/j.issn.1009-6493.2023.21.003
- Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol a Biol Sci Med Sci. 2001;56(6):M366–M372. doi:10.1093/gerona/56.6.m366
- Zhang Y, Wang LX, Lü XH, et al. Application of mini nutritional assessment-short form in nutrition screening in elderly inpatients with chronic diseases. Chin J Mult Organ Dis Elder. 2019;18(2):107–111. doi:10.11915/j.issn.1671-5403.2019.02.020
- Ma L, Tang Z, Chan P, Walston JD. Novel Frailty Screening Questionnaire (FSQ) Predicts 8-year Mortality in Older Adults in China. J. Frailty Aging. 2019;8(1):33–38. doi:10.14283/jfa.2018.38
- Liu H, Shang N, Chhetri JK, et al. A Frailty Screening Questionnaire (FSQ) to Rapidly Predict Negative Health Outcomes of Older Adults in Emergency Care Settings. J. Nutr Health Aging. 2020;24(6):627–633. doi:10.1007/s12603-020-1374-8
- McLachlan G, Peel D. Finite Mixture Models. New York, NY: John Wiley & Sons; 2000.
- Mplus, Latent Variable Mixture Modeling. What is a good value of entropy? Available from: http://www.statmodel.com/Discussion/messages/13/2562.html?1487458497. Accessed December 10, 2023.
- Berg-Warman A, Schiffman IK, Zusman SP, Natapov L. Oral health of the 65+ age group in Israel-2020. Isr. J Health Policy Res. 2021;10(1):58. doi:10.1186/s13584-021-00494-6
- Alibhai FJ, Li RK. Understanding systemic factors in aging and rejuvenation. Aging. 2020;12(21):20936–20937. doi:10.18632/aging.104213
- World Health Organization. Consultation for the draft WHO global strategy on tackling oral diseases opens. Available from: https://www.fdiworlddental.org/consultation-draft-who-global-strategr-tackling-oral-diseases-opens. Accessed December 10, 2023.
- Han AR, Shin MH, Yang JH, Choi CK, Koh JT, Kim OS. Body mass index and self-rated oral health in Korean adults in 2017. Gerodontology. 2023;40(2):183–191. doi:10.1111/ger.12624
- Chang Y, Jeon J, Kim JW, Song TJ, Kim J. Association between Findings in Oral Health Screening and Body Mass Index: a Nation-Wide Longitudinal Study. Int. J Environ Res Public Health. 2021;18(21):11062. doi:10.3390/ijerph182111062
- Suvan J, Petrie A, Moles DR, et al. Body mass index as a predictive factor of periodontal therapy outcomes. J Dent Res. 2014;93(1):49–54. doi:10.1177/0022034513511084
- Toniazzo MP, Amorim PS, Weidlich P. Relationship of nutritional status and oral health in elderly: Systematic review with meta-analysis. Clin Nutr. 2018;37(3):824–830. doi:10.1016/j.clnu.2017.03.014
- Algra Y, Haverkort E, Kok W, et al. The Association between Malnutrition and Oral Health in Older People: a Systematic Review. Nutrients. 2021;13(10):3584. doi:10.3390/nu13103584
- Joshy G, Arora M, Korda RJ, Chalmers J, Banks E. Is poor oral health a risk marker for incident cardiovascular disease hospitalisation and all-cause mortality? Findings from 172 630 participants from the prospective 45 and Up Study. BMJ Open. 2016;6(8):e012386. doi:10.1136/bmjopen-2016-012386
- Poudel P, Paudel G, Acharya R, George A, Borgnakke WS, Rawal LB. Oral health and healthy ageing: a scoping review. BMC Geriatr. 2024;24(1):33. doi:10.1186/s12877-023-04613-7
- Khoury W, Glogauer J, Tenenbaum HC, Glogauer M. Oral inflammatory load: neutrophils as oral health biomarkers. J Periodontal Res. 2020;55(5):594–601. doi:10.1111/jre.12758
- Kotronia E, Wannamethee SG, Papacosta AO, et al. Poor Oral Health and Inflammatory, Hemostatic, and Cardiac Biomarkers in Older Age: results From Two Studies in the UK and USA. J. Gerontol a Biol Sci Med Sci. 2021;76(2):346–351. doi:10.1093/gerona/glaa096
- Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative Stress in Oral Diseases: understanding Its Relation with Other Systemic Diseases. Front Physiol. 2017;8:693. doi:10.3389/fphys.2017.00693
- Teixeira FB, Saito MT, Matheus FC, et al. Periodontitis and Alzheimer’s Disease: a Possible Comorbidity between Oral Chronic Inflammatory Condition and Neuroinflammation. Front Aging Neurosci. 2017;9:327. doi:10.3389/fnagi.2017.00327
- Forman DE, Maurer MS, Boyd C, et al. Multimorbidity in Older Adults With Cardiovascular Disease. J Am Coll Cardiol. 2018;71(19):2149–2161. doi:10.1016/j.jacc.2018.03.022
- National Health Commission of People’s Republic of China. Report on Chinese residents’ chronic diseases and nutrition. Beijing: People’s Medical Publishing House; 2022.
- Paul O, Arora P, Mayer M, Chatterjee S. Inflammation in Periodontal Disease: possible Link to Vascular Disease. Front Physiol. 2021;11:609614. doi:10.3389/fphys.2020.609614
- Pruntel SM, van Munster BC, de Vries JJ, Vissink A, Visser A. Oral Health as a Risk Factor for Alzheimer Disease. J. Prev Alzheimers Dis. 2024;11(1):249–258. doi:10.14283/jpad.2023.82
- Guo D, Shi Z, Luo Y, Ding R, He P. Association between oral health behavior and chronic diseases among middle-aged and older adults in Beijing. China BMC Oral Health. 2023;23(1):97. doi:10.1186/s12903-023-02764-y
- Carramolino-Cuéllar E, Tomás I, Jiménez-Soriano Y. Relationship between the oral cavity and cardiovascular diseases and metabolic syndrome. Med Oral Patol Oral Cir Bucal. 2014;19(3):e289–e294. doi:10.4317/medoral.19563
- D’Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84(3):269–273. doi:10.1177/154405910508400312
- Chew J, Chia JQ, Kyaw KK, et al. Association of oral health with frailty, malnutrition risk and functional decline in hospitalized older adults: a cross-sectional study. J Frailty Aging. 2023;12(4):277–283. doi:10.14283/jfa.2023.33
- Kugimiya Y, Watanabe Y, Ueda T, et al. Rate of oral frailty and oral hypofunction in rural community-dwelling older Japanese individuals. Gerodontology. 2020;37(4):342–352. doi:10.1111/ger.12468
