Abstract
The complex nature of the surgical treatment of adolescent idiopathic scoliosis (AIS) requires a wide variety of health care providers. A well-coordinated, multidisciplinary team approach to the care of these patients is essential for providing high-quality care. This review offers an up-to-date overview of the numerous interventions and safety measures for improving outcomes after AIS surgery throughout the perioperative phases of care. Reducing the risk of potentially devastating and costly complications after AIS surgery is the responsibility of every single member of the health care team. Specifically, this review will focus on the perioperative measures for preventing surgical site infections, reducing the risk of neurologic injury, minimizing surgical blood loss, and preventing postoperative complications. Also, the review will highlight the postoperative protocols that emphasize early mobilization and accelerated discharge.
Introduction
Adolescent idiopathic scoliosis (AIS) is a lateral and rotational deformity of the spine defined by a radiographic Cobb angle of >10° affecting otherwise healthy children around the age of puberty. An estimated 1%–3% of children between the ages of 10 and 16 are affected by AIS with a 7:1 female to male ratio.Citation1,Citation2 While the vast majority of these children will not require any intervention for their spinal curvature, ~0.26% of children with AIS will be treated with either bracing or surgery.Citation2
In the US, over 4,500 surgeries were performed for the treatment of AIS in 2000.Citation3 Data compiled from the Scoliosis Research Society Morbidity and Mortality Committee estimate the complication rate for AIS surgery to be 5.7%. The most common complications for posterior instrumentation and fusion included wound infection (1.35%), pulmonary complications excluding pulmonary embolism (0.95%), neurologic injury (0.32%), and other medical complications.Citation4
The complex nature of the surgical treatment of AIS requires a wide variety of different health care providers. During the perioperative period, the average pediatric spine patient will interact with many providers including preoperative nurses, anesthetist, anesthesiologists, scrub technicians, circulating nurses, spine surgeons, neurologists and neuromonitoring technicians, cell-saver and radiology technicians, intensive care/floor nurses, physical and occupational therapists, and hospitalists. While each provider may have a different sphere of influence in patient care (eg, preoperative, intraoperative, postoperative), safe and effective surgical treatment of AIS is the primary goal of every provider.
Multidisciplinary teams that focus on establishing and implementing surgical site infection (SSI) prevention protocols have successfully reduced infection in pediatric spine patients.Citation5 Expanding the scope of these teams to reduce all known complications for the entire perioperative period may have the same effect. The purpose of this paper is to outline the evidence-based interventions involved in safely caring for the AIS surgical patient.
Reducing the risk of infection
SSI can be a devastating complication for pediatric patients undergoing spinal surgery for AIS. Perioperative infection is the most common acute complication in AIS surgery with an incidence ranging between 0.5% and 6.7%.Citation6 Infections after orthopedic surgery can negatively affect patient outcomes, result in greater physical limitations, and significantly reduce the quality of life. In addition to the impact on the patient, SSI prolongs hospital stay, results in more rehospitalizations, and increases direct health care costs.Citation7 Postoperative infections are estimated to increase costs by 300% in orthopedic patients in general, with costs ranging from $26,977 to $961,722 (mean $155,000) for spine implant-related infections.Citation5,Citation7,Citation8
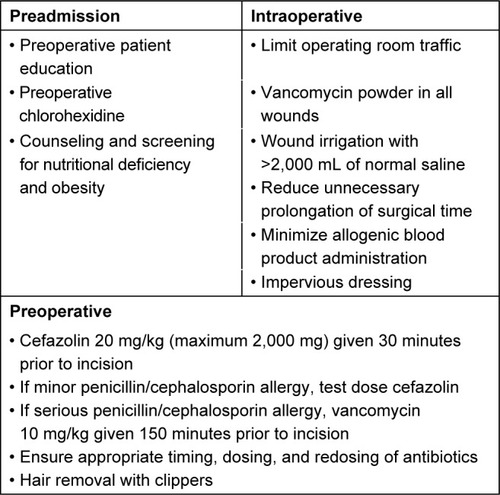
Prevention of infection is the responsibility of the entire health care team. Standardized protocols have been adopted in many centers to reduce the risk of SSI. Ballard et al reported a 50% relative risk reduction in pediatric spine surgery infections after the implementation of a multidisciplinary task force for SSI prevention.Citation5 Their team consisted of delegates from orthopedic surgery, infectious disease, epidemiology, pharmacy, anesthesia, quality improvement, and operative staff who met monthly to update their protocols and track their effectiveness.Citation5
Preadmission baths with chlorohexidine reduce the concentration of cutaneous bacteria.Citation9 While there have been no studies in pediatric spine patients, preoperative chlorohexidine bathing prior to arrival at the hospital has been shown to be effective in reducing SSI in knee and hip arthroplasty.Citation10,Citation11 In the neuromuscular pediatric population, optimization of preoperative nutrition also lowers SSI. Jevsevar and Karlin found that maintaining serum albumin >35 g/L and total blood lymphocyte count >1.5 g/L resulted in a significant decrease in SSI and the length of hospital stay.Citation12 Obesity has also been identified as a potential, modifiable SSI risk factor.Citation13 In a retrospective case–control study, Linam et al found that a body mass index of greater than the 95th percentile correlated with an increase in postoperative SSI and readmission.Citation14 Also, providing patients with preoperative informational handouts on SSI prevention has been recommended as a low-cost, low-risk infection control method.Citation15
Appropriate perioperative antimicrobial prophylaxis is a fundamental intervention that has greatly reduced SSI in pediatric spine deformity surgery.Citation16 Coagulase-negative Staphylococcus aureus remains the most common organism responsible for SSI in AIS surgery. However, polymicrobial flora and gram-negative bacteria have also been linked to SSI in the AIS population.Citation17 In two retrospective reviews of over 1,400 pediatric spine deformity surgeries which included both AIS and neuromuscular scoliosis patients, gram-negative organisms accounted for over 50% of SSI, with a growing incidence of infection from Pseudomonas aeruginosa.Citation13,Citation14 First-generation cephalosporins, namely, cefazolin, are the recommended antimicrobial prophylaxis for all spine patients.Citation15 The use of clindamycin as the sole agent for perioperative antibiosis was an independent risk factor for SSI after pediatric scoliosis surgery.Citation14 Some centers have shown a decrease in SSI after spine surgery for AIS with the use of routine ceftazidime and vancomycin instead of cefazolin.Citation17 Current consensus-driven best practice guidelines for high-risk pediatric spine patients recommend routine perioperative intravenous (IV) cefazolin use, addition of gram-negative coverage, and adherence to timing, dosing, redosing, and cessation regimens.Citation15 While these guidelines are directed toward high-risk patients, the infection control principles are based on the best available evidence and should be applied to the AIS patient.
Appropriate delivery of antibiotic prophylaxis is the responsibility of the entire operative team. Optimal dosing and timing are essential for an effective SSI prevention; cefazolin should be given within 30 minutes of incision at a dose 20 mg/kg (maximum 2,000 mg) and vancomycin should be given within 150 minutes of incision at a dose of 10 mg/kg (maximum 1,000 mg).Citation18 Labbe et al demonstrated that appropriate dosing and timing reduced SSI in pediatric spine patients with an odds ratio of 5.5.Citation18 Dosing and timing of IV antibiotic prophylaxis should be included in the “time-out” protocol as it has been shown to be a cheap and effective way to ensure appropriate treatment.Citation19 In addition to IV antibiotic prophylaxis, several studies have demonstrated the effectiveness and safety of routine use of vancomycin powder in the bone graft and/or the surgical site.Citation20–Citation22 Sweet et al retrospectively studied the use of 2 g of vancomycin powder in 911 of 1,732 AIS spine surgeries and found a reduction in deep infection rate from 2.6% to 0.2%.Citation21
Increased operating room (OR) traffic, including frequent opening and closing of OR doors, disrupts the laminar airflow, increases the number of microbes surrounding the surgical field, and contributes to increased risk of infection.Citation23 Panahi et al found that there was an average of 60 door openings during a primary total joint replacement lasting 92 minutes and an average of 135 openings during revision cases.Citation23 In addition to limiting OR access, Vitale et al recommended several perioperative measures to prevent SSI in their 2013 Best Practice Guidelines for high-risk pediatric spine surgery that are applicable to AIS surgery.Citation15 When hair is removed from the surgical site, clippers should be used as opposed to shaving. Impervious dressings are preferred and changes to the dressing should be minimized before discharge. Furthermore, they recommend routine intraoperative wound irrigation; both >2,000 mL of normal saline and dilute betadine irrigation have been shown to reduce SSI after adult spine surgery, with neither solution proven to be superior.Citation15
Several other factors may alter the risk of SSI in AIS surgery. In a systematic review, Vitale et al found that there is conflicting or poor-quality evidence that volume of blood loss, rates of blood transfusion, greater number of levels fused, and prolonged operative time increase the risk of SSI.Citation15 There is also weak evidence that drain use reduces SSICitation24; however, there is also evidence that drain use does not impact complication rate or SSI.Citation25 The authors’ recommended infection prevention measures are summarized in .
Preventing neurologic injury
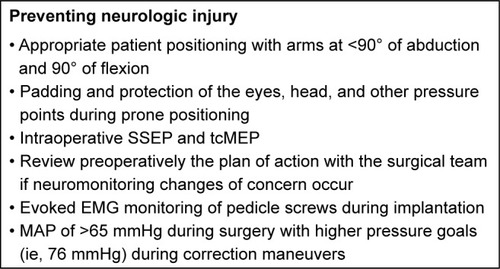
Prevention of neurologic injury begins with safe patient positioning. Commonly, surgical correction of AIS requires posterior spinal fusion (PSF) and, thus, prone positioning. Complications from prone positioning for spinal surgery include perioperative blindness and peripheral neuropathies including brachial plexus injury.
In a retrospective review of over 500,000 patients undergoing spinal fusion, the incidence of perioperative blindness is 1.9 events per 10,000 cases. Diabetes, end-organ damage, spinal deformity surgery, and paralysis were independent risk factors for perioperative blindness, with over 56% of events occurring in spinal deformity surgeries.Citation26 Perioperative blindness can result from several etiologies including ischemic optic neuropathy, central retinal artery occlusion (CRAO), and cortical blindness.Citation27 Ischemic optic neuropathy results from decreased ocular profusion and is the most common cause of perioperative blindness. It is associated with obesity, male sex, Wilson frame use, longer anesthesia time, greater estimated blood loss, and fluid resuscitation with increased ratios of crystalloids to colloids.Citation28 CRAO is the second most common form of perioperative blindness and results from either direct or indirect pressure on the eye that increases intraocular pressure to the point of ischemia. CRAO, also known as headrest syndrome, is both a preventable and an irreversible form of blindness.Citation29 Cortical blindness is caused by decreased perfusion to the visual cortex of the occipital lobes in the brain. This irreversible form of blindness is typically bilateral and associated with hypotension, prolonged hypoxia, cardiac arrest, and thromboembolic events.Citation29 Prevention of perioperative blindness should focus on reducing the risk of prolonged hypotension, repletion of surgical blood loss, and avoidance of direct compression of the eyes.Citation27,Citation29
Peripheral nerve injuries are a rare perioperative complication with an estimated frequency of 0.03%.Citation30 The most common sites of perioperative peripheral neuropathy are ulnar neuropathy, brachial plexus injury, median neuropathy, and radial neuropathy, accounting for 28%, 20%, 4%, and 3% of all anesthesia-related nerve injury malpractice claims.Citation31 Ulnar nerve injuries may result from direct compression over the cubital tunnel and/or excessive elbow flexion >90°.Citation27 The cervical and thoracic nerve roots of the brachial plexus are susceptible to injury as they pass superficially in the axilla around several bony prominences, tethered proximally at the vertebrae and distally by the axillary fascia. Improper positioning, specifically with the arm abducted >90°, places excessive tension or compression on the brachial plexus and is associated with postoperative brachial plexopathy.Citation32 Two retrospective reviews of over 800 prone positioned pediatric scoliosis surgery patients examined the rate of impending intraoperative brachial plexopathy using somatosensory cortical evoked potentials (SSEP). Between 3.6% and 6.2% of patients developed SSEP findings highly concerning for impending brachial plexopathy, with a reduction in SSEP amplitude of >30%.Citation33,Citation34 The use of neuromonitoring of the ulnar nerve with SSEP is effective for the early detection and prevention of brachial plexopathy related to patient positioning. Based on the current evidence, it is recommended that when patients are positioned prone, their arms should be abducted <90°, the elbow should not be fully extended if the shoulders are abducted, and ulnar nerve SSEP should be utilized to prevent impending brachial plexus injuries.Citation27,Citation31–Citation34 Care should also be taken when padding and positioning the pelvic bolsters to prevent compression on the lateral femoral cutaneous nerve. Lateral femoral cutaneous nerve neuropathy has been reported in up to 24% of patients undergoing posterior spine surgery, with longer surgical time being identified as a risk factor.Citation35 The pain, hypersensitivity, paresthesia, or numbness typically resolves within 2–6 months, but may require steroid injections or decompression.Citation27 In addition to preventing position-related neurologic injuries, all bony prominences should be padded to reduce the risk of pressure ulcerations. The abdomen should be allowed to hang freely; this has been shown to decrease intra-abdominal pressure, reduce the risk of abdominal compartment syndrome, and minimize blood loss during spinal surgery.Citation27,Citation31,Citation36
Neurologic complication remains a devastating, yet rare complication after surgical correction of AIS. In 2005, the Scoliosis Research Society reported a 0.3% incidence of neurologic deficits after PSF and a 1.2% incidence after combined anterior and posterior surgery.Citation4 Both the reduction in quality of life and financial costs related to neurogenic complications and spinal cord injury are enormous. The estimated lifetime cost of an injury resulting in paraplegia at age 25 is $977,142.Citation37
Correction of spinal deformity places the spinal cord and the nerve roots at risk for injury, which may result in loss of motor and/or sensory function. Intraoperative monitoring of the neurologic status allows for early identification of possible neurologic injury and possible intervention to prevent such an injury.Citation38 Successful implementation of neurophysiologic monitoring requires coordination of care between the surgeon, anesthesiologist, neurophysiologist, and operative staff. Despite the resources required for intraoperative monitoring, it still remains cost-effective.Citation37 SSEP assess the functional status of the sensory tracts through stimulation of a peripheral nerve and record the electrical responses at various locations along that neural pathway. Other modalities such as neurogenic motor evoked potentials (NMEP) and transcranial motor evoked potentials (tcMEP) have been developed to monitor intraoperative dorsolateral and ventral motor tract function.Citation39 Padburg et al demonstrated that the combined intraoperative use of SSEP and NMEP predicted neurologic status with 98.6% sensitivity, 100% specificity, a false-positive rate of 0.014%, and no false negatives. They concluded that normal combined motor and sensory monitoring findings obviated the need for intraoperative wake-up tests, the gold standard method for intraoperative assessment of neurologic injury.Citation40
Recent evidence has demonstrated that NMEP actually reflect a backfiring through the afferent sensory or dorsal column pathways, instead of testing the motor spinal pathways.Citation41 In tcMEP monitoring, the response of electrical stimulation of the motor cortex through transcutaneous leads is recorded at the target muscle groups, which tests the native motor pathway more directly.Citation42 Inhalation anesthetics can easily disrupt tcMEP; IV anesthesia may be a more favorable form of anesthesia for tcMEP monitoring.Citation39 Level I evidence based on 1,121 patients undergoing surgery for AIS demonstrated the efficacy of combined SSEP and tcMEP monitoring in both detecting impending spinal cord injuries and allowing for interventions that may preserve spinal cord function.Citation42 In 2014, Vitale et al released a list of “Best Practices” for intraoperative neuromonitoring.Citation43 The consensus based on current evidence recommends the routine use of SSEP and tcMEP or other forms of motor tract monitoring, defines a 50% degradation of SEEP amplitude or a sustained decrease in tcMEP as a significant warning criterion, and suggests that wake-up tests should be considered when patients have persistent signal changes or cannot be monitored.Citation43 Furthermore, Vitale et al recommended the implementation of a multidisciplinary checklist for how to respond to concerning neuromonitoring changes, which focuses on communication between the surgeon, anesthesiologist, perioperative nursing staff, and the neurophysiology team.
The development of intraoperative evoked electromyography (EMG) monitoring of pedicle screws provides a safe and effective way to evaluate for iatrogenic neurologic injury from aberrant screw placement. Glassman et al demonstrated that a normal EMG response predicts the absence of nerve injury, while an abnormal EMG response may or may not predict neurologic deficit requiring further clinical or radiographic investigation.Citation44 summarizes the authors’ recommended interventions to reduce the risk of perioperative neurologic injury.
Reducing perioperative blood loss
Soft tissue dissection, bone decortication, osteotomies, and instrumentation involved with surgical correction of AIS can lead to significant blood loss. Blood loss during AIS surgery varies significantly, with the average blood loss during PSF ranging from 275 to 907 mL.Citation45–Citation47 Also, estimates for blood loss during anterior spinal fusion average 323±171 mL and during combined anterior and posterior approaches average 1,277±821 mL.Citation46 Operative time, male sex, and increased preoperative kyphosis have been identified as independent predictors of increased blood loss.Citation46 Excessive perioperative blood loss places patients at risk for infection, hemodynamic instability, cardiopulmonary dysfunction, renal failure, and possible death. Also, allogenic blood transfusion presents additional risks for patients, including potential blood-borne infection, transfusion reaction, electrolyte imbalance, coagulopathy, and increased risk of SSI.Citation46,Citation48–Citation50 Allogenic blood transfusion has been associated with complications in up to 20% of patients undergoing spinal surgery.Citation51 Certain factors place patients at risk for needing allogenic red blood cell transfusion, including higher American Society of Anesthesiologists physical status classification, longer surgical duration, and increased number of levels fused.Citation52 Minimizing perioperative blood loss and reducing the need for allogenic blood products are essential for safety in AIS surgery.
Controlled hypotensive anesthesia utilizes pharmacologic agents in order to maintain a mean arterial pressure (MAP) below normal physiologic levels. Deliberate hypotension has been shown to be a safe and effective method to reduce operative time and surgical blood loss in spine surgery as well as total joint arthroplasty.Citation46,Citation49,Citation53,Citation54 A retrospective analysis of over 300 AIS patients found that by lowering MAPs to <65 mmHg at the time of incision reduced blood loss by 33% with no complications related to the use of hypotension.Citation49 When blood pressure levels were elevated at the time of incision, blood loss increased by 29% and operative time was 29 minutes greater than the hypotensive anesthesia cohort. Hypotensive anesthesia with an ideal MAP <65 mmHg at the time of incision minimizes surgical blood loss, improves visualization during exposure of the spine, and reduces operative times. Short-acting neuromuscular blockade with agents such as rocuronium may also decrease blood loss during exposure, but should be given early enough in the dissection so as to allow neuromonitoring to resume prior to instrumentation.
Lowering MAP during the surgical approach in order to reduce intraoperative blood loss must be balanced with maintaining a MAP necessary for spinal cord perfusion. MAP <60 mmHg during correction of spinal deformity has been associated with significant SSEP changes and an increased risk of spinal cord ischemia.Citation55 While high-quality data on specific MAP goals during the instrumentation and correction of deformity is lacking, it is recommended that the MAP should be >70 mmHg during this portion of the case.Citation56,Citation57 The MAP should be elevated further to a goal of >80 mmHg if there is high concern for neurologic injury, such as a complete loss of neuromonitoring signals.Citation57
Antifibrinolytic agents such as tranexamic acid and ε-aminocaproic acid are widely used perioperatively in surgical procedures with the potential for significant blood loss. These medications are synthetic analogs of lysine, which reversibly block lysine-binding sites on plasminogen, preventing the activation of plasmin and inhibiting fibrinolysis.Citation58 Several prospective, randomized, double-blind, placebo-controlled studies show that tranexamic acid is an effective, safe, and cheap method to reduce blood loss during spinal fusion.Citation59–Citation61 Verma et al found that both tranexamic acid and ε-aminocaproic acid reduced operative blood loss, but not transfusion rate.Citation60 Tranexamic acid was more effective in reducing blood loss. Of note, there was no difference between treatment and placebo groups when the MAP was >75 mmHg, suggesting the importance of MAP in surgical blood loss.Citation60 Dosing of tranexamic acid varies with loading doses ranging from 10 to 30 mg/kg and subsequent continuous infusion dosing from 1 to 10 mg/kg/h.Citation48,Citation59,Citation60 A 2008 Cochrane Review of the effectiveness of antifibrinolytic drugs on blood loss in children undergoing scoliosis surgery concluded that these agents are indeed effective in reducing surgical blood loss, but the effect on decreasing blood transfusion remains unclear.Citation62
Cell salvage systems (cell saver) can be used intraoperatively to collect blood from the surgical field, filter cellular, noncellular, and biochemical debris, and provide a source of autologous red blood cell transfusion.Citation63 The use of cell saver has been shown to reduce the need for allogenic transfusion in pediatric pelvic and spine surgery.Citation63–Citation65 While autologous transfusion is exceptionally safe, there are reports of rare transfusion reactions.Citation66 A retrospective case–control study found that cell saver use with a 150 mL collection bowl reduced allogenic transfusion rates and volumes during PSF for AIS.Citation63 Cell saver was more effective in surgeries lasting longer than 6 hours and with estimated blood losses of >30% of total blood volume. Other studies have questioned the need for routine use of cell saver in AIS surgery.Citation67,Citation68 In a nonrandomized prospective study of 95 children undergoing PSF for AIS, Weiss et al found that the use of cell saver did not reduce the risk of allogenic transfusion.Citation67 Their cell saver system required 250 mL of blood collected in order to be washed and returned, as opposed to the 150 mL required in other studies.Citation63,Citation67 outlines the measures recommended to reduce perioperative blood loss.
Reducing postoperative complications
In order to expedite discharge and return patients to a feeling of “normalcy” after surgery, it is essential to prevent and manage any medical complications. Certain AIS patients are at greater risk of sustaining a complication after surgery. A retrospective review of over 700 AIS patients who underwent PSF in 2012 found that those children with a body mass index in the 95th percentile or greater were significantly more likely to have an adverse event after PSF. Patients who had >13 levels instrumented or operative times exceeding 365 minutes were more likely to have an extended length of stay in hospital (>6 days).Citation69 Pugely et al investigated the factors that contributed to short-term mortality after spinal fusion and found that those patients with cognitive impairment, elevated American Society of Anesthesiologists classification, a history of hepatobiliary disease, prolonged operative times, and fusion to the pelvis had significantly higher complication rates.Citation70 Particular attention should be paid to patients with the above risk factors in order to minimize the complication rates.
The idea of utilizing postoperative protocols to reduce complications is not new. Wenger et al published a review article in 1992 which proposed a goal of a routine 5–7 day postoperative stay for straightforward AIS patients undergoing PSF.Citation71 This review provided recommendations that are also included in modern accelerated discharge (AD) pathways, such as frequent incentive spirometer use, elevating the head of patients’ beds to allow for better lung expansion, early Foley removal to minimize the risk of urinary tract infections, and having a low threshold for aspirating fluid collections or hematomas, to aid in early diagnosis and treatment of wound issues. Minimizing perioperative blood loss may help prevent postoperative orthostasis which can contribute to delayed mobilization and slow recovery. Monitoring patients’ wounds postoperatively can provide early signs of a developing wound hematoma or SSI. Temperature spikes are very common in the immediate postoperative period; 72% of patients had fever with temperatures >38°C and 9% had temperatures >39° in one study.Citation72 However, this has not been correlated to postoperative infection. Fevers after postoperative day (POD) 3 or 4 are likely of greater concern and should be evaluated with appropriate cultures. Another major source of infection is the urinary tract, and any organisms found in a patient’s urine before or after surgery should be treated aggressively.Citation15
Ileus is a common complication after spinal fusion surgery, with an incidence of between 0.6% and 16.7% in adult lumbar spine surgery.Citation73 Nachlas et al studied gastric, intestinal, and colonic motility by tracking the barium administered immediately postop in 160 patients in a landmark study performed in 1972. They found that, in patients having extraintestinal procedures, gastric motility returned within 24 hours, and colonic motility can be slowed for 3–5 days but return can be hastened by giving a laxative.Citation74 An aggressive bowel regimen is important for postoperative bowel function. In the AD pathway, following surgery, all patients are allowed to eat ice chips and transitioned to a clear liquid diet on POD0 followed by initiation of solid foods on POD1. While patients’ appetite may take several days to return in full, most children easily tolerate small meals starting on POD1 with minimal nausea. Unlike neuromuscular scoliosis patients, most AIS patients are well-nourished at baseline. Thus, while early resumption of an oral diet provides the much needed nutrition to patients during the early healing period, it more importantly allows for dosing of oral pain medications on POD1. Oral narcotics and antispasmodics tend to be longer acting with less sedating and other adverse side effects. Discontinuation of the patient-controlled analgesia also eliminates an additional impediment to mobilization.
Pulmonary complications can contribute to prolonged hospital stays, especially among patients with pre-existing pulmonary issues. Scoliosis is associated with progressive restrictive lung disease, and thoracic surgery impedes respiration due to postoperative pain, anesthesia, and immobilization. Pneumonia, respiratory failure requiring prolonged mechanical ventilation, bronchospasm, and atelectasis are the common complications seen following PSF; however, these pulmonary complications are much more common among neuromuscular patients.Citation75 Nonetheless, AIS patients with a history of poor exercise tolerance, a curve exceeding 80°, or a history of severe reactive airway disease stand to benefit from a preoperative pulmonary evaluation.Citation76 A retrospective review of pediatric patients who had pulmonary function testing prior to spinal fusion demonstrated that patients with preoperative forced expiratory volume in 1 second <40% predicted, vital capacity <60%, inspiratory capacity <30 mL/kg, or total lung capacity <60% were more likely to require prolonged postoperative mechanical ventilation than those patients who did not have them.Citation76 Preoperative testing can provide vital insight and allow for better planning on behalf of both physicians and patients’ families.
While the majority of AIS patients are otherwise healthy in contrast to the neuromuscular scoliosis patient population, a minority of AIS patients have severe medical comorbidities. This subpopulation of medically complex AIS patients merits more extensive preoperative workup and optimization. This may include hyperalimentation and nutritional supplementation, pulmonary function testing, cardiac assessment, and the assistance of a pediatric hospitalist both prior to and after surgery to streamline care.Citation77 While these patients stand to benefit from many of the principles included in the pathway, especially early mobilization, this population of patients is not the target of the AD pathway.
Optimizing postoperative care
Treating children with AIS is a complex undertaking. Traditionally, hospital stays after spinal fusion procedures have been prolonged. Factors that have historically contributed to length of stay are difficulty with mobilization, pain control, resumption of general diet, and residual drain output. A multidisciplinary approach that addresses these factors in hopes that an earlier return to “normalcy” both expedites discharge and reduces the complications of prolonged immobilization and lengthy hospital stays.Citation45
The author’s recommended AD protocol truly begins prior to surgery, as expectations must be explained to patients and their families (). Patients’ caregivers are informed that their children will be rapidly mobilized to allow for a short hospital stay. The importance of preoperative counseling cannot be underestimated, as many families expect prolonged hospital stays after surgery. Knowing that the patients will likely be discharged as soon as 1–2 days after surgery allows the caregivers to plan appropriately for their return home. A team-based approach to family reassurance during the hospitalization is also critical and all team members need to understand the expectations for AD.
Figure 4 Postoperative protocol for patients undergoing posterior spinal fusion for adolescent idiopathic scoliosis.

The importance of early mobilization after spine surgery has been emphasized by a number of studies. This idea was proposed as early as 1973, when Leider et al proposed that AIS patients should be encouraged to ambulate early after surgery, citing that “rapid mobilization reduces fatigue and allows more rapid return to a more normal life-style”.Citation78 The next several decades witnessed marked improvements in instrumentation systems for spinal fusion. In 1988, Heilbronner and Sussman performed an early investigation of 40 AIS patients who had undergone Harrington rod instrumentation. The goal of new spinal instrumentation systems was, in fact, to rid the need for body casts and prolonged immobilization. At that time, the length of stay ranged from 5 to 13 days and averaged 8.5 days.Citation79 This trend has continued, as instrumentation has continued to improve and provide more robust fixation to allow for faster and more extensive mobilization. A survey of Shriners hospital spinal deformity surgeons published in 2007 revealed that, within the Shriners system, therapy for AIS patients was aimed at moving patients early, with the goal of sitting on POD1, standing on POD2, and walking on POD2 or 3.Citation80 Given the fact that the majority of AIS patients are otherwise healthy, high-functioning children preoperatively, returning them to their preoperative level of function or better in a timely fashion is critically important. Tarrant et al’s prospective study of AIS patients undergoing PSF found that, on average, these patients return to school or college full-time 10 weeks postoperatively, and over 50% returned to unrestrained physical activity by 24 weeks postop.Citation81 This is markedly faster than in past decades, and many children may be able to surpass these goals. The AD protocol aims to motivate and enable children to return to their daily activities as soon as possible.
Pain control regimens vary between institutions. The AD protocol calls for the use of a patient-controlled analgesia and IV benzodiazepines on the night of POD0 with an option to include gabapentin and ketorolac. These IV medications are discontinued on the morning of POD1, assuming patients are tolerating an oral diet, with only breakthrough doses of IV narcotic continued as needed beyond this point. An acute pain service consult is placed only for those patients with an extensive chronic pain management history or those who have difficulty with pain despite the above regimen, and this is a rare occurrence. Epidural catheters and intrathecal injections have been evaluated extensively for their use in pain control, but these are not commonly employed as part of the AD protocol.Citation20,Citation82–Citation84 IV acetaminophen is often used as an adjunct in multimodal pain regimen. While it is still not a routinely given medication due to availability and cost, it can be given while the patient is fasting; it is nonsedating and may serve to decrease narcotic requirements.Citation85
Wound drains and Foley catheters are all removed on POD1 in the AD protocol. The potential advantages and disadvantages of using drains after PSF have been evaluated in several studies. Theoretically, drains promote egress of fluids away from the cutaneous incision and minimize the formation of hematoma which can serve as a medium for bacterial growth; however, they also create the potential for drain contamination, impeded mobilization, increased discomfort, and increased care needs (ie, drain stripping or reservoir emptying). Diab et al evaluated 324 drained patients and 176 undrained patients and found that the mean time to drain removal was 57 hours, with half of the surgeons using drain output as a criterion to determine when to remove their drains and the other half leaving them for a predetermined amount of time (15 surgeons removed the drain after 48 hours, 2 after 72 hours, and 1 after 24 hours).Citation25 Length of stay in hospital did not differ between these two groups. They found that drain use was beneficial, but no consensus was found regarding how long to leave drains in place. Clearly, only a minority of surgeons removed their drains during the first 24 hours after surgery. Blank et al randomized drain use in 30 AIS patients undergoing PSF.Citation86 Dressings were examined at 4, 12, 24, and 48 hours post-operatively, and all drains were removed at 48 hours. They found that closed suction reduced the frequency of needed dressing changes, and it also reduced the rate of wound complications including superficial hematoma or infection and the ultimate need for implant removal.Citation86 Early drain removal on POD1 has been found to expedite mobilization and decrease patient discomfort without a resultant increase in wound complications.Citation45
Summary
The surgical care for AIS patients requires a multidisciplinary approach to minimize complications and maximize outcomes. Multidisciplinary teams have successfully been shown to improve the quality of care for AIS patients by reducing infection rates.Citation5 There are several evidence-based methods which have been shown to reduce the risk of infection, prevent neurologic injury, minimize surgical blood loss, and optimize postoperative care. It is the responsibility of every member of the perioperative team to take ownership of the patient and to be actively involved in improving patient care.
Acknowledgments
Medtronic Spine, Zimmer/Biomet, and Orthopaediatric are acknowledged for the consulting services. Harrison Foundation is acknowledged for the grant support.
Disclosure
The authors report no conflicts of interest in this work.
References
- WeinsteinSLDolanLAChengJCYDanielssonAMorcuendeJAAdolescent idiopathic scoliosisLancet200837196231527153718456103
- AsherMABurtonDCAdolescent idiopathic scoliosis: natural history and long term treatment effectsScoliosis200611216759428
- DolanLAWeinsteinSLSurgical rates after observation and bracing for adolescent idiopathic scoliosis: an evidence-based reviewSpine (Phila Pa 1976)20073219 SupplS91S10017728687
- CoeJDArletVDonaldsonWComplications in spinal fusion for adolescent idiopathic scoliosis in the new millennium. A report of the Scoliosis Research Society Morbidity and Mortality CommitteeSpine (Phila Pa 1976)200631334534916449909
- BallardMRMillerNHNyquistACEliseBBauleshDMEricksonMAA multidisciplinary approach improves infection rates in pediatric spine surgeryJ Pediatr Orthop201232326627022411332
- LiYGlotzbeckerMHedequistDSurgical site infection after pediatric spinal deformity surgeryCurr Rev Musculoskelet Med Epub201229
- WhitehouseJDFriedmanNDKirklandKBRichardsonWJSextonDJThe impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra costInfect Control Hosp Epidemiol200223418318912002232
- HedequistDHaugenAHreskoTEmansJFailure of attempted implant retention in spinal deformity delayed surgical site infectionsSpine (Phila Pa 1976)2009341606419077923
- PopovichKJLylesRHayesRHotaBTrickWWeinsteinRAHaydenMKRelationship between chlorhexidine gluconate skin concentration and microbial density on the skin of critically ill patients bathed daily with chlorhexidine gluconateInfect Control Hosp Epidemiol201233988989622869262
- JohnsonAJKapadiaBHDaleyJAMolinaCBMontMAChlorhexidine reduces infections in knee arthroplastyJ Knee Surg201326321321823288739
- KapadiaBHJohnsonAJDaleyJAIssaKMontMAPre-admission cutaneous chlorhexidine preparation reduces surgical site infections in total hip arthroplastyJ Arthroplasty201328349049323114192
- JevsevarDSKarlinLIThe relationship between preoperative nutritional status and complications after an operation for scoliosis in patients who have cerebral palsyJ Bone Joint Surg Am19937568808848314827
- CroftLDPottingerJMChiangHYZieboldCSWeinsteinSLHerwaldtLARisk factors for surgical site infections after pediatric spine operationsSpine (Phila Pa 1976)2015402E112E11925569528
- LinamWMMargolisPAStaatMABrittoMTHornungRCassedyAConnellyBLRisk factors associated with surgical site infection after pediatric posterior spinal fusion procedureInfect Control Hosp Epidemiol200930210911619125680
- VitaleMGRiedelMDGlotzbeckerMPBuilding consensus: development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high-risk pediatric spine surgeryJ Pediatr Orthop201333547147823752142
- TransfeldtELonsteinJWinterRBradfordDMoeJMayfieldJWound infections in reconstructive spinal surgeryOrthop Trans19859128
- MyungKSGlassmanDMToloVTSkaggsDLSimple steps to minimize spine infections in adolescent idiopathic scoliosisJ Pediatr Orthop2014341293323812142
- LabbeACDemersAMRodriguesRArletVTanguayKMooreDLSurgical-site infection following spinal fusion: a case-control study in a children’s hospitalInfect Control Hosp Epidemiol200324859159512940580
- RosenbergADWamboldDKraemerLEnsuring appropriate timing of antimicrobial prophylaxisJ Bone Joint Surg Am200890222623218245579
- BorkhuuBBorowskiAShahSALittletonAGDabneyKWMillerFAntibiotic-loaded allograft decreases the rate of acute deep wound infection after spinal fusion in cerebral palsySpine (Phila Pa 1976)200833212300230418827695
- SweetFARohMSlivaCIntrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomesSpine (Phila Pa 1976)201136242084208821304438
- O’NeillKRSmithJGAbtahiAMArcherKRSpenglerDMMcGirtMJDevinCJReduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powderSpine J201111764164621600853
- PanahiPStrohMCasperDSParviziJAustinMSOperating room traffic is a major concern during total joint arthroplastyClin Orthop Relat Res2012470102690269422302655
- HoCSucatoDJRichardsBSRisk factors for the development of delayed infections following posterior spinal fusion and instrumentation in adolescent idiopathic scoliosis patientsSpine (Phila Pa 1976)200732202272227717873822
- DiabMSmucnyMDormansJPUse and outcomes of wound drain in spinal fusion for adolescent idiopathic scoliosisSpine (Phila Pa 1976)2012371196697322037527
- NandyalaSVMarquez-LaraAFinebergSJSinghRSinghKIncidence and risk factors for perioperative visual loss after spinal fusionSpine J20141491866187224216394
- DePasseJMPalumboMAHaqueMEbersonCPDanielsAHComplications associated with prone positioning in elective spinal surgeryWorld J Orthop20156335135925893178
- Postoperative Visual Loss StudyGRisk factors associated with ischemic optic neuropathy after spinal fusion surgeryAnesthesiology20121161152422185873
- StamboughJLDolanDWernerRGodfreyEOphthalmologic complications associated with prone positioning in spine surgeryJ Am Acad Orthop Surg200715315616517341672
- WelchMBBrummettCMWelchTDTremperKKShanksAMGuglaniPMashourGAPerioperative peripheral nerve injuries: a retrospective study of 380,680 cases during a 10-year period at a single institutionAnesthesiology2009111349049719672188
- KamelIBarnetteRPositioning patients for spine surgery: Avoiding uncommon position-related complicationsWorld J Orthop20145442544325232519
- UribeJSKollaJOmarHDakwarEAbelNMangarDCamporesiEBrachial plexus injury following spinal surgeryJ Neurosurg Spine201013455255820887154
- SchwartzDMDrummondDSHahnMEckerMLDormansJPPrevention of positional brachial plexopathy during surgical correction of scoliosisJ Spinal Disord200013217818210780696
- LabromRDHoskinsMReillyCWTredwellSJWongPKClinical usefulness of somatosensory evoked potentials for detection of brachial plexopathy secondary to malpositioning in scoliosis surgerySpine (Phila Pa 1976)200530182089209316166901
- YangSHWuCCChenPQPostoperative meralgia paresthetica after posterior spine surgery: incidence, risk factors, and clinical outcomesSpine (Phila Pa 1976)20053018E547E55016166883
- KweeMMHoYHRozenWMThe prone position during surgery and its complications: a systematic review and evidence-based guidelinesInt Surg2015100229230325692433
- SalaFDvorakJFaccioliFCost effectiveness of multimodal intraoperative monitoring during spine surgeryEur Spine J200716Suppl 2S229S23117659365
- NashCLJrLorigRASchatzingerLABrownRHSpinal cord monitoring during operative treatment of the spineClin Orthop Relat Res1977126100105598095
- Neuromonitoring information statementSRS information statement, 20092009 Available from: https://www.srs.org/about-srs/quality-and-safety/position-statements/neuromonitoring-information-statementAccessed February 24, 2016
- PadburgAMWilson-HoldenTJLenkeLGBridwellKHSomatosensory-and motor-evoked potential monitoring without a wake-up test during idiopathic scoliosis surgery: an accepted standard of careSpine (Phila Pa 1976)19982312139214009654631
- SuCFHaghighiSSOroJJGainesRW“Backfiring” in spinal cord monitoring. High thoracic spinal cord stimulation evokes sciatic response by antidromic sensory pathway conduction, not motor tract conductionSpine (Phila Pa 1976)19921755045081621148
- SchwartzDMAuerbachJDDormansJPNeurophysiological detection of impending spinal cord injury during scoliosis surgeryJ Bone Joint Surg Am200789112440244917974887
- VitaleMGSkaggsDLPaceGIBest practices in intraoperative neuromonitoring in spine deformity surgery: development of an intraoperative checklist to optimize responseSpine Deformity201425333339
- GlassmanSDDimarJRPunoRMJohnsonJRShieldsCBLindenRDA prospective analysis of intraoperative electromyographic monitoring of pedicle screw placement with computed tomographic scan confirmationSpine (Phila Pa 1976)19952012137513797676335
- FletcherNDShourbajiNMitchellPMOswaldTSDevitoDPBruceRWClinical and economic implications of early discharge following posterior spinal fusion for adolescent idiopathic scoliosisJ Child Orthop20148325726324770995
- IalentiMNLonnerBSVermaKDeanLValdevitAErricoTPredicting operative blood loss during spinal fusion for adolescent idiopathic scoliosisJ Pediatr Orthop201333437237623653024
- FletcherNDAndrasLMLazarusDEUse of a novel pathway for early discharge was associated with a 48% shorter length of stay after posterior spinal fusion for adolescent idiopathic scoliosisJ Pediatr Orthop Epub2015724
- GrantJAHowardJLuntleyJHarderJAleissaSParsonsDPerioperative blood transfusion requirements in pediatric scoliosis surgery: the efficacy of tranexamic acidJ Pediatr Orthop200929330030419305284
- VermaKLonnerBDeanLVecchioneDLafageVReduction of mean arterial pressure at incision reduces operative blood loss in adolescent idiopathic scoliosisSpine Deformity201312115122
- SchwarzkopfRChungCParkJJWalshMSpivakJMSteigerDEffects of perioperative blood product use on surgical site infection following thoracic and lumbar spinal surgerySpine (Phila Pa 1976)201035334034620075776
- TateDEJrFriedmanRJBlood conservation in spinal surgery: review of current techniquesSpine19921712145014561471002
- WongJEl BeheiryHRampersaudYRTranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgeryAnesth Analg200810751479148618931202
- LawhonSMKahnA3rdCrawfordAHBrinkerMSControlled hypotensive anesthesia during spinal surgery. A retrospective studySpine (Phila Pa 1976)1984954504536495010
- PaulJELingELalondeCThabaneLDeliberate hypotension in orthopedic surgery reduces blood loss and transfusion requirements: a meta-analysis of randomized controlled trialsCan J Anaesth2007541079981017934161
- OwenJHThe application of intraoperative monitoring during surgery for spinal deformitySpine19992424264910635528
- MooneyJFIIIBernsteinRHennrikusWLJrMacEwenGDNeurologic risk management in scoliosis surgeryJ Pediatr Orthop200222568368912198475
- PahysJMGuilleJTD’AndreaLPSamdaniAFBeckJBetzRRNeurologic injury in the surgical treatment of idiopathic scoliosis: guidelines for assessment and managementJ Am Acad Orthop Surg200917742643419571298
- OrtmannEBesserMWKleinAAAntifibrinolytic agents in current anaesthetic practiceBr J Anaesth2013111454956323661406
- ElwatidySJamjoomZElgamalEZakariaATurkistaniAEl-DawlatlyAEfficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled studySpine (Phila Pa 1976)200833242577258019011538
- VermaKErricoTDiefenbachCThe relative efficacy of antifibrinolytics in adolescent idiopathic scoliosis: a prospective randomized trialJ Bone Joint Surg Am20149610e8024875032
- SethnaNFZurakowskiDBrustowiczRMBacsikJSullivanLJShapiroFTranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgeryAnesthesiology2005102472773215791100
- TzortzopoulouACepedaMSSchumannRCarrDBAntifibrinolytic agents for reducing blood loss in scoliosis surgery in childrenCochrane Database Syst Rev20083CD00688318646174
- BowenREGardnerSScadutoAAEaganMBecksteadJEfficacy of intraoperative cell salvage systems in pediatric idiopathic scoliosis patients undergoing posterior spinal fusion with segmental spinal instrumentationSpine (Phila Pa 1976)201035224625120081521
- LennonRLHoskingMPGrayJRKlassenRAPopovskyMAWarnerMAThe effects of intraoperative blood salvage and induced hypotension on transfusion requirements during spinal surgical proceduresMayo Clin Proc19876212109010943682953
- NicolaiPLeggetterPPGlitheroPRBhimarasettyCRAutologous transfusion in acetabuloplasty in childrenJ Bone Joint Surg Br200486111011214765876
- DomenREAdverse reactions associated with autologous blood transfusion: evaluation and incidence at a large academic hospitalTransfusion19983832963009563411
- WeissJMSkaggsDTannerJToloVCell saver: is it beneficial in scoliosis surgery?J Child Orthop20071422122719308514
- CopleyLARichardsBSSafaviFZNewtonPOHemodilution as a method to reduce transfusion requirements in adolescent spine fusion surgerySpine (Phila Pa 1976)199924321922210025016
- BasquesBABohlDDGolinvauxNSSmithBGGrauerJNPatient factors are associated with poor short-term outcomes after posterior fusion for adolescent idiopathic scoliosisClin Orthop Relat Res2015473128629425201091
- PugelyAJMartinCTGaoYIlgenfritzRWeinsteinSLThe incidence and risk factors for short-term morbidity and mortality in pediatric deformity spinal surgery: an analysis of the NSQIP pediatric databaseSpine (Phila Pa 1976)201439151225123424732856
- WengerDRMubarakSJLeachJManaging complications of posterior spinal instrumentation and fusionClin Orthop Relat Res199228424331395301
- BlumsteinGWAndrasLMSeehausenDAHarrisLRossPASkaggsDLFever is common postoperatively following posterior spinal fusion: infection is an uncommon causeJ Pediatr2015166375175525575423
- KielyPDMountLEDuJYThe incidence and risk factors for post-operative ileus after spinal fusion surgery: a multivariate analysisInt Orthop20164061825820838
- NachlasMMYounisMTRodaCPWitykJJGastrointestinal motility studies as a guide to postoperative managementAnn Surg197217545105020667
- KangGRSuhSWLeeIOPreoperative predictors of postoperative pulmonary complications in neuromuscular scoliosisJ Orthop Sci201116213914721311930
- YuanNSkaggsDLDoreyFKeensTGPreoperative predictors of prolonged postoperative mechanical ventilation in children following scoliosis repairPediatr Pulmonol200540541441916145695
- RappaportDIAdelizzi-DelanyJRogersKJOutcomes and costs associated with hospitalist comanagement of medically complex children undergoing spinal fusion surgeryHosp Pediatr20133323324124313092
- LeiderLLJrMoeJHWinterRBEarly ambulation after the surgical treatment of idiopathic scoliosisJ Bone Joint Surg Am1973555100310154586405
- HeilbronnerDMSussmanMDEarly mobilization of adolescent scoliosis patients following Wisconsin interspinous segmental instrumentation as an adjunct to Harrington distraction instrumentation. Preliminary reportClin Orthop Relat Res198822952583349691
- KingRTrottierTVariation in care among spinal deformity surgeons: results of a survey of the Shriners hospitals for childrenSpine (Phila Pa 1976)200732131444144917545914
- TarrantRCO’LoughlinPFLynchSTiming and predictors of return to short-term functional activity in adolescent idiopathic scoliosis after posterior spinal fusion: a prospective studySpine (Phila Pa 1976)201439181471147824875955
- MilbrandtTASinghalMMinterCA comparison of three methods of pain control for posterior spinal fusions in adolescent idiopathic scoliosisSpine (Phila Pa 1976)200934141499150319525843
- Van BoerumDHSmithJTCurtinMJA comparison of the effects of patient-controlled analgesia with intravenous opioids versus epidural analgesia on recovery after surgery for idiopathic scoliosisSpine (Phila Pa 1976)200025182355235710984788
- KlattJWMickelsonJHungMDurcanSMillerCSmithJTA randomized prospective evaluation of 3 techniques of postoperative pain management after posterior spinal instrumentation and fusionSpine (Phila Pa 1976)201338191626163123715024
- BlancoJSPerlmanSLChaHSDelpizzoKMultimodal pain management after spinal surgery for adolescent idiopathic scoliosisOrthopedics2013362 Suppl333523379574
- BlankJFlynnJMBronsonWThe use of postoperative subcutaneous closed suction drainage after posterior spinal fusion in adolescents with idiopathic scoliosisJ Spinal Disord Tech200316650851214657746