Abstract
Chronic widespread pain (CWP) including fibromyalgia syndrome (FMS) has a high prevalence and is associated with prominent negative consequences. CWP/FMS exhibits morphological and functional alterations in the central nervous system. The importance of peripheral factors for maintaining the central alterations are under debate. In this study, the proteins from biopsies of the trapezius muscle from 18 female CWP/FMS patients and 19 healthy female controls were analyzed. Pain intensity and pressure pain thresholds (PPT) over the trapezius muscles were registered. Twelve proteins representing five different groups of proteins were important regressors of pain intensity in CWP/FMS (R2=0.99; P<0.001). In the regression of PPT in CWP/FMS, it was found that 16 proteins representing six groups of proteins were significant regressors (R2=0.95, P<0.05). Many of the important proteins were stress and inflammation proteins, enzymes involved in metabolic pathways, and proteins associated with muscle damage, myopathies, and muscle recovery. The altered expression of these proteins may reflect both direct and indirect nociceptive/inflammatory processes as well as secondary changes. The relative importance of the identified proteins and central alterations in CWP need to be investigated in future research. Data from this and the previous study concerning the same cohorts give support to the suggestion that peripheral factors are of importance for maintaining pain aspects in CWP/FMS.
Introduction
Chronic widespread pain (CWP; population prevalence 5%–10%)Citation1–Citation4 including fibromyalgia syndrome (FMS; prevalence 2%–4%)Citation5–Citation7 is associated with pronounced negative personal implications, often treatment resistance and societal consequences. CWP/FMS exhibits morphological and functional alterations in the central nervous system – eg, the pain matrix in the brain and in the descending control of nociception – with clinical signs of central hyperexcitability.Citation5,Citation8–Citation11 Disturbances in neuroendocrine and autonomic nervous systems have also been reportedCitation12,Citation13 as well as signs of systemic oxidative stress.Citation14,Citation15 Patients with CWP/FMS generally perceive their pain as originating from the musculoskeletal system, eg, tendons and muscles. The importance of peripheral factors for maintaining the central alterations is under debate; alterations in muscles and nociceptors (ie, small-fiber pathology)Citation16–Citation24 may support a suggestion that peripheral factors act as tonic nociceptive generators and contribute to the maintenance of pain in CWP/FMS.
From a microdialysis study of the painful trapezius muscle in women with CWP/FMS, it was reported that the interstitial concentrations of glutamate and lactate correlated positively with pain intensity and negatively with pressure pain threshold (PPT) of trapezius.Citation25 However, the explained variations (R2) in these regressions were relatively low (15% and 18%, respectively), although significant, which opens up the possibility that the levels of other peripheral factors such as muscle proteins also can be altered in CWP/FMS. Two-dimensional gel electrophoresis (2-DE)Citation26 and mass spectrometry are used for separating and quantifying the protein content of a tissue. Multivariate data analysis (MVDA) including advanced principal component analysis (PCA) and partial least square (PLS) regression are important tools in the field of omics (eg, proteomics and metabolomics). Data sets from the research fields concerning omics are characterized by low subject-to-variables ratios and a large number of intercorrelated molecules/proteins. MVDA has the potential to capture, eg, complex proteome changes that may be present in chronic pain; another advantage is the reduction of multiple testing issues. Even though there are muscle proteomic studies covering different important physiological aspects – eg, aging,Citation27–Citation29 hypoxia,Citation30,Citation31 and exerciseCitation32–Citation36 – few studies have explored proteomic alterations in chronically painful muscles.Citation37 Almost a third of the 97 identified proteins from extracellular fluid of the trapezius muscle were at least twofold up- or downregulated in patients with CWP/FMS compared to healthy controls.Citation38 Recently, we reported that certain proteins of muscle biopsies from the trapezius muscle clearly differentiated between CWP/FMS and healthy controls (explained significant variation 85%) in a multivariate manner.Citation39 Registrations of pain intensity and PPT are routine in research and in clinical assessments of patients with pain.Citation40–Citation42 The question arises if these pain characteristics are associated with the proteome of the aching muscle. Hence, this second study of CWP/FMS and healthy controls investigates the relationships between proteins of trapezius muscle biopsies, pain intensity, and PPT.
Material and methods
Subjects
Patients with CWP were recruited for the study, including former patients with CWP at the Pain and Rehabilitation Centre of the University Hospital, Linköping, Sweden, and from an organization for FMS patients. Inclusion criteria were being a female aged between 20 and 65 years and having a diagnosis of CWP and/or FMS according to the American College of Rheumatology criteria.Citation43 Healthy controls (CON) were recruited through advertisement in the local newspaper. Their inclusion criteria were being a female aged between 20 and 65 years and being pain free.
All subjects were clinically examined by either of the two physicians. Exclusion criteria in both groups (CWP and CON) were any kind of anticoagulation use, continuous anti-inflammatory drug use, opioid or steroidal use, bursitis, tendonitis, capsulitis, postoperative conditions in the neck/shoulder area, previous neck trauma, disorder of the spine, neurological disease, rheumatoid arthritis, metabolic disease, malignancy, severe psychiatric illness, or any other systemic diseases, pregnancy, and difficulties understanding the Swedish language. At the end of the recruitment process, 18 women with CWP were included in the study, of whom 15 also fulfilled the criteria for FMS, as were 19 healthy controls. The recruitment process has been described in detail elsewhere.Citation25,Citation44
In our previous proteomic study investigating group differences,Citation39 we reported age and anthropometric data (here presented as mean ±1 standard deviation [SD]): CWP – age (years), 48.6±9.7; height (cm), 167.6±5.1; weight (kg), 76.8±17.3; body mass index (BMI) (kg/m2), 27.2±5.5; CON – age, 41.2±10.6; height, 168.7±7.7; weight, 68.5±12.8; BMI, 23.9±3.1. Significant group differences existed for age (P=0.035) and BMI (P=0.034). Data concerning the included subjects with respect to psychological aspects (anxiety, depression, and catastrophizing) and quality of life have essentially (ie, not exactly equal number of subjects in the two groups in the two studies) been presented elsewhere;Citation25 according to these variables, the CWP group had more intensive psychological symptoms, even though at the group level CWP did not show definite signs of depression or anxiety according to the instrument (Hospital Anxiety and Depression Scale; cut-off values for both subscales depression and anxiety >10) used for capturing this. No significant group difference in quality of life was found.
The number of subjects needed to achieve sufficient power was based on the concentration of interstitial lactate in the trapezius muscle in healthy controls and in patients with chronic trapezius myalgia reported in one of our previous studies.Citation45 Hence, using Power and Sample Size Calculation, version 3.0.2,Citation46 based on the following parameters: α=0.05, power =0.8, difference between groups =1.7, and SD =1.7, we found that 17 subjects in each group were needed. Generally, the number of subjects per group is relatively small in studies within the field of proteomics; for instance, human pain proteomic cerebrospinal fluid studies that report biomarker candidates typically have about ten subjects per group and have hitherto used traditional univariate statistics.Citation47,Citation48
After receiving verbal and written information about the study, all participants signed a consent form that was in accordance with the Declaration of Helsinki. The study was granted ethical clearances by the Regional Ethical Review Board of Linköping (Dnr: M10-08, M233-09, Dnr: 2010/164-32).
Pain intensity
Each subject rated the pain intensity in the neck–shoulder region approximately 2 hours prior to the biopsy using an 11 grade (0–10) numeric rating scale with 2 end points: 0 indicating no pain at all and 10 indicating worst possible pain.Citation49
Pressure pain thresholds
As a part of the clinical examination, PPTs were determined using an electronic pressure algometer (Somedic, Hörby, Sweden). Algometry was conducted approximately 5–7 days before the microbiopsy. The diameter of the contact area was 10 mm, and the pressure was applied perpendicularly to the skin at a speed of 30 kPa/s. The participants were instructed to mark their threshold by pressing a button as the sensation of pressure changed to pain. Algometry was performed bilaterally over the medial, middle, and lateral part of the descending part of the trapezius muscle to determine the PPTs. All measurements were conducted twice in approximately 5-minute intervals. The PPT values were calculated as the mean of these two measurements of lateral, middle, and medial site on the right and left trapezius muscle. In the regression analyses, the mean values of right and left trapezius measurements were used; ie, two Y-variables simultaneously (see “Statistics” section). Before the actual testing, the participants were given instructions and allowed to examine the testing procedure. Note that the PPTs essentially (not exactly the same number of subjects) have been presented elsewhere.Citation25,Citation44
Biopsy collection, preparation, and proteomic analysis
For a full detailed description, see our previous cohort study.Citation39 In short, biopsies were taken using Monopty BARD® microbiopsy instrument (BARD Norden, Helsingborg, Sweden) from the upper trapezius muscle at the midpoint between the seventh cervical vertebra and the acromion for the most painful side; generally the dominant side. If no differences existed the dominant side was used. The tissues were quickly frozen by immersion in isopentane precooled with dry ice and stored at –80°C until analysis. On the day of analysis, the muscle tissues were heat stabilized with Denator Stabilizer T1 (Denator, Göteborg, Sweden), placed in a tube containing urea sample buffer solution, homogenized by sonication, incubated for 2 hours in 4°C, followed by 1 hour centrifugation at 20,000× g. Protein concentration was measured by 2-D Quant Kit (GE Healthcare, Little Chalfont, UK) according to the manufacturer’s instructions.
One hundred micrograms of proteins from each of the samples was analyzed using 2-DE. Separated proteins were detected by silver staining, described elsewhere,Citation50 with a detection limit of about 5 ng/spot.Citation51 The protein patterns were analyzed as digitized images using a charge-coupled device camera (VersaDoc™ Imaging System 4000 MP, Bio-Rad, Hercules, CA, USA) in combination with a computerized imaging 12-bit system designed for evaluations of 2-DE patterns (PDQuest 8.0.1, Bio-Rad). The amount of protein in a spot was assessed as background-corrected optical density, integrated over all pixels in the spot, and expressed as integrated optical density. To correct for differences in total silver stain intensity between different 2-DE images, the amounts of the compared protein spots were quantified as optical density for individual spot per total protein intensity of all valid spots in the same gel. Thereby, ppm values (parts per million) for all proteins were generated and were statistically evaluated.
Significant protein spots were analyzed by mass spectrometry for protein identification using MALDI-TOF (Voyager De Pro, Applied Biosystems, Foster City, CA, USA) and Linear Trap Quadropole Orbitrap Velos Pro hybrid (Thermo Fisher Scientific, Waltham, MA, USA) in conjunction with nanoflow high-performance liquid chromatography system (EASY-Nlc II, Thermo Fisher Scientific). Generated mass spectra were analyzed with MaxQuant Version 1.5 and searched against the human taxonomy of the SwissProt database (released August 2014). Two missed cleavages were allowed, and N-terminal acetylation and methionine oxidation were selected as variable modifications. Fixed modification was carbamidomethylation of cysteine. For mass spectra (MS), an initial mass accuracy of 6 ppm was allowed, and the MS/MS tolerance was set to 0.5 Da. The false discovery rate at the peptide spectrum matched, and protein level was set to 0.01.
Statistics
For comparison of group differences regarding background data, pain thresholds, and pain intensity, one-way analysis of variance (ANOVA) and the nonparametric Mann–Whitney U-test were applied using IBM SPSS v.21.0 (IBM, Armonk, NY, USA) for normal distributed data and for nonnormally distributed data respectively; P<0.05 was considered significant. Effect sizes (Cohen’s d) were calculated using a calculator available on the internet (http://www.uccs.edu/~lbecker/).
The development of Omics methods (ie, large-scale data analysis for the characterization and quantification of biological molecules) has been paralleled by the development of statistical methods like MVDA, which is capable of handling a high number of intercorrelated substances in relatively few individuals.Citation52,Citation53 Traditional univariate statistical methods can quantify level changes of individual substances but disregard interrelationships between them and thereby ignore system-wide aspects. Therefore, when investigating the multivariate correlations between the proteins (X-variables) and pain intensity and pain thresholds (Y-variables), orthogonal partial least squares (OPLS) regression analysis was applied using SIMCA-P+ v.13.0 (UMETRICS, Umeå, Sweden).Citation54 When applying MVDA, we followed the recommendations concerning omics data presented by Wheelock and Wheelock.Citation53
PCA was used prior to this analysis to check for multivariate outliers. PCA can be used to extract and display systematic variation in the data matrix. A cross-validation (CV) technique was used to identify nontrivial components. Variable loading upon the same component is correlated, and variables with high loadings but with different signs are negatively correlated. Significant variables with high loadings (positive or negative) are more important for the component under consideration than variables with lower absolute loadings.Citation54 Two powerful methods are available in SIMCA-P+ for identifying multivariate outliers: 1) score plots in combination with Hotelling’s T Citation2 (identifies strong outliers) and 2) distance to model in X-space (identifies moderate outliers).
OPLSCitation54 was used for the regression analyses using the detected proteins as regressors (X-variables). OPLS separates the systemic variation in X-variables into two parts; one part is correlated and predictive to Y-variable/variables and one is uncorrelated (orthogonal) to Y-variable/variables. Variables were mean centered, scaled for unified variance (UV-scaling), and transformed (log) if necessary. The VIP variable (variable influence on projection) indicates the relevance of each X-variable pooled over all dimensions and Y-variables – the group of variables that best explain Y. VIP>1.0 combined with jack-knifed 95% confidence intervals in the regression coefficients plot not including zero were considered significant. Coefficients (PLS scaled and centered regression coefficients) were used to note the direction of the relationship (positive or negative). In this study, the analysis was made in two steps. First, all proteins were included, and then from this analysis were selected proteins with VIP >1.0 combined with the jack-knifed confidence intervals in the coefficients plot not including zero and used in a new regression presented in the results. The important/significant (VIP >1) proteins were identified. To determine the relative importance of these significant proteins, a separate regression was made only including these proteins as regressors. In the tables, P(corr) for each significant variable is also presented. This is the loading of each variable scaled as a correlation coefficient, and thus standardizing the range from –1 to +1.Citation53 P(corr) is stable during iterative variable selection and comparable between models. An absolute P(corr) >0.4–0.5 is generally considered as significant;Citation53 in the tables absolute P(corr) is presented.
R2 describes the goodness of fit – the fraction of sum of squares of all the variables explained by a principal component.Citation54 Q2 describes the goodness of prediction – the fraction of the total variation of the variables that can be predicted by a principal component using CV methods. R2 should not be considerably higher than Q2. A difference greater than 0.2–0.3 implies overfitting, meaning that the robustness of the model is poor.Citation53 To validate the model, we used cross validated analysis of variance (CV-ANOVA). The returned P-value is indicative of the statistical significance of the investigated model. The presentation of parameters from the MVDA of this study is in accordance with the guidelines presented by Wheelock and Wheelock.Citation53
Multiple linear regression (MLR) could possibly have been an alternative when regressing pain intensity and PPT, but it assumes that the regressor (X) variables are independent. If multicolinearity (ie, high correlations) occurs among the X-variables, the regression coefficients become unstable and their interpretability breaks down. MLR also assumes that a high subject-to-variables ratio is present (eg, >5), and such requirements are not required for PLS; in fact, PLS can handle subject-to-variables ratios <1. Moreover, PLS can, in contrast to MLR, handle several Y-variables simultaneously.
Results
Pain intensity and pain thresholds – univariate statistics
An expected, significant difference in pain intensity with higher levels in CWP was noted (P<0.001). Significantly lower PPTs both at right and left sides of the trapezius in CWP compared to CON (P<0.01) were found (). PPT of right and left sides showed a high and significant correlation (r=0.94, P<0.001) in all subjects taken together. Cohen’s d clearly showed that PPT was an important variable for separating the two groups of subjects. No significant correlation existed between the two PPTs and pain intensity in CWP (r: 0.23–0.25; nonsignificant).
Table 1 Pain intensity (NRS) and PPT in patients with CWP (n=18) and in CON (n=19)
Multivariate regression analyses
An unsupervised PCA was performed to check for outliers (three principal components, R2=0.34, Q2=0.08). According to the Hotelling’s T Citation2, T2Crit (0.99%), and the DModX plot (distance to the model in X-space), neither strong nor moderate outliers were found. To evaluate if correlations existed between protein expression (X-variables) and pain characteristics (pain intensity or pain thresholds; Y-variables), OPLS regressions were performed in two steps as already described; in the initial analyses, 216 proteins were included as X-variables (regressors). All analyses except for pain intensity (not possible to perform since CON had no pain.) were done both for all subjects and for the two groups of subjects separately.
To increase the interpretability of the obtained results, proteins of importance for the regressions were schematically divided according to the UniProt database (http://web.expasy.org) definition on biological process in either of six groups: 1) stress and inflammatory (S & I), 2) contractile (C), 3) metabolic (M), 4) structural (S), 5) transport (T), and 6) other (O) proteins.
Regression of pain intensity in CWP
A significant regression – with one predictive component – was found when regressing pain intensity in CWP (R2=0.99, Q2=0.91, CV-ANOVA: P<0.001; ); in the final regression, 12 proteins out of 46 proteins representing five groups (M, C, S, T, and S & I) of proteins were the important regressors. It was found as expected that the majority of the explained variation in pain intensity was due to the significant proteins; when only the significant proteins presented in were included in the OPLS regression, a highly significant regression was again obtained (R2=0.79, Q2=0.66, CV-ANOVA: P=0.007).
Table 2 important/significant proteins found in the significant OPLS model of pain intensity in the CWP group
Regression of PPTs of trapezius right and left side
A significant regression – with one predictive component – of PPTs bilaterally (ie, 2 Y-variables) was obtained for all subjects taken together (CWP and CON) (R2=0.56, Q2=0.49, CV-ANOVA: P<0.001; ). Hence, in the final model, 11 out of 31 proteins were important regressors representing four different groups of proteins (ie, M, S, T, and S & I). For this regression, it was found that all of the explained variation in PPT was due to the significant proteins; ie, when only the significant proteins () were included in the regression, a highly significant regression was obtained (R2=0.56, Q2=0.51, CV-ANOVA: P<0.001).
Table 3 important/significant proteins found in the significant OPLS regression of PPT of trapezius bilaterally (ie, 2 Y-variables) in all subjects taken together (CWP and CON)
A significant regression – with two predictive components – was found for PPTs bilaterally in CWP (R2=0.95, Q2=0.81, CV-ANOVA; P<0.05); the main part (>90%) of the explained variation was explained by the first component. In the final regression, 16 out of 45 proteins were significant regressors and represented all six groups of proteins (ie, M, C, S, T, S & I, and O; ). The majority of the explained variation in PPT in CWP was due to the significant proteins; when only the significant proteins () were included in an OPLS, a highly significant regression was obtained (R2=0.66, Q2=0.50, CV-ANOVA: P=0.004).
Table 4 important/significant proteins found in the significant OPLS model of PPT of trapezius bilaterally (ie, 2 Y variables) in the CWP group
It was not possible to obtain a significant regression of PPT in CON separately.
Discussion
Proteomics in combination with MVDA were used to determine possible correlations between proteins and pain intensity and pain thresholds for pressure; the following important results were found:
Pain intensity correlated strongly with 12 proteins from the muscle biopsies of trapezius in CWP ().
In all subjects taken together (CWP and CON), 11 proteins representing different functions had the largest importance when regressing PPT ().
The variability in PPT of CWP was strongly associated with 16 muscle proteins ().
In the clinical examination of patients with chronic pain condition pain intensity ratings, basic tests of pain sensitivity, eg, manual palpation of the aching area and tender point examinations, are applied. Psychophysical assessments such as pain thresholds, eg, for pressure, are used additionally for a more detailed assessment of pain sensitivity. Identification of objective biomarkers such as proteins in different tissues has been highlighted as a necessity to facilitate and improve diagnosis of chronic pain conditions.Citation55 It is interesting to note the strong and significant correlations between the muscle protein expressions and the investigated pain characteristics (–). Our results do not exclude that central factors are important for pain intensity and sensitivity; the relative importance of central factors and peripheral protein factors has to be investigated. Even though very strong regressions were obtained in CWP, it must be noted that not only the significant proteins contributed to R2/Q2 (–), but also that the majority of the explained variations were due to the significant proteins. The question may arise if the obtained significant regressions are overfitted. It has been stated that if R2 is substantially greater than Q2 (a difference >0.3 is mentioned in the literatureCitation52), the robustness of the regression is poor, implying overfitting.Citation53 In this study, only small differences existed between R2 and Q2 (–). Our results must be critically viewed, and there is certainly a need for confirmatory studies both in men and women, which also include possible confounders such as physical fitness, eg, strength and endurance. When interpreting the results, it must be kept in mind that this is a cross-sectional study and that the significant proteins may represent both direct and indirect nociceptive and inflammatory processes as well as secondary changes due to deconditioning, disuse, etc. This study had an explorative approach, and in future studies it is important to understand the biological constructs underlying the significant proteins. Furthermore, as exemplified below, the characterization of proteins according to the UniProt database, even though good for an overview, do not take into consideration that a certain protein can have several functions.
Regression of PPT in CWP and CON
Marked group differences in PPTs existed as reported elsewhere,Citation25,Citation44 with large effect sizes (Cohen’s d) for PPT (), ie, PPTs to a large extent can differentiate the two groups of subjects. An assumption that similar patterns of important proteins exist for the regression of group membershipCitation39 and the present regression of PPTs in all subjects was confirmed since eight of the significant proteins in were also important for group differentiating.Citation39 In the regression of group membership was found eleven upregulated (ATP synthase subunit β, mitochondrial; triosephosphate isomerase; fructose-bisphosphate aldolase A; keratin, type II cytoskeletal 1; fructose-bisphosphate aldolase A; myosin light chain 1/3, skeletal muscle isoform; alpha-crystallin B chain; pyruvate kinase PKM; carbonic anhydrase 3; glyceraldehyde-3-phosphate dehydrogenase; myosin light chain 3.) and six downregulated (Creatine kinase B-type; protein disulfide-isomerase; adenylate kinase isoenzyme 1; desmin; glutathione S-transferase Mu 2; heat shock protein β-1.) proteins in CWP,Citation39 which indicated alterations in stress and inflammation, in metabolic pathways, and in processes associated with muscle damage and recovery.Citation39 In contrast to group membership, PPTs are continuous variables, which may explain the lower explained variation (R2=0.56 vs R2=0.85). Possibly related to these variations, three other proteins had importance, and two of these had greater importance, ie, ankyrin repeat domain-containing protein 2 and myoglobin (). The former was the most important regressor; low quantities of this protein was associated with low PPTs. Muscle ankyrin repeat proteins are primarily involved in the defense of the cells against injuries ().Citation56 Ankyrin repeat domain-containing protein 2 is a negative regulator of myocyte differentiation.Citation57,Citation58 When the muscle cell is induced to oxidative stress, a posttranslational modification, phosphorylation at Ser-99 by AKT2 signaling pathway, is triggered, which in turn induces a translocation of the protein from the sarcomere to the nucleus of myofibers, thus preventing the outcome of muscle differentiation ().Citation57,Citation58 Ankyrin repeat domain-containing protein 2 has also been identified as a potent repressor/regulator of inflammatory responses through NF-κB repressor subunit p50.Citation59 The recruitment of p50 by the ankyrin protein is dependent on the AKT2-mediated phosphorylation of ankyrin repeat domain-containing protein 2 upon oxidative stress during myogenic differentiation (). Activation of NF-κB results in an increased expression of cytokines, chemokines, growth factors, and adhesion molecules.Citation60,Citation61 Dysregulation of NF-κB pathways is associated with diseases such as arthritis, autoimmunity, and cancer.Citation61 Increased activation of NF-κB has been reported in muscles of FMS patients.Citation60 NF-κB is also an important signaling pathway linked to the loss of skeletal muscle mass.Citation62 Triosephosphate isomerase acts as an enzyme of glycolysis and is important for efficient energy production in the cell (). Carbonic anhydrase 3Citation63–Citation65 and heat shock protein β-1Citation66 are suggested to have antioxidative effects and were also important regressors ( and ). Carbonic anhydrase 3 is also a specific marker for skeletal muscle damage and is released into circulationCitation63 ().
Figure 1 an overview of the significant proteins from the regression model of PPT in CWP and CON taken together.
Abbreviations: CON, controls; CWP, chronic widespread pain; PPT, pressure pain threshold.
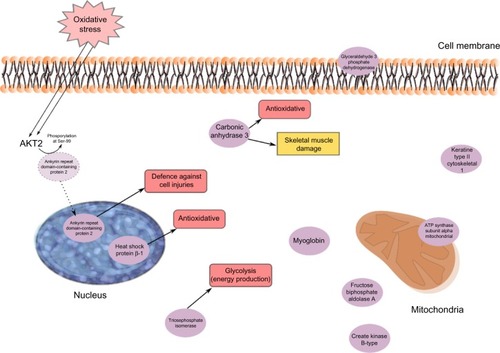
To summarize, the regression of PPTs in all subjects to a great extent confirms the results recently reported for group membershipCitation39 and links alterations in stress and inflammatory and metabolic muscle proteins to low PPT.
Regression of PPT in CWP
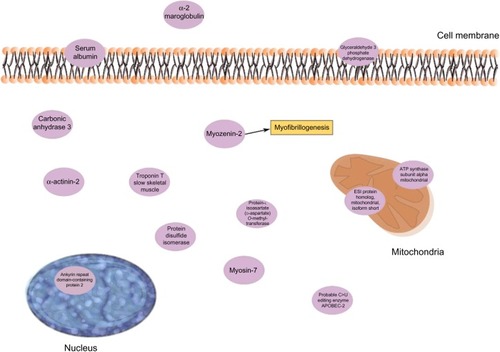
Contractile, structural, and stress/inflammatory proteins were important for PPTs in CWP separately (). Two isoforms of ankyrin repeat domain-containing protein 2 (as discussed in the “Regression of PPT in CWP and CON” section) were positively correlated with PPT in CWP. α-2-macroglobulin (negatively correlated) is an acute-phase protein and acts as a protease inhibitor and carrier for several growth factors and cytokines, including TNF-α, IL-1β, IL-6, and TGF-βCitation67 (). Protein disulfide-isomerase (positively correlated) catalyzes the sulfide bonds in proteins and also functions as a chaperone, which inhibits the aggregation of other proteins.Citation68 Other important proteins in this regression were myozenin-2 (positively correlated) and α-actinin-2 (negatively correlated). The skeletal muscle isoform α-actinin-2 is mainly concentrated in the Z-band together with proteins like myozenin and is involved in binding myofibrillar actin filaments. Myozenin-2 has an active role in the regulation of calcineurin (a T-cell activator) signaling and has been suggested as being a part of the myofibrillogenesisCitation69 (). To conclude, several of the important proteins for PPT in CWP are involved in stress and inflammatory aspects of muscles. How these proteins are related to the contractile proteins warrants further investigations.
Regression of pain intensity in CWP
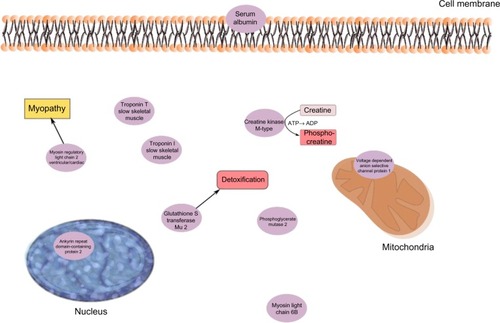
Also, in this regression, ankyrin repeat domain-containing protein 2 was a significant protein and correlated positively with pain intensity in CWP (). Glutathione S- transferase Mu 2 (positively correlated) is involved in the detoxification of products from oxidative stressCitation70 (). Creatine kinases are expressed in a wide variety of tissue and often those with high energy demands such as skeletal muscle. It catalyzes the conversion of creatine to phosphocreatine, a reaction that consumes a phosphate by reducing adenosine triphosphate (ATP) to adenosine diphosphate (ADP)Citation63 (). Decreased levels of one creatine kinase (B-type) in CWP was previously found,Citation39 which may be associated both with lower levels of ATP and low muscle pH. Low ATP and low phosphocreatine levels have been reported from the quadriceps muscle of FMS patients.Citation71 Myosin light chain 6B has been associated with skeletal muscle atrophy in chronic obstructive pulmonary disease.Citation72 Mutations of myosin light chains have also been associated with rare human myopathiesCitation73 (). Several skeletal isoforms of troponin T together with troponin I were significant regressors of pain intensity. Increased levels of skeletal troponin I and skeletal troponin T have been found in chronic muscle diseases, eg, polymyositis and dermatomyositis.Citation74,Citation75 Three isoforms of troponin T were significant, and those with highest correlations were positively correlated with pain intensity. Furthermore, on measurement of plasma, elevated levels of troponin T have been found in patients with chronic muscle disease.Citation75 To summarize, a complex pattern of stress and inflammatory proteins together with contractile and metabolic proteins correlated with pain intensity in CWP.
Figure 3 The significant proteins from the regression model of pain intensity in the CWP group.
Abbreviations: aDP, adenosine diphosphate; aTP, adenosine triphosphate; CWP, chronic widespread pain.
Conclusions and possible clinical implications
Proteomics in combination with MVDA is a potent tool for identifying potential biomarkers or bioclusters in the chronic pain research area; several proteins of the myalgic trapezius muscle were identified that correlated with pain intensity and pain sensitivity in CWP. These proteins were stress and inflammation proteins, enzymes involved in metabolic pathways, and proteins associated with muscle damage, myopathies, and muscle recovery. The altered expression of these proteins reasonably reflects both direct and indirect nociceptive/inflammatory processes as well as secondary changes. Confirmatory studies both in women and men are needed, taking into account possible confounders, and studies of the relative importance of the important proteins and central alterations for pain characteristics in CWP are also needed. Chronic pain is a complex phenomenon, and a biopsychosocial model is applied in clinical practice. This study adds information about the biological aspects, especially in the periphery (muscle). Such information can be of importance for designing treatment and rehabilitation interventions.
Acknowledgments
We gratefully appreciate our tissue donors for this study. Furthermore, we thank research nurse Eva-Britt Lind, Pain and Rehabilitation Centre, UHL, County Council of Östergötland, SE-581 85 Linköping, Sweden, for valuable help with the sample collection. This study was supported by the Swedish Council for Working Life and Social Research, the Swedish Research Council Medical Research Council of Southeast Sweden and AFA Insurance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
- BergmanSHerrstromPHogstromKPeterssonIFSvenssonBJacobssonLTChronic musculoskeletal pain, prevalence rates, and sociodemographic associations in a Swedish population studyJ Rheumatol20012861369137711409133
- CroftPRigbyASBoswellRSchollumJSilmanAThe prevalence of chronic widespread pain in the general populationJ Rheumatol19932047107138496870
- CimminoMAFerroneCCutoloMEpidemiology of chronic musculoskeletal painBest Pract Res Clin Rheumatol201125217318322094194
- MouraoAFBlythFMBrancoJCGeneralised musculoskeletal pain syndromesBest Pract Res Clin Rheumatol201024682984021665129
- StaudRPeripheral pain mechanisms in chronic widespread painBest Pract Res Clin Rheumatol201125215516422094192
- LindellLBergmanSPeterssonIFJacobssonLTHerrstromPPrevalence of fibromyalgia and chronic widespread painScand J Prim Health Care200018314915311097099
- GerdleBGronlundCKarlssonSHoltermannARoeleveldKAltered neuromuscular control mechanisms of the trapezius muscle in fibromyalgiaBMC Musculoskelet Disord20101114220205731
- StaudRCraggsJGPerlsteinWMRobinsonMEPriceDDBrain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controlsEur J Pain20081281078108918367419
- StaudRBrain imaging in fibromyalgia syndromeClin Exp Rheumatol2011296 Suppl 69S109S11722243558
- SchweinhardtPBushnellMCPain imaging in health and disease – how far have we come?J Clin Invest2010120113788379721041961
- NapadowVKimJClauwDJHarrisREDecreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgiaArthritis Rheum20126472398240322294427
- AdlerGKGeenenRHypothalamic-pituitary-adrenal and autonomic nervous system functioning in fibromyalgiaRheum Dis Clin North Am2005311187202xi15639063
- DesseinPHShiptonEAStanwixAEJoffeBINeuroendocrine deficiency-mediated development and persistence of pain in fibromyalgia: a promising paradigm?Pain200086321321510812250
- FatimaGDasSKMahdiAASome oxidative and antioxidative parameters and their relationship with clinical symptoms in women with fibromyalgia syndromeInt J Rheum Dis Epub7142015
- EisingerJGandolfoCZakarianHAyavouTReactive oxygen species, antioxidant status and fibromyalgiaJ Musculoskelet Pain19975515
- BengtssonAEditorial, the muscle in fibromyalgiaRheumatol200241721724
- SandbergMLarssonBLindbergLGGerdleBDifferent patterns of blood flow response in the trapezius muscle following needle stimulation (acupuncture) between healthy subjects and patients with fibromyalgia and work-related trapezius myalgiaEur J Pain20059549751016139178
- SandbergMLindbergLGGerdleBPeripheral effects of needle stimulation (acupuncture) on skin and muscle blood flow in fibromyalgiaEur J Pain20048216317114987626
- BengtssonAHenrikssonKGLarssonJMuscle biopsy in primary fibromyalgia. Light-microscopical and histochemical findingsScand J Rheumatol1986151162421398
- SprottHSalemiSGayREIncreased DNA fragmentation and ultrastructural changes in fibromyalgic muscle fibresAnn Rheum Dis200463324525114962957
- VisserBvan DieenJHPathophysiology of upper extremity muscle disordersJ Electromyogr Kinesiol200616111616099676
- OaklanderALHerzogZDDownsHMKleinMMObjective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgiaPain2013154112310231623748113
- SerraJColladoASolaRHyperexcitable C nociceptors in fibromyalgiaAnn Neurol201475219620824243538
- UceylerNZellerDKahnAKSmall fibre pathology in patients with fibromyalgia syndromeBrain2013136Pt 61857186723474848
- GerdleBLarssonBForsbergFChronic widespread pain: increased glutamate and lactate concentrations in the trapezius muscle and plasmaClin J Pain201430540942023887335
- GorgADrewsOLuckCWeilandFWeissW2-DE with IPGsElectrophoresis200930Suppl 1S122S13219441019
- MarxJOKraemerWJNindlBCLarssonLEffects of aging on human skeletal muscle myosin heavy-chain mRNA content and protein isoform expressionJ Gerontol A Biol Sci Med Sci2002576B232B23812023259
- TermanABrunkUTLipofuscin: mechanisms of formation and increase with ageAPMIS199810622652769531959
- BoffoliDScaccoSCVergariRSolarinoGSantacroceGPapaSDecline with age of the respiratory chain activity in human skeletal muscleBiochim Biophys Acta19941226173828155742
- GelfiCDe PalmaSRipamontiMNew aspects of altitude adaptation in Tibetans: a proteomic approachFASEB J200418361261414734630
- ViganoARipamontiMDe PalmaSProteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxiaProteomics20088224668467918937252
- HollowayKVO’GormanMWoodsPProteomic investigation of changes in human vastus lateralis muscle in response to interval-exercise trainingProteomics20099225155517419834892
- HodySLeprincePSergeantKHuman muscle proteome modifications after acute or repeated eccentric exercisesMed Sci Sports Exerc201143122281229621606878
- BurgomasterKAHowarthKRPhillipsSMSimilar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humansJ Physiol2008586115116017991697
- BurnistonJGChanges in the rat skeletal muscle proteome induced by moderate-intensity endurance exerciseBiochim Biophys Acta200817847–81077108618482594
- TakahashiMKubotaSExercise-related novel gene is involved in myoblast differentiationBiomed Res2005262798515889621
- HadreviJGhafouriBLarssonBGerdleBHellstromFMultivariate modeling of proteins related to trapezius myalgia, a comparative study of female cleaners with or without painPLoS One201389e7328524023854
- OlaussonPGerdleBGhafouriNLarssonBGhafouriBIdentification of proteins from interstitium of trapezius muscle in women with chronic myalgia using microdialysis in combination with proteomicsPLoS One2012712e5256023300707
- OlaussonPGerdleBGhafouriNSjostromDBlixtEGhafouriBProtein alterations in women with chronic widespread pain – an explorative proteomic study of the trapezius muscleSci Rep201551189426150212
- VanderweeenLOostendorpRAVaesPDuquetWPressure algometry in manual therapyMan Ther19961525826511440515
- FruhstorferHLindblomUSchmidtWCMethod for quantitative estimation of thermal thresholds in patientsJ Neurol Neurosurg Psychiatry1976391110711075188989
- GoranssonKEHeilbornUSelbergJvon ScheeleSDjarvTPain rating in the ED – a comparison between 2 scales in a Swedish hospitalAm J Emerg Med201533341942225624078
- WolfeFSmytheHYunusMThe American college of rheumatology 1990 Criteria for the classification of fibromyalgia – report of the multicenter criteria committeeArthritis Rheum1990331601722306288
- GhafouriNGhafouriBLarssonBStenssonNFowlerCJGerdleBPalmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivityPain201315491649165823707281
- RosendalLLarssonBKristiansenJIncrease in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercisePain2004112332433415561388
- DupontWDPlummerWDJrPower and sample size calculations. A review and computer programControl Clin Trials19901121161282161310
- ContiARicchiutoPIannacconeSPigment epithelium-derived factor is differentially expressed in peripheral neuropathiesProteomics20055174558456716196102
- LiuXZengBXuJZhuHXiaQProteomic analysis of the cerebrospinal fluid of patients with lumbar disk herniationProteomics2006631019102816372267
- Ferreira-ValenteMAPais-RibeiroJLJensenMPValidity of four pain intensity rating scalesPain2011152102399240421856077
- ShevchenkoAWilmMVormOMannMMass spectrometric sequencing of proteins silver-stained polyacrylamide gelsAnal Chem19966858508588779443
- SwainMRossNWA silver stain protocol for proteins yielding high resolution and transparent background in sodium dodecyl sulfate-polyacrylamide gelsElectrophoresis19951669489517498141
- ErikssonLByrneTJohanssonETryggJVikströmCMulti- and Megavariate Data Analysis: Basic Principles and Applications3rd revised edMalmö, SwedenMKS Umetrics AB2013
- WheelockAMWheelockCETrials and tribulations of ‘omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicineMol Biosyst20139112589259623999822
- ErikssonLJohanssonEKettaneh-WoldNTryggJWikströmCWoldSMulti- and Megavariate Data analysis; Part I and II2nd edUmeå, SwedenUmetrics AB2006
- ChizhBAGreenspanJDCaseyKLNemenovMITreedeRDIdentifying biological markers of activity in human nociceptive pathways to facilitate analgesic drug developmentPain2008140224925318950938
- ProskeUMorganDLMuscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applicationsJ Physiol2001537Pt 233334511731568
- BelgranoARakicevicLMittempergherLMulti-tasking role of the mechanosensing protein Ankrd2 in the signaling network of striated musclePLoS One2011610e2551922016770
- CenniVBavelloniABerettiFAnkrd2/ARPP is a novel Akt2 specific substrate and regulates myogenic differentiation upon cellular exposure to H2O2Mol Biol Cell201122162946295621737686
- BeanCVermaNKYamamotoDLAnkrd2 is a modulator of NF-[kappa]B-mediated inflammatory responses during muscle differentiationCell Death Dis20145e100224434510
- RusterMFrankeSSpathMPongratzDESteinGHeinGEDetection of elevated N epsilon-carboxymethyllysine levels in muscular tissue and in serum of patients with fibromyalgiaScand J Rheumatol200534646046316393769
- OeckinghausAGhoshSThe NF-kappaB family of transcription factors and its regulationCold Spring Harb Perspect Biol200914a00003420066092
- LiHMalhotraSKumarANuclear factor-kappa B signaling in skeletal muscle atrophyJ Mol Med (Berl)200886101113112618574572
- BrancaccioPLippiGMaffulliNBiochemical markers of muscular damageClin Chem Lab Med201048675776720518645
- ZimmermanU-JPWangPZhangXBogdanovichSForsterREAnti-oxidative response of carbonic anhydrase III in skeletal muscleIUBMB Life200456634334715370882
- CabiscolELevineRLCarbonic anhydrase III. Oxidative modification in vivo and loss of phosphatase activity during agingJ Biol Chem19952702414742147477782339
- MatsumotoTUrushidoMIdeHSmall heat shock protein beta-1 (HSPB1) is upregulated and regulates autophagy and apoptosis of renal tubular cells in acute kidney injuryPLoS One2015105e012622925962073
- FeinmanRDThe proteinase-binding reaction of alpha 2MAnn N Y Acad Sci19947372452667524400
- LumbRABulleidNJIs protein disulfide isomerase a redox-dependent molecular chaperone?EMBO J200221246763677012485997
- TakadaFWoudeDLVTongH-QMyozenin: an α-actinin- and γ-filamin-binding protein of skeletal muscle Z linesProc Natl Acad Sci U S A20019841595160011171996
- BaezSSegura-AguilarJWiderstenMJohanssonASMannervikBGlutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processesBiochem J1997324Pt 125289164836
- GerdleBForsgrenMFBengtssonADecreased muscle concentrations of ATP and PCR in the quadriceps muscle of fibromyalgia patients – a 31P-MRS studyEur J Pain20131781205121523364928
- GuoWFuWPYangYDaiLMPreliminary proteomic analysis of peripheral skeletal muscle atrophy in chronic obstructive pulmonary diseaseZhonghua Yi Xue Za Zhi20129214948951 Chinese22781565
- PoetterKJiangHHassanzadehSMutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscleNat Genet199613163698673105
- KielyPDBrucknerFENisbetJADaghirASerum skeletal troponin I in inflammatory muscle disease: relation to creatine kinase, CKMB and cardiac troponin IAnn Rheum Dis200059975075111023449
- PrellwitzWHafnerGRupprechtHJMeyerJDiagnostic and differential diagnostic value of troponinsMed Klin (Munich)1996916336342 German8767305