Abstract
Background
Pregabalin is approved by the US Food and Drug Administration for the treatment of fibromyalgia (FM), diabetic peripheral neuropathy (DPN), postherpetic neuralgia (PHN), and neuropathic pain due to spinal cord injury (SCI). Approval was based on clinical trial data demonstrating statistically significant differences in pain scores versus placebo. However, statistically significant pain relief may not always equate to clinically meaningful pain relief. To further characterize the clinical benefit of pregabalin, this analysis examined shifts in pain severity categories in patients with FM, DPN/PHN (pooled in this analysis), and SCI treated with pregabalin.
Methods
Data were pooled from 23 placebo-controlled trials in patients with FM (1,623 treated with pregabalin, 937 placebo), DPN/PHN (2,867 pregabalin, 1,532 placebo), or SCI (181 pregabalin, 175 placebo). Pain scores were assessed on an 11-point numeric rating scale and categorized as mild (0 to <4), moderate (4 to <7), or severe (7 to 10). Only patients with mean score ≥4 at baseline were randomized to treatment. The percentage of patients shifting pain category from baseline to endpoint for pregabalin and placebo was analyzed using a modified ridit transformation with the Cochran–Mantel–Haenszel procedure.
Results
A higher proportion of patients shifted to a less severe pain category at endpoint with pregabalin compared with placebo. With flexible-dose pregabalin, the percentage of patients improving from: severe to mild (pregabalin versus placebo) was 15.8 versus 13.4 in FM patients, 36.0 versus 16.6 in DPN/PHN patients, 14.3 versus 7.7 in SCI patients; severe to moderate was 28.7 versus 28.2 in FM patients, 32.5 versus 28.2 in DPN/PHN patients, 35.7 versus 28.2 in SCI patients; and moderate to mild was 38.3 versus 26.4 in FM patients, 59.5 versus 41.4 in DPN/PHN patients, 38.6 versus 27.2 in SCI patients.
Conclusion
Compared with placebo, pregabalin is more often associated with clinically meaningful improvements in pain category in patients with FM, DPN, PHN, or SCI.
Introduction
The pain intensity experienced by patients with chronic pain conditions is often delineated into the categories of mild, moderate, and severe.Citation1 These categories attempt to reflect the degree of interference a patient’s pain has on their ability to function and can be linked to patient outcomes and health resource utilization.Citation1–Citation3 It is important to note that the relationship between a pain severity score and the degree of interference on patient function is not always linear, meaning that an equivalent change in pain score may not always equate to the same degree of change in function.Citation1,Citation2 As a result, while statistical difference from placebo (or from other treatment) is a necessary and important outcome measure, taken on its own it may not always represent clinically meaningful pain relief, while pain relief may not always represent clinically meaningful changes in function.
Pregabalin is an α2δ ligand indicated in the United States for the treatment of a range of chronic pain conditions including fibromyalgia (FM) and neuropathic pain associated with diabetic peripheral neuropathy (DPN), postherpetic neuralgia (PHN), and spinal cord injury (SCI).Citation4 Pregabalin is also indicated as adjunctive therapy for partial seizures.Citation4 The efficacy of pregabalin in these chronic pain conditions was demonstrated in a number of randomized, placebo-controlled trials in which pregabalin treatment improved mean pain score when compared with placebo.Citation5–Citation26 However, how directly improvements in mean pain score relate to clinical and functional benefits for patients is not always clear.
In this pooled analysis of patient-level data from all chronic pain conditions for which pregabalin is a US Food and Drug Administration (FDA)-approved treatment option, shifts in pain severity category following treatment were examined. Shifts in pain severity with pregabalin were compared with placebo to further understand the clinical impact of pregabalin treatment.
Methods
Study design
This was an analysis of 23 randomized, placebo-controlled, parallel-group, double-blind trials of pregabalin. Patient-level data were pooled into three groups: patients with FM, patients with DPN or PHN, and patients with neuropathic pain due to SCI. FM patient data were from five trials (ClinicalTrials.gov identifiers: NCT00645398, NCT00230776, NCT00333866, NCT00830167):Citation22–Citation26 conducted between September 1999 and May 2011; ranging from 8 to 15 weeks in duration; including doses of 300 mg/day, 450 mg/day, and flexible dosing (optimized to 300 or 450 mg/day during the first 3 weeks of the trial after which patients continued on their optimized dose). DPN and PHN patient data were pooled from nine studies in patients with DPN (NCT00156078, NCT00159679, NCT00143156, NCT00553475),Citation5–Citation7,Citation10–Citation13 five studies in patients with PHN (NCT00159666),Citation7,Citation14–Citation17 and two studies in patients with either painful DPN or PHN (NCT00301223):Citation18,Citation19 conducted between March 1998 and March 2009; ranging in duration from 5 to 16 weeks; including doses of 150 mg/day, 300 mg/day, 450 mg/day, and flexible dosing (150–600 mg/day in which dosage adjustments, based on tolerability, were allowed for the first 3 weeks after which the patient remained on their optimized dose for the remainder of the trial). SCI patient data were from two trials of 12-weekCitation20 and 16-week (NCT00407745) Citation21 duration conducted between June 2002 and February 2011, with flexible dosing (150–600 mg/day dosage adjustments for the first 3 or 4 weeks). This includes all Pfizer-sponsored randomized, placebo-controlled, parallel-group, double-blind trials of pregabalin in these patient populations completed before this analysis was initiated.
All patients were ≥18 years of age and had an average pain score ≥4 on the 11-point numeric rating scale (NRS), where 0= no pain and 10= worst possible pain, during the study screening period, with four or more pain diaries completed. Patients in the FM trials had a primary diagnosis of FM according to the 1990 American College of Rheumatology criteria for FMCitation27 (widespread pain present for ≥3 months and pain in ≥11 of 18 tender points). Patients in the DPN trials had a diagnosis of type 1 or type 2 diabetes mellitus with painful, symmetrical sensorimotor polyneuropathy for ≥6 months (or, in one trial, ≥3 months).Citation5 Patients in the PHN trials had experienced neuropathic pain for ≥3 months following healing of the herpes zoster viral rash (or, in one trial, ≥6 months).Citation15 Patients in the SCI trials had a complete or incomplete SCI of ≥12-months’ duration, with chronic pain experienced continuously for ≥3 months or with remissions and relapses for ≥6 months.
The protocols for all trials adhered to the International Ethical Guidelines for Biomedical Research Involving Human Subjects, the International Conference on Harmonisation Good Clinical Practice guidelines, and the Helsinki Declaration. All trials were approved by the appropriate institutional review board and all patients provided written informed consent prior to participation.
Efficacy measures
Patients rated their pain over the previous 24 hours on the NRS (0 = no pain and 10 = worst possible pain). Pain scores were categorized as mild (0 to <4), moderate (≥4 to <7), or severe (≥7 to ≤10).Citation2,Citation28 The percentage of patients shifting, from baseline to endpoint, from one pain severity category to another was calculated for placebo- and pregabalin-treated patients (patients with mild pain at baseline were excluded from the analysis). Patients shifting from severe to mild were considered “very much improved” (2 category shift). Patients shifting from severe to moderate or from moderate to mild were considered “much improved” (1 category shift). Patients remaining in the same pain category were classified as “no change”. Patients shifting to a more severe pain category were classified as “worsened”.
Change in Fibromyalgia Impact Questionnaire (FIQ) total score was assessed in four of the five FM trials (one of the trials did not record FIQ).Citation23 The FIQ is a 20-item self-administered, psychometrically validated questionnaire designed to assess health status, progress, and outcomes in patients with FM.Citation29 The FIQ total score assesses the effect of FM symptoms with a total score range of 0–100, with higher scores indicating greater impairment.Citation29 FIQ total scores were categorized as mild (0 to <39), moderate (39 to <59), or severe (≥59 to 100).Citation30 The percentage of patients shifting, from baseline to endpoint, from one FIQ severity category to another was calculated as described above for pain scores.
Statistical analysis
Pregabalin and placebo groups were compared and statistical significance was assessed by using a Cochran–Mantel–Haenszel test with modified ridit (modridit) transformation of the calculated ordinal shift scales. Missing data were imputed by baseline observation carried forward.
Results
Study population
Patient-level data were pooled into the following groups: 2,560 patients with FM (1,623 pregabalin and 937 placebo), 4,399 patients with DPN/PHN (2,867 pregabalin and 1,532 placebo), and 356 patients with SCI (181 pregabalin and 175 placebo). Baseline demographic characteristics were similar for different treatment arms within each condition ().
Table 1 Patient demographics at baseline
Shift in pain category
Patients with FM, DPN/PHN, and SCI were more likely to improve in pain category at endpoint with pregabalin than with placebo ().
Table 2 Patients in each pain severity category at endpoint
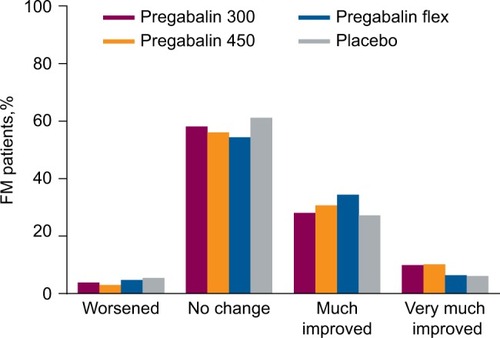
In patients with FM, there was a trend toward improvement in pain category compared with placebo with pregabalin at 300 mg/day (P=0.013), pregabalin 450 mg/day (P<0.001), and flexible-dose pregabalin (P=0.138) (). When compared with pregabalin at 300 or 450 mg/day, flexible-dose pregabalin was more likely to result in patients being “much improved” at endpoint but less likely to result in patients being “very much improved” (). While there were differences with different doses of pregabalin, when all pregabalin-treated patients were combined the improvement in pain category at endpoint was statistically significant compared with placebo (P<0.001).
Figure 1 Shift in pain severity category from baseline to endpoint for FM patients.
Abbreviations: flex, flexible dosing; FM, fibromyalgia; d, day.
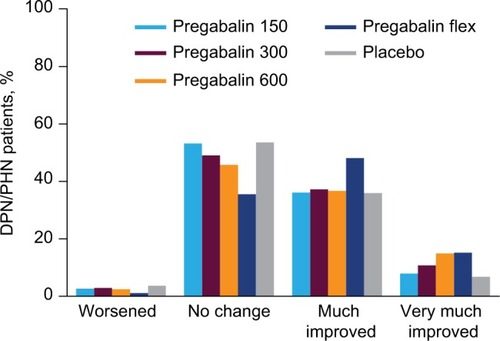
In patients with DPN/PHN, pain category at endpoint compared with placebo was improved with pregabalin 150 mg/day (P=0.001), 300 mg/day (P<0.001), 600 mg/day (P<0.001), and flexible-dose pregabalin (P<0.001) (). A shift to an improved pain category was more common with flexible-dose pregabalin than with any of the fixed doses, but pregabalin at 300 mg/day and 600 mg/day also resulted in shifts to improved pain categories ().
Figure 2 Shift in pain severity category from baseline to endpoint for DPN/PHN patients.
Abbreviations: DPN, diabetic peripheral neuropathy; flex, flexible dosing; PHN, postherpetic neuralgia.
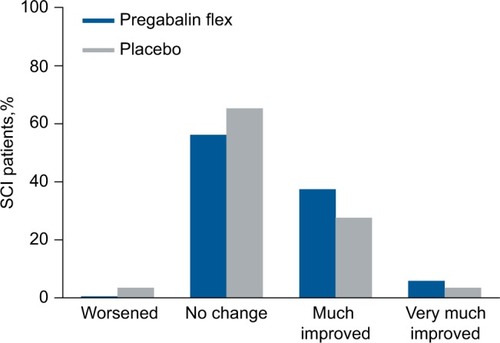
In patients with SCI, pain category at endpoint was improved with flexible-dose pregabalin compared with placebo (P=0.010) (). As with patients with FM or DPN/PHN, patients with SCI were more likely to shift to a “much improved” category than a “very much improved” category ().
Figure 3 Shift in pain severity category from baseline to endpoint for SCI patients.
Abbreviations: flex, flexible dosing; SCI, spinal cord injury.
Patients were generally more likely to shift pain category if they were classified as severe at baseline. For example, shows that for pregabalin 450 mg/day in FM patients, 49.0% of patients with severe pain at baseline remained in the severe category at endpoint, notably lower than the 63.6% of patients with moderate pain at baseline remaining in the moderate category at endpoint. There was a similar trend in each of the chronic pain conditions examined, for example, flexible-dose pregabalin in patients with DPN/PHN (31.5% severe, 38.5% moderate) and SCI (50.0% severe, 60.4% moderate).
Shift in FIQ total score category
While shifts in FIQ category from baseline to endpoint occurred in 36.6%–42.2% of patients with FM, shifts were not significantly different from placebo for pregabalin 300 mg/day (P=0.965), pregabalin 450 mg/day (P=0.136), or flexible-dose pregabalin (P=0.313). There was a trend toward a greater proportion of patients being “very much improved” (shifting from severe to mild) with pregabalin 300 mg/day (10.1%) and pregabalin 450 mg/day (13.2%), compared with placebo (7.6%) and flexible-dose pregabalin (6.8%), but this was not significant.
Discussion
Chronic pain conditions such as neuropathic pain associated with DPN, PHN, and SCI, together with FM, are often challenging to treat and have a significant impact on patients and their health-related quality of life.Citation31–Citation33 This analysis assessed the clinical impact of pregabalin treatment in each of these conditions as they represent all of the chronic pain conditions for which pregabalin is an FDA-approved treatment option.Citation4 The analysis sought to further assess the clinical impact of pregabalin treatment.
The use of pain categories mild, moderate, and severe were initially characterized in patients with chronic cancer pain,Citation1 and they have since been beneficial in research and in developing treatment approaches/algorithms in many chronic pain conditions.Citation34–Citation36 These categories are based on the impact of pain on patient function but also reflect patients’ own assessment of their pain.Citation2 As such, changes in pain category can be considered both clinically meaningful and as having a real impact for patients.
In this analysis, DPN/PHN, SCI, and FM patients with severe pain at baseline were nearly twice as likely to shift to mild pain at endpoint (ie, were “very much improved”) if they received treatment with pregabalin as opposed to placebo. Patients were also more likely to shift to moderate from severe, or mild from moderate, with pregabalin than with placebo. Overall, patients with DPN/PHN were more likely to shift to an improved pain category than patients with SCI or FM. The most notable change was in patients with DPN/PHN with severe baseline pain treated with flexible dose pregabalin, where 68.5% shifted to an improved pain category. In patients with SCI or FM, a small majority of patients did not shift pain category. This was also true for patients with DPN/PHN treated with placebo or low-dose pregabalin (150 mg/day). With higher doses of pregabalin (450 mg/day and flexible dosing), the majority of patients with DPN/PHN shifted to an improved pain category.
In patients with FM in this analysis, flexible-dose pregabalin was less likely to result in a shift in pain category than fixed doses of 300 mg/day and 450 mg/day. This was, perhaps, unexpected given the optimal approach is typically to carefully titrate pregabalin to the highest tolerable dose,Citation37–Citation39 which in this analysis would be more closely represented by the flexible dosing group. This observation was not consistent with the assessment of patients with DPN/PHN where flexible-dose pregabalin had a greater effect than fixed doses. The trials in patients with SCI included in this analysis only used flexible-dose pregabalin, which had a significantly greater effect than placebo. The average daily pregabalin dose in one study in patients with DPN/PHN over the full treatment period was 372.2 mg/day.Citation18 In patients with SCI, the average daily dose of pregabalin over both studies was 370.0 mg/day.Citation40 In the study in patients with FM, 178 of 250 patients (71.2%) received pregabalin 450 mg/day and 72 patients (28.8%) received 300 mg/day during the maintenance phase (ie, an average dose of ~400 mg/day).Citation25 As such, the difference in the effect of flexible-dose pregabalin between patient groups is unlikely to be due to differences in the average dose of pregabalin.
It should be noted that all patients with FM receiving flexible-dose pregabalin in this analysis were from a single studyCitation25 conducted solely in Japanese patients. The majority of patients with FM in the other trials in this analysis were from the United States: Three trials were conducted in patients in the United States exclusively,Citation22–Citation24 while one trial was in patients in Europe, Canada, Mexico, India, Korea, Australia, and Venezuela.Citation26 There may be cultural or racial characteristics of Japanese patients with FM that make them less likely to shift pain category than patients with FM in the United States or Europe.
Alternatively, the differences in the flexible-dose group may be related to differences in baseline pain. In general, patients were more likely to shift pain category if they had severe pain at baseline. The mean (standard deviation) baseline pain scores were lower in the flexible-dose FM population: 6.5 (1.3) in the flexible-dose pregabalin group compared with 7.0 (1.3) and 6.8 (1.4) in the pregabalin 300 mg/day and 450 mg/day groups, respectively. In addition, a lower proportion of patients were categorized as severe at baseline in the flexible-dose pregabalin group (40.4%) than in the pregabalin 300 mg/day (54.2%) or pregabalin 450 mg/day (50.5%) groups.
Previous analyses have supported the suggestion that pregabalin has a greater impact on pain, and symptoms, in patients with severe pain at baseline. These include an analysis of pregabalin clinical trials in patients with DPN that showed a greater mean change in pain score at endpoint versus placebo with pregabalin in patients with severe baseline pain compared with moderate baseline pain.Citation41 The analysis also revealed greater improvements with pregabalin in patients’ pain-related sleep interference and global impression of change.Citation41 In patients with FM, pregabalin (at 300 mg/day and 450 mg/day) also led to a greater mean change in pain score at endpoint versus placebo in patients with severe pain at baseline than in patients with moderate pain at baseline.Citation42 The data reported here demonstrate that this observation extends to changes in pain category, supporting the concept that this difference is clinically meaningful.
In this analysis, a change in FIQ category at endpoint was as common as a change in pain category. Across all treatment groups, 36.6%–42.2% of patients with FM changed FIQ category at endpoint compared with 38.9%–45.6% of patients with FM who changed pain category. However, there was no difference in the proportion of patients changing pain category with pregabalin than with placebo. It is not clear why significant changes in pain did not translate to significant changes in FIQ category. Pregabalin significantly improved FIQ total score at endpoint compared with placebo in the majority of trials in this analysis.Citation22,Citation24,Citation25 Severity categories based on FIQ score are perhaps less well established than those for pain scores, and it may be that the category divisions currently used do not always directly relate to clinically important changes. As such, this analysis of FIQ scores may be of limited benefit.
Another limitation of this analysis is that the data were pooled from a large number of studies conducted under different conditions and in different countries with the trials grouped by pain categories. At the same time, this also suggests that these findings may be broadly applicable. Finally, this was a post hoc analysis of these data with the trials not designed with the primary aim to assess changes in pain severity category.
The data in this analysis suggest that the established efficacy of pregabalin for improving pain scores in patients with neuropathic pain due to DPN, PHN, and SCI, and in patients with FM also extends to clinically meaningful improvements in pain category.
Acknowledgments
This study was sponsored by Pfizer. Medical writing support was provided by Joshua Fink, PhD, of Engage Scientific Solutions, and funded by Pfizer.
Disclosure
BP, AC, and BE are employees of Pfizer and hold stock options in Pfizer. CEA has received research grants for his institution as a principal investigator from Endo, Forest, and Lilly; has received honoraria as a consultant and advisor to Pfizer and Teva; has received honoraria as a consultant to Daiichi Sankyo, Purdue, and Nextar; has received honoraria as an advisor and speaker from Depomed, XenoPort, and Iroko; and has received honoraria as a speaker from Millenium Labs, Janssen, and Allergan. The authors report no other conflicts of interest in this work.
References
- SerlinRCMendozaTRNakamuraYEdwardsKRCleelandCSWhen is cancer pain mild, moderate or severe? Grading pain severity by its interference with functionPain19956122772847659438
- ZelmanDCDukesEBrandenburgNBostromAGoreMIdentification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathyPain20051151–2293615836967
- SchaeferCSadoskyAMannRPain severity and the economic burden of neuropathic pain in the United States: BEAT neuropathic pain observational studyClinicoecon Outcomes Res2014648349625378940
- Lyrica® (pregabalin)Prescribing InformationNew York, NY, USAPfizer Inc2011 Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=561Accessed March 9, 2016
- ArezzoJCRosenstockJLamoreauxLPauerLEfficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trialBMC Neurol200883318796160
- LesserHSharmaULaMoreauxLPooleRMPregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trialNeurology200463112104211015596757
- SharmaUGriesingTEmirBYoungJPJrTime to onset of neuropathic pain reduction: a retrospective analysis of data from nine controlled trials of pregabalin for painful diabetic peripheral neuropathy and postherpetic neuralgiaAm J Ther201017657758520393345
- Pfizer IncA 14-week, double-blind, randomized, placebo-controlled, multicenter study to evaluate the safety and efficacy of pregabalin (150mg–600mg/day) using a flexible optimal dose schedule in patients with painful diabetic peripheral neuropathy (DPN) Available from: https://clinicaltrials.gov/ct2/show/NCT00156078. NLM identifier: NCT00156078Accessed July 12, 2016
- Pfizer IncA randomized, double-blind, placebo-controlled, parallel-group, multi-center trial of pregabalin versus placebo in the treatment of neuropathic pain associated with diabetic peripheral neuropathy Available from: https://clinicaltrials.gov/ct2/show/NCT00143156. NLM identifier: NCT00143156Accessed July 12, 2016
- RichterRWPortenoyRSharmaULamoreauxLBockbraderHKnappLERelief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trialJ Pain20056425326015820913
- RosenstockJTuchmanMLaMoreauxLSharmaUPregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trialPain2004110362863815288403
- SatohJYagihashiSBabaMEfficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trialDiabet Med201128110911621166852
- TölleTFreynhagenRVersavelMTrostmannUYoungJPJrPregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind studyEur J Pain200812220321317631400
- DworkinRHCorbinAEYoungJPJrPregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trialNeurology20036081274128312707429
- SabatowskiRGálvezRCherryDAPregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trialPain20041091–2263515082123
- StaceyBRBarrettJAWhalenEPhillipsKFRowbothamMCPregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain reliefJ Pain20089111006101718640074
- van SeventerRFeisterHAYoungJPJrStokerMVersavelMRigaudyLEfficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trialCurr Med Res Opin200622237538416466610
- FreynhagenRStrojekKGriesingTWhalenEBalkenohlMEfficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimensPain2005115325426315911152
- GuanYDingXChengYEfficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in ChinaClin Ther201133215916621444113
- SiddallPJCousinsMJOtteAGriesingTChambersRMurphyTKPregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trialNeurology200667101792180017130411
- CardenasDDNieshoffECSudaKA randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injuryNeurology201380653353923345639
- ArnoldLMRussellIJDiriEWA 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgiaJ Pain20089979280518524684
- CroffordLJRowbothamMCMeasePJPregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trialArthritis Rheum20055241264127315818684
- MeasePJRussellIJArnoldLMA randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgiaJ Rheumatol200835350251418278830
- OhtaHOkaHUsuiCOhkuraMSuzukiMNishiokaKA randomized, double-blind, multicenter, placebo-controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgiaArthritis Res Ther2012145R21723062189
- PauerLWinkelmannAArsenaultPAn international, randomized, double-blind, placebo-controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgiaJ Rheumatol201138122643265221965636
- WolfeFSmytheHAYunusMBThe American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria CommitteeArthritis Rheum19903321601722306288
- HanleyMAMasedoAJensenMPCardenasDTurnerJAPain interference in persons with spinal cord injury: classification of mild, moderate, and severe painJ Pain20067212913316459278
- BurckhardtCSClarkSRBennettRMThe fibromyalgia impact questionnaire: development and validationJ Rheumatol19911857287331865419
- BennettRMBushmakinAGCappelleriJCZlatevaGSadoskyABMinimal clinically important difference in the fibromyalgia impact questionnaireJ Rheumatol20093661304131119369473
- DothAHHanssonPTJensenMPTaylorRSThe burden of neuropathic pain: a systematic review and meta-analysis of health utilitiesPain2010149233834420227832
- MurrayRFAsghariAEgorovDDImpact of spinal cord injury on self-perceived pre- and postmorbid cognitive, emotional and physical functioningSpinal Cord200745642943617228355
- ArnoldLMCroffordLJMeasePJPatient perspectives on the impact of fibromyalgiaPatient Educ Couns200873111412018640807
- DworkinRHTurkDCWyrwichKWInterpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendationsJ Pain20089210512118055266
- RitchlinCTKavanaughAGladmanDDTreatment recommendations for psoriatic arthritisAnn Rheum Dis20096891387139418952643
- KapstadHHanestadBRLangelandNRustoenTStavemKCutpoints for mild, moderate and severe pain in patients with osteoarthritis of the hip or knee ready for joint replacement surgeryBMC Musculoskelet Disord200895518426591
- AttalNCruccuGBaronREFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revisionEur J Neurol20101791113e118820402746
- FreemanRDurso-DecruzEEmirBEfficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of dosesDiabetes Care20083171448145418356405
- FreynhagenRSerpellMEmirBA comprehensive drug safety evaluation of pregabalin in peripheral neuropathic painPain Pract2015151475724279736
- ParsonsBSaninLYangREmirBJuhnMEfficacy and safety of pregabalin in patients with spinal cord injury: a pooled analysisCurr Med Res Opin201329121675168323998397
- ParsonsBLiCThe efficacy of pregabalin in patients with moderate and severe pain due to diabetic peripheral neuropathyCurr Med Res Opin201632592993726854578
- ClairAEmirBThe efficacy of pregabalin for treating fibromyalgia patients with moderate or severe baseline widespread painArthritis Rheumatol201466S10S1879 Abstract
