Abstract
Objective
Despite the many complications of succinylcholine, it is still widely used as a superior muscle relaxant for rapid sequence induction. One of these complications is postoperative myalgia (POM). The aim of this study was to investigate the prophylactic effect of low-dose ketamine on the incidence and severity of POM.
Materials and methods
In this double-blind clinical study, a total of 148 patients scheduled for general anesthesia were randomly divided into two equal groups. Initially, in Group K, 0.5 mg/kg of ketamine was injected intravenously, whereas in Group N, the same volume (5 mL) of normal saline was injected. Thereafter, anesthesia was induced in all patients, by injecting 1.5 mg/kg of fentanyl and 2 mg/kg of propofol intravenously. Following the loss of eyelid reflex, 1.5 mg/kg of succinylcholine was injected intravenously as a muscle relaxant and then the patients were intubated. POM was defined as a pain with no surgical interferences, and its intensity was graded based on a four-point scale. The incidence and severity of myalgia were assessed by a blinded observer 24 hours after surgery.
Results
In terms of demographic data, the results of this study showed that there is no significant difference between patients in both groups (P>0.05). Overall, the incidence of POM in Group K was significantly less, when compared with Group N (P<0.05), but both groups were comparable based on the grade 2 of POM. After the induction of anesthesia, the systolic and diastolic blood pressure values were found to reduce in both groups (P<0.05). However, the changes were somehow similar, and repeated measures of variance analysis showed no significant difference in the two study groups (P>0.05).
Conclusion
The addition of 0.5 mg/kg of ketamine to propofol for the induction of anesthesia can be effective in reducing the incidence of low-grade POM.
Introduction
For >60 years, succinylcholine is still being administered as a selective relaxant for rapid sequence intubation by anesthesiologists in many countries.Citation1 It has been shown to possess unique features such as low cost, fast-acting, short half-life, safe metabolites, and causing excellent muscle relaxation for intubation;Citation2 however, it has many side effects as well. Postoperative myalgia (POM), with an incidence rate of ~41%–92%, is one of the most common side effects of this drug and can take several days to cause significant discomfort in patients.Citation3 However, its effect is felt more in the throat, neck, shoulder, and abdominal muscles and is common among patients with outpatient surgery.Citation4 Due to its unknown real context of pathogenesis and in an effort to reduce the incidence and severity of succinylcholine-induced myalgia, various medications including nondepolarizing muscle relaxants, benzodiazepines, magnesium sulfate, opioids, gabapentin, and nonsteroidal anti-inflammatory drugs have been tested, with varying degrees of success.Citation5,Citation6
Ketamine is an N-methyl-d-aspartate (NMDA) receptor antagonist with excellent analgesic activity, and at subanesthetic doses it is capable of preventing central sensitization, hyperalgesia, drug resistance creation, and reduction of postoperative pain.Citation7 Although, ketamine administration could produce unpleasant psychogenic complications, no disagreeable effects have been recorded in patients who received a low dose of ketamine. The effects of ketamine on postelectroconvulsive therapy (ECT) myalgia have been studied earlier, and the results showed that ketamine could not prevent this type of myalgia.Citation8
To the best of our knowledge, the effect of ketamine on the prevention of POM is yet to be determined. Therefore, in this randomized, double-blind study, the prophylactic effect of low-dose ketamine on the incidence and severity of myalgia caused by succinylcholine injection in patients undergoing outpatient surgery was investigated.
Materials and methods
This study was approved by the Ethics Committee of Kurdistan University of Medical Sciences and was registered in the Iranian Registry of Clinical Trials (IRCT2014062412789N5). A complete description of the study was also presented to each participant, and written informed consent was obtained. The sample size was calculated based on the assumption that the incidence of POM in outpatient cases is ~70%, and intervention that can cause 25% reduction in incidence of POM will be interesting. To consider this difference and type I error equal to 5% and 90% power of the study, 72 patients were required to be in each group (α=0.05 and β=90%), but in order to avoid possible loss of samples during the study, the number of patients in each group was increased to 74. Thus, 148 patients, who were scheduled for outpatient surgery under general anesthesia, belonging to the American Society of Anesthesiologists physical status I and II within the age of 18–70 years, were included in this double-blind randomized clinical trial. Patients with a history of allergy to medications; substance abuse; malignant hyperthermia; myopathy; cardiovascular, liver, and advanced kidney diseases; and the risk of difficult intubation based on physical examination were excluded from the study.
Before surgery, patients were evaluated by the anesthesiologists. The study protocol and evaluation of myalgia based on Kararmaz’s criteria were described to the patients.Citation4 Based on a computer-generated random sequence, the 148 patients were enrolled into one of the two groups (ketamine or saline). The patients did not receive premedication.
In the operating room, standard monitoring of the noninvasive blood pressure, electrocardiogram, heart rate, and pulse oximetry was done, and the preliminary values were recorded. Thereafter, a venous cannula (G=18) was placed on the dorsum of a patient’s hand. Before the induction of anesthesia in patients of Group K, 0.5 mg/kg of ketamine (a 5 mL volume was reached by adding distilled water) was injected intravenously and 5 mL of normal saline was injected intravenously slowly into patients in Group N. Drugs were prepared in 5 mL syringes by an anesthesia nurse who was unaware of the grouping. After injecting the study drugs, 1.5 mg/kg of fentanyl was injected intravenously within 60 seconds and subsequently 2 mg/kg of propofol was administered intravenously within 30 seconds for the induction of anesthesia. Following the loss of eyelid reflex, 1.5 mg/kg of succinylcholine was injected intravenously and patients were ventilated using bag and mask and with 100% oxygen. After fasciculation, the values of heart rate and blood pressure were measured and recorded, and tracheal intubation was performed. The maintenance of anesthesia continued using a mixture of oxygen, N2O (40/60), and isoflurane 1 MAC. After 5 minutes of tracheal intubation, the values of heart rate and blood pressure were obtained and recorded again. For maintenance of muscle relaxation, 0.2 mg/kg of atracurium was used as suggested by the surgeon. At the end of the surgery, muscle relaxation was reversed using neostigmine and atropine. After the desired spontaneous ventilation, the patients were extubated. The patients were transferred to the recovery room and complications, if any, were recorded during recovery. After meeting the discharge criteria, the patients were discharged to be taken home and cared for by a responsible adult. The incidence and severity of myalgia in the patients were determined 24 hours after surgery by a medical student who was unaware of the grouping. Myalgia is defined as “a pain with no surgical interference” and is graded based on Kararmaz et al’sCitation4 four-point scale as follows: 0= no muscle pain, 1= muscle stiffness limited to one area of the body, 2= muscle pain or stiffness noticed spontaneously by a patient who requires analgesics, and 3= incapacitating generalized, severe muscle stiffness or pain.
Statistical analysis was conducted using SPSS Version 12.0 software (IBM Corporation, Armonk, NY). The comparison profile of the patients was performed using the analysis of variance test. The incidence and severity of myalgia were compared using the chi-square and independent t-tests. The value of P<0.05 was considered statistically significant.
Results
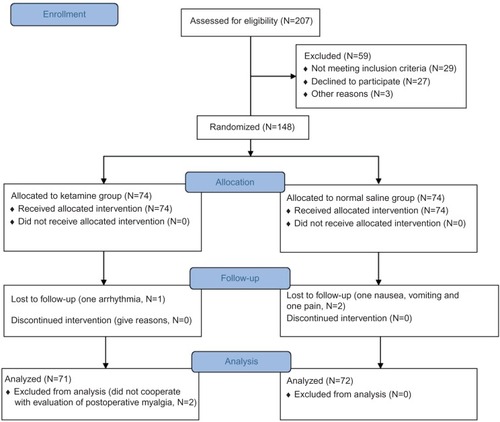
In this study, a total of 207 patients were scheduled for outpatient surgery from July 2013 to August 2014. Of them, 29 patients could not meet the entry criteria. Twenty-seven patients had no willingness to participate in the study, and the surgery of three patients was canceled. The remaining 148 patients were randomly assigned into two groups. Two patients in Group N due to nausea, vomiting, and pain and one patient in Group K due to arrhythmia were turned from outpatient to inpatient. Two persons from Group K did not cooperate with the evaluation of POM. The data associated with the 72 patients in Group N and 71 patients in Group K were analyzed ().
There were no significant differences in terms of age, sex, and duration of surgery between both groups (). In Group K, 13 (18.1%) out of the 71 patients had myalgia, whereas 36 (50%) out of the 72 patients had myalgia in Group N (P=0.001). Grade 1 POM was lower in Group K when compared with Group N (nine in Group K versus 33 in Group N; P<0.001), whereas the incidence of grade 2 POM was comparable among patients of the two groups (). The baseline values of systolic and diastolic blood pressure and heart rate in both groups were similar. After the induction of anesthesia, the values of systolic and diastolic blood pressure decreased in both groups (P<0.05). However, these changes were somehow similar in the two groups, and repeated measures of variance analysis showed no significant difference in both groups (P>0.05). Changes in heart rate after induction and intubation between the two groups were compared and repeated measures of variance analysis showed no significant difference in the two groups (P>0.05; ).
Table 1 Patients’ demographic data and duration of surgery for the normal saline (5 mL of saline IV) and ketamine (0.5 mg/kg of ketamine IV) groups
Table 2 Incidence and grade of myalgia in the normal saline (5 mL of saline IV) and ketamine (0.5 mg/kg of ketamine IV) groups
Table 3 Cardiovascular parameters in the normal saline (5 mL of saline IV) and ketamine (0.5 mg/kg of ketamine IV) groups
Discussion
The results of this study showed that ketamine significantly reduced the incidence of succinylcholine-induced myalgia.
Succinylcholine is a muscle relaxant that is commonly used due to deep neuromuscular blocks for intubation in patients undergoing ambulatory anesthesia, but associated myalgia has been shown to occur in 41%–92% of patients.Citation9,Citation10 Since ambulation increases the possibility of myalgia and its intensity, ambulatory patients are suitable for investigating this complication. Therefore, this group of patients was chosen for the study. Myalgia is most prevalent following the use of succinylcholine in the first day of surgery.Citation11 As shown in a study, 92% of patients reported myalgia in the first 24 hours of study and the incidence of myalgia did not differ at 24 hours and 48 hours after surgery.Citation8 In this study, myalgia was evaluated only in the first 24 hours after surgery.
It seems that the intrafusal contraction of muscle fibers caused damage to spindles and subsequently myalgia.Citation12 In vitro studies have shown that active, excessive, and continuous muscle contractions increase the uptake of calcium, activation of phospholipase A2, arachidonic acid, and prostaglandins production, thereby increasing the risk of muscle damage and pain.Citation13 The results of this study showed that 50% of the patients in Group N experienced myalgia after anesthesia was induced with propofol. In a meta-analysis, the average incidence of myalgia in the first 24 hours with thiopental was 49.2% and with propofol was 65.4%.Citation14 The incidence of myalgia in the present study was slightly lower when compared with the aforementioned meta-analysis. It appears that participants in this study belonged to low-level POM in general.
Ketamine creates a complex combination of both antinociceptive and pronociceptive actions. In fact, based on the mechanisms of analgesia with a direct receptor, the analgesic effect of ketamine is associated with the plasma levels of drugs and pain reduces with subanesthetic doses of ketamine.Citation15,Citation16 In general, it seems that the NMDA receptor plays an important role in the pathophysiology of pain, and the Supra spinal block of the NR2B NMDA subunit by ketamine has an important antinociceptive effect.Citation17 Recent studies have shown the role of NMDA receptors in facilitating the process of pain in the central nervous system, this receptor is also responsible for central sensitization and windup phenomenon. The administration of NMDA receptor antagonists prevents the development of sensitization, hyperalgesia, and drug resistance.Citation18 Ketamine is an antagonist of this receptor and has excellent analgesic activity at doses under anesthesia.Citation19 It strengthens the performance of mu and kappa opioid receptors and also has direct effects on the delta opioid receptor. As such, ketamine certainly modulates the response of opioid receptors and reduces tolerance to opioids.Citation20 It also strengthens the endogenesis of antinociceptive systems, in part, by its aminergic (serotonergic and noradrenergic) activation and inhibition of reuptake.Citation21 It inhibits the synthesis of nitric oxide, which probably contributed to the analgesic effect.Citation22 Also, there is evidence suggesting that ketamine interferes with nicotinic, muscarinic, and monoaminergic receptors.
As a result of the undesirable and unwanted side effects, ketamine usage is controversial in nonanesthetized patients, but when surgery is done under general anesthesia, these side effects are barely seen.Citation19 In this study, surgery was performed under general anesthesia and none of the patients experienced delirium and nightmare. In our previous study,Citation8 the effect of prophylactic ketamine on myalgia after ECT in 50 patients with major depression was demonstrated, in which 1 mg/kg of propofol and 0.3 mg/kg of ketamine were used for the induction of anesthesia, and muscle relaxation was induced using 0.5 mg/kg of succinylcholine. The results showed that the addition of ketamine to propofol had no effect on the incidence of myalgia after ECT, and only 12% of the patients had myalgia within 24 hours after ECT. However, our previous studyCitation8 is different in some aspects from the present study. In our previous study, ECT patients were investigated and the cause of myalgia was found to be different in surgical patients. Second, unlike the surgery, myalgia intensity in ECT is not related to fasciculation and motor activities.Citation23 Third, the dose of anesthetic drugs for the induction of anesthesia and muscle relaxation was less than that of the present study. The result of our previous study, which was carried out on patients undergoing ECT, is not in line with the present study.
Cardiovascular changes were similar after the induction of anesthesia in both groups. In theory, hemodynamic stability can be predicted based on the induction of anesthesia with propofol and ketamine, when compared with propofol alone. This is because ketamine increases blood pressure and heart rate; therefore, the addition of this drug to propofol during anesthesia induction can inhibit the reduction of these parameters before the stimulation of laryngoscopy and tracheal intubation. Also, following laryngoscopy and tracheal intubation, the synergistic effect of these two drugs can increase the depth of anesthesia and result in smoother cardiovascular response to airway stimulation. It appears that 0.5 mg/kg of ketamine could not produce such an impact on the hemodynamic system, thereby resulting in a significant effect.
Study limitations
One of the limitations of this study is the insufficient time for myalgia evaluation (24 hours). Failure to assess the incidence and severity of fasciculation is another limitation of this study. In this study, ketamine was diluted with sterile water, although this is not a wrong practice, but further addition of water, as carried out in this study, could produce ketamine with concentration below the physiological osmotic pressure, which may interfere with the effects of ketamine.
Conclusion
The addition of 0.5 mg/kg of ketamine to propofol for the induction of anesthesia can be effective in reducing the incidence of POM, without any change in hemodynamic indices.
Author contributions
KN and SA participated in the research design, data gathering, data analysis, and writing and finalizing the manuscript.
Acknowledgments
This article has been extracted from general medicine thesis of Doctor Sanaz Arvin. The authors appreciate the Medical Faculty Research Council for approval and support of the project. We would like to gratefully and sincerely thank Doctor Faiegh Youssefi, who helped us with statistical analysis of results.
Disclosure
The authors report no conflicts of interest in this work.
References
- NasseriKArastehMTAfkhamzadehAHakhamaneshiSEvaluation of prophylactic effect of remifentanil on succinylcholine-induced myalgia in humansTJPR20141320272030
- KaranoviNCarevMKardumGSuccinylcholine use in adult anesthesia –A multinational questionnaire surveyColl Antropol201135Suppl 1183190
- PandeyCKTripathiMJoshiGKarnaSTSinghNSinghPKProphylactic use of gabapentin for prevention of succinylcholine-induced fasciculation and myalgia: a randomized, double-blinded, placebo-controlled studyJ Postgrad Med2012581192222387644
- KararmazAKayaSTurhanogluSOzyilmazMAEffects of high-dose propofol on succinylcholine-induced fasciculations and myalgiaActa Anaesthesiol Scand200347218018412631047
- PandeyCKKarnaSTTandonMPandeyVKSinghAComparative evaluation of prophylactic use of pregabalin, gabapentin and diclofenac sodium for prevention of succinylcholine-induced myalgia: a randomized, double-blinded studyJ Postgrad Med2014601162024625934
- KumarMTalwarNGoyalRShuklaUSethiAEffect of magnesium sulfate with propofol induction of anesthesia on succinylcholine-induced fasciculations and myalgiaJ Anaesthesiol Clin Pharmacol2012281818522345952
- KaurSSaroaRAggarwalSEffect of intraoperative infusion of low-dose ketamine on management of postoperative analgesiaJ Nat Sc Biol Med20156237838226283834
- NasseriKShahsawariSZiaeeZComparing the effects of propofol-ketamine to propofol-saline on myalgia post electroconvulsive therapyJ Mazandaran Univ Med Sci2013233540
- SmithIDingYWhitePFMuscle pain after outpatient laparoscopy – influence of propofol versus thiopental and enfluraneAnesth Analg1993766118111848498652
- NewnamPTLoudonJMMuscle pain following administration of suxamethonium: the aetiological role of muscular fitnessBr J Anaesth19663875335405943809
- KimKNKimKSChoiHIJeongJSLeeHJOptimal precurarizing dose of rocuronium to decrease fasciculation and myalgia following succinylcholine administrationKorean J Anesthesiol201466645145625006369
- ErkolaOEffects of precurarisation on suxamethonium-induced postoperative myalgia during the first trimester of pregnancyActa Anaesthesiol Scand199034163671968694
- JacksonMJJonesDAEdwardsRHExperimental skeletal muscle damage: the nature of the calcium-activated degenerative processesEur J Clin Invest19841453693746437835
- SchreiberJLysakowskiCFuchs-BuderTTramerMRPrevention of succinylecholine-induced fasciculations and myalgia. A meta analysis of randomized trialsAnesthesiology2005103487788416192781
- NoppersINiestersMAartsLSmithTSartonEDahanAKetamine for the treatment of chronic non-cancer painExpert Opin Pharmacother201011142417242920828267
- DahanAOlofsenESigtermansMPopulation pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief of chronic painEur J Pain201115325826720638877
- PetrenkoABYamakuraTBabaHShimojiKThe role of N-methyl-D-aspartate (NMDA) receptors in pain: a reviewAnesth Analg20039741108111614500166
- EliaNTramèrMRKetamine and postoperative pain – a quantitative systematic review of randomised trialsPain20051131–2617015621365
- CarstensenMMøllerAMAdding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: a qualitative review of randomized trialsBr J Anaesth2010104440140620207747
- SartonETeppemaLJOlievierCThe involvement of the mu-opioid receptor in ketamine-induced respiratory depression and antinociceptionAnesth Analg20019361495150011726430
- KoizukaSObataHSasakiMSaitoSGotoFSystemic ketamine inhibits hypersensitivity after surgery via descending inhibitory pathways in ratsCan J Anaesth200552549850515872129
- De KockMFLavand’hommePMThe clinical role of NMDA receptor antagonists for the treatment of postoperative painBest Pract Res Clin Anaesthesiol2007211859817489221
- DinwiddieSHHuoDGottliebOThe course of myalgia and headache after electroconvulsive therapyJ ECT201026211612019710619