Abstract
Background
Nerve injury may induce neuropathic pain. In studying the mechanisms of orofacial neuropathic pain, attention has been paid to the plastic changes that occur in the trigeminal ganglia (TGs) and nucleus in response to an injury of the trigeminal nerve branches. Previous studies have explored the impact of sciatic nerve injury on dorsal root ganglia (DRGs) and it has shown dramatic changes in the expression of multiple biomarkers. In large, the changes in biomarker expression in TGs after trigeminal nerve injury are similar to that in DRGs after sciatic nerve injury. However, important differences exist. Therefore, there is a need to study the plasticity of biomarkers in TGs after nerve injury in the context of the development of neuropathic pain-like behaviors.
Aim
The aim of this study was to investigate the plasticity of biomarkers associated with chronic persistent pain in TGs after trigeminal nerve injury.
Materials and methods
To mimic the chronic nature of the disorder, we used an intraoral procedure to access the infraorbital nerve (ION) and induced a nerve injury in mice. Immunohistochemistry and quantification were used for revealing the expression level of each biomarker in TGs after nerve injury.
Results
Two weeks after partial ION injury, immunohistochemistry results showed strongly upregulated expressions of activating transcription factor 3 and neuropeptide Y (NPY) in the ipsilateral TGs. Microglial cells were also activated after nerve injury. In regard to positive neuronal profile counting, however, no significant difference in expression was observed in galanin, substance P, calcitonin gene-related peptide, neuronal nitric oxide synthase, phosphorylated AKT, or P2X3 in ipsilateral TGs when compared to contralateral TGs.
Conclusion
In this study, the expression and regulation of biomarkers in TGs have been observed in response to trigeminal nerve injury. Our results suggest that NPY and Iba1 might play crucial roles in the pathogenesis of orofacial neuropathic pain following this type of injury. Further investigations on the relevance of these changes may help to target suitable treatment possibilities for trigeminal neuralgia.
Introduction
Trigeminal neuralgia is regarded as being one of the most painful diseases known.Citation1 Pain resulting from this disease impairs patients’ mood, quality of life, daily life activities, and work performance.Citation2 Known factors that can cause trigeminal neuralgia are injury, inflammation, and vascular compression of the trigeminal nerve.Citation3,Citation4 However, the mechanisms of trigeminal neuralgia are still poorly understood.
Sensory neuron plasticity after peripheral injury is considered important for understanding the development of chronic persistent pain.Citation5 Studies have revealed that the impact that sciatic nerve injury has on dorsal root ganglia (DRGs) causes dramatic changes in the expression of certain peptides, more specifically excitatory peptides such as substance P (SP) and calcitonin gene-related peptide (CGRP), as well as inhibitory and excitatory neuropeptides such as galanin (GAL) and neuropeptide Y (NPY).Citation6,Citation7 Neuronal nitric oxide synthase (nNOS) and P2X3 are also known to play a role in nociception.Citation8,Citation9 Phosphorylated AKT (pAKT) is believed to have neuroprotective effects and can be regulated in sensory ganglia after injury.Citation5,Citation10,Citation11 All of these changes may have implications in the generation and modulation of neuropathic pain.Citation12 To our knowledge, only a limited number of studies have been conducted to examine neuronal plasticity of the trigeminal ganglion (TG) following trigeminal nerve injury. These studies show that the changes in neuropeptide expression of TGs after trigeminal nerve injury are similar to DRGs after sciatic nerve injury.Citation13,Citation14 However, important differences exist. For example, GAL is markedly upregulated in DRGs after sciatic nerve injury,Citation15 whereas GAL expression in TGs has been inconclusive, with results showing an increase, no change, or even decrease in expression after facial nerve injury.Citation13,Citation16 Interestingly, systematic studies have not been conducted on the expression of neuropeptides in TGs after nerve injury in the context of the development of neuropathic pain-like behaviors, as in the case of sciatic nerve injury.Citation17
Recently, several animal models have been developed to examine trigeminal nerve injury, inflammation, and trigeminal nerve root compression.Citation18–Citation21 Among these models, the infraorbital nerve (ION) constriction model of trigeminal neuralgia has been performed in both rats and mice.Citation22–Citation24 Interestingly, animal models of trigeminal neuralgia obtained by a chronic constriction injury of ION are shown to share many characteristics with clinical disorders in humans.Citation25,Citation26
Because pain-related biomarkers give accurate signals, observing and understanding their changes may help us find potential targets for treating painful diseases. In this study, we investigated the expression of several biomarkers in TGs following trigeminal nerve injury. Expression and regulation of activating transcription factor 3 (ATF3), NPY, Iba1, GAL, CGRP, SP, nNOS, pAKT, and P2X3 were examined in a modified mouse model of trigeminal neuralgia.
Materials and methods
Animals
Adult male C57BL/6J Bommince mice (A/S Bomholtgaard, Ry, Denmark) weighing 28–30 g were used in this study. All animals were kept under standard conditions on a 12-h day/night cycle with free access to food and water. The experiments were carried out according to the International Association for the Study of Pain Guidelines for the Use of Animals in Research and were approved by Karolinska Institutet ethics committee (registration number: N48/13).
Operation
Mice were anesthetized with isoflurane, received a transection to the left ION, and the contralateral side (right side) was used as a control. The surgery was performed intraorally, which allowed the hair on the snout and the vibrissae to be kept intact. A transection of ~0.5 cm was made unilaterally along the left gingivobuccal margin. The transection began at ~0.1 cm proximal to the first molar. The modification of surgery is based on two previously published studies.Citation27,Citation28 The mice were sacrificed 2 weeks after surgery.
Immunohistochemistry
All operated animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and transcardially perfused with 20 mL of warm saline (0.9%; 37°C), followed by 20 mL of a warm mixture of paraformaldehyde (4%; 37°C) with 0.4% picric acid in 0.16 M phosphate buffer (pH 7.2) and then by 50 mL of the same, but ice-cold fixative. Both the ipsilateral and contralateral TGs were dissected out and postfixed in the same fixative for 90 min at 4°C and subsequently stored in 10% sucrose in phosphate-buffered saline (PBS; pH 7.4) containing 0.01% sodium azide (Sigma-Aldrich Co., St Louis, MO, USA) and 0.02% bacitracin (Sigma-Aldrich Co.) at 4°C for 2 days. Tissues were embedded with optimum cutting temperature compound (Tissue-Tek; Miles Laboratories, Elkhart, IN, USA), frozen and cut in a cryostat (Microm, Heidelberg, Germany) at 14 μm thickness, and then mounted onto chrome-alum-gelatin-coated slides. Thaw-mounted sections were dried at room temperature (RT) for 30 min and rinsed with PBS for 15 min. Sections were incubated for 24 h at 4°C in a humid chamber with rabbit anti-ATF3 (1:4,000; D1610, Santa Cruz Biotechnology, Dallas, TX, USA), NPY (1:8,000),Citation29 rabbit anti-GAL (1:8,000),Citation30 rabbit anti-SP (1:4,000),Citation31 rabbit anti-CGRP (1:10,000),Citation32 sheep anti-nNOS (1:4,000),Citation32 rabbit anti-pAKT (1:4,000; G7441, Promega Corporation, Madison, WI, USA), and rabbit anti-P2X3 (1:4,000)Citation33 antisera diluted in PBS containing 0.2% (w/v) bovine serum albumin and 0.03% Triton X-100. Immunoreactivity was visualized using the tyramide signal amplification system (TSA Plus; NEN Life Science Products, Boston, MA, USA). Briefly, the slides were rinsed with Tris-HCL/NaCL/Tween-20 (TNT) buffer (0.1 M Tris–HCl, pH 7.5; 0.15 M NaCl; 0.05% Tween-20) for 15 min at RT, blocked with Tris-HCL/NaCL/DuPont blocking reagent (TNB) buffer (0.1 M Tris–HCl; pH 7.5; 0.15 M NaCl; 0.5% DuPont blocking reagent) for 30 min at RT followed by a 30-min incubation with horseradish peroxidase-labeled swine anti-rabbit and/or anti-sheep antibody (1:200; Dako Denmark A/S, Glostrup, Denmark) diluted in TNB buffer. After a quick wash (15 min) in TNT buffer, all sections were exposed to biotinyl tyramide-fluorescein (1:100) diluted in amplification diluent for 10 min, and finally washed in TNT buffer for 30 min (all steps performed at RT). Sections to be used for profile counts (with nucleus) were counterstained for 8–10 min in 0.001% (w/v) propidium iodide (PI; Sigma-Aldrich Co.) in PBS, rinsed twice for 5 min in PBS, and mounted with glycerol/PBS (9:1) containing 2.5% DABCO (Sigma-Aldrich Co.).
Image analysis and quantification
Specimens were analyzed on a Bio-Rad Radiance Plus confocal scanning microscope (Bio-Rad, Hemel Hempstead, UK) installed on a 710 LSM laser-scanning microscope equipped with ×10 (0.5 numerical aperture [NA]), ×20 (0.75 NA), and ×60 (1.40 NA) oil objectives. Fluorescein labeling was excited using the 488 nm line of the argon ion laser and detected after passing a HQ 530/60 (Bio-Rad Laboratories Inc.) emission filter. All the slides were scanned in a series of 1 μm thick optical sections.
To determine the percentage of immunoreactive (IR) neuronal profiles (NPs), the counting was performed on 14-µm-thick sections, and every fifth section was selected. The total number of immunopositive NPs was divided by the total number of PI-stained NPs in the TG sections, and the percentage of positive NPs was calculated. Four to eight sections of each TG from five animals in each group (i.e., ipsilateral and contralateral groups) were included in the analysis, and 1,200–1,800 NPs were counted in each ganglion.
Statistical analysis
Unpaired Student’s t-test was used for the comparison of difference between ipsilateral and contralateral expression of each biomarker. p<0.05 was taken as the criterion for statistical significance (*p<0.05, **p<0.01, and ***p<0.001).
Results
ATF3 expression in TGs after ION injury
ATF3-IR NPs were almost undetectable in the contralateral TGs 2 weeks after partial ION injury. However, a strong upregulation of ATF3-IR NPs was seen in the ipsilateral TGs, as shown in versus C and (0.9±0.2% vs. 30.1±5.8%; p<0.001), mainly in the maxillary infraorbital neurons (; red box).
Figure 1 Expression of ATF3-LI in TGs 2 weeks after partial ION transection.
Abbreviations: ATF3, activating transcription factor 3; ION, infraorbital nerve; IR, immunoreactive; LI, like immunoreactivity; TGs, trigeminal ganglia.
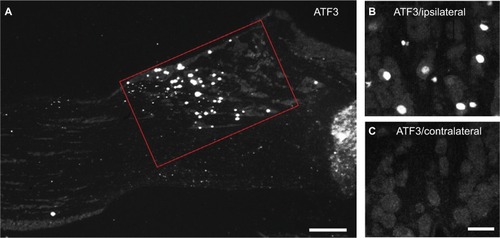
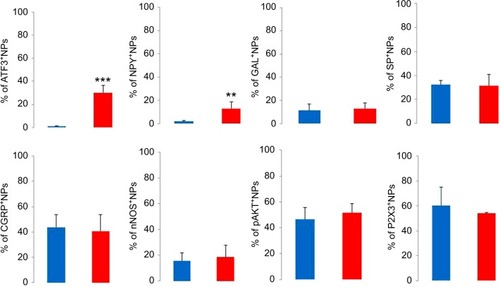
Figure 2 Percentage of positive NPs in contralateral and ipsilateral TGs 2 weeks after partial ION transection.
Abbreviations: ATF3, activating transcription factor 3; CGRP, calcitonin gene-related peptide; GAL, galanin; ION, infraorbital nerve; nNOS, neuronal nitric oxide synthase; NPs, neuronal profiles; NPY, neuropeptide Y; pAKT, phosphorylated AKT; SP, substance P; TGs, trigeminal ganglia.
Expression of biomarkers in TGs after ION injury
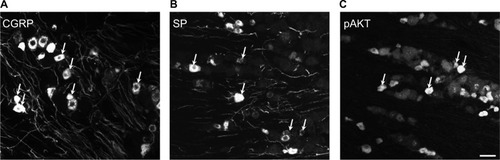
In the contralateral TGs, very few NPs were NPY positive (1.8±0.9%), while a dramatic increased expression of NPY-positive NPs was seen in the ipsilateral TGs (12.9±5.6%; p<0.01; –). This increase iŝ10-fold when compared to the control. Regarding GAL expression, more positive NPs were found in the ipsilateral TGs, but this increase in expression was not statistically significant (11.5±5.8% vs. 13±4.8%; p>0.05; and ). No significant difference was detected between the ipsilateral and contralateral TGs for CGRP (43.7±9.9% vs. 40.9±13.0%; , , and ), SP (32.2±3.7% vs. 31.6±9.1%; , , and ), P2X3 (60.4±15.8% vs. 54.0±1.3%; and ), or nNOS (15.9±6.0% vs. 18.6±9.3%; and ), respectively. pAKT-like immunoreactivity (pAKT-LI) was seen in many neurons in intact TGs (), especially small-sized ones. pAKT had a relatively high intensity in ipsilateral TGs but the percentage of positive-NPs remained the same 2 weeks after ION injury (46.6±9.2% vs. 51.5±7.1%; and ). Iba1, a marker for activated microglial cells, was activated in the ipsilateral TGs as compared to contralateral ones 2 weeks after partial ION injury ( and ). The expression of Iba1-LI in the contralateral TGs presented a typical resting microglial morphology ( and ). By contrast, the enlarged and amoeboid morphological features of activated microglia cell bodies were seen in the ipsilateral TGs ( and ), which are consistent with previous findings.Citation34
Figure 3 Immunofluorescent micrographs show the expression of biomarkers in contralateral (top panel) and ipsilateral (bottom panel) TGs 2 weeks after partial ION transection.
Abbreviations: CGRP, calcitonin gene-related peptide; ION, infraorbital nerve; nNOS, neuronal nitric oxide synthase; NPY, neuropeptide Y; pAKT, phosphorylated AKT; SP, substance P; TGs, trigeminal ganglia.
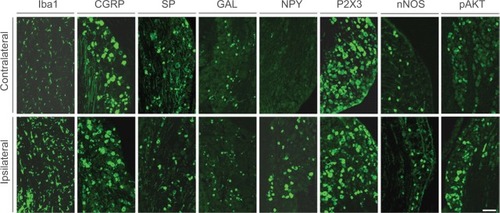
Figure 4 Expression of NPY and Iba1 in ipsilateral and contralateral TGs 2 weeks after partial ION transection.
Abbreviations: ION, infraorbital nerve; LI, like immunoreactivity; NPY, neuropeptide Y; PI, propidium iodide; TGs, trigeminal ganglia.
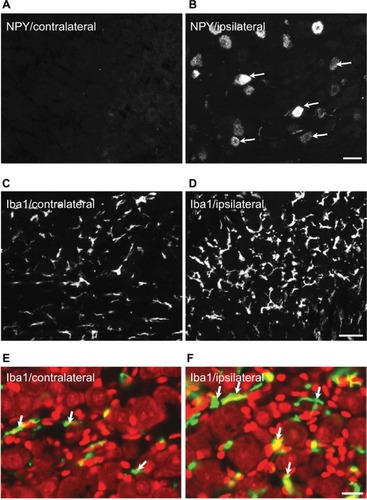
Figure 5 Immunofluorescent micrographs show the expression of biomarkers in contralateral TGs 2 weeks after partial ION transection.
Abbreviations: CGRP, calcitonin gene-related peptide; ION, infraorbital nerve; LI, like immunoreactivity; SP, substance P; TGs, trigeminal ganglia; pAKT, phosphorylated AKT.
Discussion
In this study, we investigated the expression and regulation of several biomarkers in TGs 2 weeks after partial ION injury in mice. Following the ION transection and based on the NP counting method, dramatic changes of ATF3, Iba1, and NPY-LIs were observed but minor to no changes of SP, CGRP, nNOS, pAKT, or P2X3 were found in injured TGs as compared with contralateral uninjured controls. These findings suggest that distinct mechanisms of adaptation to nerve injury may exist in TGs when compared to spinal ganglia, and that NPY and activated glial cells may play a more important role in trigeminal neuralgia.
Several animal models have been developed for mimicking the injured condition of the human trigeminal nerve.Citation35,Citation36 Regarding the model of ION injury, the surgery has mostly been performed on rats by incising the skin above the unilateral eye.Citation37,Citation38 It is known that many nerve fibers of the facial nerve are distributed around eyes, both in superficial and deep layers. Therefore, exposing the ION through the extraoral approach can inevitably cause damage to the facial nerve and other tissues. This extra tissue damage may disturb the results of behavioral studies and make it more difficult to determine the origin of pain. The model used in this study was modified from a previous report, i.e., the surgery was carried out intraorally, and the damage of other cranial nerves and tissues was greatly limited.Citation27
ATF3 is often used as a marker of damaged neurons, as its upregulation indicates subtle damage to the neurons and/or their axons.Citation39,Citation40 Here, we report that ATF3 is significantly upregulated in the ipsilateral TGs vs. the contralateral TGs 2 weeks after partial injury of ION. ATF3-LI was seen in the axotomized neurons, especially in the nucleus and sometimes cytoplasm. In addition, most maxillary infra-orbital neurons were ATF3 positive in the ipsilateral TGs, indicating that the axons of these neurons projecting to ION were injured following partial ION transection. This result further demonstrates that the transection of ION can induce a specific nocuous stimulation on the corresponding territory innervated by ION afferents, triggering dramatic plasticity changes in neuronal receptive fields in the TGs, and thus further reflects that the partial ION injury model of trigeminal neuralgia in mouse used in this study has achieved an injured specificity. These results are in line with previous findings that an increase of ATF3-LI was seen in the ipsilateral TG neurons, in the ION-innervated region, after partial ION ligation.Citation41
Recent studies suggest that microglial cells are vital players in the immune response to injury and are largely implicated in chronic pain conditions. In most cases, activation of microglial cells accompanies an increased expression of a biomarker, i.e., Iba1.Citation42,Citation43 Moreover, microglial activation in the spinal cord contributes to pain hypersensitivity, and it has been shown that pharmacological modulation of spinal microglial responses can effectively mitigate chronic pain.Citation44 In the present study, microglial activation accompanying phenotype changes was observed in the ipsilateral TGs stained with anti-Iba1 antisera following ION injury, indicating that activated microglial cells may contribute to the initiation and maintenance of neuropathic orofacial pain after trigeminal nerve injury.
NPY is widely distributed in the central and peripheral nervous systems.Citation45 Both inhibitory and excitatory effects have been reported for NPY, depending on which receptor is involved.Citation46,Citation47 Our findings that NPY is low in the contralateral TGs, but significantly upregulated in the ipsilateral TGs after ION injury, are similar to NPY expression in DRG neurons after sciatic nerve injury.Citation16,Citation48–Citation50 Our data are also in agreement with several previous reports, which indicate that NPY was upregulated in TGs following inferior alveolar nerve (IAN) injury.Citation49,Citation51,Citation52 A very recent study using a mental nerve injury model in rats also showed an increased expression of NPY in ipsilateral TGs.Citation53 Thus, upregulation of NPY in injured TG neurons indicates a role of NPY in persistent abnormal sensations.
GAL, a 29-amino acid peptide, appears to play a role in pain modulation.Citation54 GAL and its mRNA are found in TGs. After peripheral injury, no change, upregulation, or downregulation of GAL in TGs has been reported.Citation16,Citation55,Citation56 Our data here are in agreement with previous reports that GAL is also expressed in mouse TGs. However, in this study, no significant change of GAL was found in injured TGs after ION injury, indicating that peripheral nerve injury has less influence on GAL synthesis in the TGs than in the DRGs. The quantitative differences in GAL expression from these studies may be related to variations in time periods, models, or species.
It has been established that CGRP and SP have excitatory effects on DRG and spinal neurons.Citation57 Regarding regulation in the DRGs, peripheral nerve injury reduces the expression of CGRP and SP, while peripheral inflammation induced by complete Freund’s adjuvant or carrageenan instead increases their expressions.Citation58–Citation60 Interestingly, in ferret TGs, the expression of CGRP and SP was either downregulated after IAN transection or showed no change after IAN ligation.Citation13,Citation16 Here, we report no significant changes of CGRP or SP in mouse TGs following partial ION injury. Our data suggest that these two neuropeptides may have less impact on ION injury-induced abnormal sensations in mice.
Recent studies indicate that purinergic signaling plays important roles in physiological and pathophysiological processes.Citation61,Citation62 Purinergic receptors are widely distributed in mammalian tissues, and these receptors are generally divided into three classes: P1, P2X, and P2Y receptors. P2X receptors are ionotropic and ATP sensitive and have been shown to be involved in nociceptive processing.Citation63 P2X3 is mainly expressed in small-sized, nonpeptidergic neurons but also seen in some medium- and large-sized neurons in DRGs and can be regulated in different animal models.Citation64–Citation66 In rat TGs, transient expression of P2X3 has been detected in response to trigeminal nerve injury.Citation64,Citation67 Although the proportion of P2X3-positive NPs in mouse TGs remains unchanged at 2 weeks postoperation in the present study, a role of P2X3 in mediating the abnormal nociceptive process may still exist via enhanced sensitivity of P2X3 receptors as suggested by a previous study.Citation63
It is known that nNOS produces nitric oxide (NO) in the nervous system.Citation68 As a neuronal neurotransmitter and/or neuromodulator, NO is involved in multiple biological processes, including modulation of pain.Citation69–Citation71 nNOS is regulated following the peripheral nerve injury in both DRGs and TGs,Citation72,Citation73 and nerve injury-induced hypersensitivity or allodynia can be attenuated by a pharmacological inhibition of nNOS.Citation74–Citation76 In the present study, we quantified the number of nNOS-positive NPs in TGs 2 weeks after ION transection. We did not find a significant difference in nNOS expression between the ipsilateral and contralateral TGs. In contrast to our findings, a significant upregulation of nNOS-IR neurons was seen in ipsilateral TGs 7 days after IAN injury, accompanied by significantly increased levels of two NO indicators, nitrate and nitrite.Citation75 Interestingly, the same group also demonstrated that the levels of the two indicators in ipsilateral TGs went back to the control levels 14 days after injury, suggesting that a transient expression pattern of nNOS exists in injured TGs. Furthermore, the dramatic increase of nNOS in TGs 7 days, but not 14 days, after injury implies that NO may play a role at the early stage of neuropathic pain.
Phosphatidylinositol 3-kinase (PI3-K)/AKT signaling is believed to be important for promoting neuronal cell survival and axonal outgrowth.Citation77 We have previously investigated the expression of pAKT in mouse DRGs and spinal cord following sciatic nerve axotomy or carrageenan-induced inflammation.Citation5 In normal control animals, pAKT was found in both peptidergic and nonpeptidergic DRG neurons. After nerve injury, pAKT-IR neurons were seen in ipsilateral DRG neurons coexpressed with GAL but not NPY. In addition, we also found that the intensity of pAKT-LI in DRGs was increased in axotomized animals. To our knowledge, pAKT expression in TGs has been much less characterized. In this study, we reported that peripheral ION injury did not change the proportion of pAKT-positive NPs. However, even without quantification, an increased intensity of pAKT-LI was detected in the ipsilateral TGs. Our findings suggest that there is a PI3-K/AKT signaling pathway in trigeminal system.
Conclusion
Trigeminal nerve injury causes marked plasticity of biomarkers in TGs. Significant changes observed in injured TGs suggest that NPY and Iba1 may play crucial roles in the pathogenesis of orofacial neuropathic pain, in response to peripheral nerve injury.
Author contributions
RL, CL, GL, and T-JSS performed experiments, analyzed the data, and wrote the manuscript. XS, AR, and KM analyzed the data and wrote the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the China Scholarship Council (CSC) award to Dr Chuang Lyu (HIT, 2013) and the Summer Course Supervision Foundation, Karolinska Institutet, Sweden. We especially thank Doctor Tomas Hökfelt and Doctor Kaj Fried for kindly providing all antibodies and materials used in this study, as well as their valuable advice.
Disclosure
The authors report no conflicts of interest in this work.
References
- JeonHJHanSRParkMKYangKYBaeYCAhnDKA novel trigemi-nal neuropathic pain model: compression of the trigeminal nerve root produces prolonged nociception in ratsProg Neuropsychopharmacol Biol Psychiatry201238214915822449477
- GilronIWatsonCPCahillCMMoulinDENeuropathic pain: a practical guide for the clinicianCMAJ2006175326527516880448
- WangXLiangHZhouCXuMXuLSensitization induces hypersensitivity in trigeminal nerveClin Exp Allergy201242111638164223106664
- MaFZhangLLyonsDWestlundKNOrofacial neuropathic pain mouse model induced by Trigeminal Inflammatory Compression (TIC) of the infraorbital nerveMol Brain201254423270529
- ShiTJHuangPMulderJCeccatelliSHokfeltTExpression of p-Akt in sensory neurons and spinal cord after peripheral nerve injuryNeurosignals200917320321219346757
- ShiTJHuaXYLuXSensory neuronal phenotype in galanin receptor 2 knockout mice: focus on dorsal root ganglion neurone development and pain behaviourEur J Neurosci200623362763616487144
- MulderHZhangYDanielsenNSundlerFIslet amyloid polypeptide and calcitonin gene-related peptide expression are down-regulated in dorsal root ganglia upon sciatic nerve transectionBrain Res Mol Brain Res1997471–23223309221931
- CunhaTMSouzaGRDominguesACStimulation of peripheral kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kgamma/AKT/nNOS/NO signaling pathwayMol Pain201281022316281
- WangQZhuHZouKSensitization of P2×3 receptors by cystathionine beta-synthetase mediates persistent pain hypersensitivity in a rat model of lumbar disc herniationMol Pain2015111525885215
- LiuBNHanBXLiuFNeuroprotective effect of pAkt and HIF-1 alpha on ischemia ratsAsian Pac J Trop Med20147322122524507644
- SaitoANarasimhanPHayashiTOkunoSFerrand-DrakeMChanPHNeuroprotective role of a proline-rich Akt substrate in apoptotic neuronal cell death after stroke: relationships with nerve growth factorJ Neurosci20042471584159314973226
- NavarroXVivoMValero-CabreANeural plasticity after peripheral nerve injury and regenerationProg Neurobiol200782416320117643733
- ElcockCBoissonadeFMRobinsonPPChanges in neuropeptide expression in the trigeminal ganglion following inferior alveolar nerve section in the ferretNeuroscience2001102365566711226702
- HiroseKIwakuraNOritaSEvaluation of behavior and neuropeptide markers of pain in a simple, sciatic nerve-pinch pain model in ratsEur Spine J201019101746175220490875
- ZhangXXuZOShiTJRegulation of expression of galanin and galanin receptors in dorsal root ganglia and spinal cord after axotomy and inflammationAnn N Y Acad Sci19988634024139928186
- ElcockCBoissonadeFMRobinsonPPNeuropeptide expression in the ferret trigeminal ganglion following ligation of the inferior alveolar nerveArch Oral Biol200146872974311389865
- ZimmermannMPathobiology of neuropathic painEur J Pharmacol20014291–3233711698024
- TakedaMTsuboiYKitagawaJNakagawaKIwataKMatsumotoSPotassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory painMol Pain20117521219657
- ScholzJWoolfCJCan we conquer pain?Nat Neurosci20025suppl1062106712403987
- CostiganMScholzJWoolfCJNeuropathic pain: a maladaptive response of the nervous system to damageAnnu Rev Neurosci20093213219400724
- LuoDSZhangTZuoCXAn animal model for trigeminal neuralgia by compression of the trigeminal nerve rootPain Physician201215218719622430657
- LuizAPSchroederSDRaeGACalixtoJBChichorroJGContribution and interaction of kinin receptors and dynorphin A in a model of trigeminal neuropathic pain in miceNeuroscience201530018920025982562
- HenryMAFairchildDDPatilMJEffect of a novel, orally active matrix metalloproteinase-2 and -9 inhibitor in spinal and trigeminal rat models of neuropathic painJ Oral Facial Pain Headache201529328629626244437
- LuizAPKopachOSantana-VarelaSWoodJNThe role of Nav1.9 channel in the development of neuropathic orofacial pain associated with trigeminal neuralgiaMol Pain2015117226607325
- VosBPStrassmanAMMaciewiczRJBehavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerveJ Neurosci1994145 pt 1270827238182437
- Idanpaan-HeikkilaJJGuilbaudGPharmacological studies on a rat model of trigeminal neuropathic pain: baclofen, but not carbamazepine, morphine or tricyclic antidepressants, attenuates the allodynia-like behaviourPain1999792–328129010068174
- ImamuraYKawamotoHNakanishiOCharacterization of heat-hyperalgesia in an experimental trigeminal neuropathy in ratsExp Brain Res19971161971039305818
- BennettGJXieYKA peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in manPain1988331871072837713
- StanicDParatchaGLeddaFHerzogHKopinASHokfeltTPeptidergic influences on proliferation, migration, and placement of neural progenitors in the adult mouse forebrainProc Natl Acad Sci U S A200810593610361518305161
- TheodorssonERugarnORadioimmunoassay for rat galanin: immunochemical and chromatographic characterization of immunoreactivity in tissue extractsScand J Clin Lab Invest200060541141811003261
- ShiTJLiJDahlstromADeletion of the neuropeptide Y Y1 receptor affects pain sensitivity, neuropeptide transport and expression, and dorsal root ganglion neuron numbersNeuroscience2006140129330416564642
- LyuCMulderJBardeSG protein-gated inwardly rectifying potassium channel subunits 1 and 2 are down-regulated in rat dorsal root ganglion neurons and spinal cord after peripheral axotomyMol Pain2015114426199148
- SuJGaoTShiTPhenotypic changes in dorsal root ganglion and spinal cord in the collagen antibody-induced arthritis mouse modelJ Comp Neurol2015523101505152825631752
- TavesSBertaTChenGJiRRMicroglia and spinal cord synaptic plasticity in persistent painNeural Plast2013201375365624024042
- AlvarezPBrunALabertrandieAAntihyperalgesic effects of clomipramine and tramadol in a model of posttraumatic trigeminal neuropathic pain in miceJ Orofac Pain201125435436322247931
- PozzaDHCastro-LopesJMNetoFLAvelinoASpared nerve injury model to study orofacial painIndian J Med Res2016143329730227241642
- KernisantMGearRWJasminLVitJPOharaPTChronic constriction injury of the infraorbital nerve in the rat using modified syringe needleJ Neurosci Methods20081721434718501433
- DeseureKHansGBehavioral study of non-evoked orofacial pain following different types of infraorbital nerve injury in ratsPhysiol Behav201513829229625455862
- TsujinoHKondoEFukuokaTActivating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injuryMol Cell Neurosci200015217018210673325
- ShortlandPJBaytugBKrzyzanowskaAMcMahonSBPriestleyJVAverillSATF3 expression in L4 dorsal root ganglion neurons after L5 spinal nerve transectionEur J Neurosci200623236537316420444
- XuMAitaMChavkinCPartial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: behavioral, neural, and glial reactionsJ Pain20089111036104818708302
- MarchandFPerrettiMMcMahonSBRole of the immune system in chronic painNat Rev Neurosci20056752153215995723
- Romero-SandovalAChaiNNutile-McMenemyNDeleoJAA comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic painBrain Res2008121911612618538310
- JakenRJvan GorpSJoostenEANeuropathy-induced spinal GAP-43 expression is not a main player in the onset of mechanical pain hypersensitivityJ Neurotrauma201128122463247321671799
- YalamuriSMBrennanTJSpoffordCMNeuropeptide Y is analgesic in rats after plantar incisionEur J Pharmacol20136981–320621223123350
- MoloshAISajdykTJTruittWAZhuWOxfordGSShekharANPY Y1 receptors differentially modulate GABAA and NMDA receptors via divergent signal-transduction pathways to reduce excitability of amygdala neuronsNeuropsychopharmacology20133871352136423358240
- GotzscheCRWoldbyeDPThe role of NPY in learning and memoryNeuropeptides201655798926454711
- ItotagawaTYamanakaHWakisakaSAppearance of neuropeptide Y-like immunoreactive cells in the rat trigeminal ganglion following dental injuriesArch Oral Biol19933887257288215997
- WakisakaSSasakiYKurisuKTemporal analysis of neuropeptide Y expression in the rat trigeminal ganglion following peripheral axotomy of the inferior alveolar nerveNeurosci Lett1995188149527783977
- WakisakaSKajanderKCBennettGJEffects of peripheral nerve injuries and tissue inflammation on the levels of neuropeptide Y-like immunoreactivity in rat primary afferent neuronsBrain Res19925981–23493521486499
- FristadIHeyeraasKJKvinnslandIHNeuropeptide Y expression in the trigeminal ganglion and mandibular division of the trigeminal nerve after inferior alveolar nerve axotomy in young ratsExp Neurol199614222762868934559
- KhullarSMFristadIBrodinPKvinnslandIHUpregulation of growth associated protein 43 expression and neuronal co-expression with neuropeptide Y following inferior alveolar nerve axotomy in the ratJ Peripher Nerv Syst199832799010959241
- MagnussenCHungSPRibeiro-da-SilvaANovel expression pattern of neuropeptide Y immunoreactivity in the peripheral nervous system in a rat model of neuropathic painMol Pain2015113126012590
- TofighiRBardeSPalkovitsMGalanin and its three receptors in human pituitary adenomaNeuropeptides201246519520122889491
- ZhangXJiRRArvidssonJExpression of peptides, nitric oxide synthase and NPY receptor in trigeminal and nodose ganglia after nerve lesionsExp Brain Res199611133934048911933
- HenkenDBMartinJRThe proportion of galanin-immunoreactive neurons in mouse trigeminal ganglia is transiently increased following corneal inoculation of herpes simplex virus type-1Neurosci Lett199214021771801380144
- HokfeltTZhangXWiesenfeld-HallinZMessenger plasticity in primary sensory neurons following axotomy and its functional implicationsTrends Neurosci199417122307511846
- HaneschUPfrommerUGrubbBDHeppelmannBSchaibleHGThe proportion of CGRP-immunoreactive and SP-mRNA containing dorsal root ganglion cells is increased by a unilateral inflammation of the ankle joint of the ratRegul Pept1993461–22022037692491
- WeiheENohrDSchaferMKCalcitonin gene related peptide gene expression in collagen-induced arthritisCan J Physiol Pharmacol1995737101510198846393
- CalzaLPozzaMZanniMManziniCUManziniEHokfeltTPeptide plasticity in primary sensory neurons and spinal cord during adjuvant-induced arthritis in the rat: an immunocytochemical and in situ hybridization studyNeuroscience19988225755899466462
- Baroja-MazoABarbera-CremadesMPelegrinPThe participation of plasma membrane hemichannels to purinergic signalingBiochim Biophys Acta201318281799322266266
- SeiffertKDingWWagnerJAGransteinRDATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signalingJ Invest Dermatol200612651017102716410784
- ChenMGuJGA P2X receptor-mediated nociceptive afferent pathway to lamina I of the spinal cordMol Pain20051415813988
- TsuzukiKKondoEFukuokaTDifferential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory gangliaPain200191335136011275393
- KageKNiforatosWZhuCZLynchKJHonorePJarvisMFAlteration of dorsal root ganglion P2×3 receptor expression and function following spinal nerve ligation in the ratExp Brain Res2002147451151912444483
- XiangZXiongYYanNFunctional up-regulation of P2×3 receptors in the chronically compressed dorsal root ganglionPain20081401233418715715
- ErikssonJBongenhielmUKiddEMatthewsBFriedKDistribution of P2×3 receptors in the rat trigeminal ganglion after inferior alveolar nerve injuryNeurosci Lett1998254137409780086
- KaracayBBonthiusDJThe neuronal nitric oxide synthase (nNOS) gene and neuroprotection against alcohol toxicityCell Mol Neurobiol201535444946125672665
- CuryYPicoloGGutierrezVPFerreiraSHPain and analgesia: the dual effect of nitric oxide in the nociceptive systemNitric Oxide201125324325421723953
- FreireMAGuimaraesJSLealWGPereiraAPain modulation by nitric oxide in the spinal cordFront Neurosci20093217518120011139
- HancockCMRiegger-KrughCModulation of pain in osteoarthritis: the role of nitric oxideClin J Pain200824435336518427234
- KimKHKimJIHanJAChoeMAAhnJHUpregulation of neuronal nitric oxide synthase in the periphery promotes pain hypersensitivity after peripheral nerve injuryNeuroscience201119036737821664432
- DaviesSLLoescherARClaytonNMBountraCRobinsonPPBoissonadeFMnNOS expression following inferior alveolar nerve injury in the ferretBrain Res200410271–2111715494152
- Tesser-ViscainoSADenadai-SouzaATeixeiraSAPutative antinociceptive action of nitric oxide in the caudal part of the spinal trigeminal nucleus during chronic carrageenan-induced arthritis in the rat temporomandibular jointBrain Res20091302859619769951
- SugiyamaTShinodaMWataseTNitric oxide signaling contributes to ectopic orofacial neuropathic painJ Dent Res201392121113111724130220
- GuanYYasterMRajaSNTaoYXGenetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in miceMol Pain200732917922909
- AskvigJMWattJAThe MAPK and PI3K pathways mediate CNTF-induced neuronal survival and process outgrowth in hypothalamic organotypic culturesJ Cell Commun Signal20159321723125698661
