Abstract
Thanks to advances in neuroscience, biopsychosocial models for diagnostics and treatment (including physical, psychological, and pharmacological therapies) currently have more clinical support and scientific growth. At present, a conservative treatment approach prevails over surgery, given it is less aggressive and usually results in satisfactory clinical outcomes in mild–moderate temporomandibular disorder (TMD). The aim of this review is to evaluate the recent evidence, identify challenges, and propose solutions from a clinical point of view for patients with craniofacial pain and TMD. The treatment we propose is structured in a multi-modal approach based on a biobehavioral approach that includes medical, physiotherapeutic, psychological, and dental treatments. We also propose a new biobehavioral model regarding pain perception and motor behavior for the diagnosis and treatment of patients with painful TMD.
Introduction
According to health sciences definitions, temporomandibular disorder (TMD) comprises a variety of conditions affecting the anatomy and functional characteristics of the TM joint (TMJ). Factors contributing to TMD complexity are related to dentition, clenching, and other related systems that frequently provoke symptoms of muscular, articular, and periarticular pain.Citation1
Orofacial pain is defined as a pain manifested in the face or oral cavity, including such disorders as TMD, which are a major cause of nonodontogenic orofacial pain.Citation2,Citation3 TMD has considerable prevalence, with significant impact on physical and psychosocial factors.Citation2 Its prevalence has been reported to be between 3.7% and 12%, and is three to five times more frequent in women.Citation4 TMD also contributes to a high proportion of socioeconomic costs, which are usually associated with comorbidities, such as depression and other psychological factors.Citation5–Citation7 Also, the loss of work and work productivity is a major issue to consider in TMD patients being treated early on, and it requires significant public education.
Before 2000
Although before the 1980s, malocclusion and other related factors were considered fundamental and key causes of TMD, during this decade authors began to publish critical articles on these subjects.Citation8 In current clinical practice, orthodontic treatments are still used to treat TMD; however, it was established in the 1990s that the role of malocclusion in TMD is very limited or nonexistent,Citation9 and thus these disorders should not be treated with orthodontics.Citation10
Decade 2000 to 2010
During the 2000–2010 decade, invasive treatments and surgical options for TMD came into use. However, by the end of this decade, clinical experience and several studies included in systematic reviews, such as Guo et al, reported a lack of evidence supporting the use of arthrocentesis or arthroscopy for TMD treatment.Citation11
From 2010
Although the cognitive and behavioral profiles of patients with TMD have been debated for some years,Citation11 it was in the present decade that health professionals began to propose behavior-based therapiesCitation12 as a promising treatment related to cost-effectiveness.Citation13 This paradigm has since been changing and developing a wider focus, leaving behind the biomedical structural model. Thanks to advances in neuroscience,Citation14,Citation15 biopsychosocial models for diagnostics and treatment (including physical, psychological, and pharmacological therapies) currently have more clinical support and scientific growth.Citation16,Citation17
Classification and research diagnostic criteria for temporomandibular disorders
International classifications have been updated in recent decades, thus adapting to new clinical diagnoses and research. The etiology of TMD is multifactorial, which is due to related functional, structural, and psychological factors.Citation18–Citation20 In regard to the clinical presentation of TMD, one of the most frequent symptoms is pain. Pain can affect such areas as ears, eyes, and/or throat, producing neck pain, facial pain, and headaches.Citation21 Among the physical factors, inflammatory problems, such as traumatic secondary synovitis, infection, and irritation, can be found. TMD can also be associated with disk dysfunction, with or without reduction.Citation22
The Research Diagnostic Criteria for TMD (RDC/TMD) have been one of the most commonly used and recognized classifications by the international scientific community for diagnosis, evaluation, and categorization of TMD to date. Their importance is highlighted by the fact that they have been translated into various languages.Citation23,Citation24 The RDC/TMD are based on a biobehavioral model of pain, including two main axes: physical signs and symptoms (axis I) and psychological and disability factors (axis II). Included in axis I are painful myofascial disorders, disk subluxation, or luxation and arthritis or arthrosis,Citation25 given painful myofascial disorders are included in the most frequent diagnoses observed in the literature.Citation25 The most recent published version of this classification was in 2014, named Diagnostic Criteria for TMD (DC/TMD),Citation26 and aimed to improve the sensibility and specificity of the previous RDC/TMD through more comprehensive instruments for axis I and axis II that can be used by researchers and clinicians.
Neurophysiology of trigeminal sensory system
The trigeminal nerve, or cranial V nerve, is considered a mixed-function nerve (sensory, motor, and autonomic)Citation27 and a major cranial nerve.Citation28 This nerve is termed “trigeminal” due to its three main branches: the ophthalmic nerve (V1), the maxillary nerve (V2), and the mandibular nerve (V3). Sensitive axons of the trigeminal nerve innervate the majority of cranial and facial tissues, except the posterior area of the cranium, the mandibular angle, part of the external auditory canal and pavilion, and part of the pharynx.Citation29
Although the primary innervation patterns and the nerve signals are similar throughout the body, the craniofacial region has some particularities. Craniofacial innervation depends on several anatomic and functional structures of primary afferent neurons emanating from the trigeminal ganglion (and other cranial nerves). There are certain differences in the neurons and the dorsal root ganglia of the spinal cord. For example, the relationship between myelinated afferent fibers (A) and unmyelinated afferent fibers (C) is closer in the trigeminal nerve than in the spinal nerves. Spinal nerves present relatively few C fibers.Citation30 This situation could generate a higher mean velocity of conduction in trigeminal areas. In addition, cranial region distances to the neuronal body are shorter than in the rest of the body.Citation31
On the other hand, cranial peripheral nerves have fewer efferent sympathetic axons than spinal nerves. Some authors have postulated that this peculiarity could have relative influence on painful states maintained by the sympathetic nervous system itself in the trigeminal region.Citation32 Other sympathetic differences between the trigeminal area and the rest of the body are the intracranial and cutaneous blood vessels. In the trigeminal area, these vessels receive both parasympathetic and sympathetic innervation; however, in the segmental levels parasympathetic innervation is infrequent or nonexistent.Citation33
Physiopathology of TMD
Trigeminal primary afferent fibers are present in the craniofacial tissues as free nerve endings functioning as nociceptors that can activate through mechanical, thermal, or chemical stimuli. Their activation can result in the excitation of small-diameter and slow fibers (αδ or C).Citation34–Citation36 Some neurochemical components (eg, substance P, 5-HT, prostaglandins, and bradykinins) are involved in this peripheral activation by nociceptive stimulation. These substances are present in the peripheral sensitization process, and could thus enhance nerve sensitivity after minimum injury. This sensitization of nociceptive endings is a peripheral mechanism that as an alert system helps to protect injured tissues from repeated stimuli.Citation37,Citation38
The fact that “nociceptive-specific” and “wide dynamic range” neurons located in the trigeminal subnucleus caudalis are excited by nociceptive stimuli (in both cases) and non-nociceptive stimuli (in wide dynamic range) should be taken into account.Citation36 The majority of these neurons can also be excited by other inputs from the meninges, vessels, oral tissues, TMJ, and masticatory muscles.Citation25,Citation27,Citation29 These inputs have widely convergent patterns, explaining a poor and deep pain location, as well as the diffusion of referred pain, which is a typical condition in TMJ pain and its associated muscles.Citation36,Citation38,Citation39 The aim of this review is to evaluate the recent evidence, identify challenges, and propose solutions from a clinical point of view for patients with craniofacial pain and TMD.
Management of TMD
A suitable therapeutic approach for TMD should be aimed at alleviating the main signs and symptoms of this condition.Citation40 The most relevant signs of TMD are the presence of joint sounds (clicking and crepitation), reduced mouth opening, and disrupted jaw movements.Citation21,Citation41 However, pain is the primary problem of this pathology, and it is typically the reason these patients request medical care.Citation17,Citation42 Also, it is likely the reason that most studies have been aimed at evaluating the effectiveness of various intervention measures related to pain as the main variable.Citation43
Conservative treatments for TMD include medication, physiotherapy, occlusal splints, self-management strategies, and interventions based on cognitive behavioral approaches.Citation44–Citation49 At present, a conservative treatment approach prevails over surgery, given it is less aggressive and usually results in satisfactory clinical outcomes in mild–moderate TMD.Citation48,Citation50–Citation52 In fact, the evidence for the greatest effectiveness of surgical versus conservative intervention to reduce short-term pain in arthrogenic TMD is controversial and inconclusive.Citation53–Citation55 Indications for the application of each of the interventions, as well as their potential effects for the treatment of patients with TMD, are described in the following sections.
Oral and topical pharmacotherapy
The pharmacological treatment of the patients with TMD is usually empirical. Although several medications are typically prescribed for the treatment of TMD, many lack evidence for this specific pathology;Citation56 however, they have proven their effects in other musculoskeletal conditions. The most commonly used drugs include nonsteroidal anti-inflammatory drugs (NSAIDs), corticoids, analgesics, muscle relaxants, anxiolytics, opiates, tricyclic antidepressants (TCAs), gabapentin, and lidocaine patches.Citation57–Citation60 Some of these medications are used to treat the joint pain of the TMD, and others are more effective for treating muscle pain.
NSAIDs have proven their effect in reducing pain in the TMJ. One of the most frequently used NSAIDs is sodium diclofenac, which can reduce joint pain at a dose of 50 mg twice/thrice daily.Citation49,Citation61 Another NSAID used is naproxen sodium, which has been demonstrated to reduce joint pain compared with placebo. Significant differences have been shown after 3 weeks of treatment (500 mg twice a day), and a significant improvement in clinical signs and symptoms of TMD was obtained compared with celecoxib and placebo.Citation62 Piroxicam 20 mg once a day for 10 days results in greater TMJ pain reduction at 30-day follow-up.Citation63 Another substance not very well known but well tolerated is palmitoylethanolamide (300–1,200 mg daily up to 120 days),Citation64 which appears to have an analgesic and anti-inflammatory effect in patients with TMD.Citation65,Citation66
These results suggest that long-term treatment is needed to obtain the maximal effects of all these drugs, which sometimes become evident only after several weeks of treatment. NSAIDs have-well known adverse effects, however, such as exacerbation of hypertension, gastrointestinal effects ranging from dyspepsia to ulceration, and worsening of renal function, which makes analyzing the clinical situation of each patient extremely important to establish the best individual treatment.
A different approach to NSAID intake for avoiding its systemic absorption is its topical administration in creams or ointments over the TMJ to reduce pain. The application of four doses a day of topical diclofenac combined with dimethyl sulfoxide to improve its absorption is recommended.Citation67 Topical diclofenac has been suggested to achieve local concentrations significant enough to inhibit proinflammatory prostaglandin E2 production and also competitively inhibit the NMDA subtype of the glutamate receptor found in TMJ nociceptors.Citation68
For the treatment of muscle pain in myofascial TMD muscle, such relaxants as diazepam and cyclobenzaprine are commonly used.Citation61,Citation69 Diazepam has shown better effects than ibuprofen for chronic orofacial muscle painCitation71 and the same effects as placebo for reducing TMD pain.Citation71 A recent meta-analysis concluded that cyclobenzaprine had a positive effect on TMD muscle pain in the short termCitation61 through its effect over local spasms and associated acute pain; it was even more effective than clonazepam in improving jaw pain upon awakening.Citation72 The NSAID sodium diclofenac, both by itself and in combination with acetaminophen, carisoprodol, and caffeine, has been proven to have a more rapid positive effect on masticatory muscle pain compared with placebo.Citation73
TCAs have been proposed by some authors for myofascial masticatory chronic pain, particularly amitriptyline and nortriptyline, as first-line treatments for myofascial pain with referral, with low doses of 10–35 mg per day.Citation74,Citation75 Others propose a second-line treatment of gabapentin for nonresponders or for those who do not tolerate TCAs.Citation75,Citation76
Injected pharmacotherapy
In a recent review, results supported the use of injections of the corticosteroid β-methasone or sodium hyaluronate for TMJ pain.Citation53,Citation61 The corticosteroid might have an anti-inflammatory effect on the joint, and the hyaluronate could improve the joint’s lubrication, but both could also help to dilute local inflammatory substances. Inferior or double TMJ-space injection is recommended over the superior-space injection technique.Citation77
Regarding botulinum toxin (BTX) injections to the masticatory muscles, a systematic review revealed controversial results for BTX therapy. Of the five studies included, two obtained a significant reduction in pain, one showed equal effects compared with masticatory manual therapy, and two showed no significant differences for BTX compared with placebo.Citation78 More research is needed to assess the possible long-term negative effects of BTX on the infiltrated muscles. Basic research has shown that the size of the muscle recovered, but not the contractile function, after 1 year of BTX injections.Citation79 Also, when comparing BTX with placebo injection for trapezius muscle pain, there were no differences in pain-intensity measurement.Citation80
Surgical interventions
Among the surgical options, two of the most frequently used techniques for internal derangements of the TMJ or degenerative pathology are arthrocentesis based on articular lavage with or without injection of pharmaceuticals and arthroscopy. There are no differences regarding pain and mandibular function when comparing arthroscopy with arthrocentesis;Citation81,Citation82 however, there is a lack of evidence to support arthrocentesis as a better therapeutic intervention than nonsurgical interventions.Citation83,Citation84 For internal derangement of the TMJ, medical management or rehabilitation is recommended over other surgical options;Citation85 patients with symptomatic disk displacement without reduction should be treated with the simplest and least invasive intervention.Citation86 Furthermore, there is growing evidence supporting the benefit of platelet-rich plasma injections over hyaluronate combined with arthrocentesis for TMJ osteoarthritis; however, more clinical trials are needed.Citation87–Citation89
Dental management
Two approaches are usually proposed by odontologists to treat their TMD patients: orthopedic stabilization therapy and occlusal therapy. Splint therapy is frequently used for the first method group; in the second method, orthodontics and occlusal adjustment are commonly used to achieve a definite correct stable occlusion. According to Varga, signs and symptoms of TMD could not be associated with specific types of malocclusion.Citation90 This statement, together with published reports stating insufficient evidence, precludes us from recommending to our patients an orthodontic intervention or occlusal adjustment to treat TMD.Citation91,Citation92
On the other hand, splint therapy is one of the most commonly proposed conservative treatments for TMD pain associated with bruxism and also for internal derangements. It is not clear whether the use of a stabilization splint can be beneficial for reducing pain in TMD,Citation93 given its therapeutic effect remains controversial; however, it appears to have an undeniable placebo effect for pain management.Citation94 A transient effect of reduction in electromyographic activity of the masticatory muscles has been demonstrated, which did not last more than 2 weeks.Citation95,Citation96 Occlusal splints are recommended to prevent dentition damage from tooth grinding.Citation97,Citation98
Physical therapy
Physical therapy plays a prominent role in the treatment of TMD.Citation45,Citation46,Citation99 This therapeutic discipline aims to relieve pain, reduce inflammation, and restore motor function using a wide range of techniques, including manual therapy (eg, joint mobilization/manipulations, soft-tissue mobilization), therapeutic exercise, electrotherapy (eg, low-level laser therapy [LLLT], transcutaneous electrical nerve stimulation [TENS], therapeutic ultrasound, shortwave), dry needling (DN), and acupuncture.Citation45,Citation47
Manual therapy
Manual therapy for TMD, regardless of its origin, should include joint mobilization and soft-tissue techniques, with the aim of improving function and reducing pain symptoms.Citation52,Citation100 These techniques can trigger neurophysiological mechanisms responsible for pain relief and reduction of muscle activity.Citation101–Citation103
According to the literature, some authors also consider it relevant to apply these types of techniques to the cervical region, especially in the upper cervical spine.Citation52,Citation100 The demonstrated efficacy of the articular upper cervical mobilizations in reducing pain and increasing mandibular range of motion (ROM)Citation52,Citation100 could be due to the neuroanatomical connection between these two segments at the trigeminal–cervical complexCitation36,Citation104 or the biomechanical relationship between the cervical and orofacial regions.Citation105,Citation106
A debate among manual therapists concerns which approach is the best articular technique for treating the cervical spine. Authors have recommended cervical mobilizations, which have been shown to be more effective in reducing orofacial pain over manipulations.Citation52,Citation100,Citation107 They are safer, and produce similar effects at the cervical spine.Citation108–Citation110
Therapeutic exercise
Exercise focused on improving motor control and endurance of masticatory muscles is effective in alleviating the symptoms of patients with TMD.Citation52 However, exercise does not produce greater pain relief than other interventions,Citation52 such as TENS,Citation111 occlusal splints,Citation112–Citation114 patient education,Citation115,Citation116 and acupuncture.Citation117 It is important to keep in mind, however, that the exercises used in randomized controlled trials have been heterogeneous, including stretching, lingual and masticatory relaxation, and coordination exercises, among others.Citation45,Citation52 This aspect, added to the lack of a clear dosage regarding intensity, duration, and frequency, and the low methodological quality of the randomized controlled trials makes it difficult to draw conclusions.Citation45,Citation52 Nevertheless, although the superiority of the exercises cannot be assured, there is a favorable tendency when compared with other active treatments,Citation52 which might justify its use.
Therefore, we consider that therapeutic exercise could obtain superior results to other treatments if a program with motor-control exercises and endurance of the cervical and masticatory muscles is applied. Although some studies included cervical exercises, most were aimed at increasing the ROM (mobility and stretching exercises), but none intended to improve the resistance of cervical spine stabilizers.Citation52 Stabilizing muscles are essential to maintaining good postural control and helping to prevent the adoption of a forward head position.Citation118–Citation120
Manual therapy and exercise
A combined intervention of manual therapy and exercise is effective in alleviating the symptoms of patients with TMD, further enhancing the effects of both interventions in isolation.Citation52 These findings match those observed for cervical pain,Citation121 which can be explained by the summation of the hypoalgesic effects of manual therapyCitation104 along with the benefits of exercise, including improvements in physical condition, and the adoption of an active role by the patient in their treatment.Citation122–Citation125 On the other hand, although it is effective to administer this combined intervention in the cervical region, greater benefits are obtained when applied in both the orofacial and cervical regions.Citation52 Therefore, we consider it fundamental that physiotherapy treatment combine manual therapy with a program of therapeutic exercises aimed at restoring motor control and resistance of the masticatory and cervical musculature to improve the clinical condition of patients with TMD.
Dry needling and acupuncture
Few studies were found that applied dry needling (DN) for TMD.Citation126–Citation129 This intervention is used for treating local and referred pain produced by myofascial trigger points.Citation130 From these few studies, we conclude that DN results in a reduction in pain and improvements in mandibular function of patients with myofascial TMD.Citation126–Citation129 The effects of DN are comparable to the effects obtained by injection of the trigger points with lidocaine and corticosteroid.Citation131
Acupuncture is a good therapeutic modality for short-term pain relief in patients with myofascial TMD, but not in those cases in which there is a limitation of mandibular movement.Citation132,Citation133 At present, the mechanisms responsible for the analgesia produced by acupuncture are unknown, but appear to be based on the spinal and supraspinal release of serotonin,Citation134,Citation135 endogenous opioids,Citation136–Citation138 and other neurotransmitters with anti-inflammatory actions.Citation139,Citation140 The application of acupuncture is preferable by selecting points in the orofacial region, rather than distal to it, because enhanced effects are obtained in this manner,Citation141,Citation142 and not necessarily by selecting the standard acupuncture points.Citation143 These findings can be explained by the participation of peripheral opioid receptors in the analgesic process, given these receptors generate blockage of the painful input locally and unsystemically, and by the noxious stimulus itself independently of where it is applied.Citation143,Citation144 In fact, there are no differences when acupuncture and DN are compared with placebo that includes skin perforation.Citation132,Citation133,Citation145
Electrotherapy
Current evidence does not support the use of electrotherapy for pain relief in patients with TMD.Citation45,Citation53 In particular, various types of electrotherapy, such as pulsed radiofrequency energy, TENS, and LLLT, show no better results than their respective placebos in the treatment of TMD.Citation45,Citation53 However, the application criteria need to be homogenized in order to establish definitive conclusions, especially for the application of LLLT, given contradictory results are observed depending of the type of TMD, as well as the choice of parameters, such as intensity and frequency.Citation146 Regarding functional improvement, LLLT has proven effective in increasing mandibular ROM.Citation146 This effect could be due to a reduction in inflammation by suppressing cyclooxygenase, which would allow greater mobility to the joint.Citation147 However, LLLT’s mechanisms of action are not yet fully understood.
Cognitive behavioral therapy
Patients with chronic TMD usually present associated psychological factors that should be managed with specific interventions. Cognitive behavioral therapy is one of the treatments proposed to manage patients’ thoughts, behaviors, and/or feelings that might exacerbate pain symptoms. It is a noninvasive therapy and unlikely to have adverse effects.Citation148 The literature reports that cognitive behavioral therapy alone is not better than other interventions, but it is a good complement, especially when adapting the treatment to the psychological characteristics of the patient.Citation149–Citation151
Education and self-management strategies
Education and self-management are useful strategies to include in the management of patients with TMD. When comparing these interventions with occlusal splints, a slight benefit was obtained with education.Citation152 However, when compared with other interventions, such as manual therapy or therapeutic exercise, no additional benefits were observed.Citation115,Citation116,Citation152,Citation153 Nevertheless, it is assumed that education and self-management strategies are good to combine with other techniques, as observed by Wright et alCitation154 and Michelotti et al.Citation152 Most studies performed with education and self-management have included only patients with myofascial TMD, leading to a lack of evidence regarding other types of TMD. There is a need to define better what should be included in patients’ education and which self-management strategies are best according to the various types of TMD and regarding the psychosocial impairments that frequently affect patients with TMD. However, the authors consider that public and patient education could be much promising in patients with TMD, especially those based on neuroscience education, because this approach has been shown to reduce pain, disability, and psychological factors in chronic musculoskeletal disorders.Citation154
Relaxation techniques
Relaxation therapy involves self-regulation techniques aimed at reducing pain-induced stress and muscle tension. Relaxation interventions include such techniques as Jacobson’s relaxation. These techniques can be reinforced by external feedback using electromyography and/or biofeedback systems for training. Relaxation interventions included in a multimodal treatment could have a positive influence on pain intensity and maximal mouth opening, but there is scarce and controversial evidence.Citation148,Citation155
From a biomedical to a biobehavioral approach
The biomedical model has been an approach used widely in research on the etiological factors involved in TMD. This model has been based on functional theories and structural or morphological–pathological theories that attempt to explain TMD through theoretical concepts on dysfunctions of the condyle–disk complex, traumas, degenerative processes, occlusal concepts, and alterations related to masticatory muscles.Citation21 Some theories on the structural and functional biomedical model related to TMD have been useful and some concepts are still valid today, because they consistently explain the disorder from a dysfunctional point of view.
Diagnostic criteria for the classification of TMD based on physical signs and symptoms have had great impact in clinical practice and research, and have provided a standardized means of classification into various subtypes. It is important to highlight that instruments for the classification and evaluation of the psychological components involved in TMD have been included (emotional and cognitive factors);Citation26 however, analysis of the research on TMD reveals most studies that classification related to emotional and cognitive factors intended to define the psychological state and disability associated with patients’ pain has not had much impact on its use for the inclusion criteria or classification of patients.
The basis of the biomedical model is limited when we want to understand in depth the pathophysiology and perpetuation factors related to chronic pain in patients with TMD. A broader view on chronic pain is provided by neuroscientific studies from the last decade. There is strong evidence to suggest that neuroplastic changes and hyperexcitation of the central nervous system (CNS) would be part of those responsible for the central sensitization phenomenon.Citation156 Findings present in patients with TMD with chronic pain, such as generalized mechanical hyperalgesia,Citation157 structural and functional changes at the brain level,Citation14 alteration in pain modulation, comorbidities with other chronic diseases, increased expansion of pain areas, and presence of associated psychological factors would indicate a clinical profile compatible with a central sensitization process.Citation156,Citation158 It is important to mention that cognitive aspects, such as memory and learning, are heavily involved in the encoding of affective/emotional aversive stimuli that feed and perpetuate the sensitization process at the central level.Citation159
Current literature suggests that psychological and psychosocial factors have an important association with the duration of symptoms and their perpetuation in cases of chronic pain.Citation158,Citation160,Citation161 Psychological factors, such as pain catastrophizing,Citation162,Citation163 psychological distress,Citation161,Citation164 fear-avoidance beliefs,Citation165,Citation166 beliefs related to painful perception,Citation167 depressed or anxious mood,Citation168–Citation170 self-efficacy,Citation171 and passive coping,Citation164,Citation172–Citation174 are related to increased pain perception, increased levels of disability, and movement disorders in patients with chronic painful TMD. On the other hand, it has been noted that some psychosocial factors have also been identified as predictors of treatment outcome in patients with TMD.Citation175 We consider that somatic awareness is an important sensory-discriminative factor to be taken into account, since it has been related to an increased risk of suffering a TMD.Citation173,Citation176
The abundant current scientific evidence shows that the mechanistic biomedical model is not sufficient to establish a diagnosis or accurate treatment to manage patients with chronic painful TMD. A change in approach toward a more comprehensive and integral vision is necessary. We propose a diagnostic and therapeutic approach based on a biobehavioral approach. Many authors share this thought,Citation177–Citation179 even suggesting that from an ethical point of view a compulsory change is needed, given the application of unnecessary and irreversible interventions due to traditional management could endanger the patient’s well-being.Citation177,Citation178
The biobehavioral model for the diagnosis and treatment of patients with chronic painful TMD recognizes the importance of psychological factors, such as pain history, current emotional and cognitive status, beliefs, learned behaviors, and coping skills, in interaction with the physiological alterations that determine the pain experience. From the therapeutic point of view, the model allows the patients to acquire the ability to self-manage the pain, allowing an improvement in general functioning.Citation180
Based on the current clinical and scientific context, we propose a diagnostic and intervention model to address patients with painful TMD based on four dimensions (affective–motivational, sensory–discriminative, cognitive–evaluative, and motor behavior) integrated in a biobehavioral approach (). This model has been termed the biobehavioral model of pain perception and motor behavior, and although we have designed it to study any musculoskeletal disorder, in this article we adapt it to TMD. presents information about all of these approaches, grade of evidence, and magnitude of effects.
Figure 1 The four dimensions of the biobehavioral model of pain perception and motor behavior.
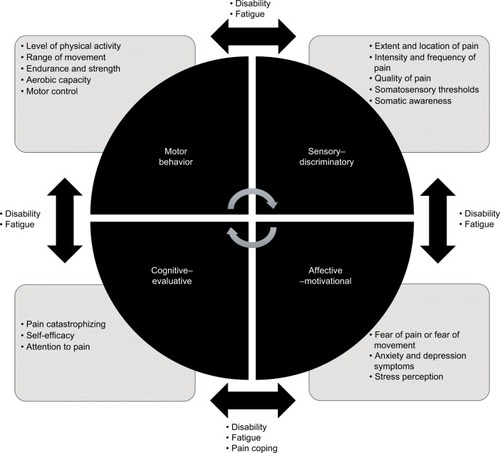
Table 1 Evidence on treatment options for pain related to TMD
Biobehavioral model of pain perception and motor behavior
A fundamental aspect of our model is the fact that musculoskeletal pain produces changes in motor behavior.Citation181 It has also been observed that pain-related movement disorders are an important cause that influences the impairment of functional capacity and the patient’s quality of life,Citation182 including the possible interaction that cognitive and emotional aspects can have on the relationship between motor behavior and pain perception. Herein, we briefly describe the theoretical aspects that underlie the biobehavioral model of pain perception and musculoskeletal pain.
Motor changes can be explained through the peripheral and central mechanisms related to the CNS.Citation183,Citation184 Experi mental studies have found that muscular pain influences motor-control strategies through central mechanisms.Citation185,Citation186 On the other hand, several studies have found functional and structural changes in motor cortical areas of patients with chronic pain.Citation187,Citation188 In this respect, activation of the supplementary motor area in patients with TMD when faced with adverse cognitive or emotional stimuli has been observed.Citation189 That same activation has been found in patients with TMD who have catastrophic helplessness.Citation190 It is important to mention that the supplementary motor area plays an important role in movement planning, and it is theorized that the pre-activation of this area found in cases of chronic pain could be due to the preparation of avoidance or anticipatory motor behaviors. We have scientific evidence revealing neurophysiological mechanisms of motor anticipation related to pain perception.Citation191,Citation192 Pain-protection behaviors can include motor activities, such as avoidance of movement and tendencies to touch the affected area of the body.Citation193 It has been proposed that the motor responses involved in the pain experience can be activated when the intensity of the pain rises beyond a certain threshold.Citation194
Emotional factors related to fear of pain play an important role in the degree of protective behaviors triggered by pain.Citation195 Recent research has shown that high levels of fear of pain are associated with being less physically active,Citation196,Citation197 limited range of motion,Citation198,Citation199 physical disability,Citation200 and strategies for adopting alternative movements.Citation201 It should be noted that behaviors associated with psychological distress, interruption of activity, and avoidance of activity are essential components in pain-related disability.Citation202
Motor behavior related to painful experiences can vary according to the case. Some patients with chronic pain use passive motor strategies to avoid pain; however, other patients use active self-regulation strategies to cope with pain.Citation203 Simmonds et al reported that movement dysfunction was not only a consequence of anticipating and minimizing pain. The motor component involved is an even more complicated problem that involves social, environmental, and psychological factors (cognitions and emotions) that can influence motor activity as a complex multidimensional construct.Citation204 Several motor programs have been proposed for the various forms of pain behavior. These can be organized at various levels of the CNS, and can be influenced by social and psychological factors.Citation195
Current evidence holds that in addition to fear of pain, other psychosocial factors might contribute to generating pain-related functional alterations.Citation205–Citation207 In this regard, Sullivan suggested that certain psychological factors, such as pain catastrophizing, fear, and depression can influence pain by reducing the threshold of activation of motor programs related to pain perception.Citation195
In summary, the biobehavioral model of pain perception and motor behavior presents a specific framework to help us understand the mechanisms involved in chronic painful TMD. Basically, we propose that sustained pain perception produces neuroplastic changes in the CNS that have implications for motor behavior that are directly and indirectly influenced by cognitive and emotional factors. In this model, the motor behavior is an essential element, given its alteration would increase levels of disability, leading to poorer quality of life, and would increase the perception of pain intensity.
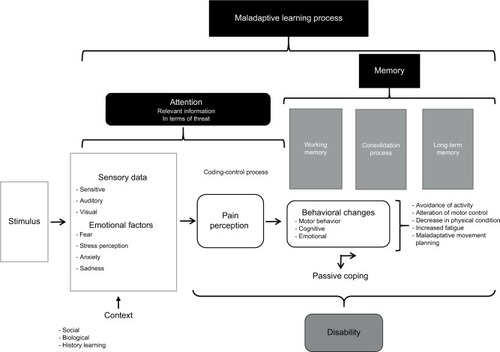
Poor motor behavior can be influenced by fear-avoidance beliefs, a decrease in self-efficacy expectations, catastrophic cognition, and an increase in depressive symptoms. Furthermore, pain-related movement disorders are a means of learning maladaptation that increases the attention to and memory of pain, favoring the perpetuation of the painful experience (). Behavioral changes associated with the experience of the perception of maintained pain can cause various movement dysfunctions, mainly when a passive coping strategy is used. The result of this situation is an increase in disability levels ().
Figure 2 Mechanisms involved in the biobehavioral model of pain perception and motor behavior.
To perform adequate clinical reasoning and undertake a good diagnostic approach using this model, it is necessary to evaluate the four dimensions and try to discern how they interact with one another, and especially to evaluate what factors are relevant (). We recommend performing an assessment that objectifies sensorimotor variables through physical tests and an evaluation of cognitive and emotional factors using self-reports to quantify them. In , we suggest the variables that should be evaluated to work with the biobehavioral model of pain perception and motor behavior.
The therapeutic approach we suggest attempts to provide a comprehensive framework for the treatment of patients with chronic painful TMD. The main variable to achieve optimum functional recovery is disability: we propose that if we improve the motor behavior, we will decrease the disability and in turn the painful perception. Therefore, we consider the possibility of a bidirectional relationship through which the treatment that reduces the painful perception can also favor the recovery of motor behavior while decreasing the disability. In order to achieve this change, it is necessary to eliminate erroneous beliefs and negative cognitions that could alter the treatment results. It will also be necessary to use motivational strategies that promote good adherence and compliance with the various types of treatments. It is important to mention that the treatment proposed herein has as a central therapeutic axis the movement to reduce pain and improve function. In relation to this, Luomajoki et al found that treatment with therapeutic exercise to improve motor performance also resulted in an improvement in pain and disability in patients with low-back pain.Citation208 In our model, we also integrate therapeutic strategies, such as therapeutic exercise, that can specifically reduce pain and would make treatment more effective.
Current scientific evidence shows the ability of therapeutic exercise to modulate pain in experimental conditions.Citation209–Citation211 In addition, we have strong scientific clinically relevant evidence that demonstrates the effectiveness of therapeutic exercise in reducing disability and pain intensity in other chronic musculoskeletal conditions.Citation212–Citation217
Multimodal treatment based on a biobehavioral approach
The treatment we propose has a multimodal point of view, but could also be structured in a multidisciplinary way, including the therapeutic interventions of physiotherapists, dentists, psychologists, and physicians. Based on current scientific evidence, we can say that a conservative approach appears to be the best option for the management of chronic painful TMD.
The treatment methods included in the therapeutic approach of this model are structured to achieve three objectives: reduction in pain perception, improvement of motor behavior, and improvement of cognitive and emotional factors related to the experience of pain. To reduce pain intensity, we propose the use of manual therapy, DN, and pharmacology. For improvements in pain and mandibular function, it is relevant to apply a combined intervention of manual therapy and therapeutic exercise directed to the orofacial, craniomandibular, and upper cervical regions. Although it is not yet a sufficiently investigated aspect, we consider that the prescription of generalized exercise in both aerobic and anaerobic modalities could be beneficial for patients with chronic conditions, which could favor the activation of the descending inhibitory system of pain, improve physical condition, and decrease the attentional focus on pain perception. In addition, splints appear to play a prominent role in the protection of dentition.
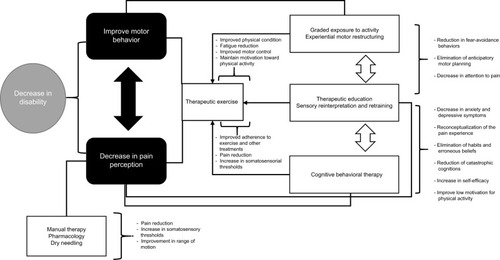
In order to improve the effectiveness of the aforementioned treatments, it is necessary to apply them in combination with biobehavioral treatments and strategies, in which we would emphasize therapeutic education, cognitive behavioral therapy, experiential motor restructuring, graded exposure to activity, sensory reinterpretation and retraining, counseling, and methods of physiological self-regulation, such as training in relaxation and biofeedback. These treatments should aim to improve adherence to therapeutic exercise and self-management techniques, eradicate counterproductive habits, encourage positive behaviors, reduce catastrophic cognition, reconceptualize erroneous beliefs about pain and movement, reduce fear-avoidance behaviors, improve stress management, and improve the patients’ knowledge of therapeutic exercise benefits. In , we present relevant aspects and recommendations to be taken into account for the approach and development of treatment from the biobehavioral model of pain perception and motor behavior. It is also important to mention that the treatment that we propose is applicable to patients with chronic painful TMD. For cases of acute or subacute pain, it is possible that less complex unimodal or bimodal approaches would have good effectiveness.
Figure 3 Representation of the therapeutic approach according to the biobehavioral model of pain perception and motor behavior.
Disclosure
The authors report no conflicts of interest in this work.
References
- IbsenOAPhelanJAOral Pathology for the Dental Hygienist6th edSaint LouisSaunders2013
- ContiPCPinto-FiamenguiLMCunhaCOContiACOrofacial pain and temporomandibular disorders: the impact on oral health and quality of lifeBraz Oral Res201226Suppl 112012323318754
- ZakrzewskaJMTemporomandibular disorders, headaches and chronic painJ Pain Palliat Care Pharmacother2015291616325643229
- MagnussonTEgermarkICarlssonGEA longitudinal epidemiologic study of signs and symptoms of temporomandibular disorders from 15 to 35 years of ageJ Orofac Pain200014431031911203765
- GiannakopoulosNNKellerLRammelsbergPKronmüllerKTSchmitterMAnxiety and depression in patients with chronic temporomandibular pain and in controlsJ Dent201038536937620079799
- ReiterSEmodi-PerlmanAGoldsmithCFriedman-RubinPWinocurEComorbidity between depression and anxiety in patients with temporomandibular disorders according to the research diagnostic criteria for temporomandibular disordersJ Oral Facial Pain Headache201529213514325905531
- Gil-MartínezAGrande-AlonsoMLa ToucheRLara-LaraMLópez-LópezAFernández-CarneroJPsychosocial and somatosensory factors in women with chronic migraine and painful temporomandibular disordersPain Res Manag20162016394567327818609
- GreeneCSOrthodontics and temporomandibular disordersDent Clin North Am19883235295383042477
- BalesJMEpsteinJBThe role of malocclusion and orthodontics in temporomandibular disordersJ Can Dent Assoc199460108999057953994
- McNamaraJATürpJCOrthodontic treatment and temporomandibular disorders: is there a relationship? Part 1: clinical studiesJ Orofac Orthop199758274899114557
- GuoCShiZRevingtonPArthrocentesis and lavage for treating temporomandibular joint disordersCochrane Database Syst Rev20094CD00497319821335
- OrlandoBManfrediniDSalvettiGBoscoMEvaluation of the effectiveness of biobehavioral therapy in the treatment of temporomandibular disorders: a literature reviewBehav Med200733310111818055333
- StowellAWGatchelRJWildensteinLCost-effectiveness of treatments for temporomandibular disorders: biopsychosocial intervention versus treatment as usualJ Am Dent Assoc2007138220220817272375
- LinCSBrain signature of chronic orofacial pain: a systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disordersPLoS One201494e9430024759798
- OonoYWangKBaad-HansenLConditioned pain modulation in temporomandibular disorders (TMD) pain patientsExp Brain Res2014232103111311924897946
- SuvinenTIKemppainenPLe BellYValjakkaAVahlbergTForssellHResearch diagnostic criteria Axis II in screening and as a part of biopsychosocial subtyping of Finnish patients with temporomandibular disorder painJ Orofac Pain201327431432424171181
- GhuryeSMcMillanRPain-related temporomandibular disorder: current perspectives and evidence-based managementDent Update201542653353653954254554626506809
- EpkerJGatchelRJCoping profile differences in the biopsychosocial functioning of patients with temporomandibular disorderPsychosom Med2000621697510705913
- LeRescheLEpidemiology of temporomandibular disorders: implications for the investigation of etiologic factorsCrit Rev Oral Biol Med1997832913059260045
- SenaMFMesquitaKSSantosFRSilvaFWSerranoKVPrevalence of temporomandibular dysfunction in children and adolescentsRev Paul Pediatr201331453854524473961
- SuvinenTIReadePCKemppainenPKönönenMDworkinSFReview of aetiological concepts of temporomandibular pain disorders: towards a biopsychosocial model for integration of physical disorder factors with psychological and psychosocial illness impact factorsEur J Pain20059661363315978854
- Graff-RadfordSBTemporomandibular disorders and headacheDent Clin North Am200751112914417185063
- JohnMTHirschCReiberTDworkinSFTranslating the research diagnostic criteria for temporomandibular disorders into German: evaluation of content and processJ Orofac Pain2006201435216483020
- KhooSPYapAUChanYHBulgibaAMTranslating the research diagnostic criteria for temporomandibular disorders into Malay: evaluation of content and processJ Orofac Pain200822213113818548842
- JohnMTDworkinSFManclLAReliability of clinical temporomandibular disorder diagnosesPain20051181–2616916154702
- SchiffmanOhrbachRTrueloveEDiagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest GroupJ Oral Facial Pain Headache201428162724482784
- KajiAMaedaTWatanabeSParasympathetic innervation of cutaneous blood vessels examined by retrograde tracing in the rat lower lipJ Auton Nerv Syst19913221531582030261
- SandersRDThe trigeminal (V) and facial (VII) cranial nerves: head and face sensation and movementPsychiatry (Edgmont)2010711316
- BathlaGHegdeANThe trigeminal nerve: an illustrated review of its imaging anatomy and pathologyClin Radiol201368220321322889460
- YoungRFKingRBFiber spectrum of the trigeminal sensory root of the baboon determined by electron microscopyJ Neurosurg197338165724629883
- PellegriniJJHornAKEvingerCThe trigeminally evoked blink reflex – I: neuronal circuitsExp Brain Res199510721661808773237
- HoffmannKDMatthewsMAComparison of sympathetic neurons in orofacial and upper extremity nerves: implications for causalgiaJ Oral Maxillofac Surg19904877207272358949
- UddmanRTajtiJMöllerSSundlerFEdvinssonLNeuronal messengers and peptide receptors in the human sphenopalatine and otic gangliaBrain Res1999826219319910224296
- TakemuraMSugiyoSMoritaniMKobayashiMYoneharaNMechanisms of orofacial pain control in the central nervous systemArch Histol Cytol20066927910016819148
- SessleBJPeripheral and central mechanisms of orofacial pain and their clinical correlatesMinerva Anestesiol200571411713615756153
- SessleBJNeural mechanisms and pathways in craniofacial painCan J Neurol Sci199926Suppl 3S7S11
- SessleBJAcute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlatesCrit Rev Oral Biol Med2000111579110682901
- SessleBJPeripheral and central mechanisms of orofacial inflammatory painInt Rev Neurobiol20119717920621708311
- BursteinRYamamuraHMalickAStrassmanAMChemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neuronsJ Neurophysiol19987929649829463456
- GauerRLSemideyMJDiagnosis and treatment of temporomandibular disordersAm Fam Physician201591637838625822556
- DworkinSFHugginsKHLeRescheLEpidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controlsJ Am Dent Assoc199012032732812312947
- OralKBal KüçükBEbeoğluBDinçerSEtiology of temporomandibular disorder painAgri2009213899419779999
- FrictonJROuyangWNixdorfDRSchiffmanELVellyAMLookJOCritical appraisal of methods used in randomized controlled trials of treatments for temporomandibular disordersJ Orofac Pain201024213915120401352
- IngawaléSGoswamiTTemporomandibular joint: disorders, treatments, and biomechanicsAnn Biomed Eng200937597699619252985
- McNeelyMLOlivoSAMageeDJA systematic review of the effectiveness of physical therapy interventions for temporomandibular disordersPhys Ther200686571072516649894
- MedlicottMSHarrisSRA systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorderPhys Ther200686795597316813476
- PaçoMPeleteiroBDuarteJPinhoTThe effectiveness of physiotherapy in the management of temporomandibular disorders: a systematic review and meta-analysisJ Oral Facial Pain Headache201630321022027472523
- ScrivaniSJKeithDAKabanLBTemporomandibular disordersN Engl J Med2008359252693270519092154
- ListTAxelssonSManagement of TMD: evidence from systematic reviews and meta-analysesJ Oral Rehabil201037643045120438615
- SyropSBInitial management of temporomandibular disordersDent Today20022185257
- DimitroulisGGremillionHADolwickMFWalterJHTemporomandibular disorders – 2: non-surgical treatmentAust Dent J19954063723768615742
- Armijo-OlivoSPitanceLSinghVNetoFThieNMichelottiAEffectiveness of manual therapy and therapeutic exercise for temporomandibular disorders: systematic review and meta-analysisPhys Ther201696192526294683
- ListTAxelssonSManagement of TMD: evidence from systematic reviews and meta-analysesJ Oral Rehabil201037643045120438615
- DıraçoğluDSaralİBKeklikBArthrocentesis versus nonsurgical methods in the treatment of temporomandibular disc displacement without reductionOral Surg Oral Med Oral Pathol Oral Radiol Endod200910813819272808
- StegengaBde BontLGDijkstraPUBoeringGShort-term outcome of arthroscopic surgery of temporomandibular joint osteoarthrosis and internal derangement: a randomized controlled clinical trialBr J Oral Maxillofac Surg19933113148431411
- MujakperuoHRWatsonMMorrisonRMacfarlaneTVPharmacological interventions for pain in patients with temporomandibular disordersCochrane Database Syst Rev201010CD00471520927737
- AgiusAMJonesNSMuscatRA randomized controlled trial comparing the efficacy of low-dose amitriptyline, amitriptyline with pindolol and surrogate placebo in the treatment of chronic tension-type facial painRhinology201351214315323671895
- WhiteAPArnoldPMNorvellDCEckerEFehlingsMGPharmacologic management of chronic low back painSpine (Phila Pa 1976)20113621 SupplS131S14321952185
- GewandterJSMcDermottMPMcKeownAReporting of crossover clinical trials of analgesic treatments for chronic pain: Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks systematic review and recommendationsPain2016157112544255127437786
- FirmaniMMirallesRCasassusREffect of lidocaine patches on upper trapezius EMG activity and pain intensity in patients with myofascial trigger points: a randomized clinical studyActa Odontol Scand201573321021825428627
- Häggman-HenriksonBAlstergrenPDavidsonTPharmacological treatment of orofacial pain: health technology assessment including a systematic review with network meta-analysisJ Oral Rehabil2017441080082628884860
- TaLEDionneRATreatment of painful temporomandibular joints with a cyclooxygenase-2 inhibitor: a randomized placebo-controlled comparison of celecoxib to naproxenPain20041111–2132115327804
- de CarliMLGuerraMBNunesTBPiroxicam and laser phototherapy in the treatment of TMJ arthralgia: a double-blind randomised controlled trialJ Oral Rehabil201340317117823252583
- GabrielssonLMattssonSFowlerCJPalmitoylethanolamide for the treatment of pain: pharmacokinetics, safety and efficacyBr J Clin Pharmacol201682493294227220803
- MariniIBartolucciMLBortolottiFGattoMRBonettiGAPalmitoylethanolamide versus a nonsteroidal anti-inflammatory drug in the treatment of temporomandibular joint inflammatory painJ Orofac Pain20122629910422558609
- GabrielssonLGouveia-FigueiraSHäggströmJAlhouayekMFowlerCJThe anti-inflammatory compound palmitoylethanolamide inhibits prostaglandin and hydroxyeicosatetraenoic acid production by a macrophage cell linePharmacol Res Perspect201752e0030028357126
- BusincoLRBusincoARD’EmiliaMLaurielloMTirelliGCTopical versus systemic diclofenac in the treatment of temporomandibular joint dysfunction symptomsActa Otorhinolaryngol Ital200424527928315871609
- DongXDSvenssonPCairnsBEThe analgesic action of topical diclofenac may be mediated through peripheral NMDA receptor antagonismPain20091471–3364519766393
- CairnsBEPathophysiology of TMD pain: basic mechanisms and their implications for pharmacotherapyJ Oral Rehabil201037639141020337865
- SingerEDionneRA controlled evaluation of ibuprofen and diazepam for chronic orofacial muscle painJ Orofac Pain19971121394610332320
- PramodGShashikanthMShambulingappaPLeleSAnalgesic efficacy of diazepam and placebo in patients with temporomandibular disorders: a double blind randomized clinical trialIndian J Dent Res201122340440922048580
- HermanCRSchiffmanELLookJORindalDBThe effectiveness of adding pharmacologic treatment with clonazepam or cyclobenzaprine to patient education and self-care for the treatment of jaw pain upon awakening: a randomized clinical trialJ Orofac Pain2002161647011889661
- VaroliFKPitaMSSatoSIssaJPdo NascimentoCPedrazziVAnalgesia evaluation of 2 NSAID drugs as adjuvant in management of chronic temporomandibular disordersScientificWorldJournal2015201535915225874243
- PleshOAdamsSHGanskySATemporomandibular joint and muscle disorder-type pain and comorbid pains in a national US sampleJ Orofac Pain201125319019821837286
- HavivYRettmanAAframianDSharavYBenolielRMyofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentinJ Oral Facial Pain Headache201529214415125905532
- KimosPBiggsCMahJAnalgesic action of gabapentin on chronic pain in the masticatory muscles: a randomized controlled trialPain20071271–215116017030096
- LiCZhangYLvJShiZInferior or double joint spaces injection versus superior joint space injection for temporomandibular disorders: a systematic review and meta-analysisJ Oral Maxillofac Surg2012701374421824703
- ChenYWChiuYWChenCYChuangSKBotulinum toxin therapy for temporomandibular joint disorders: a systematic review of randomized controlled trialsInt J Oral Maxillofac Surg20154481018102625920597
- WardSRMinamotoVBSuzukiKPHulstJBBremnerSNLieberRLRecovery of rat muscle size but not function more than 1 year after a single botulinum toxin injectionMuscle Nerve Epub2017526
- KwanchuayPPetchnumsinTYiemsiriPPasukNSrikanokWHathaiareerugCEfficacy and safety of single botulinum toxin type A (Botox) injection for relief of upper trapezius myofascial trigger point: a randomized, double-blind, placebo-controlled studyJ Med Assoc Thai201598121231123627004309
- Al-MoraissiEAOpen versus arthroscopic surgery for the management of internal derangement of the temporomandibular joint: a meta-analysis of the literatureInt J Oral Maxillofac Surg201544676377025701306
- RigonMPereiraLMBortoluzziMCLoguercioADRamosALCardosoJRArthroscopy for temporomandibular disordersCochrane Database Syst Rev20115CD00638521563153
- BouchardCGouletJPEl-OuazzaniMTurgeonAFTemporomandibular lavage versus nonsurgical treatments for temporomandibular disorders: a systematic review and meta-analysisJ Oral Maxillofac Surg20177571352136228132759
- VosLMHuddleston SlaterJJStegengaBLavage therapy versus nonsurgical therapy for the treatment of arthralgia of the temporomandibular joint: a systematic review of randomized controlled trialsJ Orofac Pain201327217117923630689
- SchiffmanELLookJOHodgesJSRandomized effectiveness study of four therapeutic strategies for TMJ closed lockJ Dent Res2007861586317189464
- Al-BaghdadiMDurhamJAraujo-SoaresVRobalinoSErringtonLSteeleJTMJ disc displacement without reduction managementJ Dent Res2014937 Suppl37S51S24659775
- KiliçSCGüngörmüşMSümbüllüMAIs Arthrocentesis plus platelet-rich plasma superior to arthrocentesis alone in the treatment of temporomandibular joint osteoarthritis? A randomized clinical trialJ Oral Maxillofac Surg20157381473148325976690
- HancıMKarameseMTosunZAktanTMDumanSSavaciNIntra-articular platelet-rich plasma injection for the treatment of temporomandibular disorders and a comparison with arthrocentesisJ Craniomaxillofac Surg201543116216625491276
- HegabAFAliHEElmasryMKhallafMGPlatelet-rich plasma injection as an effective treatment for temporomandibular joint osteoarthritisJ Oral Maxillofac Surg20157391706171325882438
- VargaMLOrthodontic therapy and temporomandibular disordersMed Sci2010347585
- KohHRobinsonPGOcclusal adjustment for treating and preventing temporomandibular joint disordersJ Oral Rehabil200431428729215089931
- LutherFLaytonSMcDonaldFOrthodontics for treating temporomandibular joint (TMJ) disordersCochrane Database Syst Rev20107CD00654120614447
- Al-AniZGrayRJDaviesSJSloanPGlennyAMStabilization splint therapy for the treatment of temporomandibular myofascial pain: a systematic reviewJ Dent Educ200569111242125016275687
- KlasserGDGreeneCSOral appliances in the management of temporomandibular disordersOral Surg Oral Med Oral Pathol Oral Radiol Endod2009107221222319138639
- HaradaTIchikiRTsukiyamaYKoyanoKThe effect of oral splint devices on sleep bruxism: a 6-week observation with an ambulatory electromyographic recording deviceJ Oral Rehabil200633748248816774505
- NascimentoLLAmorimCFGiannasiLCOcclusal splint for sleep bruxism: an electromyographic associated to Helkimo Index evaluationSleep Breath200812327528017987334
- MotamediMHNaviFPourshahabMOutcomes of management of early temporomandibular joint disorders: how effective is nonsurgical therapy in the long-term?Natl J Maxillofac Surg20101210811122442579
- MacedoCRSilvaABMachadoMASaconatoHPradoGFOcclusal splints for treating sleep bruxism (tooth grinding)Cochrane Database Syst Rev20074CD00551417943862
- SturdivantJFrictonJRPhysical therapy for temporomandibular disorders and orofacial painCurr Opin Dent1991144854961802010
- CalixtreLBMoreiraRFFranchiniGHAlburquerque-SendínFOliveiraABManual therapy for the management of pain and limited range of motion in subjects with signs and symptoms of temporomandibular disorder: a systematic review of randomised controlled trialsJ Oral Rehabil2015421184786126059857
- WeerapongPHumePAKoltGSThe mechanisms of massage and effects on performance, muscle recovery and injury preventionSports Med200535323525615730338
- BishopMDTorres-CuecoRGayCWLluch-GirbésEBeneciukJMBialoskyJEWhat effect can manual therapy have on a patient’s pain experience?Pain Manag20155645546426401979
- VigotskyADBruhnsRPThe role of descending modulation in manual therapy and its analgesic implications: a narrative reviewPain Res Treat2015201529280526788367
- BartschTGoadsbyPJIncreased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura materBrain2003126Pt 81801181312821523
- ZafarHNordhEErikssonPOTemporal coordination between mandibular and head-neck movements during jaw opening-closing tasks in manArch Oral Biol200045867568210869479
- ErikssonPOZafarHNordhEConcomitant mandibular and head-neck movements during jaw opening-closing in manJ Oral Rehabil199825118598709846906
- La ToucheRParís-AlemanyAMannheimerJSDoes mobilization of the upper cervical spine affect pain sensitivity and autonomic nervous system function in patients with cervico-craniofacial pain? A randomized-controlled trialClin J Pain201329320521522874091
- GrossARKayTMKennedyCClinical practice guideline on the use of manipulation or mobilization in the treatment of adults with mechanical neck disordersMan Ther20027419320512419654
- AssendelftWJBouterLMKnipschildPGComplications of spinal manipulation: a comprehensive review of the literatureJ Fam Pract19964254754808642364
- GrossAMillerJD’SylvaJManipulation or mobilisation for neck painCochrane Database Syst Rev20101CD00424920091561
- CrockettDJForemanMEAldenLBlasbergBA comparison of treatment modes in the management of myofascial pain dysfunction syndromeBiofeedback Self Regul19861142792913607094
- NiemeläKKorpelaMRaustiaAYlöstaloPSipiläKEfficacy of stabilisation splint treatment on temporomandibular disordersJ Oral Rehabil2012391179980422809314
- MaloneyGEMehtaNForgioneAGZawawiKHAl-BadawiEADriscollSEEffect of a passive jaw motion device on pain and range of motion in TMD patients not responding to flat plane intraoral appliancesCranio2002201556611831346
- MagnussonTSyrénMTherapeutic jaw exercises and interocclusal appliance therapy: a comparison between two common treatments of temporomandibular disordersSwed Dent J1999231273710371003
- MichelottiASteenksMHFarellaMParisiniFCiminoRMartinaRThe additional value of a home physical therapy regimen versus patient education only for the treatment of myofascial pain of the jaw muscles: short-term results of a randomized clinical trialJ Orofac Pain200418211412515250431
- CraaneBDijkstraPUStappaertsKDe LaatAOne-year evaluation of the effect of physical therapy for masticatory muscle pain: a randomized controlled trialEur J Pain201216573774722337211
- RaustiaAMPohjolaRTAcupuncture compared with stomatognathic treatment for TMJ dysfunction – part III: effect of treatment on mobilityJ Prosthet Dent19865656166233464742
- LeeMHParkSJKimJSEffects of neck exercise on high-school students’ neck-shoulder postureJ Phys Ther Sci201325557157424259804
- GuptaBDAggarwalSGuptaBGuptaMGuptaNEffect of deep cervical flexor training vs. conventional isometric training on forward head posture, pain, neck disability index in dentists suffering from chronic neck painJ Clin Diagnostic Res201371022612264
- KimJYKwagKIClinical effects of deep cervical flexor muscle activation in patients with chronic neck painJ Phys Ther Sci201628126927326957772
- MillerJGrossAD’SylvaJManual therapy and exercise for neck pain: a systematic reviewMan Ther201015433435420593537
- TakaiYYamamoto-MitaniNAbeYSuzukiMLiterature review of pain management for people with chronic painJpn J Nurs Sci201512316718325407249
- O’RiordanCCliffordAvan de VenPNelsonJChronic neck pain and exercise interventions: frequency, intensity, time, and type principleArch Phys Med Rehabil201495477078324333741
- GeneenLJMooreRAClarkeCMartinDColvinLASmithBHPhysical activity and exercise for chronic pain in adults: an overview of Cochrane reviewsCochrane Database Syst Rev20174CD01127928436583
- RhodesREJanssenIBredinSSWarburtonDEBaumanAPhysical activity: health impact, prevalence, correlates and interventionsPsychol Health201732894297528554222
- Fernández-CarneroJLa ToucheROrtega-SantiagoRShort-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disordersJ Orofac Pain20102411061220213036
- González-IglesiasJClelandJANetoFHallTFernández-de-las-PeñasCMobilization with movement, thoracic spine manipulation, and dry needling for the management of temporomandibular disorder: a prospective case seriesPhysiother Theory Pract201329858659523687913
- Gonzalez-PerezLMInfante-CossioPGranados-NunezMUrresti-LopezFJLopez-MartosRRuiz-Canela-MendezPDeep dry needling of trigger points located in the lateral pterygoid muscle: efficacy and safety of treatment for management of myofascial pain and temporomandibular dysfunctionMed Oral Patol Oral Cir Bucal2015203e326e33325662558
- Blasco-BonoraPMMartín-Pintado-ZugastiAEffects of myofascial trigger point dry needling in patients with sleep bruxism and temporomandibular disorders: a prospective case seriesAcupunct Med2017351697427697769
- SimonsDGTravellJGMyofascial Pain and Dysfunction: The Trigger Point Manual1BaltimoreWilliams & Wilkins1999
- VenâncioRAAlencarFGZamperiniCDifferent substances and dry-needling injections in patients with myofascial pain and headachesCranio20082629610318468269
- JungAShinBCLeeMSSimHErnstEAcupuncture for treating temporomandibular joint disorders: a systematic review and meta-analysis of randomized, sham-controlled trialsJ Dent201139534135021354460
- WuJYZhangCXuYPAcupuncture therapy in the management of the clinical outcomes for temporomandibular disordersMedicine (Baltimore)2017969e606428248862
- ChengRSPomeranzBElectroacupuncture analgesia could be mediated by at least two pain-relieving mechanisms; endorphin and non-endorphin systemsLife Sci1979252319571962160969
- TsaiHYLinJGInokiRFurther evidence for possible analgesic mechanism of electroacupuncture: effects on neuropeptides and serotonergic neurons in rat spinal cordJpn J Pharmacol19894921811852471859
- HanZJiangYHWanYWangYChangJKHanJSEndomorphin-1 mediates 2 Hz but not 100 Hz electroacupuncture analgesia in the ratNeurosci Lett19992742757810553941
- MendelsonGThe possible role of enkephalin in the mechanism of acupuncture analgesia in manMed Hypotheses197734144145895589
- PomeranzBChiuDNaloxone blockade of acupuncture analgesia: endorphin implicatedLife Sci1976191117571762187888
- LuJShaoRHHuLTuYGuoJYPotential antiinflammatory effects of acupuncture in a chronic stress model of depression in ratsNeurosci Lett2016618313826921452
- KavoussiBRossBEThe neuroimmune basis of anti-inflammatory acupunctureIntegr Cancer Ther20076325125717761638
- La ToucheRAngulo-Díaz-ParreñoSde-la-HozJLEffectiveness of Acupuncture in the treatment of temporomandibular disorders of muscular origin: a systematic review of the last decadeJ Altern Complement Med201016110711220038262
- La ToucheRGoddardGDe-La-HozJLAcupuncture in the treatment of pain in temporomandibular disorders: a systematic review and meta-analysis of randomized controlled trialsClin J Pain201026654155020551730
- GoddardGKaribeHMcNeillCVillafuerteEAcupuncture and sham acupuncture reduce muscle pain in myofascial pain patientsJ Orofac Pain2002161717611889662
- SekidoRIshimaruKSakitaMDifferences of electroacupuncture-induced analgesic effect in normal and inflammatory conditions in ratsAm J Chin Med200331695596514992547
- DıraçoğluDVuralMKaranAAksoyCEffectiveness of dry needling for the treatment of temporomandibular myofascial pain: a double-blind, randomized, placebo controlled studyJ Back Musculoskelet Rehabil201225428529023220812
- ChenJHuangZGeMGaoMEfficacy of low-level laser therapy in the treatment of TMDs: a meta-analysis of 14 randomised controlled trialsJ Oral Rehabil201542429129925491183
- SakuraiYYamaguchiMAbikoYInhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblastsEur J Oral Sci20001081293410706474
- AggarwalVRLovellKPetersSJavidiHJoughinAGoldthorpeJPsychosocial interventions for the management of chronic orofacial painCochrane Database Syst Rev2011911CD00845622071849
- LiuHXLiangQJXiaoPJiaoHXGaoYAhmetjiangAThe effectiveness of cognitive-behavioural therapy for temporomandibular disorders: a systematic reviewJ Oral Rehabil2012391556221827522
- KotirantaUSuvinenTForssellHTailored treatments in temporomandibular disorders: where are we now? A systematic qualitative literature reviewJ Oral Facial Pain Headache2014281283724482785
- RandhawaKBohayRCôtéPThe effectiveness of noninvasive interventions for temporomandibular disordersClin J Pain201632326027825924094
- MichelottiAIodiceGVollaroSSteenksMHFarellaMEvaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw musclesJ Am Dent Assoc20121431475322207667
- WrightEFDomenechMAFischerJRUsefulness of posture training for patients with temporomandibular disordersJ Am Dent Assoc2000131220221010680388
- LouwAZimneyKPuenteduraEJDienerIThe efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literaturePhysiother Theory Pract201632533235527351541
- ZhangYMontoyaLEbrahimSHypnosis/relaxation therapy for temporomandibular disorders: a systematic review and meta-analysis of randomized controlled trialsJ Oral Facial Pain Headache201529211512525905529
- HarperDESchrepfAClauwDJPain mechanisms and centralized pain in temporomandibular disordersJ Dent Res201695101102110827422858
- La ToucheRParis-AlemanyAHidalgo-PérezALópez-de-Uralde-VillanuevaIAngulo-Diaz-ParreñoSMuñoz-GarcíaDEvidence for central sensitization in patients with temporomandibular disorders: a systematic review and meta-analysis of observational studiesPain Pract Epub2017529
- JonesGTPsychosocial vulnerability and early life adversity as risk factors for central sensitivity syndromesCurr Rheumatol Rev201612214015326717947
- MansourARFarmerMABalikiMNApkarianAVChronic pain: the role of learning and brain plasticityRestor Neurol Neurosci201432112913923603439
- AdamsLMTurkDCPsychosocial factors and central sensitivity syndromesCurr Rheumatol Rev20151129610826088211
- SoaresGMRizzatti-BarbosaCMChronicity factors of temporomandibular disorders: a critical review of the literatureBraz Oral Res Epub2015113
- La ToucheRParis-AlemanyAGil-MartínezAPardo-MonteroJAngulo-Díaz-ParreñoSFernández-CarneroJMasticatory sensory-motor changes after an experimental chewing test influenced by pain catastrophizing and neck-pain-related disability in patients with headache attributed to temporomandibular disordersJ Headache Pain2015162025902781
- VellyAMLookJOCarlsonCThe effect of catastrophizing and depression on chronic pain: a prospective cohort study of temporomandibular muscle and joint pain disordersPain2011152102377238321871734
- FillingimRBOhrbachRGreenspanJDPsychological factors associated with development of TMD: the OPPERA prospective cohort studyJ Pain20131412 SupplT75T9024275225
- Gil-MartínezANavarro-FernándezGMangas-GuijarroMAComparison between chronic migraine and temporomandibular disorders in pain-related disability and fear-avoidance behaviorsPain Med201718112214222328575454
- Gil-MartínezAGrande-AlonsoMLópez-de-Uralde-VillanuevaILópez-LópezAFernández-CarneroJLa ToucheRChronic temporomandibular disorders: disability, pain intensity and fear of movementJ Headache Pain201617110327812883
- GalliUEttlinDAPallaSEhlertUGaabJDo illness perceptions predict pain-related disability and mood in chronic orofacial pain patients? A 6-month follow-up studyEur J Pain201014555055819875320
- SuNLobbezooFvan WijkAvan der HeijdenGJVisscherCMAssociations of pain intensity and pain-related disability with psychological and socio-demographic factors in patients with temporomandibular disorders: a cross-sectional study at a specialised dental clinicJ Oral Rehabil201744318719628036120
- LuoXPietrobonRSunSXLiuGGHeyLEstimates and patterns of direct health care expenditures among individuals with back pain in the United StatesSpine (Phila Pa 1976)2004291798614699281
- DıraçoğluDYıldırımNKSaralİTemporomandibular dysfunction and risk factors for anxiety and depressionJ Back Musculoskelet Rehabil201629348749126519118
- BristerHTurnerJAAaronLAManclLSelf-efficacy is associated with pain, functioning, and coping in patients with chronic temporomandibular disorder painJ Orofac Pain200620211512416708829
- TurnerJAWhitneyCDworkinSFMassothDWilsonLDo changes in patient beliefs and coping strategies predict temporomandibular disorder treatment outcomes?Clin J Pain19951131771888535036
- FillingimRBOhrbachRGreenspanJDPotential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control studyJ Pain20111211 SupplT46T6022074752
- RileyJLMyersCDCurrieTPSelf-care behaviors associated with myofascial temporomandibular disorder painJ Orofac Pain200721319420217717958
- GrossiMLGoldbergMBLockerDTenenbaumHCReduced neuropsychologic measures as predictors of treatment outcome in patients with temporomandibular disordersJ Orofac Pain200115432933912400401
- BairEOhrbachRFillingimRBMultivariable modeling of phenotypic risk factors for first-onset TMD: the OPPERA prospective cohort studyJ Pain20131412T102T11524275218
- ReidKIGreeneCSDiagnosis and treatment of temporomandibular disorders: an ethical analysis of current practicesJ Oral Rehabil201340754656123691977
- KlasserGDGreeneCSThe changing field of temporomandibular disorders: what dentists need to knowJ Can Dent Assoc2009751495319239744
- PallaSBiopsychosocial pain model crippled?J Orofac Pain201125428929022247923
- CarlsonCRPsychological considerations for chronic orofacial painOral Maxillofac Surg Clin North Am200820218519518343324
- LundJPDongaRWidmerCGStohlerCSThe pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activityCan J Physiol Pharmacol19916956836941863921
- FeuersteinMBeattiePBiobehavioral factors affecting pain and disability in low back pain: mechanisms and assessmentPhys Ther19957542672807899485
- SterlingMJullGWrightAThe effect of musculoskeletal pain on motor activity and controlJ Pain20012313514514622823
- CoteJNBementMKUpdate on the relation between pain and movement: consequences for clinical practiceClin J Pain201026975476220664335
- Le PeraDGraven-NielsenTValerianiMInhibition of motor system excitability at cortical and spinal level by tonic muscle painClin Neurophysiol200111291633164111514246
- KorotkovALjubisavljevicMThunbergJChanges in human regional cerebral blood flow following hypertonic saline induced experimental muscle pain: a positron emission tomography studyNeurosci Lett2002335211912312459513
- MaihöfnerCBaronRDeColRThe motor system shows adaptive changes in complex regional pain syndromeBrain2007130Pt 102671268717575278
- VallenceAMSmithATaborARolanPERiddingMCChronic tension-type headache is associated with impaired motor learningCephalalgia201333121048105423598373
- Weissman-FogelIMoayediMTenenbaumHCGoldbergMBFreemanBVDavisKDAbnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasksPain2011152238439621167644
- SalomonsTVMoayediMWeissman-FogelIPerceived helplessness is associated with individual differences in the central motor output systemEur J Neurosci20123591481148722564074
- TuckerKLarssonAKOknelidSHodgesPSimilar alteration of motor unit recruitment strategies during the anticipation and experience of painPain2012153363664322209423
- MoseleyGLNicholasMKHodgesPWDoes anticipation of back pain predispose to back trouble?Brain2004127Pt 102339234715282214
- KeefeFBlockADevelopment of an observational method for assessing pain behavior in chronic pain patientsBehav Ther198213363375
- PrkachinKCraigKExpressing pain: the communication and interpretation of pain signalsJ Nonverbal Behav199519191192
- SullivanMJToward a biopsychomotor conceptualization of pain: implications for research and interventionClin J Pain200824428129018427226
- VerbuntJASiebenJMSeelenHADecline in physical activity, disability and pain-related fear in sub-acute low back painEur J Pain20059441742515979022
- LeeuwMGoossensMELintonSJCrombezGBoersmaKVlaeyenJWThe fear-avoidance model of musculoskeletal pain: current state of scientific evidenceJ Behav Med2007301779417180640
- BahatHSWeissPLSprecherEKrasovskyALauferYDo neck kinematics correlate with pain intensity, neck disability or with fear of motion?Man Ther201419325225824291364
- GeisserMEHaigAJWallbomASWiggertEAPain-related fear, lumbar flexion, and dynamic EMG among persons with chronic musculoskeletal low back painClin J Pain2004202616914770044
- GeorgeSZFritzJMMcNeilDWFear-avoidance beliefs as measured by the fear-avoidance beliefs questionnaire: change in fear-avoidance beliefs questionnaire is predictive of change in self-report of disability and pain intensity for patients with acute low back painClin J Pain200622219720316428956
- ThomasJSFranceCRPain-related fear is associated with avoidance of spinal motion during recovery from low back painSpine (Phila Pa 1976)20073216E460E46617632385
- VlaeyenJWLintonSJFear-avoidance and its consequences in chronic musculoskeletal pain: a state of the artPain200085331733210781906
- Van DammeSKindermansHA self-regulation perspective on avoidance and persistence behavior in chronic pain: new theories, new challenges?Clin J Pain201531211512224662496
- SimmondsMJMoseleyGLVlaeyenJWPain, mind, and movement: an expanded, updated, and integrated conceptualizationClin J Pain200824427928018427225
- SullivanMJRodgersWMWilsonPMBellGJMurrayTCFraserSNAn experimental investigation of the relation between catastrophizing and activity intolerancePain20021001–2475312435458
- AsghariANicholasMKPain self-efficacy beliefs and pain behaviour: a prospective studyPain20019418510011576748
- SardáJNicholasMKAsghariAPimentaCAThe contribution of self-efficacy and depression to disability and work status in chronic pain patients: a comparison between Australian and Brazilian samplesEur J Pain200913218919518448371
- LuomajokiHKoolJde BruinEDAiraksinenOImprovement in low back movement control, decreased pain and disability, resulting from specific exercise interventionSports Med Arthrosc Rehabil Ther Technol201021120416091
- KoltynKFExercise-induced hypoalgesia and intensity of exerciseSports Med200232847748712076175
- KoltynKFAnalgesia following exercise: a reviewSports Med2000292859810701712
- KoltynKFBrellenthinAGCookDBSehgalNHillardCMechanisms of exercise-induced hypoalgesiaJ Pain201415121294130425261342
- AmiriSABandpeiMAJavanshirKRezasoltaniABiglarianAThe effect of different exercise programs on size and function of deep cervical flexor muscles in patients with chronic nonspecific neck painAm J Phys Med Rehabil201796858258828225440
- BertozziLGardenghiITuroniFEffect of therapeutic exercise on pain and disability in the management of chronic nonspecific neck pain: systematic review and meta-analysis of randomized trialsPhys Ther20139381026103623559524
- SaragiottoBTMaherCGYamatoTPMotor control exercise for chronic non-specific low-back painCochrane Database Syst Rev20161CD01200426742533
- ShireARStæhrTAOverbyJBDahlMBJacobsenJSChristiansenDHSpecific or general exercise strategy for subacromial impingement syndrome: does it matter? A systematic literature review and meta analysisBMC Musculoskelet Disord201718115810.1186/s12891-017-1518-028416022
- Gil-MartínezAKindelan-CalvoPAgudo-CarmonaDMuñoz-PlataRLópez-de-Uralde-VillanuevaILa ToucheRTherapeutic exercise as treatment for migraine and tension-type headaches: a systematic review of randomised clinical trialsRev Neurol20135710433443 Spanish24203665
- López-de-Uralde-VillanuevaIMuñoz-GarcíaDGil-MartínezAA systematic review and meta-analysis on the effectiveness of graded activity and graded exposure for chronic nonspecific low back painPain Med2015171172188
- de FreitasRFFerreiraMABarbosaGACalderonPSCounselling and self-management therapies for temporomandibular disorders: a systematic reviewJ Oral Rehabil2013401186487424102692
