Abstract
Treatment of trigeminal neuralgia (TN) is achieved by using adjuvant analgesics like antiepileptics, with carbamazepine (CBZ) being the first-line approach for TN patients, although side effects may be present. Other approaches using gabapentin, namely when associated with peripheral analgesic block of TN trigger points with the local anesthetic ropivacaine (ROP), resulted in decreased pain and daily drug intake (reduced side effects). This study evaluates if the association between CBZ and the peripheral block with ROP reinforces the clinical value of CBZ. In this parallel, double-blinded study, idiopathic TN patients were randomized to receive during 4 weeks either CBZ (CBZ; n = 21) or CBZ associated with the peripheral analgesic block using ROP (CBZ + ROP; n = 24). The primary outcome measures were the following: i) pain intensity, evaluated by the numerical rating scale; ii) number of pain crises; and iii) number needed to treat. Evaluation points were at the beginning (day 1) and end (day 29) of treatment and after a follow-up of 5 months (month 6). Both protocols resulted in a decrease of pain intensity and number of pain crises, but only the association CBZ + ROP showed i) a significant stronger reduction in pain intensity at month 6 and ii) a significant decrease in the daily dose of CBZ given to patients (both at day 29 and month 6). In contrast, the daily dose in CBZ-only patients remained constant or even increased. The number needed to treat for the association CBZ + ROP over the CBZ protocol reduced from 5 at the end of the 4-week treatment to 3 after the 5-month follow-up. Data reinforce the use of CBZ as a primary tool to control pain in TN patients, as the association CBZ + ROP i) improves the clinical qualities of CBZ, ii) strongly reduces the daily dose of CBZ, and iii) reduces the potential side effects attributed to high doses of CBZ.
Introduction
Neuropathic pain is a form of pain caused by a lesion or disease of the peripheral or central nervous system.Citation1,Citation2 It is a challenging condition to treat because of the following reasons: i) the heterogeneity of etiologies, symptoms, and underlying mechanisms; ii) poor response to conventional analgesics; and iii) the tendency for treatment being performed in a uniform fashion across the patient population.Citation3 Trigeminal neuralgia (TN) (annual incidence of 4–5/100,000)Citation4 is a type of neuropathy characterized by periods of intense paroxystic pain, usually of short duration and triggered by innocuous stimuli, although resulting in excruciating pain.Citation5,Citation6 A large number of cases of TN are idiopathic (primary or asymptomatic TN), usually with no detectable structural nerve lesion (includes the potential vascular compression of the fifth nerve in 15% of these patients) and a normal neurological evaluation.Citation7,Citation8 Like in other neuropathies, classic analgesics most frequently have no beneficial effects in controlling TN pain, even in secondary (symptomatic) TN, when it is associated with identifiable structural lesions, like a tumor or multiple sclerosis. Treatment is achieved by using adjuvant analgesics like antiepileptics (AE) and antidepressives.Citation6,Citation8 Contrary to other neuropathies, which use gabapentin as first-line treatment,Citation1,Citation9,Citation10 the AE carbamazepine (CBZ) has for a long time been and still is considered the first-line pharmacological approach for TN patients.Citation5–Citation8,Citation11,Citation12 Several drawbacks are associated with CBZ intake. It produces a toxic epoxide metabolite and regular blood tests are thus recommended; it is also associated with 10% incidence of rashes, has a negative effect on bone density, may induce abnormal liver function, may result in interstitial pneumonitis, and presents significant interactions with other drug classes.Citation3,Citation6,Citation8,Citation12,Citation13 Oxcarbazepine may be used in TN patients unresponsive to CBZCitation6,Citation14 and, as second-line drugs, baclofen, lamotrigine,Citation8 and pregabalinCitation15/gabapentinCitation12,Citation16–Citation21 are at front line.
In cases of CBZ intolerance, hypersensitivity, drug interactions or a narrower therapeutic index, and a higher degree of adverse side effects, gabapentin can be used as a second-line treatment.Citation12,Citation16–Citation21 Recently, a combination of different drugs have been used to treat TN.Citation7 When gabapentin is associated with the peripheral analgesic block of TN trigger points with the local anesthetic ropivacaine (ROP), the result is a significant decrease of pain intensity scores, number of paroxystic pain crises, and daily drug intake.Citation21 As a consequence of smaller gabapentin doses during the combination gabapentin + ROP, a reduction of adverse side effects is obtained when compared with gabapentin in monotherapy, the latter presenting already a much lighter pattern of side effects than CBZ in monotherapy.Citation12 Finally, one main objective of the clinical approach to TN, the functional capacity for the patients, was shown to be significantly improved when associating the oral intake of gabapentin with the peripheral block of TN trigger points with ROP.Citation21 Following these data,Citation21 the objective of the present study is to evaluate if a similar association between CBZ and the peripheral analgesia of TN trigger points with ROP reinforces the clinical value of CBZ as major therapy for TN, by reducing pain intensity scores, daily drug doses, and adverse side effects.
Methods
The methodology followed in the present study is reported as possible to the recent recommendations of the CONSORT group for improving the quality of reports of parallel-group randomized trials.Citation22
Patients – inclusion and exclusion criteria
Out of 48 patients with idiopathic TN and uncontrolled pain arriving at the Fafe Pain Unit of the Hospital Center of Alto Ave (Fafe, Portugal), 45 were randomly treated during 4 weeks with the traditional approach of CBZ in monotherapy (CBZ protocol; n = 21) or with the new protocol CBZ associated with the peripheral analgesic block of TN trigger points with ROP (CBZ + ROP protocol; n = 24) (). Two patients were excluded before allocation due to the presence of multiple sclerosis, and one patient allocated to CBZ protocol was excluded at day 1 due to an allergic reaction to CBZ (). Patients were eligible for the study if they presented a pain intensity measured by the numerical rating scale (NRS) with a score ≥6 (from a 0–10 scale) and met the consensus criteria for the diagnosis of primary (idiopathic) TN.Citation6,Citation7,Citation23 The inclusion criteria were the following: i) occurrence of episodes of facial paroxysmal pain in territory innervated by one or more branches of the trigeminal nerve (NRS score ≥6); ii) trigger areas (if touched lightly, will provoke an episode of pain);Citation19,Citation21 iii) normal neurological examination; iv) normal neuroimaging analysis following a CT scan or MRI; and v) symptoms not attributed to another disorder.
Figure 1 Flowchart of the steps followed by TN patients along the experimental design of the study. Note that out of 48 TN patients who were assessed to participate in this study, 2 were excluded before allocation owing to exclusion criteria, and 1 CBZ patient was excluded during day 1.
On the other hand, the following exclusion criteria were also considered: i) patients refuse to participate; ii) psychological instability (clinical depressive condition); iii) atypical pain location (eg, no specific trigger points); iv) secondary (symptomatic) TNCitation6 (multiple sclerosis, temporomandibular joint disorders, and neoplasms); v) altered neurological profile (hypoesthesia, dysesthesia, anesthesia, and paresis); vi) association with other cranial nerve neuralgias (eg, glossopharyngeal neuralgia); vii) imagiological alterations (neoplasms, abnormal vasculature, or intracranial pathology) observed in CT scan or MRI; and viii) proposed surgical intervention (compression of the Gasser ganglion confirmed by imagiology; preference of the patient in cases of uncontrolled pain and adverse side effects).
The therapeutic protocols used were accepted by the Hospital Ethical Committee and the patients were informed that i) they were going to be submitted to one of two different treatment protocols to solve their pain problem and ii) they could drop or change treatment if no pain control was achieved. All patients signed an informed consent.
Random allocation and treatment protocols
The 45 TN patients entering the study were the first arriving to the Chronic Pain Unit and fulfilling the inclusion criteria. There were 2 days in the week (Monday and Thursday) for pain consult at the unit, with those patients arriving in the first day being assigned to one treatment group and those arriving in the second day of the week being attributed to the second therapeutic protocol. Thus, there was no sequential attribution of protocols CBZ or CBZ + ROP, with patient random allocation being solely dependent on the day of presentation at the Pain Unit. Patients were recruited between January 2006 and October 2008. None of the patients included in the present study had participated in the previous TN study of our group.Citation21 Patients were allocated to one of the following treatment protocols (): i) protocol CBZ + ROP and ii) protocol CBZ.
Protocol CBZ + ROP
Treatment using CBZ given orally plus ROP administered superficially at TN facial trigger points. These were pointed by the patients as the exact area of the face that usually induces pain when touched. The peripheral analgesic block with ROP was performed at the Pain Unit under sterile conditions, using a 27-gauge needle for administering subcutaneously 2 mL of a 2 mg/mL ROP solutionCitation21,Citation24 in each trigger point. Each local block was performed once a weekCitation21,Citation25 during the 1 month treatment (days 1, 8, 15, 22, and 29). When patients arrived to the Pain Unit (day 1) who had been referred from other health institutions, they presented uncontrolled pain under a CBZ dose of 400–1000 mg/day. From the first day, the CBZ dose taken by each patient could increase gradually until 1200 mg/day if the pain intensity reached or kept an NRS score ≥6, or gradually reduced if pain control was regained. Every 7 days, during their visit to the Pain Unit, the NRS score of the patient was recorded and CBZ dose adjusted if necessary, each alteration being performed in steps of 200 mg/day.Citation6
Protocol CBZ
This treatment is done using only CBZ in monotherapy. Patients entering this protocol received additionally a control injection of saline (the vehicle of ROP administered in the other protocol, CBZ + ROP) at facial trigger points, every 7 days of treatment (days 1, 8, 15, 22, and 29). Although the usual CBZ effective dosage ranges between 400–600 and 1000–1200 mg/day,Citation6,Citation26 when patients with uncontrolled pain referred from other health institutions arrived at the Fafe Pain Unit (day 1), their CBZ dose (whatever it was) was increased by 200 mg/day;Citation6 thus, no titulation of the drug was performed in order to avoid clinical instability of patients and ethical issues. Every 7 days, during their visit to the Pain Unit, the NRS score of the patients was recorded and CBZ dose adjusted if necessary.
Experimental sequence
During the 29 day treatment,Citation21 all patients were evaluated by the hospital staff at day 1 and then periodically at days 8, 15, 22, and 29 (1-month treatment). During the periods between days 2 and 7, 9 and 14, 16 and 21, and 23 and 28, patients were at home and were requested to record their NRS pain intensity score in an individual pain diary provided by the staff plus the CBZ dose, the hour when medication was taken, and side effects observed.
Paracetamol (acetaminophen) was used in this study for breakthrough pain in those cases where patients needed pain control between CBZ doses, or if the medication prescribed in the protocol was not having an analgesic effect. They were instructed to take it as needed every 8 hours with a maximum of 3000 mg/day, in order to avoid a potentiation of the toxic effect of CBZ at the hepatic level.
After the 1-month period of treatment, patients from both protocols were requested to continue their treatment at home, using the same CBZ dose used at day 29. If patients during the 5-month follow-up experienced a new pain episode, they were instructed to return to the Pain Unit for evaluation and readjustment of the treatment or, alternatively, they were provided with the most adequate conventional treatment.
Double-blinded study
This study was blinded to both the authors and patients. In what concerns the authors, firstly, the application of each protocol treatment to the patients was performed by a researcher who was blinded i) to the content of the peripheral injections (saline or ROP), which were prepared by another member of the Pain Unit; ii) to the NRS scores evaluation of pain intensity; and iii) to the number of daily pain crisis of each patient. Secondly, NRS scores and number of pain crisis were evaluated by a second researcher, who was blinded to the protocol assigned to each patient. Thirdly, the statistical evaluation of the data was performed by a third researcher, who was not a health service professional and was not aware of the clinical implications of protocols CBZ and CBZ + ROP. All these precautions resulted in a study blinded to the authors. In what concerns the patients, as already stated for the informed consent of the patients, they were not aware of which protocol was being applied to them, as all of them were taking i) oral CBZ and ii) an injection (saline or ROP). In a previous study,Citation21 a third group of patients has been submitted to a protocol of ROP only (which implied absence of a blinded study to the patients) besides a gabapentin monotherapy group and a gabapentin + ROP protocol. However, the clinical insecurity of a ROPonly protocolCitation21 resulted, in the present study, in the evaluation of only two protocols, which allowed a study blinded also to the patients: CBZ monotherapy (with injection of saline) and CBZ + ROP (with injection of ROP).
Primary outcome measures
The predef ined primary outcome measures were the following:
Evaluation of pain intensity using the NRS scale: Evaluation points for statistical analysis were at the arrival to the Pain Unit (day 1), at the end of the treatment (day 29), and after a follow-up of 5 months (month 6). Patients classified their pain between 0 (no pain) and 10 (the worst pain imaginable). The number corresponding to the pain felt was chosen by each patient in the interview with the nurse. The evaluation of pain intensity at days 1 and 29 was performed in the Pain Unit, whereas at month 6 it was performed during a phone interview (see the following paragraph). A pain reduction of 2 points in the NRS scale when compared with the baseline pain score (day 1) was considered clinically significant.Citation27–Citation31
Daily number of paroxysmal pain episodes: Only data obtained at day 1 and 5 months after the end of the treatment (follow-up; month 6) were used for statistical analysis. The follow-up evaluation was performed at the end of the day of performing month 6, during a phone interview to each patient, who was asked i) how many pain attacks he or she had suffered during that day (and the pain intensity in the NRS scale) or, in case of no pain, ii) how many pain crises he or she had suffered in the worst day of the last week before interview (and the highest pain intensity in the NRS scale). If no pain was recorded following these two questions, the staff recorded 0 (zero) crisis for the patient at month 6 (and 0 in the NRS scale).
Number needed to treat (NNT): The NNT is an estimate of the number of patients that would need to be given a treatment for one of them to achieve a desired outcome.Citation31,Citation32 Following the rationale of a previous study,Citation21 we compared the therapeutic result between a new proposed therapy (GBP + ROP protocol) and a conventional treatment (CBZ protocol), as suggested by Altman.Citation33 This allows a comparison of efficacy between the two clinical treatments.Citation33 Accordingly, in the present study, NNT is defined as 1/[the proportion of patients successfully treated with CBZ + ROP (with at least 50% pain relief) – the proportion of patients successfully treated with the standard CBZ monotherapy], as expressed in the equation below. The NNT of protocol CBZ + ROP over protocol CBZ was determined for day 29 (comparing with the baseline values at day 1) and month 6 (comparing with baseline values at day 29). The 95% confidence interval (CI) for each NNT result was obtained using the free calculator at the site of the University of Manchester: www.phsim.man.ac.uk/nnt/
Secondary outcome measure
A secondary outcome measure of this study was the evolution of daily dosage of CBZ following the 1-month treatment under protocols CBZ + ROP and CBZ (day 1 and day 29) and after a follow-up of 5 months (month 6). Changes in the daily dose of CBZ would reflect the necessity for pain control and the potential presence of adverse side effects.
Statistics
Data are presented as average ± standard deviation (SD) along the several variables under study. The chi-square test was used to compare the distribution of the cases by treatment versus age, sex, pain location, and facial side. The normal distribution of the results was verified using the Kolmogorov–Smirnov test, whereas the equality of variances was evaluated by the Levene’s test. A comparison of the means of NRS values, number of pain crises, and CBZ dosages of protocols CBZ + ROP and CBZ was performed at each statistical evaluation point (day 1, day 29, month 6 for NRS and CBZ dosage data; day 1 and month 6 for the number of pain crisis) using the Student’s t-test for equality of means.
Results
Patient baseline characteristics
Out of 48 patients assessed for eligibility, 46 patients were randomly allocated to one of the two therapeutical protocols (). Two patients were excluded because they had multiple sclerosis. Twenty-four were assigned to protocol CBZ + ROP and 22 to protocol CBZ. However, one CBZ patient had to be excluded at day 1 due to an allergic reaction to CBZ. Thus, only 21 patients followed the CBZ protocol (; ). summarizes the flow of patients throughout the experimental protocol of this study. The baseline data for the demographic characteristics of patients selected for both protocols, their comorbidities, and their concomitant drug therapies are given in . No statistical significant differences were present between CBZ + ROP and CBZ groups of patients in what concerns age, sex, pain location (number of trigeminal branches affected), and facial side affected (P > 0.05 in all comparisons), showing that similar sets of patients were randomly allocated to both treatments. No differences were also observed in the percentage of CBZ + ROP and CBZ patients without any known comorbidity (42% and 33%, respectively) and in the percentage of those presenting hypertension, the most frequent comorbidity (42% and 38%) observed in these TN patients (). Occasional cases occurred of diabetes mellitus type 2, stroke, epilepsy, depression, and cardiac or thyroid pathology. In what concerns concomitant drug therapies, the most common drugs used in addition to CBZ + ROP/CBZ were antihypertensors (18 of the 45 TN patients): three CBZ patients were taking anticoagulants, two antiarrhythmics or antihypothyroidism drugs, and one another anticonvulsivant, whereas 1 CBZ + ROP patient was taking an antidepressant ().
Table 1 Baseline characteristics of TN patients
Effect of CBZ + ROP and CBZ protocols in pain control
No differences were found between patients from protocol CBZ + ROP (NRS1 = 9.1 ± 1.1) and protocol CBZ (NRS1 = 9.1 ± 1.4) (P = 0.887) (; ) in pain intensity at the beginning of the treatment (day 1). This result reinforces the homogeneity of the participants and the similarity between patients allocated to the two protocols. At the end of the treatment (day 29), both protocols significantly reduced pain intensity (CBZ + ROP(1–29), NRS difference = 6.3, P < 0.0001; CBZ(1–29), NRS difference = 5.2, P < 0.001), but CBZ + ROP therapy resulted in a significantly stronger pain reduction than patients following CBZ protocol (CBZ + ROP, NRS29 = 2.8 ± 0.8; CBZ, NRS29 = 3.8 ± 1.0; P < 0.001) (; ). After 5 months, both protocols significantly reduced pain intensity when compared with day 1 (CBZ + ROP(1–6m), NRS difference = 7.0; P < 0.0001; CBZ(1–6m), NRS difference = 5.1; P < 0.0001). However, significant differences were observed again between the two protocols, with CBZ + ROP inducing a significantly stronger reduction in pain intensity than CBZ alone (CBZ + ROP, NRS6m = 2.0 ± 0.7; CBZ, NRS6m = 3.9 ± 1.4; P < 0.0001) (; ).
Figure 2 Effect of the 2 protocols (CBZ + ROP and CBZ) on the pain intensity of patients at the end of the 4-week treatment (day 29) and after a 5-month follow-up (month 6). No baseline differences were observed in the pain intensity between patients allocated to the two protocols (day 1). Note that both after the 4-week treatment and the 5-month follow-up, CBZ + ROP patients showed a stronger pain reduction than CBZ-only patients. For statistical comparisons and significances, see the Results section.
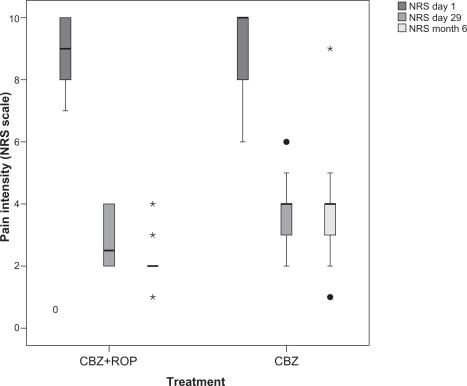
Table 2 Outcome results between groups and their statistical significance
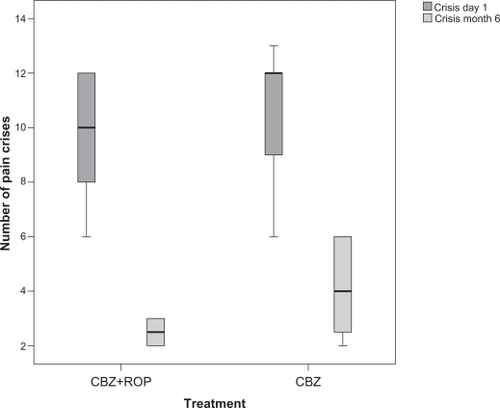
The baseline number of daily crises of paroxysmal sudden and intense pain was similar between patients of both protocols (day 1: CBZ + ROP, ncrises = 9.6 ± 2.3; CBZ, ncrises = 10.6 ± 2.2; P = 0.131) (; ). It was not possible to obtain data from the end of the 4-week treatment (day 29). After a follow-up of 5 months, both protocols reduced the number of daily crises (CBZ + ROP(1–6m), difference = 7.0; P < 0.0001; CBZ(1–6m), difference = 6.5; P < 0.0001), with patients treated with CBZ + ROP protocol showing a significantly stronger reduction than those under CBZ monotherapy (month 6: CBZ + ROP, ncrises = 2.5 ± 0.5; CBZ, ncrises = 4.1 ± 1.7; P < 0.0001) (; ).
Figure 3 Number of daily episodes of pain before (day 1) and after a 5-month follow-up (month 6) in patients submitted to CBZ + ROP and CBZ treatments. No baseline differences were observed in the number of pain crisis between patients allocated to the two protocols (day 1). After 6 months, CBZ + ROP patients showed a stronger reduction in the number of pain crises than those under CBZ protocol. For statistical comparisons and significances, see the Results section.
NNT
When comparing the clinical benefit obtained by CBZ + ROP, the NNT for the treatment associating CBZ + ROP over the CBZ protocol was 5.25 (95% CI: 2.48–27.95) at the end of the 4-week period of treatment (day 29), but reduced to 3.11 (95% CI: 1.84–15.33) after a follow-up of 5 months (month 6). Thus, 5 and 3 are the estimated number of patients (at day 29 and at month 6, respectively) who need to be treated with the new treatment (CBZ + ROP protocol) rather than the standard treatment (CBZ protocol) for one additional patient to benefit.Citation21,Citation33
CBZ daily dose
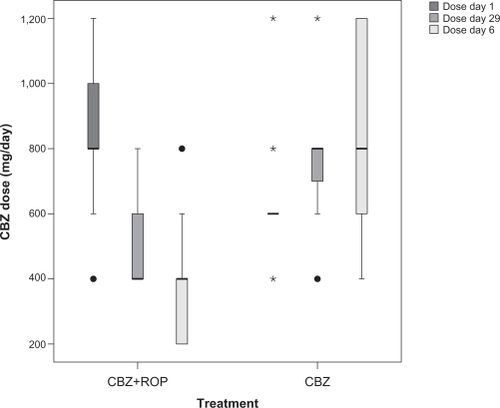
When arriving at the Pain Unit from other health center institutions (day 1), patients beginning the CBZ + ROP protocol were taking 836 ± 253 mg/day of CBZ, and patients starting the CBZ protocol were taking 626 ± 163 mg/day of CBZ (). At the end of the treatment, the CBZ daily dose has been significantly reduced in patients with CBZ + ROP protocol while CBZ intake even increased in patients submitted to CBZ monotherapy (day 29: CBZ + ROPdose = 525 ± 165 mg/day; CBZdose = 757 ± 200 mg/day; P < 0.0001) (; ). Finally, after the follow-up period, CBZ intake further reduced in CBZ + ROP protocol and resulted in a significantly lower final daily dose of CBZ than in patients following CBZ protocol (in this case, CBZ intake increased again) (month 6: CBZ + ROPdose = 367 ± 183 mg/day; CBZdose = 826 ± 291 mg/day; P < 0.0001) (; ).
Figure 4 Longitudinal evolution of the daily dose of CBZ taken during treatment (4-week period) protocols CBZ + ROP and CBZ. Therapy with CBZ + ROP clearly reduced successively the intake of CBZ from day 1 to day 29 and from the end of the treatment to month 6, whereas CBZ monotherapy resulted in a progressive increase of the daily dose of this antiepileptic. For statistical comparisons and significances, see the Results section.
Discussion
CBZ has been a for long time and is still considered the first-line pharmacological option for controlling pain in TN. However, CBZ often results in adverse side effects and intolerance, with these cases being solved by second-line AE or antidepressant drugs or, in case of prolonged intolerance, a surgical option. In order to improve the clinical outcome of CBZ therapy and reduce its unwanted effects, the present study evaluated the association of CBZ with the peripheral analgesic block of TN trigger points with the local anesthetic ROP. A similar approach has resulted in improved efficacy when using gabapentin in the treatment of TN.Citation21 The protocol associating CBZ + ROP resulted in a significant reduction in i) pain intensity, ii) the number of daily pain crises, and iii) the daily dose of CBZ intake, when compared with the traditional CBZ protocol in monotherapy.
Methodological considerations
The rationale of the present study was to further increase the efficacy of first-line drug CBZ in controlling TN pain and, not less important, to reduce potentially the impact of the adverse side effects associated with the therapeutic doses usually used.Citation12,Citation34,Citation35 In order to eliminate the possibility that any beneficial effect could depend on the physical action of local administration of the analgesic ROP solution by clearing adhesions or inflammatory molecules from the vicinity of the nerve,Citation25 the protocol CBZ-only was accompanied with injection of saline to TN trigger points. Thus, the improvements observed in the different outcomes analyzed resulted exclusively from the pharmacological action of CBZ + ROP and CBZ and not by the manipulation and liquid introduction at trigger points. An additional advantage of saline administration to CBZ-only patients was the possibility of performing a treatment blinded to both patients and researchers (double-blinded study for the treatment).
The frequency of ROP injections applied subcutaneously to TN patients respected the guidelines for the practice of interventional techniques.Citation25 A patient should receive an injection at intervals not smaller than 1 week, which was the period chosen to mediate between each ROP (or saline) administration. The follow-up evaluation of patients treated with both protocols (month 6) was performed by phone interview, as they were trained since the beginning of this study to classify their pain according to a 0–10 scale.
Clinical impact of the CBZ + ROP association
Because a 2-point decrease in the mean NRS scale (0–10 scale) is considered the minimum clinical relevant difference in pain intensity when comparing the effect of two treatments,Citation27–Citation31 both the CBZ + ROP and CBZ protocols were clinically effective in reducing pain after a 4-week therapy. In fact, they decreased pain intensity in 6.3 and 5.3 points (respectively) after the 4-week therapy. At the end of the treatment, the pain reduction obtained by CBZ + ROP was significantly stronger than that of CBZ. However, as NRS mean was only 1 point smaller after CBZ + ROP than CBZ (2.8 versus 3.8), it is questionable whether the statistical significant improvement of CBZ + ROP protocol reaches a really clinical importance.Citation31 After a 5-month follow-up, however, the significant reduction in pain intensity obtained by CBZ + ROP patients reached the border of a 2-point difference of clinical significance when compared with the reduction obtained by CBZ patients (NRS of 2.0 versus 3.9). Two aspects must be considered when approaching the discussion of these data. Firstly, from the end of the treatment to 5 months later, the pain intensity in CBZ + ROP patients showed a further decrease (2.8 → 2.0), whereas no changes were observed in CBZ-treated patients (3.8 → 3.9); secondly, the same authors claiming that a 2-point scale decrease is the minimum clinical benefit following a pain treatment for a ‘much better improvement’ also considered that a 1-point reduction in the NRS pain scale was felt as ‘slightly better,’Citation29 which can also be considered an improvement.Citation31 In fact, a 1-point reduction in pain intensity represented the minimal clinically important difference, as defended by the same authors. These data indicate that both at the end of the 4-week treatment (day 29) and after a follow-up 5 months later (month 6), the CBZ + ROP protocol reinforced the pain reduction resulting from the traditional CBZ-only protocol. This suggests that, as was previously demonstrated for the gabapentin and ROP association,Citation21 a potentiation or synergism between the AE and local analgesic effect occurs when CBZ and ROP are associated in the same protocol. Previous studies also indicated that combinations of CBZ + gabapentin or gabapentin + lamotrigineCitation18 can result in TN pain control.
When comparing CBZ + ROP protocol with the standard CBZ protocol, another indication of improvement in the clinical outcome is the NNT. The number of patients who must be treated by the CBZ + ROP protocol to generate one more success than would have resulted had all patients been given the comparison treatment (CBZ-only) was 5 after the 4-week treatment (day 29) and reduced to 3 after a 5-month follow-up (month 6). Again, this shows that data 5 months after the treatment are more robust in indicating an advantage of the combination of CBZ + ROP upon CBZ than immediately after the end of the treatment.
Another important therapeutical improvement in the combination of CBZ + ROP is the demonstration of a large decrease in the daily dose of CBZ intake, both at the end of the treatment (day 29) and, even further, after the 5-month follow-up. On the contrary, CBZ monotherapy observed a progressive increment in the daily CBZ dosage. The typical maintenance CBZ doses applied to TN patients seen in the literature range between 300–800 mg/day,Citation12 600–800 mg/day,Citation35 200–1200 mg/day,Citation7 400–1200 mg/day,Citation11 or 600–1200 mg/day.Citation6 In the present study, the CBZ + ROP proportioned pain control with a medium daily dose of 367 mg/day, which points clearly to the lower range of the clinical intervals referred to above; on the other hand, the CBZ monotherapy protocol resulted in a daily CBZ intake of around 800 mg/day, which is located in the middle/upper third of the typical dose range of CBZ applied to TN patients. These data show that the clinical result of TN treatment with CBZ + ROP is superior to CBZ monotherapy, because the much lower dose of CBZ needed following the CBZ + ROP protocol will decrease the potential presence/intensity of adverse side effects. The possibility of CBZ subtherapeutic treatment in the CBZ + ROP or CBZ-only protocols was excluded, as shown by the significant pain intensity decrease associated with these therapeutical approaches.
Potential mechanisms underlying the effect of CBZ + ROP association
CBZ is involved in i) the recruitment of endogenous descending antinociceptive mechanisms by inhibiting noradrenaline uptake (a mechanism in part related to the action of some antidepressants) and ii) in the suppression of spontaneous neuronal activity, stabilization of hyperexcited neural membranes, and/or reduction of propagation of synaptic impulses, due to CBZ modulation of voltage-gated sodium channels in a voltage- and frequency-dependent manner.Citation12,Citation36 Importantly, low-dose ROP has an analgesic action based, at least partly, on common mechanisms because both CBZ and ROP act on voltage-gated sodium channelsCitation37–Citation40 by reducing ectopic neuronal activity without blocking nerve conduction. Major causes of ectopic firing include patches of demyelination, which can be present in TN at the trigeminal root entry zone or in focal areas resulting from microvascular nerve compression of the trigeminal nerve;Citation12,Citation41–Citation43 the cellular mechanism that appears to underlie ectopic neuronal hyperexcitability is the remodeling of voltage-sensitive ion channels (including sodium channels), which are present at very low densities in the axonal membrane under myelin,Citation44 but largely accumulate at sites of nerve injury and demyelination.Citation45 Consequently, the ‘ignition hypothesis’ of TNCitation46 postulates that pain paroxysms begin with discharge in a small cluster of trigeminal nerve afferents upon cutaneous trigger point stimulation, which when crossed after discharge ‘ignites’ activity and the recruitment of passive uninjured neighboring neurons; the augmented activity ignites additional passive neuronal fibers; and the resulting positive feedback chain reaction triggers a paroxysmal pain crisis.Citation45 Thus, it is possible that the therapeutic value of the present CBZ + ROP combination for CBZ in monotherapy may result from additive (and synergistic) i) control of peripheral fiber depolarization at trigger points, ii) stabilization of uninjured passive neighboring neurons at the trigeminal ganglion (by both CBZ and ROP), and iii) increased action of noradrenaline at the synaptic cleft in the central nervous system (CBZ only).
Drug interactions of CBZ with concomitant drug therapies
Most patients with concomitant drug therapy were taking antihypertensors, namely angiotensin-converting enzyme inhibitors, which do not present known interactions with CBZ.Citation47–Citation49 CBZ may decrease serum concentrations of amiodarone, levothyroxine, and warfarin,Citation48,Citation49 but to the best of our knowledge, the reversal has not been reported. The only two patients (in a total of 45) under drug therapy that may increase CBZ serum concentration were those taking fluoxetine, which may inhibit the hepatic metabolism of CBZ, or sodium valproate, which may prolong the elimination half-life of CBZ epoxide.Citation48,Citation49 However, it should be reinforced that neither these two patients nor the other 43 of this study presented evident side effects. This must have been achieved by i) the intense pain felt by patients arriving at the Pain Unit, which may have masked any putative CBZ side effects, and ii) the low/medium doses of CBZ that our team managed to achieve for sufficient pain control in CBZ + ROP and CBZ patients, respectively. Only one patient allocated to CBZ protocol was excluded from the study at day 1 due to an allergic reaction to CBZ.
Limitations of the study
Some important limitations can be included in the present study. Firstly, the generalization of findings to all patients who do not tolerate drug therapy after CBZ should be made with caution because no comparisons were made with adverse effects of other drugs that can be an alternative to the main classic treatment. The exclusion criteria were extensive and 8.4% of TN patients arriving at the Pain Unit were withdrawn from the study, which indicates that the study should be confirmed in larger-scale (less homogeneous) studies. Secondly, although the effect of treatment on pain intensity and number of paroxysmal crises was still significantly different after 6 months of treatment with CBZ + ROP and CBZ, the follow-up period may not have been sufficient to determine the potentially long-term effects of the proposed treatment (unknown quiescent periods and pain-free intervals that can reach months or years). Thirdly, no plasma levels of CBZ were measured in order to correlate the oral dose given with that really present in circulation.
Conclusions
CBZ is established as the first-line drug choice for pain control in TN. However, when CBZ fails to reduce pain or the adverse side effects do not allow increasing CBZ dosage, second-line drugs like gabapentin may solve the problem. Recently, an improvement of this second alternative has been achieved by the association of gabapentin with the peripheral analgesic block of TN trigger points with ROP. The same approach has been the objective of the present study, in order to improve the clinical outcome of CBZ therapy. We demonstrate that the association of CBZ and peripheral administration of ROP (CBZ + ROP protocol) resulted in a clinically significant further improvement of the decrease in pain intensity already achieved by CBZ in monotherapy (CBZ protocol). This is accompanied with a clear decrease in the daily CBZ dosage needed for TN pain control, with a consequent potential reduction in the adverse side effects associated. Additionally, an NNT of 5 at the end of the treatment that reduces to 3 after a follow-up of 5 months indicates that in long-lasting treatments with CBZ, the advantages of its association with the peripheral block with ROP increase with time. However, large-scale CBZ + ROP studies are needed to evaluate the dimension of the improvement obtained by the association CBZ + ROP.
Acknowledgements
The authors thank the Clinical Director of the Hospital Center of Alto Ave (Fafe Unit), the Chronic Pain Unit team of the same Hospital, and Dr. Ramalho Fontes, the Director of the Department of Neurology of Hospital S. Marcos, Braga (2006–2008), for the collaboration in this study. A special acknowledgment is given to Fundação para a Ciência e Tecnologia (FCT), FEDER, and Project Nr. PTDC/SAU-NEU/108557/2008 for supporting the publication of this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- DworkinRHO’ConnorABAudetteJRecommendations for the pharmacological management of neuropathic pain: an overview and literature updateMayo Clin Proc201085Suppl 3S3S1420194146
- TreedeRDJensenTSCampbellJNNeuropathic pain: redefinition and a grading system for clinical and research purposesNeurology2008701630163518003941
- VadaloucaASiafakaIArgyraETherapeutic management of chronic neuropathic pain: an examination of pharmacologic treatmentAnn N Y Acad Sci2006108816418617192564
- KatusicSWilliamsDBBeardCMEpidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945–1984Neuroepidemiology1991102762811798430
- NurmikkoTJTrigeminal neuralgia and other facial neuralgiasCerveroFJensenTSHandbook of Clinical Neurology: Pain81 (3rd series).LondonElsevier2006574596
- CruccuGGronsethGAlksneJAAN-EFNS guidelines on trigeminal neuralgia managementEur J Neurol2008151013102818721143
- HickeyAHScrivaniSBajwaZCranial neuralgiasFishmanSMBallantyneJCRathmellJPBonica’s Management of Pain4th edLondonWolters Kluwer, Lippincott Williams & Wilkins2010953972
- GronsethGCruccuGAlksneJPractice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological SocietiesNeurology2008711183119018716236
- FinnerupNBOttoMMcQuayHJJensenTSSindrupSHAlgorithm for neuropathic pain treatment: as evidence based proposalPain200511828930516213659
- DworkinRHO’ConnorABBackonjaMPharmacologic management of neuropathic pain: evidence-based recommendationsPain200713223725117920770
- JornsTPZakrzewskaJMEvidence-based approach to the medical management of trigeminal neuralgiaBr J Neurosurg20072125326117612914
- CheshireWPTrigeminal neuralgia: for one nerve a multitude of treatmentsExpert Rev Neurother200771565157917997704
- NaritaHOzawaTNishiyamaTAn atypical case of fulminant interstitial pneumonitis induced by carbamazepineCurr Drug Saf20094303319149523
- Gomez-ArguellesJMDoradoRSepulvedaJMOxcarbazepine monotherapy in carbamazepine-unresponsive trigeminal neuralgiaJ Clin Neurosci20081551651918378142
- PérezCSaldañaMTNavarroATrigeminal neuralgia treated with pregabalin in family medicine settings: its effect on pain alleviation and cost reductionJ Clin Pharmacol20094958259019299534
- SistTFiladoraVMinerMGabapentin for idiopathic trigeminal neuralgia: report of two casesNeurology19974814679153497
- KhanOAGabapentin relieves trigeminal neuralgia in multiple sclerosis patientsNeurology1998516116149710050
- SolaroCMessmer-UccelliMUccelliALow-dose gabapentin combined with either lamotrigine or carbamazepine can be useful therapies for trigeminal neuralgia in multiple sclerosisEur Neurol200044454810894995
- CheshireWPDefining the role for gabapentin in the treatment of trigeminal neuralgia: a retrospective studyJ Pain2002313714214622800
- PandeyCKSinghNSinghPKGabapentin for refractory idiopathic trigeminal neuralgiaJ Indian Med Assoc200810612412518705259
- LemosLFloresSOliveiraPGabapentin supplemented with ropivacain block of trigger points improves pain control and quality of life in trigeminal neuralgia patients when compared with gabapentin aloneClin J Pain200824647518180639
- SchulzKFAltmanDGMoherDCONSORT 2010 statement: updated guidelines for reporting parallel group randomised trialsPLoS Med201073
- ZakrzewskaJMTrigeminal, eye, and ear painMelzackRWallPDHandbook of Pain Management: A Clinical Companion to Wall and Melzack’s Textbook of PainLondonChurchill Livingstone2003199215
- BreivikHLocal anesthetic blocks and epiduralsMcMahonSBKoltzenburgMMelzack’s Textbook of Pain5th edLondonElsevier-Churchill Livingstone2006903925
- ManchikantiLSinghVTrescotAMGuidelines for the practice of interventional techniquesBoswellMVColeBEWeiner’s Pain Management: A Practical Guide for Clinicians7th edLondonCRS Press2006847878
- AhmadMGouckeCRManagement strategies for the treatment of neuropathic pain in the elderlyDrugs Aging20021992994512495368
- FarrarJTPortenoyRKBerlinJADefining the clinically important difference in pain outcome measuresPain20008828729411068116
- FinnerupNBSindrupSHBachFWLamotrigine in spinal cord injury pain: a randomized controlled trialPain20029637538311973012
- SalaffiFStancatiASilvestriCAMinimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scaleEur J Pain2004828329115207508
- HanleyMAJensenMPEhdeDMClinically significant change in pain intensity ratings in persons with spinal cord injury or amputationClin J Pain200622253116340590
- DworkinRHTurkDCWyrwichKWInterpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendationsJ Pain2008910512118055266
- McQuayHJMooreAMethods of therapeutic trialsMcMahonSBKoltzenburgMMelzack’s Textbook of Pain5th edLondonElsevier-Churchill Livingstone2006415425
- AltmanDGConfidence intervals for the number needed to treatBMJ1998317130913129804726
- ZakrzewskaJMLopezBCTrigeminal and glossopharyngeal neuralgiaMcMahonSBKoltzenburgMMelzack’s Textbook of Pain5th edLondonElsevier-Churchill Livingstone200610011010
- CanaveroSBonicalziVDrug therapy of trigeminal neuralgiaExpert Rev Neurother2006642944016533146
- SangCNHayesKSAnticonvulsivant medications in neuropathic painMcMahonSKoltzenburgMWall and Melzack’s Textbook of Pain5th edLondonElsevier2006964976
- BurchielKJCarbamazepine inhibits spontaneous activity in experimental neuromasExp Neurol19881022492533181365
- DevorMWallPDCatalanNSystemic lidocaine silences ectopic neuroma and DRG discharge without blocking nerve conductionPain1992482612681589245
- LiuBGZhuangXLLiSTXuGHThe effects of ropivacaine on sodium currents in dorsal horn neurons of neonatal ratsAnesth Analg2000901034103810781449
- RowbothamMCPetersenKLAnticonvulsants and local anesthetic drugsLoeserJDButlerSHChapmanCRTurkDCBonica’s Management of Pain3rd edLondonLippincott Williams & Williams2001329341
- LoveSCoakhamHBTrigeminal neuralgia: pathology and pathogenesisBrain20011242347236011701590
- ArreseILagaresAAldayRTypical trigeminal neuralgia associated with brainstem white matter lesions on MRI in patients without criteria of multiple sclerosisActa Neurochir (Wien)20081501157116118958387
- PrasadSGalettaSTrigeminal neuralgia: historical notes and current conceptsNeurologist200915879419276786
- WaxmanSLKocsisJStysPKThe AxonLondonOxford University Press1995
- DevorMResponses of nerves to injury in relation to neuropathic painMcMahonSBKoltzenburgMMelzack’s Textbook of Pain5th edLondonElsevier-Churchill Livingstone2006905927
- RappaportZHDevorMTrigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglionPain1994561271388008402
- PatsalosPNPeruccaEClinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugsLancet Neurol2003247348112878435
- PeruccaEClinically relevant drug interactions with antiepileptic drugsBr J Clin Pharmacol20066124625516487217
- DíazRASanchoJSerratosaJAntiepileptic drug interactionsNeurologist2008146 Suppl 1S55S6519225371