?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The effective management of pain in chronic pancreatitis (CP) remains a therapeutic challenge. Analgesic drugs, such as opioids, and the underlying pathology can impair gut function. The autonomic nervous system influences hormone secretion and gut motility. In healthy volunteers, electrical (using noninvasive transcutaneous vagal nerve stimulation [t-VNS]) and physiological (using deep slow breathing [DSB]) modulation of parasympathetic tone results in pain attenuation and enhanced gut motility. Thus, the aims were to investigate whether t-VNS and DSB could enhance the parasympathetic tone, decrease pain sensitivity and improve gut motility in CP.
Patients and methods
A total of 20 patients (12 males, mean age=61 years, range: 50–78 years) with CP were randomized to short-term (60 minutes) t-VNS and DSB, or their placebo equivalent, in a crossover design. Cardiometrically derived parameters of autonomic tone, quantitative sensory testing of bone and muscle pain pressure, conditioned pain modulation (CPM) and assessments of gastroduodenal motility with ultrasound were performed.
Results
In comparison to sham, t-VNS and DSB increased cardiac vagal tone (CVT) (P<0.001). However, no changes in pain pressure thresholds for bone (P=0.95) or muscle (P=0.45) were seen. There was diminished CPM (P=0.04), and no changes in gastroduodenal motility were observed (P=0.3).
Conclusion
This explorative study demonstrated that t-VNS and DSB increased CVT in patients with CP. However, this short-lasting increase did not affect pain sensitivity to musculoskeletal pain or gastroduodenal motility. The chronic pain in CP patients is complex, and future trials optimizing neuromodulation for pain relief and improved motility are needed.
Introduction
Chronic pancreatitis (CP) is characterized by fibro-inflammation, and it is often associated with chronic pain.Citation1 As the disease progresses, exocrine and endocrine insufficiency may develop,Citation2,Citation3 necessitating treatment with antidiabetic medication and enzymatic supplementation. While these aforementioned complications are relatively straightforward to treat, chronic pain is more challenging and is associated with reduced quality of life and increased health resource utilization.Citation4–Citation6 In the patients where the underlying pathology is not amenable to endoscopic intervention or surgery, the treatment of pain is largely pharmacological in nature.Citation7 Analgesic monotherapy rarely provided meaningful analgesic relief and combination therapy, such as opioids and gabapentoids are therefore often needed. However, adverse effects secondary to opioids are common, and therefore, achieving a balance between analgesic benefits and such side effects is challenging, particular in patients with other complications such as duodenal stenosis, pseudocysts and ongoing pancreatic inflammation. Notwithstanding the adverse cognitive effects of opioids, they may further compromise gastrointestinal (GI) function with diminution of motility.Citation8,Citation9 Therefore, there is a pressing unmet clinical need for the development of effective alterna-tives, which ameliorate pain and enhance GI motility in CP.
Normal GI function relies on a bidirectional communication from the gut to the central nervous system, delivered in part by the autonomic nervous system (ANS). The ANS consists of two broadly opposing branches referred to as the sympathetic and the parasympathetic nervous systems,Citation10 which integrate with the enteric nervous system in the gut. The vagus nerve forms the main parasympathetic neural substrate of the ANS, while the spinal afferents form the sympathetic nerves.Citation11 Thus, in health, there is a delicate balance between sympathetic and parasympathetic influences, which is responsible for release of hormones, GI motility and the maintenance of homeostasis. A paucity of vagal activity has been identified as a pathophysiological feature in a number of disorders related to the GI tract including inflammatory bowel disease, functional dyspepsia, irritable bowel syndrome and diabetic autonomic neuropathy and gastroparesis.Citation12–Citation15 In CP, preliminary studies have postulated that autonomic dysfunction may contribute to the complex pain and GI dysmotility.Citation16–Citation18 Vagal activity can be therapeutically accentuated either physiologically, with deep slow breathing (DSB), or electrically, using vagus nerve stimulation (VNS), which can be applied invasively or noninvasively through the skin. In healthy volunteers, DSB and noninvasive transcutaneous electrical vagal nerve stimulation (t-VNS) of the auricular branch of the vagus nerve have been shown to decrease experimental pain and increase GI motility.Citation19,Citation20 Hence, combined physiological and electrical modulation of vagal tone, using DSB and t-VNS, respectively, may represent an alternative non-pharmacological treatment modality for pain and GI dysmotility in CP. We hypothesized that by increasing parasympathetic tone, with combined DSB and VNS, sensory processing of pain would be modulated and gastroduodenal motility enhanced in comparison to sham stimulation and sham breathing. The aims of the study were to study patients with CP to evaluate the effects of parasympathetic modulation using DSB and t-VNS on 1) experimental musculoskeletal pain thresholds, 2) descending pain modulation and 3) gastroduodenal motility.
Patients and methods
Study oversight
This was a randomized, single-blinded, sham-controlled, crossover study conducted at the research laboratories of Mech-Sense at the Department of Gastroenterology and Hepatology, Aalborg University Hospital, Aalborg, Denmark. All patients provided written informed consent after oral and written information and adequate consideration. The North Denmark Region Committee on Health Research Ethics approved the study (N-20090008).
Patients
Twenty patients were recruited from the outpatient clinic of our institution. Participants were eligible if diagnosed with CP according to the Mayo Clinic Diagnostic Criteria and aged 18–75 years.Citation21 Other chronic pain conditions, pregnancy and allergies to any equipment of the trials (e.g., latex gloves, electrodes) were regarded as exclusion criteria. Individual patient’s analgesic treatment regimens were not altered during the study, and rescue medication for pain flare-ups was allowed.
Randomization and blinding
Patients were randomly assigned to receive 60 minutes of t-VNS during which they undertook 2 × 10 minutes of DSB, or sham procedure, in a crossover design. Randomization was employed using a randomization list generated at randomization.com. Patients were told that they would receive two different forms of nerve stimulation at different locations on the ear. Investigators were not blinded to stimulation sequence (i.e., single-blinded study). Patients were fasted for at least 6 hours prior to each study visit. Visits were planned at identical hours of the day and with an interval of minimum 7 days to reduce any potential carryover effect.Citation22 The study protocol is illustrated in .
Outcomes measures
There were no specific thresholds predetermined due to the exploratory nature of study. The outcome measures were merged, as this was an explorative study. Pain was assessed using 1) quantitative sensory testing (QST) to document changes in pain sensitivity assessed by pressure pain thresholds in bone and muscle and 2) changes in descending pain modulation capacity using a conditioned pain modulation (CPM) paradigm.Citation17,Citation19 Finally, gastroduodenal motility parameters were assessed using 3) a modified version of a real-time ultrasonography method to demonstrate changes in motility pattern following a standardized drink test.Citation23
Study procedures
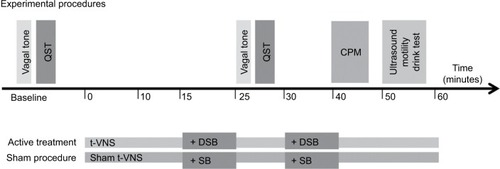
An overview of the experimental protocol is provided in .
Figure 2 Experimental procedures.
VNS
Noninvasive transcutaneous electrical VNS of the auricular branch of the vagal nerve was performed using a commercially available device (Nemos®; cerbomed GmbH, Erlangen, Germany). A bipolar stimulation electrode was connected to a pocketsize stimulator, and the electrode was placed in the left concha. The skin was cleaned with an alcohol wipe (Alkoholswabs; Mediq Danmark A/S, Brøndby, Denmark) to ensure a sufficient skin contact was made between the electrode and the skin. The stimulation intensity was individually adjusted (described later) and ranged from 0.1 mA to 10 mA with a pulse width of 250 μs and a stimulation frequency of 30 Hz. At baseline, patients were familiarized to the stimulus for 5 minutes prior to the experimental procedures, where the intensity was slowly increased until the optimal intensity was reached (i.e., a painful tingling sensation). Due to habituation, the intensity was readjusted throughout the study visit. The aim of the stimulation was continuous stimulation at the level of the intensity of the initial perceptive experience. For sham t-VNS, the device was turned 180°, stimulating the outer earlobe that does not contain parasympathetic fibers.Citation24 Patients received t-VNS, or sham equivalent, for a total of 60 minutes.
Breathing protocol
Physiological vagal stimulation was undertaken with a validated deep breathing protocol, consisting of breathing at full inspiratory capacity for 4 seconds, followed by exhaling to forced expiratory vital capacity for 6 seconds, repeated at a frequency of 0.1 Hz (i.e., six breaths per minute).Citation20 For the sham breathing procedure, patients were asked to breathe normally and count their breaths. Patients undertook two sessions of 10 minutes of DSB, or sham equivalent, at two epochs during the study, i.e., at 15 minutes and 30 minutes.
Experimental procedures
ANS Monitoring
Electrocardiogram (ECG) electrodes (Ambu® BlueSensor P; Ambu A/S, Copenhagen, Denmark) were attached at the right and left sub-clavicular areas and over the cardiac apex. Systolic blood pressure, diastolic blood pressure and mean blood pressure were continuously recorded using a photoplethysmographic sensory cuff (Nexfin®; BMEYE B.V., Amsterdam, the Netherlands) attached to the right third digit.Citation25,Citation26 The ECG was recorded using a commercially available biosignals acquisition system (Neuroscope™; Medifit Instruments Limited, Enfield, England; Portapres®; Finapres Medical Systems BV, Amsterdam, the Netherlands). Based on an R-wave detection algorithm, beat-to-beat, R-R interval and heart rate were computed. Cardiac vagal tone (CVT) was computed beat-to-beat based on detecting positive phase shifts in the R-R interval, a process called “phase-shift demodulation”. CVT is measured on a validated linear vagal scale, where 0 represents full atropinization and is a putative measure of efferent vagal activity. The Neuroscope also derives cardiac sensitivity to the baroreflex (CSB) through the incorporation of beat-to-beat R-R intervals with mean blood pressure into a 10-second cyclical and is considered to be an indirect measure of PNS afferent activity.
QST
Muscle and bone pain pressure thresholds were assessed at baseline and after 10 minutes and 25 minutes of intervention. Assessments were made on the right quadriceps muscle and the tibia bone. A handheld electronic pressure algometer (Algometer; Somedic SenseLab AB, Hörby, Sweden) was used with a probe surface area of 1 cm2 for muscle stimulation and 3 mm2 for bone stimulation. Pressure was increased at a rate of 30 kPa/s until the moderate pain threshold was reached (i.e., a score of 7 on a 11-point visual analog scale, ranging from 0 to 10).Citation27 The patients were instructed to notify the investigators when reaching the moderate pain threshold, and the corresponding pressure stimulation intensity (kPa) was noted. The muscle pressure was also used as the test stimulus before, during and after induction of CPM (described later).
CPM
The capacity of descending pain modulation was assessed after 40 minutes of intervention using a validated CPM paradigm (REF). CPM is a clinically measurable form of descending pain modulation that can be induced experimentally by a conditioning stimulus and quantified by applying a “test-pain” before and after its induction. Normally, there is a 30%–40% fall in the pain evoked by the test stimulus after the conditioned stimulation.Citation17,Citation28 We used the cold pressor test for conditioning and somatic pressure stimulation of the quadriceps muscle as test stimulus.
Cold pressor test
The right hand was immersed in cold water (2.0°C±0.3°C) continuously stirred by a pump. The patients were told to remove the hand from the water after 2 minutes of immersion or earlier if the pain was intolerable. If a patient withdrew his/her hand due to intolerable pain prior to this 120 seconds mark, the data were still included, as it was considered that descending pain control had been induced due to the intensity of the conditioning stimulus.Citation17
Pressure stimulation (test stimulus)
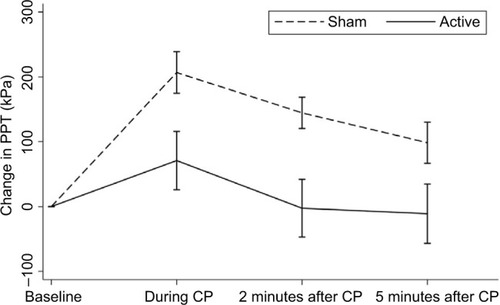
The moderate pressure pain threshold was determined on the quadriceps muscle 5 cm proximal to the patella using the pressure probe described earlier. Pressure thresholds were assessed before, during (90 seconds) and 2 minutes and 5 minutes after the cold pressor test.Citation19 The differences in pain thresholds before and after induction of cold pressor pain provide a quantitative index of the CPM capacity.
Evaluation of gastroduodenal motility parameters
Assessment of gastroduodenal motility was performed after 50 minutes of intervention. A powdered tomato soup (Knorr® Cup-a-Soup tomato; Unilever, Heilbronn, Germany) provided the basis of the liquid meal used for the drink test. Ten grams of powdered soup with an energy content of 35 kcal (1 g fat, 6 g carbohydrates, 0.5 g dietary fibers, 1.25 g protein and 0.75 g salt) was dissolved in 400 mL of boiled water. The soup was served at a temperature of 40°C. Patients were asked to consume the soup over 2 minutes via a straw to reduce any associated air swallowing. A modified validated version of a real-time ultrasonography method previously described by Kusunoki et alCitation19,Citation23 was used for gastroduodenal motility parameters. The superior mesenteric vessels and the left liver lobe were used as landmarks to obtain a standardized sagittal ultrasonic view. For ultrasound investigations, a scanner (MyLab™70 XVision; Esaote S.p.A., Genoa, Italy) with a standard abdominal probe (CA631; Esaote S.p.A.) was used. The patients were instructed in shallow breathing during ultrasonography. Still images were stored at baseline (fasting state) and after 1 minute and 15 minutes postprandial at relaxed state. Using in-built tool within the scanner, a free-hand tracing of the mucosal line of the antrum was used for the estimate of antral cross-section areas (CSAs). The gastric emptying rate (GER) was defined as
The frequency of antral contractions was defined as the number of contractions within a 3-minute interval obtained during shallow breathing within the first 5 minutes postprandial. This 3-minute interval was recorded and analyzed offline avoiding CSA measurements during inspiration. The amplitude of antral contractions was calculated from the maximal reduction in CSAs and included the mean of three full contractions during the 3-minute interval:
The motility index (MI) was defined as the mean amplitude multiplied by the frequency of antral contractions per 3 minutes:
Statistical analyses
All data are presented as mean±standard deviation (SD) unless otherwise indicated. Paired Student’s test or Wilcoxon signed-rank tests were used to analyze cardiac-derived parameters, QST parameters and gastroduodenal motility parameters; results of within-group analysis and between-group analysis are reported. Bonferroni corrections were applied for multiple comparisons. A P-value <0.05 was considered statistically significant. The software package STATA version 14.1 (StataCorp LP, College Station, TX, USA) was used for statistical calculations.
Results
All 20 enrolled patients completed the study. Patients’ demographic and clinical characteristics are reported in . The ultrasonographic results of six participants were excluded from the analysis of gastroduodenal motility parameters due to air ingestion resulting in inadequate visualization of the antrum. No adverse events were reported for sham or active t-VNS.
Table 1 Demographic and clinical characteristics of patients
ANS parameters
All cardiac-derived parameters are reported in . Compared to sham stimulation, an increase in CVT was seen after t-VNS (3.9±2.3 vs. 6.2±4.8; P=0.02) (). Furthermore, the mean blood pressure was significantly lowered during t-VNS compared to baseline (86.4±15.5 mmHg vs. 81.6±16.0 mmHg; P=0.046), while no significant effect on diastolic blood pressure was observed.
Figure 3 Changes in cardiac-derived parameters boxplot.
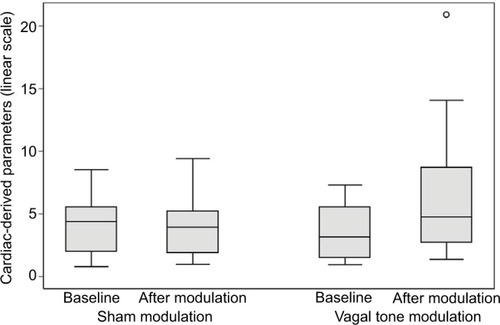
Table 2 Cardiac-derived parameters and QST in CP patients before and after vagal tone modulation (deep breathing combined with t-VNS) and sham stimulation
QST
QST parameters are reported in . Compared to sham stimulation, t-VNS induced no demonstrable differences in muscle pressure thresholds (989±336 kPa vs. 991±342 kPa; P=0.97) or bone pressure thresholds (161±63 kPa vs. 170±88 kPa; P=0.67). Also, no differences in thresholds were observed between patients on opioids vs. no opioids.
CPM
Prior to the conditioning stimulus (CPM baseline), the pressure pain thresholds were comparable between active and sham-stimulated patients (967±274 kPa vs. 887±284 kPa; P=0.37). However, a diminished CPM response was seen after t-VNS when compared to sham stimulation (7.6±22.5% vs. 26.6±18.8%; P=0.02) ( and ).
Gastroduodenal motility parameters
No significant changes in gastroduodenal motility parameters were observed between t-VNS and sham stimulation: gastric emptying rate (36.2±33.8% vs. 23.4±48.4%; P=0.55), frequency of antral contractions (4.9±2.4 per 3 minutes vs. 4.6±3.0 per 3 minutes; P=0.69), amplitude of antral contractions (42.4±17.3% vs. 42.1±18.5%; P=0.96) and MI (no unit) (4.9±3.6 vs. 4.5±3.3; P=0.31).
Discussion
We have provided evidence that in patients with CP, acute combined electrical and physiological modulation of vagal tone results in an increase in cardiometrically derived parameters of vagal tone in comparison to sham stimulation. However, this increase was not associated with any alterations in experimental somatic pain sensitivity or gastroduodenal motility parameters. In addition, a reduction in descending inhibition was seen during vagal stimulation as compared to the sham procedure.
The effects of intervention
Vagal activation
Thus, the key result of this study was that t-VNS was able to increase the cardiometrically derived parameters of parasympathetic activity in patients with a potentially sensitized pain system.Citation17,Citation29,Citation30 In a previous multicenter study of healthy subjects, the normal range for CVT was defined as 1.9–17.8.Citation25 In another study of patients with functional chest pain of presumed esophageal origin, the patients had lower CVT at baseline (5.5±0.84 vs. 11.76±1.6) in comparison to healthy subjects. By comparison with these studies, our patients had an even lower baseline CVT at baseline, thereby potentially indicating the existence of an underlying vagal neuropathy.
Motility
Hitherto, t-VNS has not been investigated in patients with CP. In a recent study of healthy volunteers, t-VNS increased MI and the frequency of antral contractions compared to sham stimulation,Citation19 although this was not an effect that we saw in this study. In part, this may be a consequence of the concomitant analgesic treatment in our patient cohort as well as the chronic neuronal changes in patients with CP, whereby neural plasticity has been demonstrated both peripherally and centrally.Citation16,Citation31,Citation32 In relation to gastroduodenal motility assessment, temporal properties and intensity of the t-VNS may also play a role with different impact on the vagal tone, and other modalities of VNS may be used in future trials.
Pain
The combined effect of acute t-VNS and DSB was insufficient to induce an analgesic effect arguably in patients with an already chronically sensitized pain system. During the recent past, a considerable research effort has been afforded to investigate the analgesic potential of VNS.Citation33 Traditionally, it is thought that visceral pain afferent information is primarily conducted via spinal afferents although there is an increasing appreciation that the ANS is involved in pain genesis in maintenance across a number of chronic pain syndromes including CP.Citation16,Citation18,Citation34 Of note, Botha et alCitation20 demonstrated that physiological accentuation of vagal tone, using DSB, could prevent the development of acid-induced esophageal hypersensitivity in a validated model in healthy volunteers. Moreover, it was shown that the analgesic effect of DSB could be abolished with coadministration of the vagolytic agent atropine. In another study of healthy volunteers investigating t-VNS, experimental somatic pain thresholds increased with short-lasting-t-VNS of the auricular branch of the vagal nerve.Citation19 The analgesic effect of VNS is postulated to be mediated through stimulation of afferent vagal nerve fibers projecting centrally to the nucleus tractus solitarius, raphe magnus, locus coeruleus, amygdala and periaqueductal gray. All these areas in the central nervous system are involved in the descending inhibitory modulation of pain, via the neurotransmitter gamma-aminobuytric acid (GABA).Citation35 Hence, the failure to induce pain attenuation in this cohort may be due to aberrant central plasticity and/or malfunction within the pain system particularly as previous studies of patients with CP have shown diminished CPM compared to healthy volunteers.Citation17,Citation29 The diminished CPM effect during VNS compared to sham stimulation was an unexpected finding. In animal studies, it has been suggested that during diabetic neuropathy and central sensitization, the GABAergic effect can shift from inhibitory to excitatory leading to disinhibition.Citation36 If this is the case, in a subpopulation of these patients having expectedly central sensitization due to chronic pain, the unexpected finding of diminished CPM may be partly explained. If this is the case, the increased parasympathetic tone should then activate the GABAergic system, which due to disinhibition responds conversely. Based on these present findings, it remains uncertain whether the findings are as consequences of peripheral and/or central nervous system changes in this cohort of patients with CP.Citation32
Methodological considerations and study limitations
CP represents a heterogeneous group of patients, with different pathophysiology and a variable number of coexisting diagnoses such as diabetes and different analgesic treatments, which evolves with disease duration.
Therefore, the inclusion of patients with both painful (n=14) and non-painful CP (n=6) may have influenced the results of this study and further investigations of an exclusive cohort of patients with painful CP, i.e., presumed sensitized pain system, may be of relevance for design of future studies. We wanted, however, to investigate CP patients, with functioning nervous system, and therefore, the investigated patients only had a mean duration of CP of 92.4 months (), as we suspected that CP patients with chronic pain would have a more complex and sensitized pain system.
Although this study enhanced parasympathetic tone, there was no change in any of the algometric end points, thereby suggesting that the analgesic potency of the short-term t-VNS may not have been powerful enough to induce clinical effects in CP patients with a potentially chronic sensitized pain system. Otherwise, structural changes in the nervous system due to CP, neuroinflammation due to coexisting diabetes and the complex nature of CP including several parallel mechanisms behind the pain and dysmotility could likely influence the outcome. Therefore, other more potent modalities such as cervical t-VNS with multiple stimulations, could potentially be considered for future studies. Finally, as our design did not allow assessment of the physiological or the electrical stimulation individually, different approaches, e.g., multiple study arms, may be advantageous in the future.
In relation to ultrasound recordings, intra-observer variance may apply. We, however, aimed to reduce this potential bias, as specialists in radiology JBF or AM supervised all procedures that were all undertaken by the same operator JJ.Citation19 Furthermore, patient instruction was imperative, as the procedure needs delicate time managements and focus on not consuming air concomitant with ingestion of the soup. Some patients, however, did ingest air, which in time obscured the visualization of the antrum during analyses of gastroduodenal motility parameters in six patients. Other authors experienced similar problems in healthy volunteers, even in the pioneering study by Kusunoki et al.Citation19,Citation23 This may imply that exclusion of patients may easily occur, in particular in patients with already impaired gut function, e.g., CP; hence, future trials should take this into consideration and power the study for potential dropouts due to impaired visualization of the antrum.
Conclusion
This study provides evidence that t-VNS may serve as a potential modulator of the parasympathetic nervous system in patients with CP. However, in this study, short-term neuromodulation was not sufficient to affect musculoskeletal pain pressure sensitivity and gastroduodenal motility parameters. This could be due to the complex pathophysiology of CP pain and dysmotility, including peripheral neuropathy, central sensitization and plasticity in central pain processing. Future trials in CP are likely to necessitate longer periods of neuromodulation and potentially should be conducted as a possible add-on to existing analgesic treatment.
Author contributions
Study design and original idea: CB, JBF, ADF and AMD; inclusion of patients: JJ; data collection and analysis: JJ, AM, CB and JBF; statistical analyses: SSO; interpretation of results: all authors contributed; drafting of the manuscript: JJ; literature search, critical review for important intellectual content and final preparation of the manuscript: all authors. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
Siemensfonden funded the Nemos® device. ADF was supported by the Danish Diabetes Academy founded by the Novo Nordisk Foundation and the Research and Development Department, University Hospitals of North Midlands, Stoke-on-Trent, UK.
Disclosure
The authors report no conflicts of interest in this work.
References
- WarshawALBanksPAFernàndez-del CastilloCAGA technical review: treatment of pain in chronic pancreatitisGastroenterology199811537657769721175
- LiebJGForsmarkCEReview article: pain and chronic pancreatitisAliment Pharmacol Ther200929770671919284407
- PasrichaPJUnraveling the mystery of pain in chronic pancreatitisNat Rev Gastroenterol Hepatol20129314015122269952
- MulladyDKYadavDAmannSTType of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort studyGut2011601778421148579
- OlesenSSJuelJNielsenAKFrøkjærJBWilder-SmithOHGDrewesAMPain severity reduces life quality in chronic pancreatitis: implications for design of future outcome trialsPancreatology201414649750225455540
- OlesenSSPoulsenJLBrobergMCHMadzakADrewesAMOpioid treatment and hypoalbuminemia are associated with increased hospitalisation rates in chronic pancreatitis outpatientsPancreatology201616580781327320721
- OlesenSSJuelJGraversenCKolesnikovYWilder-SmithOHGDrewesAMPharmacological pain management in chronic pancreatitisWorld J Gastroenterol201319427292730124259960
- VijungcoJDPrinzRAManagement of biliary and duodenal complications of chronic pancreatitisWorld J Surg200327111258127014534824
- KetwarooGAChengVLemboAOpioid-induced bowel dysfunctionCurr Gastroenterol Rep201315934423900996
- Al OmranYAzizQThe brain-gut axis in health and diseaseAdv Exp Med Biol201481713515324997032
- TakLMRieseHde BockGHManoharanAKokICRosmalenJGMAs good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disordersBiol Psychol200982210111019463887
- PellissierSDantzerCCaniniFMathieuNBonazBPsychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndromePsychoneuroendocrinology201035565366219910123
- BonazBSinnigerVPellissierSVagal tone: effects on sensitivity, motility, and inflammationNeurogastroenterol Motil201628445546227010234
- BuysschaertMDonckierJDiveAKetelslegersJMLambertAEGastric acid and pancreatic polypeptide responses to sham feeding are impaired in diabetic subjects with autonomic neuropathyDiabetes19853411118111854043558
- FarrugiaGHistologic changes in diabetic gastroparesisGastroenterol Clin North Am2015441313825667021
- BuscherHCJLvan GoorHSweepCGJLendersJWMWilder-SmithOHGIncreased sympathetic activity in chronic pancreatitis patients is associated with hyperalgesiaJ Pain Palliat Care Pharmacother201024436236621133744
- OlesenSSBrockCKrarupALDescending inhibitory pain modulation is impaired in patients with chronic pancreatitisClin Gastroenterol Hepatol20108872473020304100
- BuscherHCLendersJWWilder-SmithOHSweepCGvan GoorHBilateral thoracoscopic splanchnicectomy for pain in patients with chronic pancreatitis impairs adrenomedullary but not noradrenergic sympathetic functionSurg Endosc20122682183218822395951
- FrøkjaerJBBergmannSBrockCModulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivityNeurogastroenterol Motil201628459259826728182
- BothaCFarmerADNilssonMPreliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivityGut201564461161724870622
- LayerPYamamotoHKalthoffLClainJEBakkenLJDiMagnoEPThe different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitisGastroenterology19941075148114877926511
- LiangZ-HXieC-CLiZ-PZhuX-PLuA-PFuW-BDeqi sensation in placebo acupuncture: a crossover study on Chinese medicine studentsEvid Based Complement Alternat Med2013201362067123983794
- KusunokiHHarumaKHataJReal-time ultrasonographic assessment of antroduodenal motility after ingestion of solid and liquid meals by patients with functional dyspepsiaJ Gastroenterol Hepatol20001591022102711059931
- PeukerETFillerTJThe nerve supply of the human auricleClin Anat2002151353711835542
- FarmerADCoenSJKanoMNormal values and reproducibility of the real-time index of vagal tone in healthy humans: a multi-center studyAnn Gastroenterol201427436236825330916
- FarmerADCoenSJKanoMPsychophysiological responses to visceral and somatic pain in functional chest pain identify clinically relevant pain clustersNeurogastroenterol Motil201426113914824134072
- OlesenSSBouwenseSAWWilder-SmithOHGvan GoorHDrewesAMPregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trialGastroenterology2011141253654321683078
- YarnitskyDBouhassiraDDrewesAMRecommendations on practice of conditioned pain modulation (CPM) testingEur J Pain201519680580625330039
- BouwenseSAWOlesenSSDrewesAMPoleyJ-Wvan GoorHWilder-SmithOHGEffects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trialPLoS One201278e4209622879908
- BouwenseSAWde VriesMSchreuderLTWSystematic mechanism-orientated approach to chronic pancreatitis painWorld J Gastroenterol2015211475925574079
- BouwenseSAWBuscherHCJLvan GoorHWilder-SmithOHGS-ketamine modulates hyperalgesia in patients with chronic pancreatitis painReg Anesth Pain Med201136330330721490522
- FrøkjærJBBouwenseSAWOlesenSSReduced cortical thickness of brain areas involved in pain processing in patients with chronic pancreatitisClin Gastroenterol Hepatol2012104434.e438.e22155560
- ChakravarthyKChaudhryHWilliamsKChristoPJReview of the uses of vagal nerve stimulation in chronic pain managementCurr Pain Headache Rep201519125426493698
- DayMSympathetic blocks: the evidencePain Pract2008829810918366465
- YarnitskyDConditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain statesCurr Opin Anaesthesiol201023561161520543676
- Lee-KubliCAGCalcuttNAAltered rate-dependent depression of the spinal H-reflex as an indicator of spinal disinhibition in models of neuropathic painPain2014155225026024103402