 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chronopharmacology studies the effect of the timing of drug administration on drug effect. Here, we measured the influence of 4 timing moments on fentanyl-induced antinociception in healthy volunteers. Eight subjects received 2.1 μg/kg intravenous fentanyl at 2 pm and 2 am, with at least 2 weeks between occasions, and 8 others at 8 am and 8 pm. Heat pain measurements using a thermode placed on the skin were taken at regular intervals for 3 hours, and verbal analog scores (VAS) were then obtained. The data were modeled with a sinusoid function using the statistical package NONMEM. The study was registered at trialregister.nl under number NTR1254. A significant circadian sinusoidal rhythm in the antinociceptive effect of fentanyl was observed. Variations were observed for peak analgesic effect, duration of effect, and the occurrence of hyperalgesia. A peak in pain relief occurred late in the afternoon (5:30 pm) and a trough in the early morning hours (5:30 am). The difference between the peak and trough in pain relief corresponds to a difference in VAS of 1.3–2 cm. Only when given at 2 am, did fentanyl cause a small but significant period of hyperalgesia following analgesia. No significant changes were observed for baseline pain, sedation, or the increase in end-tidal CO2. The variations in fentanyl’s antinociceptive behavior are well explained by a chronopharmacodynamic effect originating at the circadian clock in the hypothalamus. This may be a direct effect through shared pathways of the circadian and opioid systems or an indirect effect via diurnal variations in hormones or endogenous opioid peptides that rhythmically change the pain response and/or analgesic response to fentanyl.
Keywords:
Introduction
Chronopharmacology studies the effect of the timing of drug administration (in terms of the hour in a 24 hour period, the day in a 1-month or 1-year period, or the year in a lifetime) on the drug’s pharmacokinetics and/or pharmacodynamics.Citation1,Citation2 When applied to the 24-hour circadian rhythm, it is known that numerous drugs exhibit a differential response depending on the time of administration. This also applies to drugs used in anesthesia, such as local anesthetics, barbiturates, muscle relaxants, and opioids.Citation1,Citation2 For opioids, circadian effects have been observed for drug disposition (eg, meperidine and morphine) and therapeutic sensitivity (eg, tramadol and codeine).Citation2,Citation3 However, the number of studies on opioid pharmacology is restricted; hence, knowledge on the influence of the circadian rhythm on opioid analgesic efficacy remains poor.Citation2 Evidently, further understanding and application of a chronotherapeutic approach to opioid treatment of acute and chronic pain would increase opioid efficacy and possibly improve the efficacy–safety balance.
To scrutinize the hypothesis that opioids display a diurnal antinociceptive effect, we performed a study on the influence of 4 distinct timing moments on fentanyl-induced analgesia in healthy volunteers. The analgesic effect of intravenous fentanyl, administered at 8 am, 2 pm, 8 pm, or 2 am, was examined using an experimental heat pain model.
Methods
Following approval of the protocol by the Leiden University Medical Center Human Ethics Committee, 16 healthy volunteers (12 women and 4 men; aged 18–30 years; body mass index [BMI] <28 kg/m2) were enrolled in the study. The study protocol complied with the Helsinki declaration. The study was registered at trialregister.nl (No. NTR1254). Written and oral informed consent were obtained prior to the inclusion in the study. Exclusion criteria include age <18 years, BMI >30 kg/m2, presence of underlying disease, history of drug allergy, history of psychiatric disease, history of illicit substance abuse. All female subjects were taking oral contraceptives. The subjects were instructed not to eat or drink for at least 6 hours before the study.
Heat pain was induced using the TSA-II Neurosensory Analyzer (Medoc Ltd, Ramat Yishai, Israel). Using a 3-cm2 probe, the skin on the volar side of the left or right forearm was stimulated with a gradually increasing stimulus (0.5°C/sec; baseline temperature 32°C). The volar side of the arm was divided into 6 zones and marked as previously described.Citation4 The thermode was moved from zone to zone between stimuli to avoid sensitization to heat stimulus. Following heat stimulation, the subjects scored their verbal analog scores (VAS) in pain intensity on a 10-cm long scorecard. The thermode peak temperature depended on an initial trial phase in which the subject rated the pain to 3 peak temperatures: 46°C, 48°C, and 49°C. The lowest stimulus causing a VAS >5 cm was used in the remainder of the study. The test data were discarded. Then, baseline values (ie, predrug VAS) were obtained. Baseline values were obtained on each of the 2 experimental sessions.
The subjects were randomly divided into 2 experimental groups. The first group received fentanyl at 2 pm and 2 am and the second group at 8 am and 8 pm. The experimental days were separated by a 2-week washout period. We studied 2 distinct groups to reduce the number of occasions at which the healthy volunteers were exposed to potent opioid. At the appropriate time, 2.1 μg/kg intravenous fentanyl was administrated intravenously over 90 seconds. Subsequently, heat pain measurements were taken every 10 minutes for 3 hours (first pain test at 10 minutes after the start of the fentanyl infusion). Additionally, at each testing interval, a verbal rating score of sedation using a scale ranging from 0 to10 (from 0 = fully alert to 10 = severely sedated and sleepy) and end-tidal CO2 measurements were obtained via a face mask connected to a gas monitor (Multicap, Datex, Helsinki, Finland). Arterial hemoglobin oxygen saturation was measured via a finger probe (SpO2) with a pulse oximeter (Masimo, Irvine, California). The study was powered to observe a 1-cm difference in VAS of a 10-cm scale ranging from 0 (= no pain) to 10 (= most intense pain imaginable) between 2 study groups (power = 90%; α = 0.05).
A linear mixed model was used to compare the baseline parameter values (thermode temperature to reach a VAS >5, sedation score, and end-tidal CO2) using SPSS® 16.0 software (SPSS, Chicago, Illinois). P values < 0.05 were considered significant. To quantify the effect of fentanyl on pain relief, we initially assessed the effect relative to baseline (ie, ΔVAS, by subtraction of baseline VAS at each time point), and subsequently, we calculated the area between the VAS data points and the zero line (area between the effect-time curves, AECs). Consequently, the more negative the AEC the more analgesic the response. We present the AEC data as mean change in VAS over time (ie, AEC/180 minutes; unit = cm). Then, to get an indication of the presence of a circadian effect on fentanyl analgesia, the data were modeled using a sinusoid function:
where, the A = amplitude, f = frequency (occurrence of the sinus per 24 hour), and ϕ = a phase shift. To obtain the 95% confidence interval of the sinusoid, a bootstrap analysis was performed using 1,000 reiterations with replacement. Data analysis was performed using the statistical package NONMEM® version VI (ICON Development Solutions, Ellicott City, Maryland).Citation5 For sedation and end-tidal CO2, the area under the effect-time curves were calculated (without subtraction of baseline values) and compared using a linear mixed model.
Results
No differences in baseline parameters were observed within group or between groups (). In each group, there were 6 women and 2 men. None of the subjects were nightshift workers, had passed international times zones in the 3 months before the study, or reported sleep disturbances. The subjects completed the study without major side effects. Incidental occurrences of low SpO2 (<95%) were treated by prompting the subject to take a deep breath.
Table 1 Baseline parameter values and 3-hour area under the time-effect curve for end-tidal CO2 and sedation
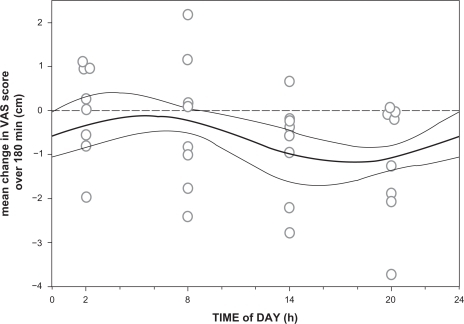
All injections were performed at the planned time of day ± 4.3 minutes (maximal range; no significant difference between groups). After injection, all volunteers reached maximal analgesia within 20 minutes and returned to within 10% of their baseline pain sensitivity levels by the end of the experiment. The influence of the time of infusion on ΔVAS is shown in and . Time-related variations are observed for peak analgesic effect (with the least effect at 2 am), duration of effect (with the shortest duration at 8 am), and the occurrence of a small hyperalgesic response (most pronounced at 2 am). Individual AEC values (all divided over 180 minutes, giving the mean change in VAS over 180 minutes) vs study time are given in together with the data fit (± 95% confidence interval). A significant sinus wave was present in the data (the wave was significantly different from a linear response line, P < 0.01). The parameter values are offset = −0.63 ± 0.25 cm, A = 0.65 ± 0.20 cm, and ϕ = 27 ± 21 degrees (all parameters P < 0.01, values are typical value ± SE). The negative value of the offset indicates that on average at all times, an analgesic response occurred. An amplitude of 0.65 means that the average VAS varied by 1.3 cm over time (recalculation for just the first 90 minutes of the experiment would yield a variation in VAS of 2 cm; note that these variations are model predictions). The value of ϕ of 27 degrees indicates that at midnight (0 hour in ), the sinus was shifted by 27 degrees. The frequency value f was fixed to 1 as we assumed that the sinus occurred once every 24 hour. Fentanyl was most analgesic in the late afternoon and early evening hours (between 2 pm and 8 pm; minimum of the sinus occurred at 5 am), whereas it was least analgesic in the early morning hours (from 2 am to 8 am; maximum of the sinus occurred at 3 pm).
Figure 1 Effect of fentanyl on heat pain scores in 2 groups of subjects. A and C, One group received intravenous 2.1 μg/kg fentanyl at 8 am and 8 pm; B and D, the other group at 2 pm and 2 am. Values are mean ± SD. Baseline values (ie, predrug values) are given at time t = 0.
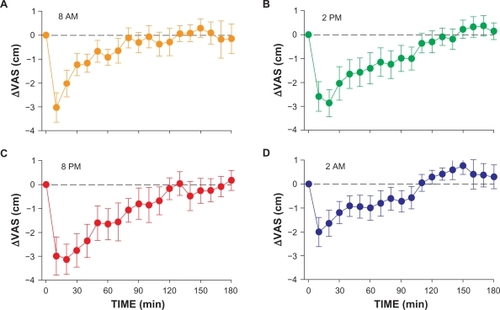
Figure 2 Mean pain scores relative to baseline (ie, ΔVAS with baseline = 0 at time t = 0) after injection of 2.1 μg/kg fentanyl observed at 2 am, 8 am, 2 pm, and 8 pm.
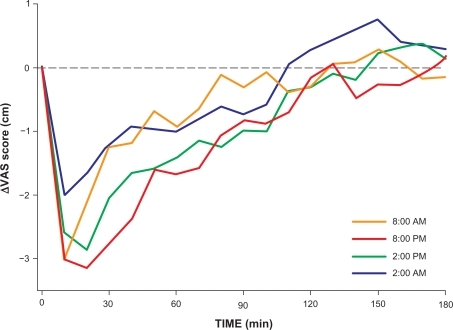
Figure 3 Data fit of analgesic effect from 2.1 μg/kg intravenous fentanyl vs time of day at which the drug was injected. Analgesic effect is defined as the mean change in VAS over the 180-minutes study period. Each circle represents the analgesic effect of one subject. The fit is a sinusoidal curve (thick continuous line) ±95% confidence interval (thin continuous lines). The broken line denotes a separation between mean analgesic responses (data below the broken line) and hyperalgesic responses (above the broken line).
Side effects showed much less of a variation over time than analgesia with no differences among observations within and between groups (analysis of variance [ANOVA]: P > 0.05; ). A significant sinus could not be demonstrated for end-tidal pCO2 or sedation.
Discussion
We observed a circadian sinusoidal rhythm in the analgesic effect of fentanyl. Variations were observed for peak analgesic effect, duration of effect, and the occurrence of hyperalgesia. When using AEC as end point, we observed a peak in pain relief late in the afternoon (5:30 pm) and a trough in the early morning hours (5:30 am). The difference between the peak and trough in pain relief corresponds to a difference in VAS of 1.3–2 cm. This indicates that the magnitude of the diurnal variation of fentanyl analgesia is significant, albeit relatively small with increased sensitivity to fentanyl in the late afternoon and early evening hours (1–11 pm).
Opioid effect on the circadian rhythm
Our study did not specifically examine the suprachiasmatic nucleus (SCN) or endogenous opioid pathways. Taken into account the current knowledge on the interaction of the circadian clock and opioid pathways, an interaction of the 2 systems seems possible, although at present this remains speculative. In mammals, the SCN in the hypothalamus is the site that controls circadian behavioral rhythmicity (ie, the master clock).Citation6,Citation7 The SCN is synchronized by external stimuli of which the light or dark cycle is the most important (the retina is directly linked to the SCN via the retinohypothalamic tract). Other synchronizers include locomotor activity, drugs (eg, benzodiazepines, opioids, and serotonin agonists), and social interaction. The SCN controls many cyclic events in the mammalian body including the synthesis and release of hormones, such as melatonin and cortisol, and body temperature. The generation of rhythmicity in the SCN is genetically determined and based on feedback loop that involves several genes, including Per1, Per2, and Clock.Citation6,Citation7 The SCN and its afferent and efferent pathways contain various neurotransmitters including neuropeptide Y, γ-amino butyric acid, and enkephalins. The role of enkephalins in the circadian system has received increasing attention as δ-opioid receptors were identified in the hamster SCN, and the μ-opioid receptor agonist fentanyl induces a phase shift in the circadian rhythm of hamsters independent of any behavioral effects of the opioid.Citation8,Citation9 We showed previously that fentanyl modifies the circadian pacemaker possibly via direct effects on SCN electrical activity and regulation of Per genes.Citation8 This suggests that pathways regulating the circadian clock intersect directly or indirectly with pathways that express opioid receptors. Then, our current study, in which a diurnal variation in fentanyl’s analgesic behavior is observed (ie, an effect opposite to fentanyl’s influence on the clock), could be interpreted as showing involvement of the opioid system in the circadian rhythm. Alternatively, other studies indicate that circadian rhythms entrained to drugs of abuse and rewarding stimuli (eg, methamphetamine-sensitive circadian oscillator, daily rhythm of food-anticipatory behavioral activity, and food or chocolate anticipatory timing systems) are mediated independently of the photic entrainment system,Citation10–Citation12 suggesting that also for opioids, entrainment may occur distinct from the known circadian clock. Evidently, further studies are needed to elucidate the interaction between the known circadian clock and opioid pathways.
We refrained from measuring plasma fentanyl concentrations in our observational study. We discussed that frequent blood sampling could interfere with the subject rating of heat pain possibly causing stress-induced analgesia that encompasses strong circadian variations.Citation13 Consequently, the variation in fentanyl’s effect may be due to a true increase in the opioid’s antinociceptive efficacy (a pharmacodynamic effect), as suggested above, but we cannot exclude a diurnal variation in fentanyl’s pharmacokinetics. An increase in plasma fentanyl concentrations in the late afternoon and early evening may well explain our findings. Variations in plasma morphine concentrations following oral administration in patients with cancer pain have been observed due to variations in absorption and/or changes in the volume of distribution over a 24-hour period.Citation14 Similarly, intramuscular meperidine injections in patients with sickle cell anemia were associated with circadian changes in drug disposition and elimination over the day.Citation2 In contrast, oral codeine and tramadol given to healthy volunteers in the morning or evening did not show any differences in pharmacokinetics.Citation3 Similarly, and of importance to our study, in 2 separate studies, in volunteers receiving intravenous fentanyl, the plasma fentanyl concentration–time profiles were independent of the time of infusion.Citation15 This then suggests that our findings are related to a circadian effect on fentanyl’s pharmacodynamics and not to its pharmacokinetics.
Circadian variations in the pain response
Several animal studies showed that the response to noxious stimuli is not constant over a 24-hour period.Citation1,Citation2,Citation13,Citation16 The results of human experimental and clinical studies are less clear with some studies finding no difference in pain over time, whereas others found more pain in the morning or evening.Citation1,Citation2,Citation17–Citation19 Experimental pain studies indicate that variations in pain sensitivity depend on the tissue tested and the nociceptive assay employed.Citation1,Citation2,Citation17–Citation19 Using a similar thermode as we did, Strian et alCitation18 did observe a variation in pain threshold values to warm and cold stimuli, but these variations were small and had no consistent pattern among subjects. Our study was not designed or powered to study variations in pain sensitivity and, as expected, we did not observe significant differences in temperature to induce VAS > 5 cm. However, at this point, we cannot exclude some effect of variations in pain sensitivity on the antinociceptive responses that we observed with less pain reporting between 1 pm and 11 pm (and hence a greater analgesic response at these times). Indeed, skin sensitivity to heat is minimal at 6 pm and maximal at 6 am, and painful stimulation of the nasal mucosa with CO2 is also increased during evening test sessions.Citation17,Citation20 Further studies are needed to investigate the complex interaction between variations in pain sensitivity and opioid treatment. An important question in this respect is, eg, whether the pain and analgesic rhythms display antagonistic or synergistic interactions.
Mechanisms of opioid circadian rhythm
The mechanism through which the circadian rhythm affects opioid analgesic efficacy remains unknown. Variations in hormones (eg, cortisol, melatonin) and endogenous opioid peptides (metaenkephalin and β-endorphins) could play an important role in interacting with the nociceptive pathways and opioid system.Citation21–Citation23 For example, the analgesic effect of melatonin is more pronounced at night.Citation24 An interesting observation in mice is that μ-opioid receptor expression displays a 24 hour rhythm.Citation25 Downregulation of the brain μ-opioid receptor was associated with a decrease in morphine analgesia. Extrapolation of these animal data to ours in humans then suggests that during the late evening, morning, and early afternoon, human μ-opioid receptors are downregulated via a direct or indirect (eg, hormonal) influence of the SCN. Our finding of enhanced analgesic fentanyl efficacy from 1 pm to 11 pm is in agreement with other human studies showing similar patterns of opioid effect. Nonlethal opioid overdose (ie, increased opioid sensitivity causing respiratory depression) shows a significant peak in the afternoon and early evening, an effect that was independent of the opioid plasma concentrations.Citation26 Oral codeine and tramadol display greater analgesic sensitivity when administered in the early evening.Citation3
Hyperalgesia
A somewhat surprising observation in our study was the occurrence of a moderate hyperalgesic response (pain sensitivity greater than baseline) following analgesia in subjects receiving fentanyl at 2 am (). This phenomenon was outspoken in 5 subjects tested at 2 am and occurred on 11 occasions in the whole study (). Hyperalgesia in response to opioids has been observed in various species, including humans. Recent data indicate that opioid-induced hyperalgesia is not related to activation of opioid receptors but possibly due to activation of N-methyl-d-aspartic acid receptors within pain pathways.Citation27,Citation28 Animal studies showed that hyperalgesia induced by opioid-receptor blockade by naloxone (ie, a nonopioid receptor phenomenon) follows a diurnal rhythm.Citation29 This then suggests that our results may have been influenced by 3 separate rhythms: an inherent pain rhythm, a fentanyl analgesic, and antianalgesic rhythm.
Critique of methods
It may be discussed that the observed rhythm is partly related to the use of 2 distinct subject groups, one of which was studied at 8 am and 8 pm, the other at 2 pm and 2 am. This could, eg, occur when the 2 groups would differ in their AECs without a within-group difference between measurement points (eg, [AEC [group 1] at 2 pm = 2 am] > [AEC [group 2] at 8 am = 8 pm]). However, this was not the case ( and ). In both groups, the data collected in the morning hours (2 am or 8 am) displayed a peak effect and AEC of lesser magnitude than the data collected in the afternoon or early evening hours (2 pm or 8 pm). This suggests that the observed rhythm was inherently present in the 2 study groups and not related to the design of the study.
We modeled the data with a symmetrical sinusoid function. This function was significantly better than a linear function. We did assess also nonsymmetrical sinusoid functions by allowing the 4 parts of the sinusoid to vary independently in amplitude (with factor FAC). However, no significant improvements in minimum objective function were observed in comparison to FAC value of 1. Furthermore, assessing the residuals of the symmetrical sinusoid functions showed the absence of any bias (means residuals per test period not different from zero). This indicates that the sinusoid chosen adequately described the data.
We subtracted the baseline pain score from the VAS–time data to allow objective assessment of the change in VAS over time (AEC). This was possible in our data set as we observed little variation in the baseline VAS (predrug) score. We cannot exclude, however, that some error in baseline values may erroneously propagate to the estimates of the model parameters. However, in our analysis, the error only propagates to the interindividual variability of the model parameter offset. We tested the variance in offset and observed that it was not different from zero, suggesting that subtraction of baseline pain scores did not affect our study outcome. In some studies, the analysis of circadian effects is sensitive to “edge effects” or the moment in time defined as the start of day or start of analysis (this is often related to the use of a smoothing function).Citation30 We chose midnight as starting point of our analysis. Our NONMEM analysis of the data with a nonsmoothed sinusoid does not have any edge effects.
Recent studies on chronopharmacology of labor analgesia with intrathecal bupivacaine indicate that one has to be careful with the interpretation of rhythmic patterns in the duration of analgesia.Citation30 This concerns patient studies in which daily routines (external rhythms such as nursing and anesthesia provider shifts) produce artifacts (suggesting a biological rhythm in intrathecal analgesia duration) that have little to do with biological rhythms.Citation30 We were aware of these pitfalls and designed our study to prevent influences from external rhythms. However, despite our efforts, we cannot exclude some albeit small effect from external sources on our study outcome.
We used intravenous fentanyl, which is used in the treatment of acute pain and in the perioperative setting to prevent pain and cardiovascular stimulation. In chronic pain treatment, the drug is commonly used orally or via a dermal path. Because it seems improbable that the mode of administration affects the chronopharmacological behavior of fentanyl (as discussed above, the effects are pharmacodynamic in nature and not pharmacokinetic), we assume that the results that we obtained also apply to the chronic pain setting.
We tested both male and female subjects. There is now ample evidence that opioids show greater analgesic effect in women compared to men.Citation31 The data obtained in the 4 male subjects fell well within the female data range, suggesting the absence of a sex effect in our data set. However, our study was neither designed nor powered to unearth sex differences, and therefore, the existence of possible sex differences in the chronopharmacological behavior of fentanyl requires further study.
Conclusion
We observed a circadian rhythm in the analgesic effect of fentanyl in human volunteers using an experimental heat pain model. Our data indicate an increase in analgesic efficacy in the late afternoon and early evening hours. We argue that the most probable cause for our findings is chronopharmacodynamic effect regulated by the circadian clock in the hypothalamus. This may be a direct effect through shared pathways of the circadian system and the opioid system or an indirect effect via diurnal variations in hormones or endogenous opioid peptides that rhythmically change the pain response and/or analgesic response to fentanyl.
Disclosure
The authors report no conflicts of interest in this work.
References
- LabrecqueGVanierMCBiological rhythms in pain and in the effects of opioid analgesicsPharm Ther199568129147
- ChassardDBruguerolleBChronobiology and anesthesiaAnesthesiology200410041342714739819
- HummelTKrateschH-GLötschJHepperMLiefholdJKobalGAnalgesic effects of dihydrocodeine and tramadol when administered either in the morning or eveningChronobiol Int19951262727750159
- OlofsenERombergRBijlHAlfentanil and placebo analgesia: no sex differences detected in models of experimental painAnesthesiology200510313013915983465
- BealBLSheinerLBBoeckmanAJNONMEM Users Guide (1989–2006).Ellicott City, MarylandIcon development Solutions2006
- HastingsMThe brain, circadian rhythms, and clock genesBr Med J1998317170417079857134
- Wager-SmithKKaySACircadian rhythm genetics: from flies to mice to humansNat Genet200026232710973243
- VansteenselMJMagnoneMCvan OosterhoutFThe opioid fentanyl affects light input, electrical activity and Per gene expression in the hamster suprachiasmatic nucleiEur J Neurosci2005212958296615978007
- MeijerJHRuijsACAlbusHFentanyl, a upsilon-opioid receptor agonist, phase shifts the hamster circadian pacemakerBrain Res200086813514010841898
- MohawkJBaerMMenakerMThe methamphetamine-sensitive circadian oscillator does not employ canonical clock genesProc Natl Acad Sci U S A20091063519352419204282
- StorchKFWeitzCJDaily rhythms of food-anticipatory behavioral activity do not require the known circadian clockProc Natl Acad Sci U S A20091066808681319366674
- Angeles-CastellanosMSalgado-DelgadoRRodríguezKBuijsRMEscobarCExpectancy for food or expectancy for chocolate reveals timing systems for metabolism and rewardNeuroscience200815529730718585440
- Puglisi-AllegraSCastellanoCOliverioACircadian variations in stress-induced analgesiaBrain Res19822523733766891282
- GourlayGKPlummerJLCherryDAChronopharmacokinetic variability in plasma morphine concentrations following oral doses of morphine solutionPain1995613753817478680
- GuptaSKSouthamMAHwangSSEvaluation of diurnal variation in fentanyl clearanceJ Clin Pharmacol1995351591627751426
- KavaliersMHirstMDaily rhythms of analgesia in mice: effects of age and photoperiodBrain Res19832793873936315182
- DahanACircadian influences, low-dose isoflurane, and the ventilatory response to hypoxiaAnesthesiology200510320720815983477
- StrianFLautenbacherSGalfeGHolzlRDiurnal variations in pain perception and thermal sensitivityPain1989361251312919090
- BrueraEMacmillanMKuehnNMillerMJCircadian distribution of extra doses of narcotic analgesics in patients with cancer pain: a preliminary reportPain1992493113141408295
- ProcacciPCorteMDZoppiMMarescaMRhythmic changes of the cutaneous pain threshold in man: a general reviewChronobiologia1974177964459047
- HamraJGKamerlingSGWolfsheimerKJBagwellCADiurnal variation in plasma ir-beta-endorphine levels and experimental pain thresholds in the horseLife Sci1993531211298515686
- WescheDLFredericksonRCAThe role of the pituitary in the durnal variation in tolerance to painful stimuli and brain enkephalin levelsLife Sci198129219922057198705
- HindmarschKWTanLSankaranKLaxdalVADiurnal rhythms of cortisol, ACTH, and β-endorphin levels in neonates and adultsWest J Med19891511531562549730
- EbadiMGovitrapongPPhansuwan-PujitoPNelsonFReiterRJPineal opioid receptors and analgesic action of melatoninJ Pineal Res1998241932009572527
- YoshidaMOhdoSTakaneHChronopharmacology of analgesic effect and its tolerance induced by morphine in miceJ Pharmacol Exp Ther20033051200120512626644
- GalleraniMManfrediRDal MonteDCaloGBrunaldiVSimonatoMCircadian differences in the individual sensitivity to opiate overdoseCrit Care Med2001299610111176167
- van DorpELAKestBKowalczykWJMorphine-6β-glucuronide rapidly increases pain sensitivity independently of opioid receptor activity in mice and humansAnesthesiology20091101356136319461298
- WaxmanARAroutCCaldwellMDahanAKestBAcute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in miceNeurosci Lett2009462687219559072
- FredricksonRHyperalgesia induced by naloxone follows diurnal rhythm in responsivity to painful stimuliScience1977198756758561998
- ShaferSLLemmerBBoselliEPitfalls in chronobiology: intrathecal bupivacaine for labor analgesiaAnesth AnalgIn press2010
- NiestersMDahanAKestBDo sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studiesPainIn press2010