Abstract
Background
A new propylene glycol-free and reduced-volume formulation of diclofenac sodium 75 mg/mL designed for intradeltoid administration has been found to be bioequivalent to a reference formulation of diclofenac sodium 75 mg/3 mL given via the intragluteal route in normal healthy volunteers. Standard needles may not reach the gluteus maximus muscle in many cases, especially in the obese. The objective of this study was to determine the pharmacokinetic parameters of the new formulation and compare the bioavailability of intradeltoid diclofenac sodium 75 mg/mL with that of the intragluteal 75 mg/3 mL reference formulation in obese volunteers.
Methods
A comparative, two-way, single-dose, bioavailability study was carried out in 10 obese (body mass index > 25) male Indian volunteers after a washout period of seven days. Blood samples were collected until six hours following drug administration and analyzed using a prevalidated high-pressure liquid chromatography method.
Results
The mean maximum plasma concentration and time to reach maximum plasma concentration for the test formulation were 1.30 μg/mL and 0.50 hours, respectively, versus 0.93 μg/ mL and 1.08 hours for the reference formulation. The mean areas under the curve from 0 to last measurable time point (AUC0–t) for the test and reference formulations were 2.71 μg·h/mL and 2.73 μg·h/mL, respectively. The mean AUCs from 0 to infinity (AUC0–∞) for the test and reference formulations were 3.71 μg·h/mL and 3.75 μg·h/mL, respectively.
Conclusion
The results suggest that the test formulation of diclofenac sodium 75 mg/mL has an AUC0–t and AUC0–∞ comparable with the reference intragluteal formulation of diclofenac sodium 75 mg/3 mL, but with an earlier time to reach maximum plasma concentration and a trend towards a higher maximum plasma concentration. This could be attributed to faster absorption from the deltoid region than from the gluteal region. The test formulation could be helpful in the management of pain in obese or overweight patients and those with dense subcutaneous fat in the gluteal area.
Introduction
Injectable nonsteroidal anti-inflammatory drugs are commonly used to reduce postoperative pain. They are inexpensive and have been tested over time. They have also been reported to lessen postoperative narcotic requirements and narcotic side effects, including respiratory depression and nausea.Citation1,Citation2
Diclofenac sodium injection is effective and well tolerated in the management of postoperative painCitation1–Citation3 and is currently marketed worldwide in a 3 mL injectable intramuscular formulation. It is generally administered intragluteally. However, the standard needles used may not reach the gluteus maximus muscle in patients who are obese.Citation4–Citation7
For optimal effect following intramuscular injection it is important that the drug be delivered to the muscle. Problems can arise if drugs designed to be absorbed from muscle are delivered into subcutaneous tissue. Increasing obesity in developed and many developing countries makes this an important concern.Citation7 Other injection sites, such as the deltoid, have been suggested and could be more suitable for these patients. The currently available 3 mL formulation of injectable diclofenac precludes intradeltoid injection due to its larger injection volume.
A new propylene glycol-free and reduced-volume injection of diclofenac sodium has been developed. By using a combination of three classes of solvents containing monohydric and polyhydric alcohols and a polyhydric alcohol ether in combination with water as the principal solvent, it has become possible to dissolve 75 mg of diclofenac in just 1 mL of injection solution without a substantial increase in viscosity. This new formulation, when administered via the deltoid muscle, was observed to have a bioavailability comparable with the intragluteal formulation of diclofenac sodium 75 mg/3 mL in healthy adult Indian subjects.Citation8
The bioavailability of this new formulation has not been defined in obese subjects. The World Health Organization has revised the body mass index (BMI) cutoff for Asian Indians, and suggested a BMI of 25 kg/m2 to define obesity rather than the 30 kg/m2 recommended for Europeans.Citation9
The objective of the present study was to determine the pharmacokinetics of the new reduced-volume intradeltoid formulation of diclofenac sodium 75 mg/mL injection and to compare its bioavailability with that of the intragluteal 75 mg/3 mL formulation in adult male Indian subjects with a BMI > 25.
Material and methods
The study was carried out in 10 volunteers with a BMI > 25 at the B.V. Patel Pharmaceutical Education and Research Development Centre, Ahmedabad, India. All subjects provided written informed consent to participate in the study prior to enrolment, and were free to withdraw at any time during the study. The study was approved by the Institutional Ethics Committee and was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki.
Subjects
The study population consisted of 10 adult male subjects with a BMI > 25 (mean 26.85, range 25.30–31.16), of mean age 32.3 (25–39) years, mean weight 76.5 (68–89) kg, and mean height 168.7 (163–173) cm.
Design
This study was an open-label, randomized, single-dose, two-way, crossover, comparative bioavailability study that assessed the two injectable formulations of diclofenac under fasting conditions, during two separate dosing periods, with a washout period of seven days between the two periods. The volunteers were administered each of the two study drugs after an overnight fast. Dose administration was performed as per the randomization schedule generated at the B.V. Patel Pharmaceutical Education and Research Development Centre, Ahmedabad, India. Subjects received single doses of the intradeltoid test formulation (diclofenac 75 mg/mL, Troikaa Pharmaceuticals Ltd., India) and the intragluteal reference formulation (Voveran®, diclofenac 75 mg/3 mL, Novartis, India). Intramuscular injections were administered using BD PrecisionGlide needles (Becton Dickinson India Pvt. Ltd., India, 23 G 0.6 × 25 mm) and BD 2 mL Discardit II™ syringes.
Blood sampling
Following administration of the test/reference formulation in each period, a total of 16 blood samples of 6 mL each were collected before dosing and at 10, 20, 30, 40, and 50 minutes, and 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, and 6.0 hours following drug administration. During each session, an indwelling catheter was inserted into a forearm vein. Samples were collected in tubes containing lithium heparinate and immediately centrifuged at 4° C. Plasma was separated and frozen at − 70° C until further analysis.
Method of analysis
After the addition of 0.05 mL internal standard (mefenamic acid) 25 μg/mL, 0.5 mL plasma samples were acidified with 0.05 mL 6% trichloroacetic acid. The drug was extracted into dichloromethane 5 mL. The dichloromethane layer was separated and evaporated under nitrogen gas, and then reconstituted in a 0.1 mL mobile phase. A 0.06 mL solution was injected into the column of a high-pressure liquid chromatography system (Jasco 900 series, Japan) equipped with a PU 980 pump, AS 950 autosampler, and UV 975 detector. Separations were achieved using a Grace Vydac 5 μm ODS (4.6 × 250 mm) column (Separations Group Inc, W.R. Grace & Co., Columbia, MD, USA) with a mobile phase consisting of acetonitrile and 0.01 M, pH 6.6 potassium dihydrogen orthophosphate buffer (40:60, % v/v) at a flow rate of 0.8 mL/min under ultraviolet detection at 282 nm. The samples were analyzed at 30° C with a linear range of 0.1–6 μg/mL (y = 0.3572x + 0.0019; r = 0.999), with an average recovery of 68%. The intraday and between-day coefficients of variation (%CV) of all the quality control samples were <5% and <4%, respectively. The accuracy of the method was between 90% and 110%. The lowest value on the calibration curve was the lower limit of quantitation, ie, 0.1 μg/mL, and the limit of detection was 0.025 μg/mL.
Pharmacokinetic analysis
The pharmacokinetic parameters measured include the observed maximum plasma concentration (Cmax), time to reach Cmax (Tmax), and the area under the plasma concentration- time curve from 0 hours to the time point of last measurable concentration (AUC0–t) and 0 hours to infinity (AUC0–∞). The Cmax and Tmax were directly determined from the plasma concentration versus time curves. The AUC0–t from time zero to the last quantifiable point (Ct) was calculated using the trapezoidal rule, and the extrapolated AUC from Ct to infinity (AUC0–∞) was determined as Ct/kl. AUC0–∞ was calculated as the sum of the AUC0–t plus the ratio of the last measurable concentration to the elimination rate constant (k). Logarithmic transformation was done before data analysis for Cmax, AUC0–t, and AUC0–∞. Analysis of variance (ANOVA) was used to assess effects. Intrasubject variability in terms of the overall %CV was evaluated from the ANOVA results for Ln-transformed data. For the pharmacokinetic parameters Cmax, AUC0–t, and AUC0–∞, 90% confidence intervals (CI) for the ratios of test and reference product averages were calculated using ANOVA of the Ln-transformed data. Consistent with the two one-sided tests for bioequivalence, 90% CIs for the ratio of both the product averages were calculated by first calculating the 90% CI for the differences in the averages of the Ln-transformed data and then taking the antilogarithms of the CI obtained.
Safety and tolerability
General clinical safety was assessed via physical examination and vital signs at screening and at the end of the study. Clinical laboratory tests and electrocardiograms were also conducted at screening, before dosing within each treatment period, and at the end of the study. Adverse events were assessed for severity and relationship to treatment throughout the study.
Results
Analytic method
A reverse-phase high-pressure liquid chromatographic method was developed to determine the bioavailability of diclofenac after administration of the two formulations. The calibration range was selected based on expected body concentrations. The method was specific and selective for the analyte, and good linearity was observed within the range. Sufficient recovery was obtained by extraction under acidic conditions in dichloromethane. The precision and accuracy of the method made it suitable for the intended use.
Pharmacokinetic parameters
The mean plasma concentration-time profiles of diclofenac sodium following administration of single doses to 10 obese volunteers are shown in , and a summary of the pharmacokinetic parameters is presented in . Mean Cmax and mean Tmax for the test formulation were 1.30 ± 0.18 μg/ mL and 0.50 ± 0.16 hours, respectively, and 0.93 ± 0.14 μg/ mL and 1.08 ± 0.31 hours for the reference formulation. Peak plasma concentrations reported in the literature have ranged from 1.89 to 2.15 μg/mL following an intramuscular injection of diclofenac 75 mg.Citation8,Citation10 Variable values of Tmax have been reported, ranging from 3.1 to 6.4 hours for diclofenac sodium with different formulations in healthy subjects.Citation11
Figure 1 Linear plot of mean plasma concentrations (μg/mL) versus time profile of diclofenac sodium for test and reference formulations in 10 obese (BMI > 25) male subjects under fasting conditions.
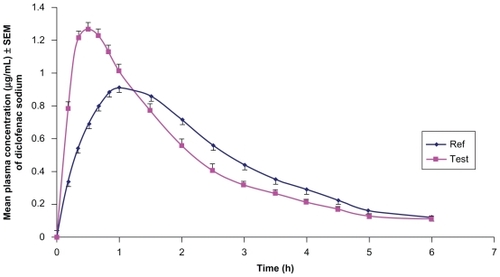
Table 1 Mean pharmacokinetic parameters in 10 obese male volunteers following intragluteal administration of the reference formulation (diclofenac sodium 75 mg/3 mL) and test formulation (diclofenac sodium 75 mg/1 mL)
The mean AUC0–t for the test and reference formulations was 2.71 ± 0.39 μg·h/mL and 2.73 ± 0.49 μg·h/mL, respectively. The mean AUC0–∞ for the test and reference formulations was 3.71 ± 0.52 μg·h/mL and 3.75 ± 0.58 μg·h/mL, respectively. AUC0–t and AUC0–∞ values are in line with those we have reported earlier for healthy volunteers.Citation8
The mean elimination rate constant kel and mean t1/2 for the test and reference formulations were 0.11 ± 0.02 h− 1 and 6.30 ± 1.27 hours, and 0.12 ± 0.02 h− 1 and 5.93 ± 1.11 hours respectively. The point estimate, 90% and 95% CI, and summary of statistics are tabulated in and , respectively.
Table 2 Point estimate, 90% and 95% confidence intervals for the ratio of the product averages of test and reference formulations
Table 3 Summary statistics of diclofenac sodium in 10 obese adult subjects under fasting conditions
The statistical analysis revealed no significant differences between the test and reference formulations for Cmax, AUC0–t, and AUC0–∞, suggesting that the pharmacokinetic profile is similar between the two formulations. The %CV corresponding to intrasubject variability was 9.18%, 5.92%, and 7.95% for Cmax, AUC0–t, and AUC0–∞, respectively. The means (90% CI) of the Cmax, AUC0–t, and AUC0–∞ for the test:reference ratios were 1.39 (129.8, 151.3), 0.993 (94.6, 104.4), and 0.989 (92.9, 106.08), respectively.
Safety and tolerability
All 10 subjects completed the study, during which there were no premature withdrawals or deaths. No serious adverse events were recorded, and there were no clinically significant changes in vital signs, clinical laboratory variables, electrocardiographic parameters, or physical examination findings during the study.
Discussion
Depending on the depth of fat, intramuscular injections using standard 35 mm and 25 mm needles may be injected subcutaneously in a significant number of patients, and not into the gluteal musculature. This could alter the pharmacokinetics of the administered medication. Hence, an alternative injection site should probably be chosen to increase the success rate of intramuscular deposition of medication.Citation7 The deltoid muscle has been suggested as an alternative site for intramuscular drug administration. Several reports have suggested that this is a better site for injection than the gluteal musculature.Citation12,Citation13
Based on deltoid fat pad thickness determination, it has been observed that for men weighing 59–118 kg, use of a 25 mm needle would result in at least 5 mm of muscle penetration in all subjects. For women weighing less than 60 kg, a 16 mm needle would be sufficient to achieve muscle penetration of 5 mm. For women weighing 60–90 kg, a 25 mm needle would be sufficient, and women weighing more than 90 kg would require a 38 mm needle to enable intramuscular administration.Citation14 Similar observations were reported by Cook et al,Citation15 who suggested that in all males and females with a BMI < 35, intramuscular injection into the deltoid could be achieved with a 25 mm needle, whilst in females with a BMI > 35, a 35 mm needle is required. Thus, standard needles would reach the muscle in patients by intradeltoid administration.
To address the need for an intradeltoid route to inject diclofenac sodium, a new formulation containing 75 mg/mL was developed. We have previously reported that the new formulation of injectable intradeltoid diclofenac 75 mg/mL is bioequivalent to the intragluteal diclofenac sodium 75 mg/3 mL reference formulation.Citation8
All 10 subjects completed the study and were included for both statistical and analytic analysis. Based on repeated-measures ANOVA, subject, period, treatment, and interaction term (period × treatment) showed a nonsignificant difference. The P values suggest that there is no statistically significant difference. The 90% CIs for all the pharmacokinetic parameters were within bioequivalence acceptance criteria, with the only exception being Cmax, for which the upper bound was above the 125% limit.
With regard to the extent of absorption, the AUC0–t and AUC0–∞ were comparable between the test and reference formulations. Mean Cmax was higher for the test formulation, but this difference was not statistically significant. Tmax was earlier for the test formulation than for the reference formulation (0.50 hours versus 1.08 hours, respectively). Earlier Tmax and slightly higher Cmax may be attributed to the depth of subcutaneous fat in the gluteal region and better blood flow to the deltoid than to the gluteus muscle.Citation16
Diclofenac has been reported to be associated with gastrointestinal, cardiovascular, and hepatic side effects.Citation17 The most common side effects following diclofenac injection are gastrointestinal and pain at site of injection.Citation18 Mild to moderate adverse events have been reported in about 5% patients with renal colic following intramuscular diclofenac.Citation19 Only minor gastrointestinal side effects have been reported following intramuscular diclofenac postoperatively.Citation20 In the present study, both formulations were well tolerated, and no adverse events were reported.
Conclusion
Our results suggest that the test formulation of diclofenac sodium 75 mg/mL has a comparable AUC0–t and AUC0–∞ but an earlier Tmax and a trend towards a higher Cmax in comparison with the reference diclofenac sodium 75 mg/3 mL formulation. This could be attributed to faster absorption from the deltoid region than from the gluteal region. The test formulation, which can be given by the intradeltoid route using standard needles, would be helpful in the management of postoperative pain and other painful conditions. This formulation would be especially useful in obese or overweight patients and those with dense subcutaneous fat in the gluteal region, in whom intramuscular injections into the gluteus musculature using standard needles may fail to reach the muscle.
Acknowledgments
We are grateful to Troikaa Pharmaceuticals Ltd., Ahmedabad, India, for providing the drug samples, and to Ms Rita Rana and the clinical staff of B.V. Patel Pharmaceutical Education and Research Development Centre, Ahmedabad, India, for their assistance with the pharmacokinetic study.
Disclosure
The authors report no conflicts of interest in this work.
References
- BourlertADiclofenac intramuscular single dose to decrease pain in post operative Caesarean section: A double blind randomized controlled trialJ Med Assoc Thai2005881151915960211
- Al-WailiNSEfficacy and safety of repeated postoperative administration of intramuscular diclofenac sodium in the treatment of post-cesarean section pain: A double-blind studyArch Med Res200132214815411343813
- RautomaPSantanenULuurilaHPreoperative diclofenac is a useful adjunct to spinal anesthesia for day-case varicose vein repairCan J Anesth200148766166411495873
- ZaybakAGünesUYTamselSDoes obesity prevent the needle from reaching muscle in intramuscular injections?J Adv Nurs200758655255617484745
- ChanVOColvilleJPersaudTIntramuscular injections into the buttocks: Are they truly intramuscular?Eur J Radiol200658348048416495027
- NisbetACIntramuscular gluteal injections in the increasingly obese population: Retrospective studyBMJ2006332754263763816524934
- BurbridgeBEComputed tomographic measurement of gluteal subcutaneous fat thickness in reference to failure of gluteal intramuscular injectionsCan Assoc Radiol J2007582727517521050
- OjhaAShepDNivsarkarMPatelSJaiswalVPadhHPharmacokinetic profile of a new formulation of injection diclofenac designed for intradeltoid useExpert Opin Pharmacother200910451752219239400
- The Asia Pacific perspective: Redefining obesity and its treatment Regional Office for the Western Pacific of the World Health OrganizationWorld Health Organization, International Association for the Study of Obesity and International Obesity Task Force.St Leonards, AustraliaHealth Communications Australia Pty Limited2000
- KurowskiMPharmacokinetics and biological availability of diclofenac preparations following intramuscular injection of 75 mg and oral administration of 150 mg of active drugZ Rheumatol198847137423369245
- NawazRNawazMButtAZafarMSaleemiUKinetics of diclofenac sodium; single oral dose disposition in male volunteersThe Professional2004114411416
- GrabinskiPYKaikoRFRogersAGHoudeRWPlasma levels and analgesia following deltoid and gluteal injections of methadone and morphineJ Clin Pharmacol198323148556841658
- SaxenaAGraceJJosieLRisperidone long-acting injections: Successful alternative deltoid muscle injections for refractory schizopheniaJ Psychiatry2008594042
- PolandGABorrudAJacobsonRMDetermination of deltoid fat pad thickness. Implications for needle length in adult immunizationJAMA199727721170917119169899
- CookIFWilliamsonMPondDDefinition of needle length required for intramuscular deltoid injection in elderly adults: An ultrasonographic studyVaccine200624793794016191454
- EvansEFProctorJDFratkinMJVelandiaJWassermanAJBlood flow in muscle groups and drug absorptionClin Pharmacol Ther197517144471122668
- AithalGPDayCPNonsteroidal anti-inflammatory drug-induced hepatotoxicityClin Liver Dis200711356357517723920
- PrabhakarHShiraoSAShelgaonkarVCComparative evaluation of intramuscular ketoprofen and diclofenac sodium for post-operative pain reliefInternet Journal of Anesthesiology2006111
- FragaAMoreira-da-SilvaVSeveroLIntramuscular etofenamate versus diclofenac in the relief of renal colic: A randomised, single-blind, comparative studyClin Drug Investig20032311701706
- HynesDMcCarrollMHiesse-ProvostOAnalgesic efficacy of parenteral paracetamol (propacetamol) and diclofenac in post-operative orthopaedic painActa Anaesthesiol Scand200650337438116480474