Abstract
Fibromyalgia appears to present in subgroups with regard to biological pain induction, with primarily inflammatory, neuropathic/neurodegenerative, sympathetic, oxidative, nitrosative, or muscular factors and/or central sensitization. Recent research has also discussed glial activation or interrupted dopaminergic neurotransmission, as well as increased skin mast cells and mitochondrial dysfunction. Therapy is difficult, and the treatment options used so far mostly just have the potential to address only one of these aspects. As ambroxol addresses all of them in a single substance and furthermore also reduces visceral hypersensitivity, in fibromyalgia existing as irritable bowel syndrome or chronic bladder pain, it should be systematically investigated for this purpose. Encouraged by first clinical observations of two working groups using topical or oral ambroxol for fibromyalgia treatments, the present paper outlines the scientific argument for this approach by looking at each of the aforementioned aspects of this complex disease and summarizes putative modes of action of ambroxol. Nevertheless, at this point the evidence basis for ambroxol is not strong enough for clinical recommendation.
Introduction
Fibromyalgia syndrome (FMS) is a chronic, undegenerate symptom complex that is characterized by chronic widespread pain and evoked pain at tender points. Other common symptoms include insomnia, depression, fatigue, stiffness, and gastrointestinal disorders.Citation1–Citation3 Approximately 2%–5.8% of the population of industrial countries suffer from FMS,Citation1,Citation4–Citation9 and 80%–90% of patients are female. Although FMS is classified as a noninflammatory disorder, there is increasing evidence for changes in inflammatory mediators,Citation10–Citation15 and a disturbed balance in pro- and anti-inflammatory cytokines is being discussed.Citation12,Citation16–Citation18 In addition, it is also considered a stress-related-disorder with dysfunction of the hypothalamic–pituitary–adrenocortical axis.Citation19–Citation21 Furthermore, increases in oxidative stress and toxic metabolites of lipid peroxidation have been shown for FMS.Citation22–Citation24 It has been proposed that fibromyalgia could be a sympathetically maintained neuropathic pain syndrome.Citation25 Moreover, it has been suggested that dorsal root ganglia and peripheral sensory neuron sodium channels may play a major role in fibromyalgia pain transmission.Citation26
In previous publications, we described the successful topical treatment of neuropathic painCitation27,Citation28 and nociceptive painCitation29 with ambroxol cream in a case series. Furthermore, not only have we observed beneficial topical and oral individual treatment results in FMS (–; Kern KU. Data on file. Personal clinical observations. 2011–2017) but also other investigators have observed similar effects using oral ambroxol,Citation30,Citation31 both of which certainly could be regarded as placebo effects at this stage. Ambroxol is a secretolytic substance, but may also potentially influence several pathophysiological mechanisms involved in fibromyalgia. First, ambroxol interferes with oxidative stress and influences cytokines and inflammation.Citation32,Citation33 Second, ambroxol blocks sodium channels,Citation34 especially the tetrodotoxin-resistant (TTX-r) channel subtype Nav1.8,Citation34–Citation36 which is expressed particularly in spinal ganglion cellsCitation37 and in nociceptive, sensory neurons.Citation37–Citation40 This should limit central sensitization in chronic widespread muscle pain,Citation41 which clearly also occurs in FMS.Citation42 Based on these effects, ambroxol may be an interesting treatment approach for FMS, even if detailed examinations concerning these single mechanisms remain to be performed and an influence of ambroxol on inhibitory descending pain pathways, important in FMS, has not yet been examined. The present paper outlines the scientific argument for the treatment of fibromyalgia using ambroxol by looking at many different aspects of this complex disease and summarizes putative modes of action (–, ).
Figure 1 Individual development of FIQ and NRS in four responders to oral ambroxol for fibromyalgia.
Abbreviations: FIQ, Fibromyalgia Impact Questionnaire; NRS, numeric rating scale (0–100).
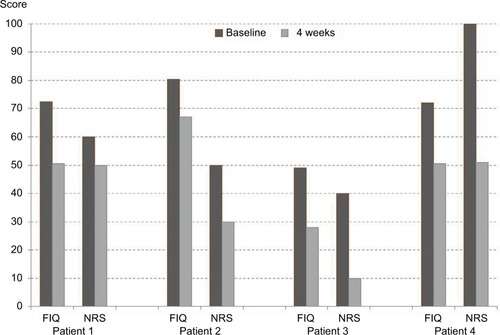
Figure 2 Passage of time of fibromyalgia pain reduction.
Abbreviation: NRS, numeric rating scale (0–10).
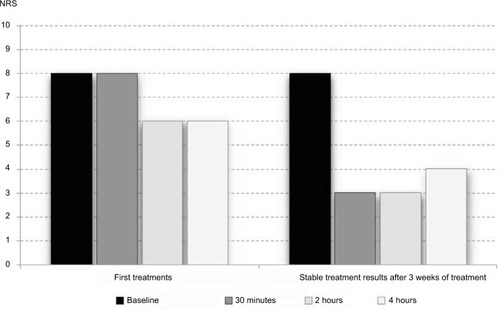
Figure 3 Passage of time of fibromyalgia pain reduction.
Abbreviation: NRS, numeric rating scale (0–10).
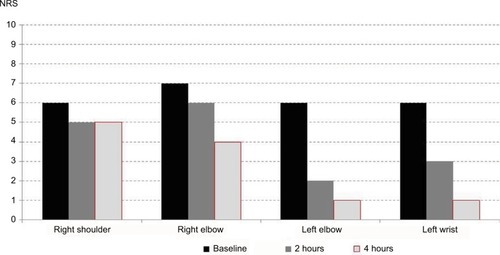
Figure 4 Mechanisms involved in fibromyalgia and influenced by ambroxol (see –).
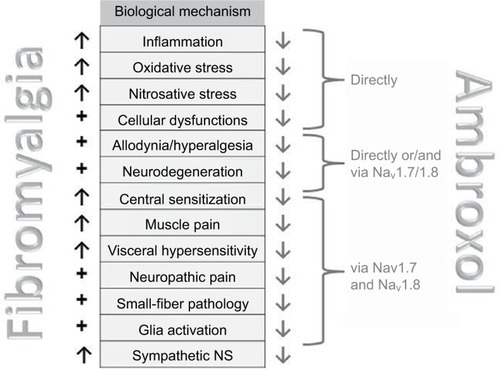
Table 1 Reported inflammatory and oxidative changes in fibromyalgia, explaining biological pain induction, and potential helpful modes of action of ambroxol
Table 2 Reported nociceptive and CNS changes, cellular dysfunction, and accompanying symptoms in fibromyalgia
Table 3 Relevance of sodium channels and corresponding therapeutic approaches
Skin, mitochondria, and mast cells
Skin condition
Salemi et alCitation43 detected IL1β, IL6, and TNFα in skin biopsies of a subgroup of approximately 30% of FMS patients, but not in control subjects. This finding was interpreted as the presence of inflammatory foci indicating neurogenic inflammation, which might be the reason for the efficacy of nonsteroidal anti-inflammatory therapy, which has occasionally been reported. IL1β,Citation44,Citation45 IL6,Citation44,Citation46,Citation47 and TNFαCitation44–Citation46,Citation48–Citation52 are inhibited by ambroxol. Blanco et alCitation53 demonstrated an increased number of mast cells in FMS patients, the secretion of which was also inhibited by ambroxol.Citation54–Citation56 Other skin biopsies have shown significant mitochondrial dysfunction and an increased level of oxidative metabolites, in conjunction with inflammatory signsCitation57,Citation58 correlated with pain.Citation57 Ambroxol also improves mitochondrial dysfunctionCitation59–Citation61 and oxidative stress.Citation44,Citation60,Citation62–Citation65 Uçeyler et alCitation66 investigated the gene expression of the proinflammatory cytokines TNFα, IL6, and IL8 and the anti-inflammatory IL10 in skin biopsies of 25 FMS patients, compared these to patients with depression and healthy controls, and found no detectable differences. The results did not support the hypothesis of these cytokines being involved in the sensitization of peripheral nerves in the skin. In one of the most comprehensive investigations with skin biopsies, FMS patients had reduced intraepidermal nerve-fiber density compared to controls, which supports the view that the pain syndrome in a subgroup of FMS patients is partially of neuropathic origin.Citation67 In vitro and in vivo investigations have demonstrated that ambroxol can relieve neuropathic pain.Citation28,Citation29,Citation68–Citation71 Our clinical practice observations have shown pain relief in FMS following some oral treatments or topical application of ambroxol 20% cream (–; Kern KU. Data on file. Personal clinical observations. 2011–2017), which according to the aforementioned relationships need not necessarily be attributed solely to the local anesthetic properties of the compound, especially when improved over time ().
Whole-body cryotherapy, beneficial in a subgroup of FMS patients,Citation72 works primarily via impact on the skin. This therapeutic approach stabilizes lysosomal membranes,Citation73 among others, and reduces the negative effects of proteins of lysosomal enzymes. Ambroxol has a comparable effect. The compound significantly enhances reduced enzyme activity of the lysosomal glucosylceramidase (in Parkinson’s disease),Citation74–Citation76 as well as α-galactosidase A (in Fabry’s disease), α-glucosidase (in Pompe’s disease),Citation77 and β-glucocerebrosidase (in Gaucher’s disease).Citation78,Citation79 At least for the aforementioned diseases, ambroxol is thus clearly an enzyme-modifying therapeutic option. Reduction of many enzymes is also present in FMS.Citation23,Citation24,Citation80–Citation82 Low activity of the enzyme prolyl endopeptidase in serum is even supposed to have predictive diagnostic value.Citation82 The possibility of enhancement of this specific enzyme activity by ambroxol should thus be investigated.
Mitochondria
Mitochondrial dysfunction in FMS has been demonstrated in skin biopsies,Citation57,Citation58 blood,Citation83 and muscle cells, and may explain muscular pain.Citation84 If such mitochondrial dysfunction also occurs in neurons of the central nervous system (CNS), this could contribute to general hypersensitivity and chronic widespread pain.Citation84 The inflammatory components of FMS have also been regarded as an expression of mitochondrial dysfunction, and thus an improvement in mitochondrial function may be a new therapeutic approach.Citation85 In turn, ambroxol has an impact on mitochondria: it inhibits lipid peroxidation in hepatic mitochondria by 96%,Citation59 prevents toxic increase in mitochondrial membrane permeability,Citation60 and in animal models improves mitochondrial oxidative damage.Citation61
Another investigation also pointed to mitochondrial dysfunction: stimulation of mononuclear cells of healthy subjects resulted, as expected, in significantly increased cytokine levels in contrast to unstimulated cultures. In FMS patients, however, the concentrations of most cytokines were lower. Behm et alCitation11 interpreted this observation as an impairment of cell-mediated immunity in FMS patients. On the other hand, there are findings that ambroxol could protect immunocompetent cells from dysfunctionCitation86 and appears to strengthen cell-mediated immunity.Citation87
Mast cells
In comparison to healthy subjects, patients with FMS have more mast cells in the skin.Citation53,Citation88 The significance of this finding for the pathogenesis of FMS has been classified as unclear by some authors,Citation88 whereas others have used this as a basis for classifying FMS as a mast cell-associated disorder.Citation53 If this latter interpretation were to hold true, the fact that ambroxol inhibits secretion from mast cellsCitation54–Citation56 would be of considerable importance. At least in pain models on ischemia/reperfusion, there is clearly a close relationship between cardiac mast cells and C-fibers.Citation89 Furthermore, mast cells play an important role in chronic urticaria, and in one study a surprising 70% of 126 urticaria patients also suffered from FMS. Torresani et alCitation90 discussed whether neuropeptides released owing to degranulation of increased numbers of mast cells in FMS patients may stimulate nerve endings, and chronic urticaria may thus occur as a result of skin neuropathology in FMS. Recently, it was demonstrated that cortiocotropin-releasing hormone and substance P are increased in FMS and stimulate release of IL6 and TNFα from mast cells.Citation91 Both IL6Citation44,Citation46,Citation47 and TNFαCitation44–Citation46,Citation48–Citation52 are reduced by ambroxol. However, there are open questions remaining: therapeutic use of the mast-cell stabilizer ketotifen does not show significant differences between groups with regard to pain and Fibromyalgia Impact Questionnaire (FIQ) scores, which raises the question whether mast cells do play a major role in FMS.Citation88
Chronic psychological, oxidative, and nitrosative stress
Chronic stress and cortisol
Since it is still not clear how chronic stress influences visceral and somatosensory pain regulation, both types of hyperalgesia were investigated in an animal model: the authors demonstrated that chronic stress also led to upregulation of the Nav1.8 channel.Citation92 It was shown that both visceral and somatosensory hyperalgesia and the increased expression of Nav1.8 normalized after 3 days without stress: this related to sodium channels in the dorsal root ganglion (DRG) neurons of those segments, which are responsible for the pelvic viscera.Citation92 This may for example explain the associated visceral symptoms in FMS, and in turn suggest a therapeutic approach using ambroxol with its selective Nav1.8 blockade. This applies even more if FMS is considered a stress-mediated disorder,Citation5,Citation93 in which the overexpression of Nav1.8 is not further downregulated and a receptor blockade would gain even greater importance.
Since pain and fatigue as core symptoms of FMS are also characteristic of disorders with reduced cortisol levels, it has been hypothesized that there may also be reduced cortisol levels (caused by fatigue?) in FMS. Although glucocorticoid tests in 12 female FMS patients showed no reductions in daytime cortisol profile in comparison to 15 controls, they did however show reduced sensitivity of glucocorticoid-receptor function; this was considered a pathophysiologically relevant finding for FMS by Geiss et al.Citation94 In this context, the fact that the anti-inflammatory potency of ambroxol is comparable to dexamethasoneCitation46,Citation51,Citation95 and beclomethasoneCitation96 without requiring glucocorticoid receptors is not necessarily relevant, but nevertheless worthy of note.
Oxidative stress
The findings concerning oxidative stress in fibromyalgia are currently still inconsistent. In particular, it is not clear whether the disease is caused by oxidative stress.Citation97 Enhanced oxidative stress mediated by free radicals is however evident in FMS and leads to increased cytokine expression. There is much evidence that suggests that increased oxidative stress leads to increased severity of FMS symptoms.Citation81,Citation97 In particular, a positive correlation has been observed between FIQ and increased lipid peroxidation.Citation81 Malondialdehyde is a toxic metabolite of lipid peroxidation, and significantly increased levels of this metabolite have repeatedly been found in patients with FMS.Citation22–Citation24 Ambroxol inhibits this harmful lipid peroxidation.Citation59,Citation98,Citation99 Furthermore, the enzyme xanthine oxidase correlates with the severity of muscular pain in FMSCitation100 and is also reduced by ambroxol.Citation45 A similar relationship has been shown for other antioxidative substances: decreased levels of catalase have been shown for FMS,Citation80,Citation81 and these levels are enhanced by ambroxol.Citation62,Citation101 The same applies to glutathione peroxidase,Citation45 which is also decreased in FMS patients and enhanced by ambroxol.Citation80,Citation81 There are apparently lower levels of the intracellular antioxidative enzyme superoxide dismutase in FMS patients;Citation23,Citation24,Citation80 ambroxol can also lead to increased levels of this enzyme.Citation45,Citation98,Citation101–Citation104 Skin biopsies of FMS patients also show increased levels of oxidative metabolites that correlated with the severity of pain and inflammation.Citation57 Both are relevant for the development of peripheral nerve damage, which has also been observed in FMS and may be the cause of allodynia. Since investigations on DRG neurons of mice recently suggested that nociceptor hyperexcitability induced by oxidative stress is primarily mediated via sensitization of the ambroxol-inhibited Nav1.8-channel type,Citation105 Schlüter and LefflerCitation106 investigated the influence of the strong oxidant chloramine T. They confirmed these findings, which were more pronounced for the Nav1.8 than for the Nav1.7 subtype, which however is also inhibited by ambroxol.Citation107
In summary, the balance of oxidants and antioxidants appears to be disturbed in FMS, and increased levels of free radicals are possibly responsible for development of the disease.Citation24,Citation80 Fibromyalgia can thus also be understood as an oxidative disorder.Citation24 Understandably, rheumatologists are requesting further investigations into the effects of radical scavengers,Citation100 and ambroxol is known to be one such scavenger.Citation44,Citation60,Citation62–Citation65
Oxidative stress and lipid peroxidation do not only occur in FMS and depression. Some of the products resulting from these processes are in addition predictors for neurode-generation; this may be the reason for associations of both indications with neuropathic pain.Citation108 Oxidative damage of DNA may be important in this context.Citation109 As a strong radical scavengerCitation44,Citation60,Citation62–Citation65 and inhibitor of lipid peroxidation,Citation59,Citation98,Citation99 ambroxol may thus counteract neurodegenerative changes during disease progression in FMS.
Both ambroxol and melatonin are able to protect from lipid peroxidation.Citation110 Melatonin levels that are too low may have a negative impact in FMS.Citation111 Since melatonin is one of the targets of the latest strategies in the development of drugs for FMS,Citation112,Citation113 and this is based on being a radical scavenger that functions like a strong antioxidant,Citation114 the same may also apply to ambroxol.
Nitrosative stress
Nitrosative stress is caused by reactive nitrogen species, eg, nitrogen monoxide (NO) and its product peroxynitrite. These harmful and highly reactive nitrogen compounds are involved in cellular dysregulation. It is assumed that nitrosative stress is involved in neurological and inflammatory disorders. This has also been demonstrated for FMS.Citation84,Citation115 It has been suggested that NO is involved in the pathophysiology of FMS,Citation97,Citation116 may be responsible for pain sensitivity,Citation117 and correlates with pain severity.Citation118 In addition, NO levels correlate with the FIQ score.Citation119 On this basis, Cimen et alCitation120 have requested the search for inhibitors of nitric oxide synthase (NOS) for FMS treatment, since this enzyme catalyzes the (unfavorable) formation of NO. The same effect, however, is also achieved with ambroxol: the compound inhibits the production and activity of NO.Citation44,Citation121–Citation123
Sex hormones
Since FMS primarily affects women, there is reason to presume that sex hormones play an important role. Estradiol (E2) has a key function in pain modulation. The effects of E2 are mediated via estrogen receptors (ERs).Citation124,Citation125 ERs (ERα, ERβ) and Nav1.8 may be expressed in DRG neurons. In knockout mice for ERβ, Nav1.8 is upregulated,Citation124 and in addition voltage-gated sodium channels are inhibited by E2.Citation125 In principle, hormone deficiency may thus contribute to hyperexcitability in fibromyalgia. Hormone-replacement therapy, however, does not lead to an improvement in symptoms,Citation126 and sex-hormone deficiency has not been demonstrated for FMS.Citation127,Citation128 Nevertheless, ambroxol is able to inhibit experimentally upregulated Nav1.8 sodium channelsCitation34–Citation36 or those sodium channels that are functionally insufficiently blocked by E2.Citation34 The compound is an approximately 12-fold stronger inhibitor of Nav1.8 than lidocaine and 40-fold stronger if neuronal sodium channels in general are considered.Citation36 Of note, lidocaine has already been used successfully for FMS.Citation129–Citation132,Citation336
Muscular pain
Both peripheral and central sensitization processes are involved in the transition from acute to chronic muscular pain.Citation133–Citation135 One of the currently leading theories suggests that acute stimulation of specific nociceptors binding isolectin B4 (IB4) may lead to long-term hypersensitivity of nociceptors. Consequently, a lasting increase in TTX-r sodium-channel activity (such as Nav1.8) is required, in order to achieve long-term changes in intracellular signalling.Citation136 Nav1.8 inhibition with ambroxol would in this case be a preventive approach. Recent studies again confirmed the importance of IB4-positive muscular nociceptors for chronic muscular pain,Citation137,Citation138 thereby confirming older and similar research results.Citation139,Citation140 Tissue hyperacidity in muscles owing to ischemia and inflammation has a decisive impact on the initiation and progression of chronic muscular pain.Citation141,Citation142 Acid-sensing ion channel (ASIC)-3 and transient receptor-potential cation-channel subfamily V, member 1 are involved in the activation of muscular nociceptors, the induction of central sensitization, and chronic muscular pain.Citation143–Citation145 ASIC3 has been demonstrated to play a major role in triggering acid-induced chronic muscular pain.Citation139,Citation146 Its activation again increased Nav1.8 activity, with essential development of long-lasting hyperalgesia and chronic widespread muscular pain in a mouse model of fibromyalgia.Citation41 Since to date, ASIC3 cannot be specifically blocked, Chen et alCitation41 considered selective blockade of Nav1.8 a good treatment option for chronic muscular pain with ischemic conditions.
According to their own reports, patients affected by FMS in the USCitation147 and GermanyCitation148 had only minor benefit from anti-inflammatory treatment. Correspondingly, in their microdialysis investigations in muscles of FMS patients, Christidis et alCitation149 detected no changes in the proinflammatory cytokines IL1β, IL6, IL8, or TNFα. In contrast, another cytokine, MCP1, not only occurs with increased levels in the blood of fibromyalgia patientsCitation150 but is also supposed to induce persistent muscular hyperalgesia and chronic sensitization.Citation151 Should this be of relevance for FMS, ambroxol may again be of therapeutic benefit, since it can contribute to a reduction in MCP1.Citation51,Citation95,Citation152 Muscular pain in FMS patients is also explained by mitochondrial dysfunction in muscular cells.Citation84 As just described, this could also be improved by ambroxol.Citation59–Citation61 Furthermore, the ambroxol-reduced oxidative–toxic enzyme xanthine oxidaseCitation45 correlates with muscular pain severity in FMS.Citation100
Neuropathic pain and small-fiber pathology
The latest research on FMS pain has shown that at least in a subgroup of patients, a neuropathic component is involved.Citation67,Citation153–Citation155 Changes in small nerve fibers and a high PainDetect score suggest this,Citation156 even though this questionnaire has not been validated for the disease.Citation155 In a comparison of diabetic polyneuropathy with FMS, approximately 30% of patients showed an overlap of sensory profiles, whereas other distinct profiles were disease-specific.Citation156 Furthermore, it is noteworthy that many drugs used for the treatment of FMSCitation157 are also used for neuropathic pain.Citation158
There is increasing knowledge in particular about changes in small nerve fibers. In this respect, Uçeyler and SommerCitation159 and Doppler et alCitation160 considered it important to use the term “small-fiber neuropathology” and distinguish this from “small-fiber neuropathy”. Interestingly, Doppler et alCitation160 demonstrated significantly reduced average axon diameters in skin biopsies of 32 FMS patients compared to 12 patients with small-fiber neuropathy and 40 healthy controls. It appears that quite different pathophysiological mechanisms lead to the development of small-fiber degeneration and/or regeneration.Citation66,Citation161 In FMS, not only changes in peripheral small fibers but also in the eye (which belongs to the CNS) occur.Citation162,Citation163 Controlled investigations with skin biopsiesCitation67 and laser-evoked potentialsCitation164 showed reduced intraepidermal nerve-fiber density in FMS patients compared to healthy controls, and thereby also support the theory of at least a partial neuropathic origin of pain. As mentioned earlier, we were able to report clinical efficacy of topical ambroxol for neuropathic pain in previous publications;Citation27–Citation29,Citation165 however, experimentally there is also no doubt that ambroxol exerts systemic effects as well.Citation34,Citation69–Citation71 In small-fiber neuropathy, primarily small unmyelinated peripheral neurons are damaged; in other words, nociceptive C-fibers of the skin primarily expressing Nav1.8.Citation37–Citation40,Citation166–Citation168 In animal models, approximately 50% of the C-fibers express just these Nav1.8 channels that are inhibited by ambroxol,Citation166 and their numbers even increase under painful conditions.Citation167,Citation168 In addition, at least in patients with pure small-fiber neuropathy, gain-of-function mutations of Nav1.8 have been detected.Citation169–Citation172 Furthermore, Nav1.8 can be increasingly expressed in case of distal degeneration of small-diameter peripheral axons and thus contribute to central sensitization.Citation171 Owing to its mechanism of action, ambroxol can be expected to provide some protection from this type of sensitization in FMS.
Finally, and as an indication for neuropathic pain involvement, patients with FMS show low tolerance of cold water,Citation173 whereas the ambroxol-inhibited Nav1.8 channel is of particular importance for cold pain.Citation38,Citation174 In the animal model, ambroxol suppressed cold allodynia by approximately 75%.Citation69
Central sensitization, allodynia, and hyperalgesia
Central sensitization
It is widely accepted among researchers that the biological component of FMS is associated with long-term or even permanent functional changes of the nociceptive nervous system.Citation175,Citation176 A systematic review on central sensitization in fibromyalgia evaluated 13 studies concerning functional changes (via functional magnetic resonance imaging). Nociceptive stimuli led to more pronounced but otherwise comparable activation of the pain matrix in FMS patients compared to controls.Citation42 Eight studies investigating structural changes (via voxel-based morphometry) provided moderate evidence for a correlation between central sensitization and a decrease in gray matter in certain regions.Citation42 In their experiments with thermal stimulation, Vierck et alCitation177 demonstrated abnormally prolonged sensitivity in FMS patients, which again was interpreted as an indication of central sensitization and a specific influence of widespread chronic pain from deep somatic tissue. Visceral hyperalgesia, somatosensory hyperalgesia, and increased expression of Nav1.8 are closely associated.Citation92 Correspondingly, Nav1.8-selective antagonists (other than ambroxol) have analgesic efficacy in acid-induced chronic widespread-pain modelsCitation178 and lead to a reduction in allodynia and hyperalgesiaCitation179 in animal models of neuropathic and inflammatory pain. Following experiments in a fibromyalgia animal model, Chen et alCitation41 thus generally considered selective Nav1.8 blockers, one of which was ambroxol, as a good choice of treatment of chronic pain and for limitation of central sensitization.
Allodynia and hyperalgesia
Allodynia and hyperalgesia are common signs in FMS.Citation180–Citation182 Sleep deprivation can cause these signs,Citation183 as well as oxidative stress, mitochondrial dysfunction, and inflammation, with the consequence of peripheral nerve damage.Citation57 Functional brain-imaging studies have provided compelling evidence for abnormal pain processing in FMS correlating with patients’ hyperalgesia or allodynia.Citation184 FMS patients experience prickling and touch-evoked allodynia at the same frequency as patients with diabetic polyneuropathy.Citation156 Furthermore, FMS patients show lower heat-pain and cold-pain thresholds than controls,Citation185,Citation186 and severe thermal allodynia following cutaneous heat exposure has been reported.Citation187 Systemic ambroxol, however, suppressed heat hyperalgesia by 100% in an animal model.Citation69
Pain symptoms in FMS animal models are more likely associated with dysfunction of biogenic amine-mediated CNS pain control compared to pain due to nerve injuries.Citation188 However, rats in an FMS model showed hypersensitivity to tactile muscle pressure and cold stimuli. Once again in an animal model, ambroxol reduced cold hyperalgesia and mechanical allodynia by approximately 75%.Citation69 The observation that ambroxol also reduces mechanical allodynia in an experimentally induced inflammation in rats by approximately two-thirdsCitation68 suggests that the antiallodynic analgesic effect is not necessarily restricted to neuropathic pain. It is indeed possible to reduce mechanical allodynia in monoarthritis pain with ambroxol by 50%.Citation69
The Nav1.8 channel is detected mainly in C- or Aδ-fibers and neurons of the posterior horn,Citation37–Citation40 although it is also expressed in Aβ-fibers.Citation68,Citation174,Citation189–Citation191 Since in chronic inflammation, which is also discussed for FMS, the excitability of Nav1.8 is shifted to hyperpolarization, this contributes to allodynia, and a blockade using ambroxol should then have a particularly pain-relieving effect. For completeness, it should not go unmentioned that the intrathecal administration of ambroxol has also led to an antiallodynic effect in animal experiments.Citation68 Furthermore, simultaneous therapy with ambroxol reduces heat and cold hyperalgesia due to oxaliplatin in an animal model, which the authors felt to be transferable to humans.Citation192 In summary, there is plenty of evidence for a reduction in FMS hyperalgesia or allodynia following ambroxol treatment.
Sympathetic nervous system, glia, and dopamine
Sympathetic nervous system
One indication of sympathetic nervous system involvement in FMS was detected in a subgroup of obese female FMS patients by Okifuji et al.Citation193 They found a strong correlation between body-mass index and levels of the sympathomimetic epinephrine and IL6. The latter agent is reduced by ambroxol.Citation44,Citation46,Citation47 Investigations into heart-rate variability have shown persistent excessive sympathetic activity in FMS.Citation194 Norepinephrine injections can induce FMS pain.Citation195
In 2009, Martinez-Lavin and SolanoCitation196 presented a hypothesis on FMS in which sodium channels play a major role, and the authors suggested that sodium-channel blockers could become a therapeutic option for FMS pain. This renders the sodium-channel blocker ambroxol interesting for therapy: sodium channels localized in DRGs have a molecular gatekeeper function for impulses from peripheral nociceptors. Trauma, infection, or other factors may induce neuroplasticity via overexpression of sympathetic fibers and sodium channels in DRGs. The authors considered enhanced DRG excitability to play a key role in FMS pain. Since DRGs are potential sites of sympathetic–nociceptive short circuits, individuals who are genetically predisposed for sympathetic hyperactivity and those with inherent sodium channelopathies would be at risk of developing FMS. In addition, stressful environmental conditions in today’s society could possibly contribute to sympathetic hyperactivity, and anti-inflammatory vagus-nerve activity might not be sufficient to counteract this. If FMS is interpreted in this context as a sympathetically maintained neuropathic pain syndrome, sodium-channel blockers gain importance as a therapeutic option for FMS pain.Citation196 At least, the sodium channel Nav1.8, which is selectively blocked by ambroxol, is of importance in the sympathetic nervous system. Schofield et alCitation197 demonstrated that Nav1.8 occurs on the sympathetic superior cervical ganglion and can be blocked. Facer et alCitation198 demonstrated the presence of Nav1.8-immunoreactive sensory nerve fibers in the human myocardium, which are – interestingly with regard to sympathetic function – frequently closely associated with small capillaries.
Glia activation and dopamine
Apart from obviously enhanced sympathetic activity, FMS patients also have increased IL8 levels in cerebrospinal fluid,Citation199,Citation200 which in principle can be reduced by ambroxol.Citation96,Citation201–Citation205 Kadetoff et alCitation200 interpreted their findings to be a result of FMS symptoms being mediated by sympathetic activity, rather than being dependent on prostaglandin-associated mechanisms, and considered this supportive of the hypothesis of glia-cell activation in response to pain mechanisms.Citation200 Interestingly, intrathecal administration of ambroxol leads to an antiallodynic effect in an animal model without having an impact on peripheral swelling caused by inflammation.Citation68 Moon et alCitation71 also concluded that after intrathecal administration of ambroxol that early treatment with an Nav1.8 inhibitor may be an important factor in the clinical management of chronic mechanical allodynia during inflammatory or ischemic pain.Citation71
Enhanced levels of IL8 have the potential to activate glia cells.Citation206 Activated glia cells in turn can also produce new IL8,Citation207 which again promotes sympathetically maintained pain.Citation208 In addition, activated glia cells can produce IL1β as a result of proinflammatory stimuli,Citation209,Citation210 and IL1β is also reduced by ambroxol.Citation44,Citation45 Recent research has shown that glia cells maintain neuronal hypersensitivity in DRGs by releasing substances that also act on the immune system.Citation211 In addition to peripheral changes, persistent glial activation with resulting central sensitization is also of importance in FMS, which in turn is activated by cytokines from repeated tissue injury.Citation17,Citation212 Albrecht et alCitation213 considered glial activation in the brains of FMS patients, which was demonstrated via imaging procedures (positron-emission tomography and magnetic resonance imaging) to be being important in the pathophysiology of the disease. In another investigation, 126 fibromyalgia patients were genotyped and subgroups formed with regard to their binding affinity to translocator protein (TSPO), which is upregulated during glial activation. Those patients with high TSPO-binding affinity reported significantly more pain and FMS symptoms, which again supports glia-related mechanisms in FMS.Citation214 This fits with the observation that naltrexone, an inhibitor of microglial activity in the CNS, reduced FMS symptoms in some patients in a small pilot study.Citation215
A permanent and robust increase in microglia population also contributes to an overexpression of α-synuclein, a small soluble protein in the brain of vertebrates which, among other actions, regulates the release of dopamine.Citation216 Su et alCitation217 demonstrated that α-synuclein in addition also activates microglia, thereby contributing to the release of proinflammatory molecules. This finding has been supported by other authors.Citation218 The release of α-synuclein from affected neurons was also increased in an animal model of CNS injury with ischemia–reperfusion, thereby mediating microglia activation.Citation219 The protein has neurotoxic effects, and not only leads to the microglia activation described but also to increased dopaminergic neurodegeneration.Citation220 Research on the pathophysiology of fibromyalgia is increasingly focusing not only on glia activation but also on the neurotransmitter dopamine. Experimental induction of FMS has demonstrated decreased dopamine levels in both the brain and the spinal cord.Citation221 Imaging procedures, however, have pointed to dopamine dysfunction as an important factor in increased pain sensitivity in FMS.Citation222 Other authors have also considered dopamine an important neurochemical moderator of FMS pain perception, since their data suggested interrupted dopaminergic neurotransmission in FMS.Citation223 It is thus plausible that dopamine receptors are investigational targets for new FMS medications.Citation113 It should be pointed out that in this respect, ambroxol leads to a reduction in α-synuclein,Citation224 ie, reduces just that protein that contributes to both glia activation and dopaminergic neurodegeneration.Citation220 For this reason, the medication has also been considered for the treatment of Parkinson’s disease.Citation74–Citation76,Citation224
Neurodegeneration and neuroregeneration
A systematic review on imaging studies revealed indications of structural changes in the CNS of fibromyalgia patients.Citation42 The neurodegenerative findings of small-fiber neuropathology mentioned earlier are not restricted just to the peripheral nervous system either, but have also been reported for the cornea (cranial nerve V)Citation162 and axonal nerve injury early in the progression of the disease in the retina of FMS patients,Citation163 which belongs to the CNS. It is generally accepted that the regenerative capacity of injured nerves in the CNS is markedly worse than in the peripheral nervous system. Therefore, it is remarkable that neuroregenerative properties in the CNS have recently been described for ambroxol.Citation225 During a systematic genetic search for suitable treatment options promoting regenerative neuronal growth, Chandran et alCitation225 found that ambroxol was not just the only one of the tested substances causing eight gene expressions in treated DRG neurons, but also enhanced axonal sprouting from these. Furthermore, they were able to demonstrate real neuroregeneration in the CNS by ambroxol in an optical nerve model in vivo: studies using knockout mice confirmed that systemically administered ambroxol significantly and morphologically improved regeneration of the optic nerve.Citation225 It has to be pointed out, though, that despite the fact that ambroxol obviously crosses the blood–brain barrier,Citation79,Citation226 brain levels could be too low to cause relevant effects under currently used therapeutic dosages.Citation227 This reduces potential side effects, and also a therapeutically desired effect. Whether the mother substance bromhexine, which definitively crosses the blood–brain barrier without CNS side effects,Citation228 could be of additional benefit remains unanswered.
At least in ischemia-induced neurodegeneration, reactive oxygen species have a key function, and ambroxol is able to contribute to the reduction of such ischemia-caused nerve injury.Citation229 Oxidative stress and lipid peroxidation occur not just in fibromyalgia and depression. Some of the products resulting from these processes are also predictors of neurodegeneration.Citation108 As a strong radical scavenger and inhibitor of lipid peroxidation, ambroxol should under these circumstances counteract neurodegenerative changes during the progression of FMS. This effect of ambroxol has been demonstrated at least for polyneuropathy caused by oxaliplatin.Citation192 Oxaliplatin also leads to an increase in inflammatory mediators and oxidative stress, and is thus peripherally neurotoxic. Simultaneous treatment with oral ambroxol in these animal models reduces relevant neuropathic pain, and as a result decreases heat and cold hyperalgesia, and both of these symptoms have also been reported for FMS.Citation154,Citation156,Citation185,Citation186 The authors considered these results transferable to humans.
Sodium channels
There is some evidence that sodium channels are important in FMS. In an investigation of 73 female FMS patients, genetic Nav1.7 polymorphism was associated with severe fibromyalgia.Citation26 The receptor is assumed to play an important role in pain transmission in DRG neurons in FMS.Citation196 Nav1.7 subtypes,Citation170,Citation230–Citation233 as well as Nav1.8 mutations,Citation171,Citation234 are also associated with small-fiber neuropathy, and at least one small-fiber pathology appears to be present in a subgroup of FMS.Citation159,Citation160 Although there have been reports of Nav1.7 gain-of-function mutations and even more evidently hypothalamic dysfunction, it is not known whether or not this channel subtype plays a functional role in the hypothalamus with regard to external stressors in man. At least experimentally, however, it can be demonstrated that Nav1.7 is upregulated in the CNS in parallel with osmotic stressCitation235 and that oxidative stress leads to sensitization of Nav1.8.Citation106 In gain-of-function mutations of the SCN10A gene, which encodes for Nav1.8, symptoms with diffuse painful sensory neuropathy, autonomic symptoms and gastrointestinal dysfunctionCitation170,Citation171,Citation234,Citation236 resemble FMS symptoms and are associated with hyperexcitability of DRG neurons.Citation230 Selective sodium-channel blockers are currently unavailable for routine clinical practice.Citation237 As presented herein, quite a few medications used for fibromyalgia cause (among other actions) sodium-channel blockade, even though this is aspecific.
More than 500 randomized controlled trials (RCTs) on the treatment of fibromyalgia were already available in 2008. The strongest recommendations of several medical societies were for various antidepressants.Citation238 It is remarkable that many tricyclic antidepressants, selective serotonin-reuptake inhibitors, and serotonin–norepinephrine reuptake inhibitors also block sodium channels.Citation239 For instance, duloxetine is beneficial for FMSCitation157,Citation238 and blocks both Nav1.7 and Nav1.8.Citation240,Citation241 The sodium-channel blockade of duloxetine is stronger than that of venlafaxine, which in turn was only attributed minimal effects in a systematic review.Citation242 Amitriptyline, which has received a strong recommendation for FMS,Citation157,Citation238 also blocks Nav1.7Citation239,Citation243,Citation244 and Nav1.8,Citation244 or rather generally TTX-r channels (to which Nav1.8 belongs) in trigeminal neurons, DRG neurons, and gastrointestinal neurons.Citation245–Citation247 On the other hand, paroxetine shows less effect in FMS,Citation157,Citation248 and in comparison to amitriptyline only blocks Nav1.7 at high concentrations.Citation239 Furthermore, gabapentin, which was recommended in a data analysis by CochraneCitation249 also blocks Nav1.7,Citation250,Citation251 and pregabalin, which was also classified as beneficial,Citation157,Citation249 reduces paroxysmal neuropathic itch in patients with a variant of the SCN9A gene, which encodes for Nav1.7.Citation252 Even ibuprofen, which is often preferred by patients,Citation157 blocks the channel subtypes Nav1.7Citation253–Citation255 and Nav1.8 after systemicCitation255 and topical administration.Citation254 Finally, tramadol, which is recommended as second-line treatment,Citation157 also blocks sodium channels.Citation256,Citation257 An interesting fact in this respect is that at least peripheral analgesia with opioids is partly mediated via µ-receptors on primary afferent Nav1.8-positive neurons.Citation258
Although much evidence points to the importance of sodium channels in FMS and promising RCTs have been conducted, the relevance of sodium channel-blocking anti-epileptic drugs cannot be confirmed: in a systematic review, Wiffen et alCitation249 found no valid indications that the sodium-channel blockers of this group of substances achieved above-average therapeutic results in FMS. It tends to be forgotten, however, that to date generally, no specific analgesics for the blockade of the main targets Nav1.7 and Nav1.8 are available for treatment, and for this very reason could not be assessed in this review. Thus far, none of the compounds used for neuropathic pain (including local anesthetics, antidepressants, and antiepileptics) shows relevant selectivity for Nav1.8 that would be comparable to ambroxol.Citation34,Citation35 Should the blockade of Nav1.8 and/or Nav1.7 be an important treatment approach for FMS, efficacy of ambroxol is very likely: not only Nav1.8 but also Nav1.7 is selectively blocked by ambroxol,Citation107,Citation259 and this blockade is even more pronounced in man than in rats.Citation107
Inflammatory mediators
Cytokines
Independent of sodium-channel blockade or antineuropathic effects, ambroxol should be able to reduce nociceptive pain via its anti-inflammatory properties. This has also been reported by us for topical ambroxol in a case series of pain patients.Citation29 In addition, it has been shown in humans for acute sore throatCitation260,Citation261 and experimentally demonstrated.Citation32,Citation33,Citation46,Citation68,Citation69,Citation262 Ambroxol exerts its comprehensive anti-inflammatory properties, for example, via inhibition of many proinflammatory cytokines.Citation32
The general importance of cytokines in the induction and maintenance of pain has been well demonstrated in both animal models and pain syndromes in humans,Citation263 including FMS.Citation12,Citation13,Citation264 Cytokines can also contribute to the origin of pain in the CNS, and spinal cytokines can exert an external impact on peripheral pathology by influencing the efferent neuronal system, with effects on peripheral tissue.Citation265 They are also important mediators of neuropathic pain,Citation266–Citation268 which is also reduced by ambroxol (as already reported). Cytokines also have an impact on changes in the hypothalamic–pituitary–adrenocortical axis,Citation269–Citation271 and thereby on clinical symptoms, such as hyperalgesia, fatigue, sleep disorders, allodynia, adrenocortical hormone-associated disorders, stress responses, anxiety, muscular pain, and cognitive dysfunction;Citation13,Citation200 all these are symptoms associated with FMS.Citation19–Citation21 The diverse and well-documented impact of ambroxol on cytokines is likely to be of major relevance.
Interleukin 6 and interleukin 8
In particular proinflammatory and thus pain-inducing IL6 and IL8, which are both reduced by ambroxol, have clinical relevance in FMS. During the past 10 years, approximately 100 of 140 studies on FMS have demonstrated changes in inflammatory mediators and associated these with the pathogenesis and clinical signs of the disease. Several studies observed increased serum levels of IL6Citation14,Citation199,Citation200,Citation272 or IL8.Citation199,Citation200,Citation273–Citation275 A systematic review conducted in 2011 reported evidence for higher serum levels of these cytokines, as well as for IL1RA in FMS, but no confirmed changes in IL1β, IL4, IL10, MCP1, or TNFα.Citation12
Even before the observation of a real correlation of the intensity of the disease with IL6 und IL8 levels, these were repeatedly reported as being associated with the clinical symptoms of FMS.Citation13,Citation276,Citation277 For instance, Mendieta et alCitation276 demonstrated that both IL6 and IL8 correlated with clinical psychiatric scores, and considered these interleukins as particularly constant inflammatory mediators in FMS, with their levels significantly correlating with the severity of symptoms.
However, serum concentrations do show large variability, as demonstrated in a systematic analysis by Uçeyler et al.Citation12 In particular, a disturbed balance of proinflammatory and anti-inflammatory cytokines is likely to play a role in the origin and maintenance of FMS-related pain.Citation263 Their pathophysiological role continues to be disputed, though.Citation12,Citation16,Citation17 In contrast to other authors, Ranzolin et alCitation278 did not discover differences in IL6 or IL8 in FMS patients compared to healthy controls in a recent prospective controlled study. Reasons for many partially contradictory findings concerning cytokines are multiple impact factors, such as circadian rhythmicity and influences from depression, physical activity, and infections, which were frequently not clearly assessed in the studies. In addition, drugs can have an impact on cytokines, such that cytokines vary in subgroups or during the progression of the disease. In a systematic investigation of ambroxol for the treatment of fibromyalgia, these factors will definitely need to be considered, at least for this partial effect of the compound.
Interleukin 6
During the last 10 years, numerous studies have demonstrated higher serum levels of IL6 in FMS,Citation14, Citation91,Citation199,Citation200,Citation272 and this has been confirmed in a systematic review and meta-analysis.Citation12 Since IL6 not only has algesic effects but is also involved in the development of hyperalgesia,Citation279 fatigue, and depression,Citation13,Citation14 it can be assumed to have a role in the modulation of FMS symptoms.Citation208 As it is very difficult to limit neuronal hyperexcitability caused by IL6, this interleukin obviously plays a major role during the chronification process and in the poor response of some pain conditions to treatment.Citation280 With robust data on increased IL6 levels in FMS, there are also equally robust data confirming that ambroxol reduces both the release and levels of IL6.Citation44,Citation46,Citation47,Citation205 In a model on acute lung injury, this was demonstrated with comparable significance to dexamethasone-treated animals.Citation46
Interleukin 8
In the aforementioned review, Uçeyler et alCitation12 also demonstrated higher serum and plasma levels of IL8. These findings were repeatedly confirmed thereafter.Citation199,Citation200,Citation273,Citation274,Citation277 Ang et alCitation150 found a significant correlation of increased IL8 levels with pain severity using the Brief Pain Inventory (BPI): they were able to correlate each change in pain intensity according to BPI with a corresponding increase in IL8. Using a highly sensitive method, Xiao et alCitation277 supported the assumption of inflammatory changes in FMS by demonstrating an elevated level of the inflammatory marker CRP in 105 FMS patients compared to 61 healthy controls. The elevated CRP values also showed a significant correlation with IL8 levels. Furthermore, Sturgeon et alCitation93 demonstrated a significant correlation between IL8 levels and pain catastrophizing, anxiety, and postmenopausal depression. IL8 was also associated with pain and sleep disorders.Citation273 In a comparison of cerebrospinal fluid findings in rheumatoid arthritis and FMS, Kosek et alCitation199 demonstrated higher IL8 levels in FMS patients. Kadetoff et alCitation200 also demonstrated higher cerebrospinal fluid and serum concentrations of IL8 in fibromyalgia, an overall constellation that the authors interpreted as an expression of sympathetic activity. In an animal model, Moon et alCitation71 correspondingly showed that intrathecal ambroxol inhibited mechanical allodynia and thermal hyperalgesia in a dose-dependent manner. It can be assumed that a reduction in IL8 is involved: in vitro as well as in vivo, both the release and detectable concentrations of IL8 are reduced by ambroxol, a fact that has been repeatedly shown.Citation47,Citation96,Citation201–Citation205 This is probably an important finding concerning this “perhaps most important interleukin” in FMS.
IL1-receptor antagonist
IL1RA is an inhibitor produced by the body that slows down and finally stops the action of the proinflammatory IL1 and IL1β by binding at their site on the IL1 receptor. Increased serum levels of IL1RA in FMS have been demonstrated in many studies;Citation12,Citation199 however, the proinflammatory interleukins “to be regulated” – IL1 and the highly reactive IL1β – do not at all appear to show elevated serum levels in FMS.Citation12,Citation200 In contrast to this, however, Imamura et alCitation281 detected similarly elevated levels of IL1β in a comparison of FMS patients to osteoarthritis patients, with comparable duration of disease and pain intensity. Using questionnaires and plasma analyses of 50 FMS patients, Menzies et alCitation19 demonstrated a negative correlation between subjective stress and IL1β levels. Therefore, possibly just the fact that no elevated levels of IL1 or IL1β can be detected is an expression of severe FMS symptoms or for long duration of the disease. For instance, this may be the reason that elevated levels of IL1β in skin have indeed been detected, but only in a subgroup of FMS patients.Citation43 The impaired balance between IL1 and IL1RA remains to be clarified. It is a fact, however, that ambroxol has a major impact: it has been well demonstrated to reduce IL1Citation44,Citation45,Citation48,Citation51 and IL1β,Citation44,Citation45 and thus should have a positive preventive effect, at least in cases of initially elevated levels, if present.
Interleukins 4 and 10
Investigations have shown decreased levels of the anti-inflammatory and thus “analgesic” cytokines IL4 and IL10 in FMS in comparison to healthy controls.Citation18 In a 2011 review, however, the same research group could not detect clear evidence of serum differences in these two interleukins.Citation12 Mendieta et alCitation276 also recently reported no relevant changes in serum levels of IL10 in FMS.
In contrast to this, other authors have demonstrated elevated IL10 levelsCitation13,Citation278,Citation281,Citation282 and a significant correlation of these with FIQ scores.Citation283 IL10 is also increased in the cerebrospinal fluid of FMS patients in comparison to patients with rheumatoid arthritis.Citation199 Under ambroxol treatment in experimental stimulation of human alveolar macrophages, IL10 was not elevated, in contrast to IL12,Citation87 and the same applied after bacterial stimulation.Citation284 Ambroxol thereby promoted a reduced cytokine response after exogenous inflammation and strengthened cell-mediated immunity by shifting the “local balance” toward IL12.Citation87 Following exposure to allergens in an artificially sensitized respiratory tract in an animal model, however, ambroxol induced an increase in IL10 in a “protective” manner.Citation285
Yigit et alCitation286 genotyped 300 FMS patients and 270 healthy controls with regard to IL4 for specific polymorphism of the IL4 gene. They detected highly significant differences, suggesting that IL4 may be a suitable genetic marker for FMS. Investigations of various authors, however, led to inconsistent results by reporting decreased IL4 levels,Citation18,Citation287 no change in IL4 levels,Citation288 or increased IL4 levelsCitation199 in FMS. In an investigation on human mast cells, even very low dosages of ambroxol inhibited anti-IgE-induced release of IL4.Citation54
Monocyte chemotactic protein 1
MCP1 (formerly called CCL2) in human monocytes acts in an anti-inflammatory fashion by inhibiting the development of proinflammatory cytokines. Not only have some investigations on fibromyalgia shown elevated levels of MCP1,Citation150,Citation274 but it also induced dose-dependent chronic mechanical hyperalgesia for up to 6 weeks in an animal model.Citation151 In their interpretation of the results, the authors suggest that MCP1 induces persistent muscular hyperalgesia and thereby chronic sensitivity toward other proalgesic substances. Ang et alCitation150 reported elevated levels of MCP1 in FMS and demonstrated significant correlations of each change with pain severity measured using the BPI. They thus presumed that MCP1 is involved in the pathogenesis of FMS. MCP1 was also elevated in 25 FMS patients with an “altered stress response” compared to healthy controls.Citation274 There is, however, possibly a negative clinical correlation with subjective, actually perceived stress.Citation19 The importance of this finding has been emphasized by genetic investigations, in which markedly elevated MCP1 levels were detected in an FMS subpopulation with a specific mutation.Citation289
If MCP1 is indeed of importance in FMS, patients might benefit from treatment with ambroxol. In an animal model, inhaled ambroxol reduced MCP1.Citation51,Citation95 The effect of ambroxol by inhalation at 7.5 mg/mL was comparable to 0.5 mg/kg intraperitoneal dexamethasone. Again, potent effects comparable to cortisone have been demonstrated.Citation95 In another animal model with several control groups, ambroxol was also able significantly to reduce experimentally elevated MCP1 for 28 days.Citation152
Inflammasomes
Recent studies identified inflammasomes, cytosolic protein complexes in macrophages and neutrophil granulocytes, as promoters of classical cytokine-mediated inflammatory processes.Citation290 The NLRP3 inflammasome is assumed to be activated in FMSCitation291,Citation292 and is considered a new therapeutic target.Citation293 Inflammasomes are obviously inhibited in their activity if reactive oxygen species (“oxygen radicals”) are reducedCitation290 and activated by mitochondrial dysfunction,Citation292 both of which are presumed to be present in FMS.Citation57 Since ambroxol is a strong radical scavengerCitation44,Citation60,Citation62–Citation65 and improves mitochondrial dysfunction,Citation59–Citation61 it should also have an impact on this newly described pathomechanism.
Interleukin 13, interleukin 5, and immunomodulation
Following secondary data analysis, Sturgill et alCitation287 reported a remarkable reduction of IL13 in FMS patients. This interleukin is produced by T lymphocytes, stimulates the differentiation of B lymphocytes, and is generally considered a central mediator in allergic reactions. In cases where decreased IL13 levels have to be discussed as missing anti-inflammatory components, ambroxol would apparently exacerbate this condition: the release of IL13 is reduced by ambroxol.Citation54 Ambroxol also reduces IL13 if administered prior to experimentally produced hyperreactivity of the airways and subsequent exposition to allergens; however, this is not the case if administered afterward. Interestingly, overall this had a beneficial and protective effect.Citation285 This applies similarly to IL5, which has a positive chemotactic action on eosinophilic granulocytes: Sturgill et alCitation287 also demonstrated a reduction of IL5 in FMS. Ambroxol also has an inhibiting effect concerning IL5, and if administered prior to provocation in an animal model, suppressed hyperreactivity and airway eosinophilia and reduced inflammation of subepithelial regions.Citation285 The relationships described raise the question of whether potent inhibition of the release of IgE-dependent mediatorsCitation294 and immunomodulatory cytokines from basophilic granulocytes by ambroxol,Citation54 as well as the immunomodulatory significance of Nav1.8 sodium-channel inhibition by ambroxol,Citation295 are important in FMS and warrant further investigation.
Symptoms associated with fibromyalgia
Patients with fibromyalgia also suffer from hypersensitive visceral organs. Symptoms of overactive bladder,Citation296 for instance, occur more frequently in fibromyalgia patients and depend on the severity of the disease. These can be assessed using the fibromyalgia bladder index.Citation297 Patients with chronic interstitial cystitis or painful bladder disorders, on the other hand, show an above-average presence of fibromyalgia.Citation298 According to investigations on rat bladders by Drewa et al,Citation299 ambroxol is also able to suppress bladder contractions; they thus considered the compound a therapeutic option for the treatment of overactive bladder.
In similar fashion, irritable bowel syndrome (IBS) is also associated with fibromyalgia: FMS patients suffer more often from this disease,Citation300 and FMS is found more often in patients with IBS.Citation301 Besides new insights concerning the potential importance of Nav1.1 for IBS,Citation302 especially the Nav1.8 receptor, which is selectively blocked by ambroxol, is again of importance: in general, investigations with Nav1.8-free mice and Nav1.8-inhibiting compounds showed lower (also visceral) hyperexcitability or a reduction of hyperexcitability under treatment.Citation68,Citation174,Citation178,Citation179,Citation303–Citation306 Knockout mice without the Nav1.8 receptor not only show little visceral pain but also no referred hyperalgesia whatsoever following stimuli in the colon.Citation307 Furthermore, Nav1.8 mutations can be associated with gastrointestinal dysfunction.Citation234 Since in animal models particularly, colon DRG neurons exhibit Nav1.8-mediated increase in activity of the sodium channels, Hu et alCitation308 considered this mechanism specific for chronic visceral pain and IBS. Selective Nav1.8 blockade (such as by ambroxol) is thus considered clinically beneficial for visceral pain.Citation309
According to a review, major influence of the sympathetic rather than the parasympathetic nervous system has been deemed responsible for fibromyalgia-associated symptoms,Citation310 again with sequelae that might be addressed with ambroxol and have already been discussed elsewhere. Another association that is clinically not quite as important, but nevertheless fits into the concept is the fact that FMS patients more frequently suffer from dry eyes, and the prevalence of FMS in patients with Sjögren’s syndrome is increased by a rate of 12%–31%.Citation311–Citation313 Ambroxol leads to an increase in tear-fluid secretionCitation314 and can improve sicca symptoms.Citation315
Safety, dosage, and onset and duration of effect
In vitro, the onset of Nav1.8 blockade by ambroxol starts within a few seconds, is concentration-dependent, and fully reversible.Citation34 In paraplegic rats, hypersensitivity to static mechanical stimuli was reduced after approximately 30 minutes for approximately 3 hours.Citation70 In earlier topical treatments, the effect reported by patients persisted for well over 6 hours.Citation27,Citation29 The anti-inflammatory effect should increase over time.
In most countries, ambroxol has been sold as an over-the-counter drug for decades, owing to its good safety profile, and in 2015 the European Medicines Agency reassessed the clinical benefit:risk ratio of the drug. The selective sodium-channel blockade of the Nav1.8 channel, which is only insignificantly expressed in the heart and the CNS, is in this case clinically beneficial. After systemic administration, ambroxol was also safe: even intravenous administration of 1 g (in order to boost prenatal lung maturation and for the treatment of atelectasis) is well tolerated.Citation316,Citation317 There are individual reports of dosages of up to 3 g per day over 53 daysCitation318–Citation320 and oral administration of 1.3 g ambroxol per day over 33 days.Citation321 In a recent pilot study, ambroxol was used orally at dosages of 25 mg/kg/day up to 1,300 mg/day for Gaucher’s disease and showed good safety and tolerability,Citation79 and in an ongoing study it is being used at 1,050 mg/day for Parkinson’s disease.Citation76 Even in an RCT with children under 1 year of age with acute respiratory distress syndrome, no adverse events were noted with ambroxol up to 40 mg/kg/day.Citation322
With its good bioavailability of about 80%Citation323 and plasma levels linearly correlated with oral dosage,Citation69 dosages very likely could be used tenfold higher (or even more) than actually used (up to 120 mg/d) for potential systemic trials for the treatment of fibromyalgia pain without risk. As many ambroxol effects apart from selective Nav1.8 sodium-channel blockade develop more intensely over a longer period, we consider treatment for more than 4–6 weeks desirable before evaluation. However, this should not be a problem, because ambroxol has already been administered clinically at 90 mg for 3 monthsCitation52 and even at 75 mg twice daily without any problems for 6 monthsCitation324 and also at 75 mg twice daily for long-term treatment of 1 year.Citation325 A clinical trial investigating the treatment of FMS with ambroxol could even use a design comparable to an ongoing study, which is designed for 52 weeks using 225–1,050 mg/day for another indication.Citation76
Conclusion
Overall, we think potential RCTs with FMS patients should examine the impact of ambroxol on pain, hypersensitivity, and inflammation at dosages higher then yet approved for the over-the-counter market and for at least 6 weeks. An increasing effect should be expected and possibly could be evident clinically not before two weeks of treatment. An impact on dysfunctional descending pain pathways should not be expected, so patients with a clear response to a medication for this (indicating this special origin of pain perception) might possibly report less benefit. As it is surprising that the single substance ambroxol has so many unexpected effects on FMS-related mechanisms, the chemical properties (eg, structure and affinity) and related substances (eg, the mother substance bromhexine) could also be worth examining further.
Summary
Fibromyalgia appears to present in subgroups concerning biological pain induction with primarily inflammatory,Citation12,Citation13,Citation264 neuropathic/neurodegenerative,Citation153–Citation155 sympathetic,Citation193,Citation194,Citation196 oxidative,Citation24,Citation81,Citation97 or muscular factorsCitation84,Citation132,Citation134 and/or central sensitization.Citation42,Citation175,Citation176 On the basis of this hypothesis, fibromyalgia treatment with ambroxol should be systematically investigated, since this compound is the only treatment option used thus far that has the potential to address not just individual but all of the aforementioned aspects of pain. Nevertheless, at this point, the evidence base for ambroxol is currently not strong enough for clinical recommendation.
Disclosure
In the past 3 years, KUK has worked as a consultant and/or speaker for the following companies (*offering ambroxol): Astellas, AstraZeneca, Bionorica, Berlin Chemie,* Boehringer Ingelheim,* Betapharm, Genzyme, Grünenthal,* Hexal,* Indivior, Kyowa Kirin, Lilly, Mundipharma,* Ratiopharm,* and Sanofi.* MS reports no conflicts of interest in this work.
References
- WolfeFSmytheHAYunusMBThe American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria CommitteeArthritis Rheum19903321601722306288
- ArnoldLMClauwDJMcCarbergBHImproving the recognition and diagnosis of fibromyalgiaMayo Clin Proc201186545746421531887
- HäuserWZimmerCFeldeEKöllnerVWas sind die Kernsymptome des Fibromyalgiesyndroms? Ergebnisse einer Umfrage der deutschen Fibromyalgie Verband. [What are the key symptoms of fibromyalgia? Results of a survey of the German Fibromyalgia Association]Schmerz2008222176183 German18210165
- WolfeFClauwDJFitzcharlesMAFibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgiaJ Rheumatol20113861113112221285161
- WolfeFBrahlerEHinzAHäuserWFibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general populationArthritis Care Res2013655777785
- BrancoJCBannwarthBFaildeIPrevalence of fibromyalgia: a survey in five European countriesSemin Arthritis Rheum201039644845319250656
- AblinJNOrenACohenSPrevalence of fibromyalgia in the Israeli population: a population-based study to estimate the prevalence of fibromyalgia in the Israeli population using the London Fibromyalgia Epidemiology Study Screening Questionnaire (LFESSQ)Clin Exp Rheumatol2012306 Suppl 743943
- LawrenceRCFelsonDTHelmickCGEstimates of the prevalence of arthritis and other rheumatic conditions in the United States: part IIArthritis Rheum2008581263518163497
- Torgrimson-OjerioBRossRLDieckmannNFPreliminary evidence of a blunted anti-inflammatory response to exhaustive exercise in fibromyalgiaJ Neuroimmunol20142771–216016725457842
- GarciaJJCidonchaABoteMEHinchadoMDOrtegaEAltered profile of chemokines in fibromyalgia patientsAnn Clin Biochem201451Pt 557658124106344
- BehmFGGavinIMKarpenkoOUnique immunologic patterns in fibromyalgiaBMC Clin Pathol2012122523245186
- UçeylerNHäuserWSommerCSystematic review with meta-analysis: cytokines in fibromyalgia syndromeBMC Musculoskelet Disord20111224522034969
- Rodriguez-PintoIAgmon-LevinNHowardAShoenfeldYFibromyalgia and cytokinesImmunol Lett2014161220020324462815
- HernandezMEBecerrilEPerezMProinflammatory cytokine levels in fibromyalgia patients are independent of body mass indexBMC Res Notes2010315620525285
- NugrahaBKorallusCKielsteinHGutenbrunnerCCD3+CD56+ natural killer T cells in fibromyalgia syndrome patients: association with the intensity of depressionClin Exp Rheumatol2013316 Suppl 79S9S15
- MenziesVLyonDEIntegrated review of the association of cytokines with fibromyalgia and fibromyalgia core symptomsBiol Res Nurs201011438739419933683
- GürAOktayogluPStatus of immune mediators in fibromyalgiaCurr Pain Headache Rep200812317518118796266
- UceylerNValenzaRStockMSchedelRSprotteGSommerCReduced levels of antiinflammatory cytokines in patients with chronic widespread painArthritis Rheum20065482656266416871547
- MenziesVLyonDEElswickRKJrMontpetitAJMcCainNLPsychoneuroimmunological relationships in women with fibromyalgiaBiol Res Nurs201315221922522174319
- BradleyLAPathophysiology of fibromyalgiaAm J Med200912212 SupplS22S30
- TakLMCleareAJOrmelJMeta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disordersBiol Psychol201187218319421315796
- TokerAKucuksenSKucukACiceklerHSerum ischemia-modified albumin and malondialdehyde levels and superoxide dismutase activity in patients with fibromyalgiaClin Lab201460101609161525651705
- AkbasAInanirABenliIOnderYAydoganLEvaluation of some antioxidant enzyme activities (SOD and GPX) and their polymorphisms (MnSOD2 Ala9Val, GPX1 Pro198Leu) in fibromyalgiaEur Rev Med Pharmacol Sci20141881199120324817295
- BagisSTamerLSahinGFree radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder?Rheumatol Int200525318819014689230
- Martinez-LavinMFibromyalgia: when distress becomes (un)sympathetic painPain Res Treat2012201298156522110948
- Vargas-AlarconGAlvarez-LeonEFragosoJMA SCN9A gene-encoded dorsal root ganglia sodium channel polymorphism associated with severe fibromyalgiaBMC Musculoskelet Disord2012132322348792
- KernKUWeiserTTopical ambroxol for the treatment of neuropathic pain: a first clinical observationSchmerz2015296632640 German26597641
- KernKUWeiserTTopical ambroxol for the treatment of neuropathic pain: an initial clinical observationSchmerz201529Suppl 3S89S9626589711
- KernUWeiserTTopical ambroxol for the treatment of neuropathic or severe nociceptive pain: first case reportsPoster presented at: 9th Congress of the European Pain Federation (EFIC)September 2–5, 2015Vienna, Austria
- Martinez-MartinezLPerezLAcostaGAmbroxol for fibromyalgia: one-group pretest-posttest open label clinical observationArthritis Rheumatol201668Suppl 1044
- Martinez-MartinezLAPerezLFBecerril-MendozaLTAmbroxol for fibromyalgia: one group pretest-posttest open-label pilot studyClin Rheumatol20173681879188428466418
- BeehKMBeierJEsperesterAPaulLDAntiinflammatory properties of ambroxolEur J Med Res2008131255756219073395
- MalerbaMRagnoliBAmbroxol in the 21st century: pharmacological and clinical updateExpert Opin Drug Metab Toxicol2008481119112918680446
- LefflerAReckzehJNauCBlock of sensory neuronal Na+ channels by the secreolytic ambroxol is associated with an interaction with local anesthetic binding sitesEur J Pharmacol20106301–3192820044988
- WeiserTNav1.8 channel blockade as an approach to the treatment of neuropathic painDrugs Future2006317597601
- WeiserTComparison of the effects of four Na+ channel analgesics on TTX-resistant Na+ currents in rat sensory neurons and recombinant Nav1.2 channelsNeurosci Lett2006395317918416293367
- AkopianANSivilottiLWoodJNA tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neuronsNature199637965622572628538791
- ZimmermannKLefflerABabesASensory neuron sodium channel Nav1.8 is essential for pain at low temperaturesNature2007447714685585817568746
- BlairNTBeanBPRoles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neuronsJ Neurosci20022223102771029012451128
- RenganathanMCumminsTRWaxmanSGContribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neuronsJ Neurophysiol200186262964011495938
- ChenWNLeeCHLinSHRoles of ASIC3, TRPV1, and NaV1.8 in the transition from acute to chronic pain in a mouse model of fibromyalgiaMol Pain2014104024957987
- CagnieBCoppietersIDeneckerSSixJDanneelsLMeeusMCentral sensitization in fibromyalgia? A systematic review on structural and functional brain MRISemin Arthritis Rheum2014441687524508406
- SalemiSRethageJWollinaUDetection of interleukin 1β (IL-1β), IL-6, and tumor necrosis factor-α in skin of patients with fibromyalgiaJ Rheumatol200330114615012508404
- JangYYSongJHShinYKHanESLeeCSDepressant effects of ambroxol and erdosteine on cytokine synthesis, granule enzyme release, and free radical production in rat alveolar macrophages activated by lipopolysaccharidePharmacol Toxicol200392417317912753420
- WangYWangFYPanZEffects of ambroxol combined with low-dose heparin on TNFα and IL-1β in rabbits with acute lung injuryZhongguo Ying Yong Sheng Li Xue Za Zhi2011272231235 Chinese21845882
- SuXWangLSongYBaiCInhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharideIntensive Care Med200430113314014504727
- JinXZZhangHThe experiment and clinical study of ambroxol against the airway inflammation of chronic hypoxic rat and patients with COPDEur Respir J200220Suppl. 381658
- BianchiMMantovaniAErroiADinarelloCAGhezziPAmbroxol inhibits interleukin 1 and tumor necrosis factor production in human mononuclear cellsAgents Actions1990313–4275279
- PfeiferSZisselGKienastKMüller-QuernheimJReduction of cytokine release of blood and bronchoalveolar mononuclear cells by ambroxolEur J Med Res1997231291329113503
- YangBYaoDFOhuchiMAmbroxol suppresses influenza-virus proliferation in the mouse airway by increasing antiviral factor levelsEur Respir J200219595295812030738
- ZhangSJJiangJXRenQQAmbroxol inhalation ameliorates LPS-induced airway inflammation and mucus secretion through the extracellular signal-regulated kinase 1/2 signaling pathwayEur J Pharmacol201677513814826872986
- XiaDHXiLXvCThe protective effects of ambroxol on radiation lung injury and influence on production of transforming growth factor β1 and tumor necrosis factor αMed Oncol201027369770119636975
- BlancoIBeritzeNArguellesMAbnormal overexpression of mastocytes in skin biopsies of fibromyalgia patientsClin Rheumatol201029121403141220428906
- GibbsBFSchmutzlerWVollrathIBAmbroxol inhibits the release of histamine, leukotrienes and cytokines from human leukocytes and mast cellsInflamm Res1999482869310202994
- Zwadlo-KlarwasserGServaisMDSchmutzlerWBrosthardtPBraamUAmbroxol inhibits histamine release from human adenoidal mast cellsInflamm Res199847Suppl 1S16S179561395
- GibbsBFWolffHHGrabbeJAmbroxol inhibits IgE-dependent mediator secretion from human skin mast cellsInflamm Res200049Suppl 1S17S1810864402
- Sánchez-DomínguezBBullónPRomán-MaloLOxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with fibromyalgiaMitochondrion201521697525662535
- CorderoMDMoreno-FernandezAMCarmona-LopezMIMitochondrial dysfunction in skin biopsies and blood mononuclear cells from two cases of fibromyalgia patientsClin Biochem20104313–141174117620599870
- StetinováVHeroutVKvetinaJIn vitro and in vivo antioxidant activity of ambroxolClin Exp Med20044315215815599665
- HongJSKoHHHanESLeeCSInhibition of bleomycin-induced cell death in rat alveolar macrophages and human lung epithelial cells by ambroxolBiochem Pharmacol20036671297130614505809
- LedwozywAJablonkaSTusińskaEWplyw ambroksolu na procesy peroksydacyjne w mitochondriach pluc psa. [The effect of ambroxol on peroxidative processes in dog lung mitochondria]Pol Arch Weter1991313–4105113 Polish1842611
- GillissenABartlingASchoenSSchultze-WerninghausGAntioxidant function of ambroxol in mononuclear and polymorphonuclear cells in vitroLung199717542352429195551
- GasparMBovairaMCarrera-HuesoFJQuerolMJimenezAMorenoLEfectividad de un protocolo de tratamiento topico con dimetilsulfoxido al 50% en el sindrome de dolor regional complejo tipo. [Efficacy of a topical treatment protocol with dimethyl sulfoxide 50% in type 1 complex regional pain syndrome]Farm Hosp2012365385391 Spanish22266201
- LeeCSJangYYSongJSSongJHHanESAmbroxol inhibits peroxynitrite-induced damage of α1-antiproteinase and free radical production in activated phagocytic cellsPharmacol Toxicol200291314014912427115
- OttonelloLArduinoNBertolottoMDapinoPManciniMDallegriFIn vitro inhibition of human neutrophil histotoxicity by ambroxol: evidence for a multistep mechanismBr J Pharmacol2003140473674214534155
- UçeylerNKewenigSKafkeWKittel-SchneiderSSommerCSkin cytokine expression in patients with fibromyalgia syndrome is not different from controlsBMC Neurol20141418525240423
- KosmidisMLKoutsogeorgopoulouLAlexopoulosHReduction of intraepidermal nerve fiber density (IENFD) in the skin biopsies of patients with fibromyalgia: a controlled studyJ Neurol Sci20143471–214314725304055
- BelkouchMDansereauMATetreaultPFunctional up-regulation of Nav1.8 sodium channel in Aβ afferent fibers subjected to chronic peripheral inflammationJ Neuroinflamm20141145
- GaidaWKlinderKArndtKWeiserTAmbroxol, a Nav1.8-preferring Na+ channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory painNeuropharmacology20054981220122716182323
- HamaATPlumAWSagenJAntinociceptive effect of ambroxol in rats with neuropathic spinal cord injury painPharmacol Biochem Behav201097224925520732348
- MoonJYSongSYoonSYThe differential effect of intrathecal Nav1.8 blockers on the induction and maintenance of capsaicin- and peripheral ischemia-induced mechanical allodynia and thermal hyperalgesiaAnesth Analg2012114121522322127815
- BettoniLBonomiFGZaniVEffects of 15 consecutive cryotherapy sessions on the clinical output of fibromyalgic patientsClin Rheumatol20133291337134523636794
- BanfiGLombardiGColombiniAMelegatiGWhole-body cryotherapy in athletesSports Med201040650951720524715
- McNeillAMagalhaesJShenCAmbroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cellsBrain2014137Pt 51481149524574503
- AmbrosiGGhezziCZangagliaRLevandisGPacchettiCBlandiniFAmbroxol-induced rescue of defective glucocerebrosidase is associated with increased LIMP-2 and saposin C levels in GBA1 mutant Parkinson’s disease cellsNeurobiol Dis20158223524226094596
- Lawson Health Research InstituteAmbroxol as a treatment for Parkinson’s disease dementia Available from: https://clinicaltrials.gov/ct2/show/NCT02914366. NLM identifier: NCT02914366Accessed July 28, 2017
- LukasJPockrandtAMSeemannSEnzyme enhancers for the treatment of Fabry and Pompe diseaseMol Ther201523345646425409744
- SuzukiYChaperone therapy update: Fabry disease, GM1-gangliosidosis and Gaucher diseaseBrain Dev201335651552323290321
- NaritaAShiraiKItamuraSAmbroxol chaperone therapy for neuronopathic Gaucher disease: a pilot studyAnn Clin Transl Neurol20163320021527042680
- La RubiaMRusAMolinaFDel MoralMLIs fibromyalgia-related oxidative stress implicated in the decline of physical and mental health status?Clin Exp Rheumatol2013316 Suppl 79S121S12724373370
- FatimaGDasSKMahdiAASome oxidative and antioxidative parameters and their relationship with clinical symptoms in women with fibromyalgia syndromeInt J Rheum Dis2015201394526177214
- CulićOCorderoMDZanić-GrubišićTSerum activities of adenosine deaminase, dipeptidyl peptidase IV and prolyl endopeptidase in patients with fibromyalgia: diagnostic implicationsClin Rheumatol201635102565257127527091
- CorderoMDDe MiguelMFernandezAMMitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: implications in the pathogenesis of the diseaseArthritis Res Ther2010121R1720109177
- MeeusMNijsJHermansLGoubertDCaldersPThe role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: peripheral and central mechanisms as therapeutic targets?Expert Opin Ther Targets20131791081108923834645
- CorderoMDDiaz-ParradoECarrionAMIs inflammation a mitochondrial dysfunction-dependent event in fibromyalgia?Antioxid Redox Signal201318780080722938055
- LazzarinALuertiMCapsoniFA study of cellular immunity in newborns after prevention of respiratory distress syndrome (RDS)Int J Tissue React1986821571653700005
- AiharaMDobashiKAkiyamaMNaruseINakazawaTMoriMEffects of N-acetylcysteine and ambroxol on the production of IL-12 and IL-10 in human alveolar macrophagesRespiration200067666267111124650
- AngDCHilligossJStumpTMast cell stabilizer (ketotifen) in fibromyalgia: phase 1 randomized controlled clinical trialClin J Pain Epub2014113
- MorreyCBrazinJSeyediNCortiFSilverRBLeviRInteraction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion: activation of a local renin-angiotensin system culminating in severe arrhythmic dysfunctionJ Pharmacol Exp Ther20103351768420668055
- TorresaniCBellafioreSDe PanfilisGChronic urticaria is usually associated with fibromyalgia syndromeActa Derm Venereol200989438939219688152
- TsilioniIRussellIJStewartJMGleasonRMTheoharidesTCNeuropeptides CRH, SP, HK-1, and inflammatory cytokines IL-6 and TNF are increased in serum of patients with fibromyalgia syndrome, implicating mast cellsJ Pharmacol Exp Ther2016356366467226763911
- ZhengGHongSHayesJMWileyJWChronic stress and peripheral pain: evidence for distinct, region-specific changes in visceral and somatosensory pain regulatory pathwaysExp Neurol201527330131126408049
- SturgeonJADarnallBDZwickeyHLProinflammatory cytokines and DHEA-S in women with fibromyalgia: impact of psychological distress and menopausal statusJ Pain Res2014770771625506243
- GeissARohlederNAntonFEvidence for an association between an enhanced reactivity of interleukin-6 levels and reduced glucocorticoid sensitivity in patients with fibromyalgiaPsychoneuroendocrinology201237567168422000300
- GeLTLiuYNLinXXInhalation of ambroxol inhibits cigarette smoke-induced acute lung injury in a mouse model by inhibiting the Erk pathwayInt Immunopharmacol201633909826881857
- RicciardoloFLSorbelloVBenedettoSPaleariDEffect of ambroxol and beclomethasone on lipopolysaccharide-induced nitrosative stress in bronchial epithelial cellsRespiration201589657258225998443
- FatimaGDasSKMahdiAAOxidative stress and antioxidative parameters and metal ion content in patients with fibromyalgia syndrome: implications in the pathogenesis of the diseaseClin Exp Rheumatol2013316 Suppl 79S128S13324373371
- ZhaoSPGuoQLWangRKWangEOxidative and anti-oxidative effects of ambroxol on acute hydrochloric acid-induced lung injury in ratsZhong Nan Da Xue Xue Bao Yi Xue Ban2004295586588 Chinese16137054
- NowakDAntczakAPietrasTBialasiewiczPKrolMProtective effect of ambroxol against heat- and hydrogen peroxide-induced damage to lung lipids in miceEur Respir J199479162916347995392
- OzgocmenSOzyurtHSogutSAkyolOArdicogluOYildizhanHAntioxidant status, lipid peroxidation and nitric oxide in fibromyalgia: etiologic and therapeutic concernsRheumatol Int200626759860316283318
- JiangKWangXMaoXAmbroxol alleviates hepatic ischemia reperfusion injury by antioxidant and antiapoptotic pathwaysTransplant Proc20134562439244523953561
- ZhangBLiuYProphylaxis against ventilator-induced lung injury by ambroxolZhonghua Yi Xue Za Zhi20008015153 Chinese11798737
- WuXLiSZhangJMeta-analysis of high doses of ambroxol treatment for acute lung injury/acute respiratory distress syndrome based on randomized controlled trialsJ Clin Pharmacol201454111199120625174313
- MaYTTianYPShiHWLvCHLiuJHSunZPEffects of high dose ambroxol on lung injury induced by paraquat in ratsZhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi2007259523526 Chinese17997885
- YangFSunWYangYSDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulationJ Neuroinflamm201512219
- SchlüterFLefflerAOxidation differentially modulates the recombinant voltage-gated Na+ channel α-subunits Nav1.7 and Nav1.8Brain Res20161648Pt A12713527450927
- WeiserTPresentation at the German Pain Conference 2005, October 19–22, 2005, Bremen, GermanySchmerz19Suppl 1S512823570068
- MaesMMihaylovaIKuberaMUytterhoevenMVrydagsNBosmansEIncreased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery diseaseJ Affect Disord20101251–328729420083310
- MaesMMihaylovaIKuberaMUytterhoevenMVrydagsNBosmansEIncreased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis/chronic fatigue syndromeNeuro Endocrinol Lett200930671572220035260
- WiktorskaJALewinskiAStussMNowakDPietrasTSewerynekEEffects of certain antioxidants on lipid peroxidation process in lung homogenates of L thyroxine-receiving ratsNeuro Endocrinol Lett201031113714620150865
- PernambucoAPSchetinoLPVianaRSCarvalhoLSd’Avila ReisDThe involvement of melatonin in the clinical status of patients with fibromyalgia syndromeClin Exp Rheumatol2015331 Suppl 88S14S19
- BlumenthalDEMalemudCJRecent strategies for drug development in fibromyalgia syndromeExpert Rev Neurother201616121407141127362466
- LawsonKPotential drug therapies for the treatment of fibromyalgiaExpert Opin Investig Drugs201625910711081
- SanchezACalpenaACClaresBEvaluating the oxidative stress in inflammation: role of melatoninInt J Mol Sci2015168169811700426225957
- BortolatoBBerkMMaesMMcIntyreRSCarvalhoAFFibromyalgia and bipolar disorder: emerging epidemiological associations and shared pathophysiologyCurr Mol Med201616211913626812920
- OzgocmenSOzyurtHSogutSAkyolOCurrent concepts in the pathophysiology of fibromyalgia: the potential role of oxidative stress and nitric oxideRheumatol Int200626758559716328420
- KimSKKimSHNahSSAssociation of guanosine triphosphate cyclohydrolase 1 gene polymorphisms with fibromyalgia syndrome in a Korean populationJ Rheumatol201340331632223322459
- SendurOFTuranYTastabanEYeniseyCSerterMSerum antioxidants and nitric oxide levels in fibromyalgia: a controlled studyRheumatol Int200929662963318853166
- RusAMolinaFGassoMCamachoMVPeinadoMAdel MoralMLNitric oxide, inflammation, lipid profile, and cortisol in normal- and overweight women with fibromyalgiaBiol Res Nurs201618213814626134428
- CimenOBCimenMYYapiciYCamdevirenHArginase, NOS activities, and clinical features in fibromyalgia patientsPain Med200910581381819523030
- SeverinaISBussyginaOGPyatakovaNVKhropovYVKrasnoperovRAAmbroxol as an inhibitor of nitric oxide-dependent activation of soluble guanylate cyclaseEur J Pharmacol20004071–2616411050291
- AnfossiGRussoIMassuccoPAdenosine increases human platelet levels of cGMP through nitric oxide: possible role in its antiag-gregating effectThromb Res20021051717811864710
- KimYKJangYYHanESLeeCSDepressant effect of ambroxol on stimulated functional responses and cell death in rat alveolar macrophages exposed to silica in vitroJ Pharmacol Exp Ther2002300262963711805226
- HuFWangQWangP17β-Estradiol regulates the gene expression of voltage-gated sodium channels: role of estrogen receptor α and estrogen receptor βEndocrine201241227428022169964
- WangQCaoJHuFEffects of estradiol on voltage-gated sodium channels in mouse dorsal root ganglion neuronsBrain Res201315121823473841
- SteningKDErikssonOHenrikssonKGHormonal replacement therapy does not affect self-estimated pain or experimental pain responses in post-menopausal women suffering from fibromyalgia: a double-blind, randomized, placebo-controlled trialRheumatology (Oxford)201150354455121078629
- DehlinMBjersingJErlandssonMCerebrospinal Flt3 ligand correlates to tau protein levels in primary Sjögren’s syndromeScand J Rheumatol201342539439923837643
- KorszunAYoungEAEnglebergNCFollicular phase hypothalamic- pituitary-gonadal axis function in women with fibromyalgia and chronic fatigue syndromeJ Rheumatol20002761526153010852283
- SchafranskiMDMalucelliTMachadoFIntravenous lidocaine for fibromyalgia syndrome: an open trialClin Rheumatol200928785385519263182
- MarksDMNewhouseADurability of benefit from repeated intravenous lidocaine infusions in fibromyalgia patients: a case series and literature reviewPrim Care Companion CNS Disord20151754088
- StaudRWeylEEBartleyEPriceDDRobinsonMEAnalgesic and anti-hyperalgesic effects of muscle injections with lidocaine or saline in patients with fibromyalgia syndromeEur J Pain201418680381224193993
- StaudRNagelSRobinsonMEPriceDDEnhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled studyPain20091451–29610419540671
- Arendt-NielsenLFernandez-de-Las-PenasCGraven-NielsenTBasic aspects of musculoskeletal pain: from acute to chronic painJ Man Manip Ther201119418619323115471
- DeSantanaJMSlukaKACentral mechanisms in the maintenance of chronic widespread noninflammatory muscle painCurr Pain Headache Rep200812533834318765138
- StaudRPeripheral pain mechanisms in chronic widespread painBest Pract Res Clin Rheumatol201125215516422094192
- JosephEKLevineJDHyperalgesic priming is restricted to isolectin B4-positive nociceptorsNeuroscience2010169143143520457222
- AlvarezPChenXBogenOGreenPGLevineJDIB4+ nociceptors mediate persistent muscle pain induced by GDNFJ Neurophysiol201210892545255322914655
- AlvarezPGearRWGreenPGLevineJDIB4-saporin attenuates acute and eliminates chronic muscle pain in the ratExp Neurol2012233285986522206923
- SlukaKAPriceMPBreeseNMStuckyCLWemmieJAWelshMJChronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1Pain2003106322923914659506
- SlukaKAKalraAMooreSAUnilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesiaMuscle Nerve2001241374611150964
- LawLASlukaKAMcMullenTLeeJArendt-NielsenLGraven-NielsenTAcidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humansPain2008140225426418835099
- IssbernerUReehPWSteenKHPain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia?Neurosci Lett199620831911948733302
- BirdsongWTFierroLWilliamsFGSensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channelsNeuron201068473974921092862
- FujiiYOzakiNTaguchiTMizumuraKFurukawaKSugiuraYTRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle sorenessPain2008140229230418834667
- HoheiselUReinöhlJUngerTMenseSAcidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the ratPain20041101–214915715275762
- YenYTTuPHChenCJLinYWHsiehSTChenCCRole of acid-sensing ion channel 3 in sub-acute-phase inflammationMol Pain20095119126241
- BennettRMJonesJTurkDCRussellIJMatallanaLAn Internet survey of 2,596 people with fibromyalgiaBMC Musculoskelet Disord200782717349056
- HäuserWJungEErbslöh-MöllerBThe German fibromyalgia consumer reports: a cross-sectional surveyBMC Musculoskelet Disord2012137422607517
- ChristidisNGhafouriBLarssonAComparison of the levels of pro-inflammatory cytokines released in the vastus lateralis muscle of patients with fibromyalgia and healthy controls during contractions of the quadriceps muscle: a microdialysis studyPloS One20151012e014385626624891
- AngDCMooreMNHilligossJTabbeyRMCP-1 and IL-8 as pain biomarkers in fibromyalgia: a pilot studyPain Med20111281154116121714842
- AlvarezPGreenPGLevineJDRole for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the ratPain201415561161116724637038
- ZhiQMYangLTSunHCProtective effect of ambroxol against paraquat-induced pulmonary fibrosis in ratsIntern Med201150181879188721921364
- BazzichiLGiacomelliCConsensiAOne year in review 2016: fibromyalgiaClin Exp Rheumatol2016342 Suppl 96S145S149
- LefaucheurJPThe “paradox” of neuropathic pain associated with small-fiber lesions in the context of fibromyalgiaPain201615761364136527183446
- GauffinJHankamaTKautiainenHHannonenPHaanpääMNeuropathic pain and use of PainDETECT in patients with fibromyalgia: a cohort studyBMC Neurol2013132123409793
- KoroschetzJRehmSEGockelUFibromyalgia and neuropathic pain – differences and similarities: a comparison of 3057 patients with diabetic painful neuropathy and fibromyalgiaBMC Neurol2011115521612589
- HäuserWWalittBFitzcharlesMASommerCReview of pharmacological therapies in fibromyalgia syndromeArthritis Res Ther201416120124433463
- FinnerupNBAttalNHaroutounianSPharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysisLancet Neurol201514216217325575710
- UçeylerNSommerCReply: Small fibre neuropathy, fibromyalgia and dorsal root ganglia sodium channelsBrain2013136Pt 9e24723729475
- DopplerKRittnerHLDeckartMSommerCReduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgiaPain2015156112319232526164586
- UçeylerNSmall fiber pathology: a culprit for many painful disorders?Pain2016157Suppl 1S60S6626780379
- RamirezMMartinez-MartinezLAHernandez-QuintelaEVelazco-CasapiaJVargasAMartinez-LavinMSmall fiber neuropathy in women with fibromyalgia: an in vivo assessment using corneal confocal bio-microscopySemin Arthritis Rheum201545221421926094164
- Garcia-MartinEGarcia-CampayoJPuebla-GuedeaMFibromyalgia is correlated with retinal nerve fiber layer thinningPloS One2016119e016157427584145
- de TommasoMNolanoMIannoneFUpdate on laser-evoked potential findings in fibromyalgia patients in light of clinical and skin biopsy featuresJ Neurol2014261346147224366650
- KernUSchneiderSBialasPTopisches Ambroxol zur Therapie des CRPS: Eine neue Option?Schmerz201630Suppl 2S59
- KinlochRACoxPJNew targets for neuropathic pain therapeuticsExpert Opin Ther Targets20059468569816083337
- CoggeshallRETateSCarltonSMDifferential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed ratsNeurosci Lett20043551–2454814729231
- StricklandITMartindaleJCWoodhamsPLReeveAJChessellIPMcQueenDSChanges in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint painEur J Pain200812556457217950013
- FaberCGLauriaGMerkiesISGain-of-function Nav1.8 mutations in painful neuropathyProc Natl Acad Sci U S A201210947194441944923115331
- HanCVasylyevDMacalaLJThe G1662S NaV1.8 mutation in small fibre neuropathy: impaired inactivation underlying DRG neuron hyperexcitabilityJ Neurol Neurosurg Psychiatry201485549950524006052
- HuangJYangYZhaoPSmall-fiber neuropathy Nav1.8 mutation shifts activation to hyperpolarized potentials and increases excitability of dorsal root ganglion neuronsJ Neurosci20133335140871409723986244
- HuangJHanCEstacionMGain-of-function mutations in sodium channel Nav1.9 in painful neuropathyBrain2014137Pt 61627164224776970
- BrusselmansGNogueiraHDe SchamphelaereEDevulderJCrombezGSkin temperature during cold pressor test in fibromyalgia: an evaluation of the autonomic nervous system?Acta Anaesthesiol Belg2015661192726103738
- AbrahamsenBZhaoJAsanteCOThe cell and molecular basis of mechanical, cold, and inflammatory painScience2008321588970270518669863
- NielsenLAHenrikssonKGPathophysiological mechanisms in chronic musculoskeletal pain (fibromyalgia): the role of central and peripheral sensitization and pain disinhibitionBest Pract Res Clin Rheumatol200721346548017602994
- DesmeulesJACedraschiCRapitiENeurophysiologic evidence for a central sensitization in patients with fibromyalgiaArthritis Rheum20034851420142912746916
- VierckCJWongFKingCDMauderliAPSchmidtSRileyJL3rdCharacteristics of sensitization associated with chronic pain conditionsClin J Pain201430211912823629594
- JarvisMFHonorePShiehCCA-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the ratProc Natl Acad Sci U S A2007104208520852517483457
- VeneroniOMajRCalabresiMFaravelliLFarielloRGSalvatiPAnti-allodynic effect of NW-1029, a novel Na+ channel blocker, in experimental animal models of inflammatory and neuropathic painPain20031021–2172512620593
- LittlejohnGNeuroinflammation in fibromyalgia and CRPS: top-down or bottom-up?Nat Rev Rheumatol2016124242
- CassisiGSarzi-PuttiniPCasaleRPain in fibromyalgia and related conditionsReumatismo2014661728624938199
- SumptonJEMoulinDEFibromyalgiaHandb Clin Neurol201411951352724365316
- ChoyEHThe role of sleep in pain and fibromyalgiaNat Rev Rheumatol201511951352025907704
- StaudRBrain imaging in fibromyalgia syndromeClin Exp Rheumatol2011296 Suppl 69S109S11722243558
- GerhardtAEichWJankeSLeisnerSTreedeRDTesarzJChronic widespread back pain is distinct from chronic local back pain: evidence from quantitative sensory testing, pain drawings, and psychometricsClin J Pain201632756857926379077
- BlumenstielKGerhardtARolkeRQuantitative sensory testing profiles in chronic back pain are distinct from those in fibromyalgiaClin J Pain201127868269021487289
- WongFRodriguesASchmidtSVierckCJJrMauderliAPExtreme thermal sensitivity and pain-induced sensitization in a fibromyalgia patientPain Res Treat2010201091251322110917
- NagakuraYTakahashiMNotoTDifferent pathophysiology underlying animal models of fibromyalgia and neuropathic pain: comparison of reserpine-induced myalgia and chronic constriction injury ratsBehav Brain Res2012226124224921945299
- RenganathanMCumminsTRHormuzdiarWNWaxmanSGα-SNS produces the slow TTX-resistant sodium current in large cutaneous afferent DRG neuronsJ Neurophysiol200084271071810938298
- LaiJGoldMSKimCSInhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8Pain2002951–214315211790477
- ShieldsSDAhnHSYangYNav1.8 expression is not restricted to nociceptors in mouse peripheral nervous systemPain2012153102017203022703890
- BhardwajHCArunachalamMKumarSLNavisSNeuroprotective and anti-nociceptive potential of ambroxol in oxaliplatin induced peripheral neuropathic pain in ratsBiol Med (Aligarh)2016821000268
- OkifujiABradshawDHOlsonCEvaluating obesity in fibromyalgia: neuroendocrine biomarkers, symptoms, and functionsClin Rheumatol200928447547819172342
- LermaCMartinezARuizNVargasAInfanteOMartinez-LavinMNocturnal heart rate variability parameters as potential fibromyalgia biomarker: correlation with symptoms severityArthritis Res Ther2011136R18522087605
- Martinez-LavinMVidalMBarbosaREPinedaCCasanovaJMNavaANorepinephrine-evoked pain in fibromyalgia: randomized pilot studyBMC Musculoskelet Disord20023211860612
- Martinez-LavinMSolanoCDorsal root ganglia, sodium channels, and fibromyalgia sympathetic painMed Hypotheses2009721646618845401
- SchofieldGGPuhlHL3rdIkedaSRProperties of wild-type and fluorescent protein-tagged mouse tetrodotoxin-resistant sodium channel (NaV1.8) heterologously expressed in rat sympathetic neuronsJ Neurophysiol20089941917192718272876
- FacerPPunjabiPPAbrariALocalisation of SCN10A gene product Nav1.8 and novel pain-related ion channels in human heartInt Heart J201152314615221646736
- KosekEAltawilRKadetoffDEvidence of different mediators of central inflammation in dysfunctional and inflammatory pain: interleukin-8 in fibromyalgia and interleukin-1 β in rheumatoid arthritisJ Neuroimmunol2015280495525773155
- KadetoffDLampaJWestmanMAnderssonMKosekEEvidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levelsJ Neuroimmunol20122421–2333822126705
- JohnMAuBTJosePJExpression and release of interleukin-8 by human airway smooth muscle cells: inhibition by Th-2 cytokines and corticosteroidsAm J Respir Cell Mol Biol199818184909448049
- BetzRGlukokortikosteroide und Ambroxol hemmen die Sekretion entzundungsfördernder Zytokine in tracheobronchialen Epithelzellen: Mögliche Rolle des Transkriptionsfaktors NF-kappa B. [Glucocorticosteroids and ambroxol inhibit secretion of inflammatory cytokines in tracheobronchial epithelial cells: possible role of the NF-κB transcription factor]Pneumologie1997515491492 German9265149
- LiWMaoBWangGA study of the mechanism of qingre huatan therapy in treatment of acute exacerbation of chronic obstructive pulmonary disease by improving airway inflammation and mucus hypersecretionZhong Xi Yi Jie He Xue Bao200868799805 Chinese18664347
- LiWMaoBWangGEffect of tanreqing injection on treatment of acute exacerbation of chronic obstructive pulmonary disease with Chinese medicine syndrome of retention of phlegm and heat in feiChin J Integr Med201016213113720473738
- YamayaMNishimuraHNadineLKOtaCKuboHNagatomiRAmbroxol inhibits rhinovirus infection in primary cultures of human tracheal epithelial cellsArch Pharm Res201437452052923856970
- WatkinsLRMaierSFImmune regulation of central nervous system functions: from sickness responses to pathological painJ Intern Med2005257213915515656873
- MilliganEDWatkinsLRPathological and protective roles of glia in chronic painNat Rev Neurosci2009101233619096368
- WallaceDJLinker-IsraeliMHalleguaDSilvermanSSilverDWeismanMHCytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot studyRheumatology (Oxford)200140774374911477278
- PinteauxEParkerLCRothwellNJLuheshiGNExpression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cellsJ Neurochem200283475476312421347
- GuoWWangHWatanabeMGlial-cytokine-neuronal interactions underlying the mechanisms of persistent painJ Neurosci200727226006601817537972
- McEwenBSKaliaMThe role of corticosteroids and stress in chronic pain conditionsMetabolism201059Suppl 1S9S1520837196
- StaudRFibromyalgia pain: do we know the source?Curr Opin Rheumatol200416215716314770104
- AlbrechtDProtsenkoEMawlaIDoes brain glial activation have a role in fibromyalgia? A PBR28 PET studyPoster presented at: 16th World Congress on PainSeptember 26–30, 2016Yokohama, Japan
- KosekEMartinsenSGerdleBThe translocator protein gene is associated with symptom severity and cerebral pain processing in fibromyalgiaBrain Behav Immun20165821822727448744
- YoungerJMackeySFibromyalgia symptoms are reduced by low-dose naltrexone: a pilot studyPain Med200910466367219453963
- BarkholtPSanchez-GuajardoVKirikDRomero-RamosMLong-term polarization of microglia upon α-synuclein overexpression in nonhuman primatesNeuroscience2012208859622342967
- SuXMaguire-ZeissKAGiulianoRPriftiLVenkateshKFederoffHJSynuclein activates microglia in a model of Parkinson’s diseaseNeurobiol Aging200829111690170117537546
- HoffmannAEttleBBrunoAAlpha-synuclein activates BV2 microglia dependent on its aggregation stateBiochem Biophys Res Commun2016479488188627666480
- QiaoHZhangQYuanHElevated neuronal α-synuclein promotes microglia activation after spinal cord ischemic/reperfused injuryNeuroreport2015261165666126103121
- ZhangWWangTPeiZAggregated α-synuclein activates microglia: a process leading to disease progression in Parkinson’s diseaseFASEB J200519653354215791003
- KleinCPSperottoNDMacielISLeiteCESouzaAHCamposMMEffects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in miceNeuropharmacology201486576624929111
- AlbrechtDSMacKiePJKarekenDADifferential dopamine function in fibromyalgiaBrain Imaging Behav201610382983926497890
- LedermannKJeneweinJSprottHRelation of dopamine receptor 2 binding to pain perception in female fibromyalgia patients with and without depression: a [11C] raclopride PET-studyEur Neuropsychopharmacol201626232033026708319
- Migdalska-RichardsADalyLBezardESchapiraAHAmbroxol effects in glucocerebrosidase and α-synuclein transgenic miceAnn Neurol201680576677527859541
- ChandranVCoppolaGNawabiHA systems-level analysis of the peripheral nerve intrinsic axonal growth programNeuron201689595697026898779
- Migdalska-RichardsAKoWKLiQBezardESchapiraAHOral ambroxol increases brain glucocerebrosidase activity in a nonhuman primateSynapse201771721967
- WeiserTAmbroxol: a CNS drug?CNS Neurosci Ther2008141172418482096
- Bromhexine [package insert]Ingelheim am Rhein, GermanyBoehringer Ingelheim2012
- RöhnertPSchröderUHZiabrevaITagerMReymannKGStriggowFInsufficient endogenous redox buffer capacity may underlie neuronal vulnerability to cerebral ischemia and reperfusionJ Neurosci Res201290119320221971686
- BagalSKChapmanMLMarronBEPrimeRStorerRISwainNARecent progress in sodium channel modulators for painBioorg Med Chem Lett201424163690369925060923
- HanCHoeijmakersJGAhnHSNav1.7-related small fiber neuropathy: impaired slow-inactivation and DRG neuron hyperexcitabilityNeurology201278211635164322539570
- HoeijmakersJGMerkiesISGerritsMMWaxmanSGFaberCGGenetic aspects of sodium channelopathy in small fiber neuropathyClin Genet201282435135822803682
- FaberCGHoeijmakersJGAhnHSGain of function Nav1.7 mutations in idiopathic small fiber neuropathyAnn Neurol2012711263921698661
- DabbyRSadehMBroitmanYYosovichKDickmanRLeshinsky-SilverEPainful small fiber neuropathy with gastroparesis: a new phenotype with a novel mutation in the SCN10A geneJ Clin Neurosci201626848826711856
- BlackJAHoeijmakersJGFaberCGMerkiesISWaxmanSGNaV1.7: stress-induced changes in immunoreactivity within magno-cellular neurosecretory neurons of the supraoptic nucleusMol Pain201393923924059
- BrouwerBAMerkiesISGerritsMMWaxmanSGHoeijmakersJGFaberCGPainful neuropathies: the emerging role of sodium channelopathiesJ Peripher Nerv Syst2014192536525250524
- EberhardtMJLefflerASchmerz und Schmerzlosigkeit : Mutationen spannungsabhängiger Natriumkanäle. [Pain and analgesia: mutations of voltage-gated sodium channels]Schmerz20173111422 German27402262
- UçeylerNHäuserWSommerCA systematic review on the effectiveness of treatment with antidepressants in fibromyalgia syndromeArthritis Rheum20085991279129818759260
- DickIEBrochuRMPurohitYKaczorowskiGJMartinWJPriestBTSodium channel blockade may contribute to the analgesic efficacy of antidepressantsJ Pain20078431532417175203
- WangSYCalderonJWangGKBlock of neuronal Na+ channels by antidepressant duloxetine in a state-dependent mannerAnesthesiology2010113365566520693878
- StoetzerCPapenbergBDollTDifferential inhibition of cardiac and neuronal Na+ channels by the selective serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxineEur J Pharmacol201678311027130441
- VanderWeideLASmithSMTrinkleyKEA systematic review of the efficacy of venlafaxine for the treatment of fibromyalgiaJ Clin Pharm Ther20154011625294655
- SuterMRKirschmannGLaedermannCJAbrielHDecosterdIRufinamide attenuates mechanical allodynia in a model of neuropathic pain in the mouse and stabilizes voltage-gated sodium channel inactivated stateAnesthesiology2013118116017223221868
- ChengKIWangHCChangLLPretreatment with intrathecal amitriptyline potentiates anti-hyperalgesic effects of post-injury intra-peritoneal amitriptyline following spinal nerve ligationBMC Neurol2012124422720761
- SongJHHamSSShinYKLeeCSAmitriptyline modulation of Na+ channels in rat dorsal root ganglion neuronsEur J Pharmacol2000401329730510936487
- BielefeldtKOzakiNWhiteisCGebhartGFAmitriptyline inhibits voltage-sensitive sodium currents in rat gastric sensory neuronsDig Dis Sci200247595996612018921
- HurYKChoiISChoJHEffects of carbamazepine and amitriptyline on tetrodotoxin-resistant Na+ channels in immature rat trigeminal ganglion neuronsArch Pharm Res200831217818218365687
- PatkarAAMasandPSKrulewiczSA randomized, controlled, trial of controlled release paroxetine in fibromyalgiaAm J Med2007120544845417466657
- WiffenPJDerrySMooreRAAntiepileptic drugs for neuropathic pain and fibromyalgia: an overview of Cochrane reviewsCochrane Database Syst Rev201311CD01056724217986
- ZhangJLYangJPZhangJRGabapentin reduces allodynia and hyperalgesia in painful diabetic neuropathy rats by decreasing expression level of Nav1.7 and p-ERK1/2 in DRG neuronsBrain Res20131493131823183040
- NatkunarajahJAthertonDElmslieFMansourSMortimerPTreatment with carbamazepine and gabapentin of a patient with primary erythermalgia (erythromelalgia) identified to have a mutation in the SCN9A gene, encoding a voltage-gated sodium channelClin Exp Dermatol2009348e640e64219549232
- DevigiliGEleopraRPierroTParoxysmal itch caused by gain-of-function Nav1.7 mutationPain201415591702170724820863
- BiRYDingYGanYHNon-steroidal anti-inflammatory drugs attenuate hyperalgesia and block upregulation of trigeminal ganglionic sodium channel 1.7 after induction of temporomandibular joint inflammation in ratsChin J Dent Res2016191354226981605
- HuangZJHsuELiHCRosnerALRupertRLSongXJTopical application of compound Ibuprofen suppresses pain by inhibiting sensory neuron hyperexcitability and neuroinflammation in a rat model of intervertebral foramen inflammationJ Pain201112114115220797917
- GouldHJ3rdEnglandJDSoignierRDIbuprofen blocks changes in Nav1.7 and 1.8 sodium channels associated with complete Freund’s adjuvant-induced inflammation in ratJ Pain20045527028015219259
- LefflerAFrankGKistnerKLocal anesthetic-like inhibition of voltage-gated Na+ channels by the partial µ-opioid receptor agonist buprenorphineAnesthesiology201211661335134622504149
- HaeselerGFoadiNAhrensJDenglerRHeckerHLeuwerMTramadol, fentanyl and sufentanil but not morphine block voltage-operated sodium channelsPain20061261–323424416949748
- WeibelRReissDKarchewskiLMu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout micePloS One201389e7470624069332
- ReckzehJDie Wirkung des Sekretolytikums Ambroxol an neuronalen Natriumkanälen durch die Interaktion mit der Lokalanästhetikumbindungsstelle [PhD thesis]ErlangenFriedrich-Alexander-Universität Erlangen-Nürnberg2010
- de MeyCKoelschSRichterEPohlmannTSousaREfficacy and safety of ambroxol lozenges in the treatment of acute uncomplicated sore throat: a pooled analysisDrug Res (Stuttg)201666738439227281448
- ChenotJFWeberPFriedeTEfficacy of ambroxol lozenges for pharyngitis: a meta-analysisBMC Fam Pract2014154524621446
- WeiserTKinderKArndtKGaidaWAmbroxol unterdrückt hocheffektiv Symptome des chronischen, neuropathischen und inflammatorischen SchmerzesSchmerz200721Suppl 197
- UçeylerNSommerCCytokine regulation in animal models of neuropathic pain and in human diseasesNeurosci Lett2008437319419818403115
- StaudRCytokine and immune system abnormalities in fibromyalgia and other central sensitivity syndromesCurr Rheumatol Rev201511210911526088214
- BoettgerMKWeberKGrossmannDSpinal tumor necrosis factor α neutralization reduces peripheral inflammation and hyperalgesia and suppresses autonomic responses in experimental arthritis: a role for spinal tumor necrosis factor α during induction and maintenance of peripheral inflammationArthritis Rheum20106251308131820213802
- DeLeoJAColburnRWNicholsMMalhotraAInterleukin-6-mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy modelJ Interferon Cytokine Res19961696957008887053
- XuXJHaoJXAndell-JonssonSPoliVBartfaiTWiesenfeld-HallinZNociceptive responses in interleukin-6-deficient mice to peripheral inflammation and peripheral nerve sectionCytokine1997912102810339417815
- SommerCSchmidtCGeorgeAHyperalgesia in experimental neuropathy is dependent on the TNF receptor 1Exp Neurol199815111381429582261
- SavinoWMendes-da-CruzDALepletierADardenneMHormonal control of T-cell development in health and diseaseNat Rev Endocrinol2016122778926437623
- MalekHEbadzadehMMSafabakhshRRazaviAZaringhalamJDynamics of the HPA axis and inflammatory cytokines: insights from mathematical modelingComput Biol Med20156711226476562
- GuptaDMorleyJEHypothalamic-pituitary-adrenal (HPA) axis and agingCompr Physiol2014441495151025428852
- OrtegaEBoteMEGiraldoEGarciaJJAquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patientsScand J Med Sci Sports201222110411220536907
- BoteMEGarciaJJHinchadoMDOrtegaEFibromyalgia: anti-inflammatory and stress responses after acute moderate exercisePloS One201389e7452424023948
- BoteMEGarciaJJHinchadoMDOrtegaEInflammatory/stress feedback dysregulation in women with fibromyalgiaNeuroimmunomodulation201219634335122986514
- WangHBuchnerMMoserMTDanielVSchiltenwolfMThe role of IL-8 in patients with fibromyalgia: a prospective longitudinal study of 6 monthsClin J Pain20092511419158539
- MendietaDde la Cruz-AguileraDLBarrera-VillalpandoMIIL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patientsJ Neuroimmunol2016290222526711564
- XiaoYHaynesWLMichalekJERussellIJElevated serum high-sensitivity C-reactive protein levels in fibromyalgia syndrome patients correlate with body mass index, interleukin-6, interleukin-8, erythrocyte sedimentation rateRheumatol Int20133351259126423124693
- RanzolinADuarteALBredemeierMEvaluation of cytokines, oxidative stress markers and brain-derived neurotrophic factor in patients with fibromyalgia: a controlled cross-sectional studyCytokine201684252827209553
- WatkinsLRWiertelakEPGoehlerLESmithKPMartinDMaierSFCharacterization of cytokine-induced hyperalgesiaBrain Res1994654115267982088
- SchaibleHGNociceptive neurons detect cytokines in arthritisArthritis Res Ther201416547025606597
- ImamuraMTarginoRAHsingWTConcentration of cytokines in patients with osteoarthritis of the knee and fibromyalgiaClin Interv Aging2014993994424959074
- NakamuraTSchwanderSKDonnellyRCytokines across the night in chronic fatigue syndrome with and without fibromyalgiaClin Vaccine Immunol201017458258720181767
- IannuccelliCDi FrancoMAlessandriCPathophysiology of fibromyalgia: a comparison with the tension-type headache, a localized pain syndromeAnn N Y Acad Sci20101193788320398011
- LiFWangWHuLLiLYuJEffect of ambroxol on pneumonia caused by Pseudomonas aeruginosa with biofilm formation in an endotracheal intubation rat modelChemotherapy201157217318021454975
- TakedaKMiyaharaNMatsubaraSImmunomodulatory effects of ambroxol on airway hyperresponsiveness and inflammationImmune Netw201616316517527340385
- YigitSInanirATekcanAInanirSTuralSAtesOAssociation between fibromyalgia syndrome and polymorphism of the IL-4 gene in a Turkish populationGene20135271626423644020
- SturgillJMcGeeEMenziesVUnique cytokine signature in the plasma of patients with fibromyalgiaJ Immunol Res2014201493857624741634
- WangHMoserMSchiltenwolfMBuchnerMCirculating cytokine levels compared to pain in patients with fibromyalgia: a prospective longitudinal study over 6 monthsJ Rheumatol20083571366137018528959
- FengJZhangZWuXDiscovery of potential new gene variants and inflammatory cytokine associations with fibromyalgia syndrome by whole exome sequencingPloS One201386e6503323762283
- ChenYWangLPitzerALLiXLiPLZhangYContribution of redox-dependent activation of endothelial Nlrp3 inflammasomes to hyperglycemia-induced endothelial dysfunctionJ Mol Med (Berl)201694121335134727783111
- CorderoMDAlcocer-GomezECulicONLRP3 inflammasome is activated in fibromyalgia: the effect of coenzyme Q10Antioxid Redox Signal20142081169118023886272
- CorderoMDAlcocer-GomezEMarin-AguilarFMutation in cytochrome B gene of mitochondrial DNA in a family with fibromyalgia is associated with NLRP3-inflammasome activationJ Med Genet201653211312226566881
- ZhangHLiFLiWWThe inflammasome as a target for pain therapyBr J Anaesth2016117669370727956668
- GibbsBFDifferential modulation of IgE-dependent activation of human basophils by ambroxol and related secretolytic analoguesInt J Immunopathol Pharmacol200922491992720074455
- KehSMFacerPSimpsonKDSandhuGSalehHAAnandPIncreased nerve fiber expression of sensory sodium channels Nav1.7, Nav1.8, and Nav1.9 in rhinitisLaryngoscope2008118457357918197135
- ChungJHKimSAChoiBYThe association between overactive bladder and fibromyalgia syndrome: a community surveyNeurourol Urodyn2013321666922674758
- BrandKLittlejohnGKristjansonLWisniewskiSHassardTThe fibromyalgia bladder indexClin Rheumatol200726122097210317476564
- NickelJCTrippDAPontariMInterstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndromeJ Urol201018441358136320719340
- DrewaTMłodzik-DanielewiczNTyrakowskiTThe influence of ambroxol and capsaicin on the isolated rabbit bladder wallActa Pol Pharm200562539940316459489
- YangTYChenCSLinCLLinWMKuoCNKaoCHRisk for irritable bowel syndrome in fibromyalgia patients: a national database studyMedicine (Baltimore)20159410e61625761187
- FengBLaJHSchwartzESGebhartGFIrritable bowel syndrome – methods, mechanisms, and pathophysiology: neural and neuroimmune mechanisms of visceral hypersensitivity in irritable bowel syndromeAm J Physiol Gastrointest Liver Physiol201230210G1085G109822403791
- OsteenJDHerzigVGilchristJSelective spider toxins reveal a role for the Nav1.1 channel in mechanical painNature2016534760849449927281198
- VillarrealCFSachsDCunhaFQParadaCAFerreiraSHThe role of NaV1.8 sodium channel in the maintenance of chronic inflammatory hypernociceptionNeurosci Lett20053862727716043287
- JoshiSKMikusaJPHernandezGInvolvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain statesPain20061231–2758216545521
- EkbergJJayamanneAVaughanCWμO-conotoxin MrVIB selectively blocks Nav1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficitsProc Natl Acad Sci U S A200610345170301703517077153
- HillsleyKLinJHStaniszADissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null miceJ Physiol2006576Pt 125726716857712
- LairdJMSouslovaVWoodJNCerveroFDeficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null miceJ Neurosci200222198352835612351708
- HuSXiaoYZhuLNeonatal maternal deprivation sensitizes voltage-gated sodium channel currents in colon-specific dorsal root ganglion neurons in ratsAm J Physiol Gastrointest Liver Physiol20133044G311G32123139220
- LiuMWoodJNThe roles of sodium channels in nociception: implications for mechanisms of neuropathic painPain Med201112Suppl 3S93S9921752183
- Martinez-MartinezLAMoraTVargasAFuentes-IniestraMMartinez-LavinMSympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studiesJ Clin Rheumatol201420314615024662556
- ChoiBYOhHJLeeYJSongYWPrevalence and clinical impact of fibromyalgia in patients with primary Sjögren’s syndromeClin Exp Rheumatol2016342 Suppl 96S9S13
- HalilogluSCarliogluAAkdenizDKaraaslanYKosarAFibromyalgia in patients with other rheumatic diseases: prevalence and relationship with disease activityRheumatol Int20143491275128024589726
- OstuniPBotsiosCSfrisoPFibromyalgia in Italian patients with primary Sjögren’s syndromeJoint Bone Spine2002691515711858357
- KimDKimHJHyonJYWeeWRShinYJEffects of oral mucolytics on tear film and ocular surfaceCornea201332793393823538621
- IchikawaYTokunagaMShimizuHMoriuchiJTakayaMArimoriSClinical trial of ambroxol (Mucosolvan) in Sjögren’s syndromeTokai J Exp Clin Med1988133165169
- ZhangZQWuQQHuangXMLuHPrevention of respiratory distress syndrome in preterm infants by antenatal ambroxol: a meta-analysis of randomized controlled trialsAm J Perinatol201330752953623229957
- ZwisslerBPharmakotherapie bei akutem LungenversagenJ Anaesth Intensivbehandl2002915967
- LuertiMLazzarinACorbellaEZavattiniGAn alternative to steroids for prevention of respiratory distress syndrome (RDS): multicenter controlled study to compare ambroxol and betamethasoneJ Perinat Med19871532272383323457
- ChiaraOBPadalinoPGuadalupiPBigalettoINespoliAThe prevention of postoperative prevention ARDS: double-blind testing about the effects of ambroxol on pulmonary gas exchangeUrgent Chirurg Comment198361–216
- SchillingsGJAmbroxol in the treatment of ARDS in polytraumatised patients: a reportKlinikarzt199221828
- KimyaYKüçükkömürcüSOzanHUncuGAntenatal ambroxol usage in the prevention of infant respiratory distress syndrome: beneficial and adverse effectsClin Exp Obstet Gynecol19952232042117554258
- BaranwalAKMurthyASSinghiSCHigh-dose oral ambroxol for early treatment of pulmonary acute respiratory distress syndrome: an exploratory, randomized, controlled pilot trialJ Trop Pediatr201561533935026130623
- VerginHBishop-FreudlingGBMiczkaMNitscheVStrobelKMatzkiesFUntersuchungen zur Pharmakokinetik und Bioaquivalenz unterschiedlicher Darreichungsformen von Ambroxol. [The pharmacokinetics and bioequivalence of various dosage forms of ambroxol]Arzneimittelforschung1985351015911595 German4074420
- ZimranAAltarescuGElsteinDPilot study using ambroxol as a pharmacological chaperone in type 1 Gaucher diseaseBlood Cells Mol Dis201350213413723085429
- MalerbaMPonticielloARadaeliABensiGGrassiVEffect of twelve-months therapy with oral ambroxol in preventing exacerbations in patients with COPD: double-blind, randomized, multicenter, placebo-controlled study (the AMETHIST trial)Pulm Pharmacol Ther2004171273414643168
- DongCWangGLiBAnti-asthmatic agents alleviate pulmonary edema by upregulating AQP1 and AQP5 expression in the lungs of mice with OVA-induced asthmaRespir Physiol Neurobiol20121811212822226856
- BazzichiLRossiAMassimettiGCytokine patterns in fibromyalgia and their correlation with clinical manifestationsClin Exp Rheumatol200725222523017543146
- SuzukiMTeramotoSMatsuseTInhibitory effect of ambroxol on superoxide anion production and generation by murine lung alveolar macrophagesJ Asthma19983532672729661679
- RamachandraRMcGrewSYBaxterJCKivericEElmslieKSTetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neuronsJ Neurophysiol201210882230224122855776
- KernNUWeiserTTopisches Ambroxol zur Behandlung neuropathischer Schmerzen: Eine erste klinische Beobachtung. [Topical ambroxol for the treatment of neuropathic pain: a first clinical observation]Schmerz2015296632640 German26597641
- TaiHWangZGongHAutophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescenceAutophagy20171319911327791464
- OrtegaEGarciaJJBoteMEExercise in fibromyalgia and related inflammatory disorders: known effects and unknown chancesExerc Immunol Rev200915426519957871
- Martinez-LavinMSmall fibre neuropathy, fibromyalgia and dorsal root ganglia sodium channelsBrain2013136Pt 9e24623729476
- AkopianANSouslovaVEnglandSThe tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathwaysNat Neurosci19992654154810448219
- RozaCLairdJMSouslovaVWoodJNCerveroFThe tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of miceJ Physiol2003550Pt 392192612824446
- StaudRIs it all central sensitization? Role of peripheral tissue nociception in chronic musculoskeletal painCurr Rheumatol Rep201012644845420882373
- GiraldesALSalomaoRLealPDBrunialtiMKSakataRKEffect of intravenous lidocaine combined with amitriptyline on pain intensity, clinical manifestations and the concentrations of IL-1, IL-6 and IL-8 in patients with fibromyalgia: a randomized double-blind studyInt J Rheum Dis2016191094695327309886
- VlainichRIssyAMSakataRKEffect of intravenous lidocaine associated with amitriptyline on pain relief and plasma serotonin, norepinephrine, and dopamine concentrations in fibromyalgiaClin J Pain201127428528821178598
