Abstract
Purpose
The aim of this study was to measure the efficacy of a single 60 min application of capsaicin 8% patch in reducing chronic amputation stump and phantom limb pain, associated hypersensitivity with quantitative sensory testing, and changes in brain cortical maps using functional MRI (fMRI) scans.
Methods
A capsaicin 8% patch (Qutenza) treatment study was conducted on 14 patients with single limb amputation, who reported pain intensity on the Numerical Pain Rating Scale ≥4/10 for chronic stump or phantom limb pain. Pain assessments, quantitative sensory testing, and fMRI (for the lip pursing task) were performed at baseline and 4 weeks after application of capsaicin 8% patch to the amputation stump. The shift into the hand representation area of the cerebral cortex with the lip pursing task has been correlated with phantom limb pain intensity in previous studies, and was the fMRI clinical model for cortical plasticity used in this study.
Results
The mean reduction in spontaneous amputation stump pain, phantom limb pain, and evoked stump pain were −1.007 (p=0.028), −1.414 (p=0.018), and −2.029 (p=0.007), respectively. The areas of brush allodynia and pinprick hypersensitivity in the amputation stump showed marked decreases: −165 cm2, −80% (p=0.001) and −132 cm2, −72% (p=0.001), respectively. fMRI analyses provided objective evidence of the restoration of the brain map, that is, reversal of the shift into the hand representation of the cerebral cortex with the lip pursing task (p<0.05).
Conclusion
The results show that capsaicin 8% patch treatment leads to significant reduction in chronic pain and, particularly, in the area of stump hypersensitivity, which may enable patients to wear prostheses, thereby improving mobility and rehabilitation. Phantom limb pain (“central” pain) and associated brain plasticity may be modulated by peripheral inputs, as they can be ameliorated by the peripherally restricted effect of the capsaicin 8% patch.
Keywords:
Introduction
Following limb amputation, more than 50% patients complain of hypersensitivity in the amputation stump and chronic pain, which may be reported in both the residual limb and the area referred to the body part removed.Citation1–Citation3 The pain referred to a missing limbCitation4 or an organCitation5–Citation12 is termed phantom pain. Chronic pain has a negative impact on quality of life, aggravating psychologic distress following the amputationCitation13 and increasing the risk of developing anxiety and depression.Citation14 Moreover, stump pain may worsen on walking,Citation15,Citation16 which affects the use of a prosthesis and rehabilitation.Citation17
Several studies have reported factors that may be related to the risk of developing chronic pain following an amputation – time elapsed since amputation,Citation18 presence of pain before amputation,Citation19 higher levels of pain soon after amputation,Citation20 psychologic factors,Citation21 intensity of concomitant nonpainful phantom limb sensations,Citation22 and the site of amputation.Citation23 Objective methods have demonstrated changes in both the peripheral and central nervous systems, including neuroimaging and neurophysiologic techniques, in patients with chronic phantom pain after an amputation. Maladaptive plasticity has been suggested as an underlying mechanism for chronic pain, from observations in patients who showed spread of activation from the neighboring cortical areas into the deprived cortical area.Citation24 This spread or shift into the deprived cortical area, for example, with the lip pursing task, was positively correlated with the presence and intensity of the phantom limb pain.Citation25–Citation27
The act of lip pursing was used in our study, during the functional magnetic resonance imaging (fMRI) assessment, to evoke brain activation. This method was used previously by Lotze et al,Citation27 and consisted of a simple lip pursing movement performed every second (rate of 1 Hz) during fMRI acquisition. The scanning paradigm consisted of a run of alternating resting and stimulation (lip pursing) blocks. Each block was initiated and terminated by a “go” and “stop” command.
Many pharmacologic and nonpharmacologic strategies have been considered for reversing the maladaptive plasticity, and recent studies have shown return toward a more normal cortical activation pattern after the restoration of the peripheral function, such as targeted reinnervationCitation28 and hand transplantation.Citation29 Huse et al,Citation30 in a small study conducted in patients with phantom and stump pain who received morphine, showed both decrease in pain and changes in cortical maps in three patients, which were correlated with the decrease in pain intensity.
Randomized clinical trials in chronic phantom limb pain have focused mainly on the drugs used for neuropathic pain.Citation31 Maier et alCitation32 conducted a trial of the N-methyl-d-aspartic acid-receptor antagonist Memantine, which did not show any beneficial effect on phantom limb pain. In one study, Gabapentin was more effective than placebo in controlling phantom limb pain;Citation33 however, another similar study did not show significant difference versus placebo.Citation34 Treatment with Amitriptyline failed to provide pain relief in a placebo-controlled study.Citation35 In another clinical trial,Citation36 Amitriptyline and Tramadol produced a minor decrease in pain.
Many authors have reported the efficacy,Citation37–Citation47 safety, and tolerabilityCitation46,Citation48–Citation53 of a single application of capsaicin 8% patch (Qutenza), a high-dose topical formulation for the treatment of peripheral neuropathic pain. Capsaicin, the pungent “hot” ingredient in chili peppers, is a natural selective agonist of the vanilloid receptor TRPV1. It is released rapidly from the capsaicin 8% patch, and leads to overstimulation of the skin TRPV1 nociceptors which are “defunctionalized” and are no longer able to respond to the stimuli that normally cause pain in patients with peripheral neuropathic pain.Citation54 A single application of capsaicin 8% patch can provide pain relief for up to 3 months or more,Citation37–Citation47 without the systemic side effects seen with other pain treatments,Citation46,Citation48–Citation53 with the maximum effect reached within 1–2 weeks from the application.Citation54 Capsaicin 8% patch has been shown in a previous study to reduce the intensity of amputation stump and phantom limb pain,Citation55 but sensory and brain mechanisms have not been studied.
The aim of this study was to measure the analgesic effect of a single application of capsaicin 8% patch and the associated changes with quantitative sensory tests in the amputation stump. An additional aim was to investigate plasticity of brain maps with the lip pursing task, using fMRI scans.
Materials and methods
This was an open-label, longitudinal study in patients with amputation stump and phantom limb pain, using the capsaicin 8% patch (Qutenza) treatment as licensed. The study was conducted at Hammersmith Hospital, Imperial College London and Imperial College NHS Trust, in collaboration with colleagues at the Royal National Orthopaedic Hospital, Stanmore. The study was approved by the Charing Cross Research Ethics Committee, London (NRES Committee London – Fulham; REC reference: 15/LO/0566), and each subject provided written informed consent. This was an investigator-led study sponsored by Imperial College London.
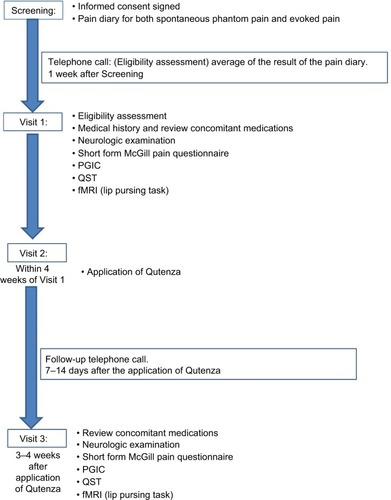
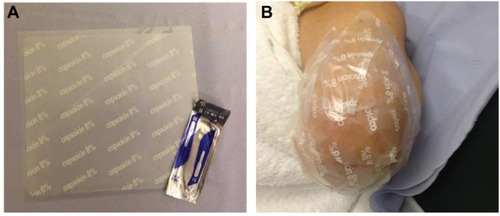
The study duration for each patient was 4 weeks, including three hospital visits and telephone calls as shown in . At the screening visit, patients were given a trial pain diary to complete for the next 7 days. The diary collected numerical pain rating score (NPRS) twice daily (ranging from 0 to 10), as the average pain over the last 12 h. An 11-point numerical rating scale (NPRS) with the 0 anchor point being “no pain” and the 10 anchor point being “pain as bad as you can imagine” was used to separately record spontaneous stump pain, evoked stump pain, and phantom limb pain. Patients with average pain intensity equal or greater than 4/10 for spontaneous stump or phantom limb pain, and who had been clinically stable on their prescribed medical treatment for a period ≥2 months, were eligible to participate further in the study. They were advised to continue with trial pain diary daily for the entire duration of the study until the end-of-study follow-up visit. All tests (Visit 1 and Visit 3) were carried out before and after a single 60 min capsaicin 8% patch application (Visit 2; ). The patch application was performed as licensed and provided as a treatment in pain clinics. The capsaicin 8% patch is a 14×20 cm (280 cm2) patch containing a high dose of capsaicin (). Each patch application delivers 179 mg capsaicin directly into the skin. Nitrile gloves were worn at all times while handling the patch and cleaning the treatment area. Before treatment, the skin was washed and dried. Hairs were clipped to promote patch adherence. The capsaicin 8% patch was cut with a scalpel prior to removal of the release liner and shaped over the surface of the stump, which was unique for each patient. The application of the patch was on intact, nonirritated, and dry skin. The patch was kept firmly in contact with the skin for 1 h (). The patch was then carefully removed, cleaning gel applied for 1 min, and the skin washed and dried.
Patients
Between 2015 and 2016, six male and eight female patients with single limb amputation were studied. The patient cohort included five with upper limb and nine lower limb amputations. The average age of subjects was 53 years (). Eight patients had amputation involving the knee joint; one had an amputation at the hind quarter level, one had a right middle finger amputation, three had an amputation at the elbow joint and one at the wrist joint. Ten amputations were traumatic, two followed a long history of chronic pain due to orthopedic problems, and two were performed because of the presence of cancer. Time since patients had the amputation ranged from 12 to 420 months (35 years), with an average of 94 months (7.8 years). The mean number (range) of capsaicin patches applied during the study was 1.5 (1–2) patches.
Table 1 Clinical characteristics of patients in the study
Clinical symptoms and pain scores
Patients described a range of symptoms, most commonly as pins and needles, tenderness of the stump, electric shocks, crushing, cold-freezing, shooting, stabbing, and burning pain. Many patients reported vivid phantom sensations. Eight patients described the missing limb with an abnormal shape, and three with an increased volume and size. All patients enrolled in the study were taking treatment for neuropathic pain at the start of the study, such as Gabapentin, Pregabalin, Duloxetine, tricyclic antidepressants, Oxycodone, Morphine, or a combination of these. Patients maintained their concomitant medications throughout the study. Details of the patients at baseline are reported in .
Clinical examination
At the initial visit, a detailed medical history was taken from the subject and the procedures were explained. Each subject was asked to describe the perceived size, shape, movement, and any other qualities of the phantom limb, as well as the location, quality, and frequency of stump and phantom pain. Clinical examination and tests were performed in both amputated and contralateral limb. Vibration, monofilament, and thermal thresholds were performed as described below. Symptoms were recorded using the short-form McGill Pain QuestionnaireCitation56 (maximum score 55, indicating severe symptoms) and by recording a numerical pain score (Likert pain score: 0=no pain; 10=maximum pain). Patients were also asked to complete a seven-point scale (Patient Clinical Global Impression of Change) before and after the treatment – as no change, improved, or worse.Citation57,Citation58
Quantitative sensory testing
Vibration perception thresholds were measured using a biothesiometer (Biomedical Instrument Company, Newbury, OH, USA) placed over a distal bony prominence. Three ascending and three descending trials were carried out, and the mean value obtained. Values >12 V were considered abnormal.Citation59,Citation60
The thresholds for light touch were measured using monofilaments made by Mr A Ainsworth, University College London, UK (No. 1, 0.0174 g to No. 20, 263.0 g). The number of the hair with the lowest force reliably detected by the patient at the center of the area of hypersensitivity was recorded. Values >No. 3 monofilament (0.0479 g) were considered abnormal.Citation59 The monofilament with the lowest force that caused pain or discomfort was also recorded. Clinical examination was carried out along the stump on its entire circumference from the distal end, to determine the area of any static (monofilament) and dynamic (brush) allodynia and also pinprick hypersensitivity. This method was used to obtain a detailed map before the application of capsaicin 8% patch, in order to localize the area requiring the treatment.
Static allodynia area was determined by a monofilament at the touch detection threshold in the contralateral intact limb, and the area of dynamic allodynia by gently stroking with a standardized brush (yellow brush; Somedic, Stockholm, Sweden). Allodynia was considered present if the sensation changed from a feeling of touch to a sensation of pain or discomfort. For pinprick testing, the sharp and dull ends of a sterile Neurotip pin were first lightly applied to the limb. The subject responded “sharp” or “dull” when the respective stimulus was detected. The surface area of increased or decreased pinprick sensation over the stump was mapped by moving the pin along the stump. Patients were asked to rate the evoked sensation as normal, diminished, absent, or increased, increased abnormal, and increased painful. The outline of both areas of allodynia and pinprick hyper sensitivity was traced onto a transparent plastic sheet and transferred to metric graph paper.Citation61 The area obtained after the examination was calculated by the weighing methodCitation62 using a Sartorius balance.
Thermal perception thresholds were performed as describedCitation63,Citation64 using the TSA-II NeuroSensory Analyzer (Medoc, Ramat Yishai, Israel). A 30×30 mm thermode was used, and thermal thresholds were determined in the center of the affected area for warm perception, cool perception, heat pain, and cold pain, from a baseline temperature of 32°C, with a change in temperature of 1°C/s. All measurements were performed by placing the thermode in an area away from the scar tissue. The mean of three consecutive tests for each modality was recorded. Values >6.4°C for warm sensation, >2.3°C for cool sensation, and >10.4°C for heat pain were considered abnormal, but variation with aging was noted.Citation59,Citation63,Citation64 Thermal thresholds were obtained from the distal site of the anterior aspect of the stump.Citation65 Sensory testing was repeated at the follow-up visit in the amputated limb and at a similar site in the intact limb.Citation65,Citation66
Functional magnetic resonance imaging
Patients were scanned twice while performing a lip pursing motor task, once prior to and once posttreatment. Patients were visually cued to perform the task in blocks of 30 s of lip pursing or resting. There were six blocks of rest and six blocks of lip pursing. The scanning protocol included a high-resolution gradient-echo T1-weighted structural anatomic volume (for registration between patients; voxel size: 1.00×1.00×1.00 mm, flip angle: 9°, repetition time [TR]/echo time: 2300/2.98 ms, 160 ascending slices, inversion time: 900 ms) acquired on a Siemens Verio 3 T scanner, as well as an Echo Planar Imaging (EPI) sequence (182 volumes of T2*-weighted gradient echo, voxel size: 3.00×3.00×3.00 mm, field of view: 192×192×105 mm, flip angle: 80°, TR/echo time: 2000/30 ms, 35 interleaved slices with 3.00 mm thickness). Each run lasted 182 TR. Data analysis was carried out with FSL (FMRIB Software Library):Citation67 1) brain extraction of the anatomic image;Citation68 2) linear registration of the anatomic image (using 12 degrees of freedom) to a standard 2 mm brain atlas (Montreal Neurological Institute [MNI]) using FLIRT (FMRIB’s Linear Image Registration Tool);Citation69 3) the functional data were registered to the anatomic image using FLIRT BBR (FMRIB’s Linear Image Registration Tool, Boundary-Based Registration); 4) the functional images were corrected using Motion Correction Linear Image Registration Tool (MCFLIRT); 5) functional images were high-pass filtered with a cutoff of 60 s; and 6) spatially smoothed using a 5 mm full width at half maximum Gaussian kernel.
Data were analyzed by convolving a canonical double-gamma hemodynamic response function with the task (30 s ON/OFF) time course. First and second scans were contrasted (First > Second) using a fixed-effect model for each patient. Subsequently, bilateral regions of interest were defined in group space for approximately the lip area (centered on MNI 52-8 36) and the hand area (centered on MNI 28–20 62) of the motor cortex and the estimated change in task-evoked blood oxygen level dependent (BOLD) signal extracted for each ROI. The signal change (First session – Second session) on the contralateral side to the amputation was then subtracted from the signal change on the ipsilateral side. One patient who did not respond to the treatment was not included in the subsequent analyses.
Statistical analysis
Data were analyzed using GraphPad Prism version 5.0 for Windows (GraphPad Prism Software, San Diego, CA, USA). The statistical test used was the paired, two-tailed Mann–Whitney test. Values were compared before and after the treatment with capsaicin 8% patch. For all statistical tests, p values <0.05 were considered significant.
Results
Clinical symptoms and pain scores
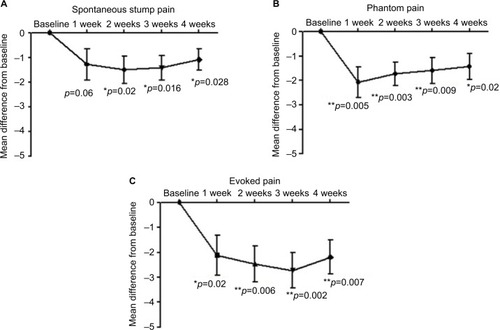
Patients (N=14) reported baseline NPRS scores (mean±SD) for spontaneous stump pain and evoked stump pain of 6.1±1.5 and 7.1±1.9, respectively, and 6.1±2.1 for phantom limb pain. After capsaicin 8% patch application, spontaneous pain, phantom and evoked pain scores all improved (). At 4 weeks following patch application, the NPRS scores for spontaneous stump pain and evoked stump pain were 5.0±2.4 and 5.0±2.5, respectively, and 4.6±2.7 for phantom limb pain. The mean difference (95% CI) for NPRS score, 4 weeks after application of capsaicin 8% patch, was −1.01 (0.12–1.89) for spontaneous stump pain (p=0.028), −2.03 (0.63–3.4) for evoked stump pain (p=0.007), and −1.41 (0.27–2.5) for phantom limb pain (p=0.018). Two patients reported complete resolution of phantom sensations after the treatment, and another reported a clear decrease in the duration of phantom limb pain episodes.
Figure 3 Pain scores at baseline and after capsaicin 8% patch treatment.
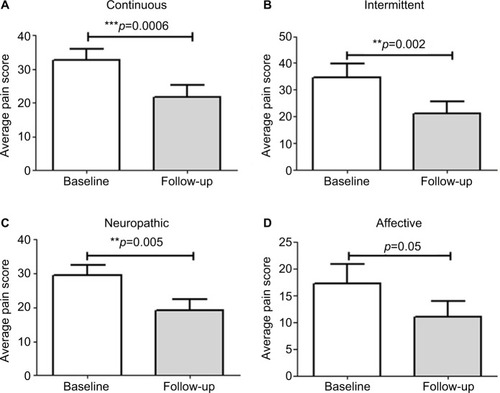
Patients also showed an improvement of the McGill pain score after capsaicin 8% patch application, with a mean difference (95% CI) at the end of the follow-up of 11.0 (5.7–16.3) for continuous pain descriptors (p=0.0006), 13.43 (6.1–20.7) for intermittent pain descriptors (p=0.002), and 10.3 (3.5–17.1) for neuropathic pain descriptors (p=0.005), while the affective descriptors showed a relatively minor, but statistically significant change (p=0.05), as shown in . After capsaicin 8% treatment, the Patient Global Impression of Change scale showed significantly marked improvement (p=0.002).
Figure 4 Short Form (SF) McGill pain score at baseline and end of study.
Quantitative sensory testing
Sensory thresholds in the contralateral (intact) limb of all patients were within normal limits.
The tactile detection threshold (with monofilaments) in the amputation stump varied from reduced to elevated thresholds, the latter particularly in proximity of the scar tissue. All subjects but one reported brush dynamic allodynia. Pinprick sensation was also reported as increased in most cases, though five patients also described pinprick as decreased or absent in the areas of the stump near scar tissue.
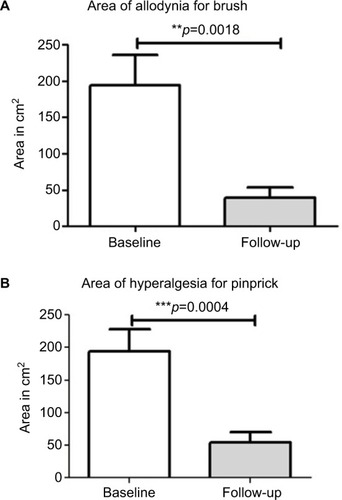
After capsaicin 8% treatment, the area of allodynia for brush and hypersensitivity for pinprick showed a statistically significant decrease ( and ). The mean difference (95% CI) at follow-up visit was −65.6 cm2 (76.98–254.2) for brush mechanical allodynia (p=0.001), 80% reduction, and −132.2 cm2 (64.85–199.6) for pinprick hypersensitivity (p=0.001), 72% reduction. No statistically significant changes in the area of monofilament static allodynia were observed after treatment.
Figure 5 Amputation stump hypersensitivity in a patient before (A) and after (B) capsaicin 8% patch treatment.
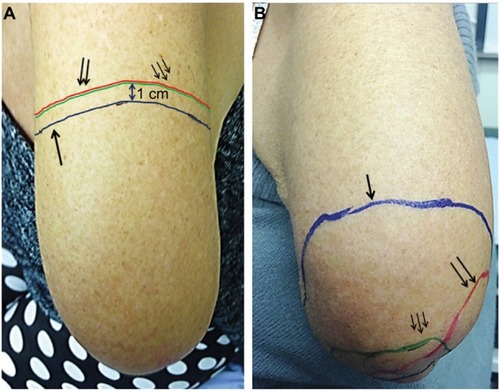
Figure 6 Areas of allodynia and hyperalgesia at baseline and end of study.
Vibration threshold tested at the amputation stump was within the normal limits in all but five patients. Four of these patients reported an elevated vibration threshold in the stump when compared to the homologous site in the intact limb, while in one patient, the vibration threshold was decreased compared to the contralateral side. These were not significantly changed after capsaicin 8% patch application.
At baseline, the mean difference in the stump from the contralateral limb for thermal thresholds was significantly elevated for cool (p=0.0017) and warm (p=0.0022) thresholds, but not for cold pain (p=0.2907) or heat pain (p=0.1788) thresholds. At follow-up, all patients showed a change with the trend toward normal values of the thermal thresholds except for two patients, while the cold and hot pain thresholds did not change. The patients who had an improvement of cool and warm thresholds showed a positive relationship between elevation of thermal thresholds at baseline and the magnitude of improvement after treatment (for cool: r=0.73, p=0.0003; for warm: r=0.63, p=0.0007).
Functional magnetic resonance imaging
Nine patients received fMRI scan before and after the treatment, as three patients had ferromagnetic objects potentially not compatible with the fMRI magnetic field, one patient was claustrophobic, and one patient was uncomfortable in keeping the supine position.
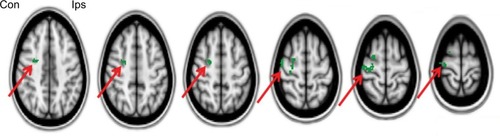
The effect of lip pursing > rest averaged across both First and Second scans and across all patients and is shown in . Analysis of data obtained in nine patients who completed the fMRI studies showed objective evidence of restoration of brain maps with the lip pursing task. The difference between the scans (First > Second scan) of lip pursing > rest averaged across completed patients is shown in , which indicates a decrease (reversal) of the spread into the hand representation area of the cerebral cortex with the lip pursing task (shown in green, arrowed).
Figure 7 Effect of lip pursing > rest averaged across both First and Second scans and across all patients (whole-brain analysis using FSL with fixed effects, cluster corrected for multiple comparisons p<0.05).
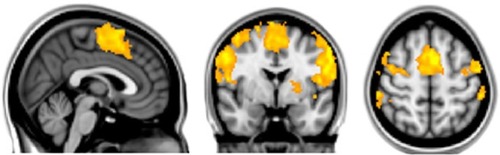
Figure 8 Difference between scans (First > Second scan) of lip pursing > rest averaged across completed patients, showing a decrease (reversal) of the shift into the hand representation of the cerebral cortex with the lip pursing task (in green, arrowed). Images flipped so that left is contralateral to the amputation and right is ipsilateral (FSL with fixed effects, cluster corrected for multiple comparisons p<0.05).
Discussion
Our results demonstrate that a single 60 min capsaicin 8% patch application to the amputation stump is effective in reducing stump and phantom limb pain for 4 weeks, without any systemic or adverse events. We observed a marked decrease in the area of allodynia for brush and pinprick hypersensitivity in the amputation stump following capsaicin 8% patch treatment, which had beneficial consequences for using a prosthesis and, hence, for the mobility and rehabilitation of the patients. Current medical treatments for neuropathic pain have not been shown to be clearly effective in reducing post-amputation stump or phantom limb pain in clinical trialsCitation33–Citation36,Citation70 and have side effects.Citation71
Our findings are in accordance with previous observational studies, as of Kern et al,Citation55 which reported relief of amputation stump and phantom limb pain in a small cohort of amputees after treatment with capsaicin 8% patch. In the larger QUEPP study,Citation53,Citation55 30% of patients with phantom pain, 25% with stump pain, and 14% with combined phantom and stump pain experienced improvement in pain intensity after patch application.
Some authors have noted that amputees may find it difficult to distinguish between phantom and stump pain, while other studies have shown that amputees are able to differentiate stump pain from the phantom pain both immediately after the amputationCitation72 and 1 year or more after the amputation.Citation73 In our study, patients were asked, after careful explanation and demonstration, to rate spontaneous and evoked stump pain twice daily and phantom limb pain. The distinctions were reiterated at clinic visits, when touch and pinprick sensation were assessed, along with any allodynia or pinprick hypersensitivity.
Our findings were consistent with previous literature showing the coexistence in the residual limb of areas of hypoesthesia and hyperpathia for pinprick and light touch,Citation66 often related to the proximity of the scar or grafted tissue.Citation65,Citation74 In our study, amputees showed elevated thresholds for warm and cool perception in the residual but not intact limb, while no differences were found for cold and hot pain thresholds. Thermal thresholds for cool and warm in the residual limb showed a trend toward normal values after the treatment, which suggests regeneration or modification of the phenotype of nerve fibers and deserves further study. Previous studies indicated similar changes in thermal thresholds in the residual limb,Citation65 though some showed differences such as decrease in cold thresholdCitation66 or increase in cold pain threshold.Citation75,Citation76 Electrical sensation and pain thresholds were found to be elevated in the residual limb in one study.Citation75 The potential sources of differences include the presence of scar or grafted tissue and the site of testing, which may account for discrepant results.
To investigate the central mechanisms following treatment with capsaicin 8% patch in amputation stump and phantom limb pain, patients in our study were also assessed with fMRI scans. Amputees who reported relief from phantom limb pain showed a decrease in the spread of lip-to-hand representation in the cortex contralateral to the amputation stump, evoked during the lip pursing task. Further studies with larger numbers of subjects are needed to replicate this proof-of-concept study and upper versus lower limb amputation effects. Sensory stimulation, for example, by a pneumatic device, would also be of interest.Citation24,Citation26 As capsaicin 8% patch effects are restricted to cutaneous nerve terminals and are generally reversible, our findings suggest that phantom limb pain may be driven or modulated by peripheral inputs.
Using neuromagnetic source imaging, Flor et al showed shift in the contralateral cortex map of traumatic amputees who had phantom limb pain,Citation24 but not in congenital amputeesCitation25 or healthy volunteers.Citation26 The cortical reorganization was confirmed by Lotze et alCitation27 and Maclver et alCitation77 using fMRI in amputees who were asked to perform voluntary movements of the lips, intact hand, and imaginary movements of the phantom limb. Based on these results, the presence of phantom pain was attributed to an increased responsivity of the area of the brain representing the body part removed, due to the spatial invasion of adjacent cortical representations. However, Raffin et alCitation78,Citation79 reported that distinct and separate brain networks are activated by active or imagined movements, and these networks are similar between amputees and healthy controls. The preservation of the connections between cortex and periphery has been largely supported by Makin et alCitation80–Citation83 in amputees. An integrative explanatory hypothesis for the mechanisms responsible for generating phantom pain suggests the potential of coexistence between reorganizational processes of the cortex with the expansion of cortical map neighboring the deafferented area and the abnormal spontaneous activity of the area now deprived of peripheral input.Citation84 The maladaptive plasticity and persistent representation models may be not mutually exclusive.Citation85,Citation86
As suggested by two studies,Citation28,Citation29 a normal cortical map representation may be restored in amputees by intervention at peripheral sites, as in our study. In these studies, both reimplantation of the missing limb and retargeting of peripheral terminals led to a large shift of cortical maps toward the expected preamputation location. Interestingly, the patient who had the transplant of both hands showed a reversal of the reorganization in a manner suggesting strengthening of preexisting connections. However, mirror therapy and mental imagery that work directly on the central nervous system have been shown to have beneficial effects with cortical remapping; thus, both central and peripheral inputs are important.Citation77,Citation87,Citation88
Conclusion
This study showed marked relief of amputation stump pain and, particularly, the area of hypersensitivity following capsaicin 8% patch application. The treatment may enable patients to wear prostheses, thereby improving mobility and rehabilitation. fMRI scan analyses provided objective evidence of the restoration brain maps, thereby demonstrating that phantom limb pain (“central” pain) may be modulated by peripheral inputs and can be reduced by the peripherally restricted effect of the capsaicin 8% patch.
Authors’ contributions
RP, MS, IRM, and PA helped recruit patients and conducted the clinical study, RL set up and analyzed the fMRI scans, RB and PA conceived the study, and all authors contributed to the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We are grateful to the Grace Anderson Trust, Edinburgh, the RNOH Charity, Stanmore and Astellas UK for financial support and helpful discussions.
Disclosure
PA has received speaker fees for symposia and meetings organized by Astellas UK, but no remuneration for this investigator-led study. The authors report no conflicts of interest in this work.
References
- EphraimPLWegenerSTMacKenzieEJDillinghamTRPezzinLEPhantom pain, residual limb pain, and back pain in amputees: results of a national surveyArch Phys Med Rehabil200586101910191916213230
- EhdeDMCzernieckiJMSmithDGChronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputationArch Phys Med Rehabil20008181039104410943752
- KernUBuschVRocklandMKohlMBirkleinFPrevalence and risk factors of phantom limb pain and phantom limb sensations in Germany. A nationwide field surveySchmerz2009235479488 German19322592
- FingerSHustwitMPFive early accounts of phantom limb in context: Paré, Descartes, Lemos, Bell, and MitchellNeurosurgery200352367568612590694
- DijkstraPURietmanJSGeertzenJHPhantom breast sensations and phantom breast pain: a 2-year prospective study and a methodological analysis of literatureEur J Pain20071119910816487732
- RameshShuklaNKBhatnagarSPhantom breast syndromeIndian J Palliative Care2009152103107
- RamachandranVSMcGeochPDOccurrence of phantom genitalia after gender reassignment surgeryMed Hypotheses20076951001100317420102
- PühseGWachsmuthJUKemperSHusstedtIWKlieschSEversSPhantom testis syndrome: prevalence, phenomenology and putative mechanismsInt J Androl2010331e216e22019765099
- OvesenPKrønerKOrnsholtJBachKPhantom-related phenomena after rectal amputation: prevalence and clinical characteristicsPain19914432892912052399
- AroseBShreeveWWBaimRSAtkinsHLPhantom gallbladder. A variant of the rim signClin Nucl Med19871264574603297459
- MarbachJJRaphaelKGPhantom tooth pain: a new look at an old dilemmaPain Med200011687715101965
- SicuteriFNicolodiMFuscoBMOrlandoSIdiopathic headache as a possible risk factor for phantom tooth painHeadache19913195775811774170
- WhyteASNivenCAPsychological distress in amputees with phantom limb painJ Pain Symptom Manage200122593894611728797
- DesmondDMMacLachlanMAffective distress and amputation-related pain among older men with long-term, traumatic limb amputationsJ Pain Symptom Manage200631436236816632084
- van der SchansCPGeertzenJHSchoppenTDijkstraPUPhantom pain and health-related quality of life in lower limb amputeesJ Pain Symptom Manage200224442943612505212
- GeertzenJHBosmansJCvan der SchansCPDijkstraPUClaimed walking distance of lower limb amputeesDisabil Rehabil200527310110415823990
- RaichleKAHanleyMAMoltonIProsthesis use in persons with lower- and upper-limb amputationJ Rehabil Res Dev200845796197219165686
- BosmansJCGeertzenJHPostWJvan der SchansCPDijkstraPUFactors associated with phantom limb pain: a 31/2-year prospective studyClin Rehabil201024544445320442256
- NikolajsenLIlkjaerSKrønerKChristensenJHJensenTSThe influence of preamputation pain on postamputation stump and phantom painPain199772339334059313280
- HanleyMAJensenMPSmithDGEhdeDMEdwardsWTRobinsonLRPreamputation pain and acute pain predict chronic pain after lower extremity amputationJ Pain20078210210916949876
- MargalitDHeledEBergerCKatzirHPhantom fighters: coping mechanisms of amputee patients with phantom limb pain: a longitudinal studyOpen J Orthopaedics20130307300305
- MontoyaPLarbigWGrulkeNFlorHTaubEBirbaumerNThe relationship of phantom limb pain to other phantom limb phenomena in upper extremity amputeesPain1997721–287939272791
- DijkstraPUGeertzenJHStewartRvan der SchansCPPhantom pain and risk factors: a multivariate analysisJ Pain Symptom Manage200224657858512551807
- FlorHElbertTKnechtSPhantom-limb pain as a perceptual correlate of cortical reorganization following arm amputationNature199537565314824847777055
- FlorHElbertTMühlnickelWPantevCWienbruchCTaubECortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputeesExp Brain Res199811922052129535570
- KnechtSHenningsenHElbertTReorganizational and perceptional changes after amputationBrain1996119Pt 4121312198813284
- LotzeMFlorHGroddWLarbigWBirbaumerNPhantom movements and pain. An fMRI study in upper limb amputeesBrain2001124Pt 112268267711673327
- ChenAYaoJKuikenTDewaldJPCortical motor activity and reorganization following upper-limb amputation and subsequent targeted reinnervationNeuroimage Clin2013349850624273732
- GirauxPSiriguASchneiderFDubernardJMCortical reorganization in motor cortex after graft of both handsNat Neurosci20014769169211426223
- HuseELarbigWFlorHBirbaumerNThe effect of opioids on phantom limb pain and cortical reorganizationPain2001901–2475511166969
- AlviarMJHaleTDungcaMPharmacologic interventions for treating phantom limb painCochrane Database Syst Rev201610CD00638027737513
- MaierCDertwinkelRMansourianNEfficacy of the NMDA-receptor antagonist memantine in patients with chronic phantom limb pain–results of a randomized double-blinded, placebo-controlled trialPain2003103327728312791434
- BoneMCritchleyPBuggyDJGabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over studyReg Anesth Pain Med200227548148612373695
- SmithDGEhdeDMHanleyMAEfficacy of gabapentin in treating chronic phantom limb and residual limb painJ Rehabil Res Dev200542564565416586190
- RobinsonLRCzernieckiJMEhdeDMTrial of amitriptyline for relief of pain in amputees: results of a randomized controlled studyArch Phys Med Rehabil20048511614970960
- Wilder-SmithCHHillLTLaurentSPostamputation pain and sensory changes in treatment-naive patients: characteristics and responses to treatment with tramadol, amitriptyline, and placeboAnesthesiology2005103361962816129989
- MouJPaillardFTurnbullBTrudeauJStokerMKatzNPEfficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials DatabasePain201315491632163923707278
- BrownSSimpsonDMMoyleGNGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trialsAIDS Res Ther2013101523351618
- IrvingGABackonjaMMDuntemanEfor NGX-4010 C117 Study GroupA multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgiaPain Med20111219910921087403
- SimpsonDMBrownSTobiasJfor NGX-4010 C107 Study GroupControlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathyNeurology200870242305231318541884
- WebsterLRMalanTPTuchmanMMMollenMDTobiasJKVanhoveGFA multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgiaJ Pain2010111097298220655809
- BabbarSMarierJFMouksassiMSPharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic painTher Drug Monit200931450251019494795
- BackonjaMWallaceMSBlonskyERfor NGX-4010 C116 Study GroupNGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind studyLancet Neurol20087121106111218977178
- BackonjaMMMalanTPVanhoveGFfor C102/106 Study GroupNGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extensionPain Med2010114600600820113411
- MouJPaillardFTurnbullBTrudeauJStokerMKatzNPQutenza (capsaicin) 8% patch onset and duration of response and effects of multiple treatments in neuropathic pain patientsClin J Pain201430428629423765045
- SimpsonDMGazdaSBrownSfor NGX-4010 C118 Study GroupLong-term safety of NGX-4010, a high-concentration capsaicin patch, in patients with peripheral neuropathic painJ Pain Symptom Manage20103961053106420538187
- SimpsonDMBrownSTobiasJKfor NGX-4010 C107 Study GroupNGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: results of a 52-week open-label studyClin J Pain201430213414223446088
- WagnerTRoth-DaniekASellAEnglandJKernKUCapsaicin 8% patch for peripheral neuropathic pain: review of treatment best practice from “real-world” clinical experiencePain Manage201223239250
- WebsterLRPeppinJFMurphyFTTobiasJKVanhoveGFTolerability of NGX-4010, a capsaicin 8% patch, in conjunction with three topical anesthetic formulations for the treatment of neuropathic painJ Pain Res2012571322328830
- WebsterLRPeppinJFMurphyFTLuBTobiasJKVanhoveGFEfficacy, safety, and tolerability of NGX-4010, capsaicin 8% patch, in an open-label study of patients with peripheral neuropathic painDiabetes Res Clin Pract201193218719721612836
- WebsterLRNunezMTarkMDTolerability of NGX-4010, a capsaicin 8% dermal patch, following pretreatment with lidocaine 2.5%/prilocaine 2.5% cream in patients with post-herpetic neuralgiaBMC Anesthesiol2011112522182397
- MaihöfnerCGHeskampMLTreatment of peripheral neuropathic pain by topical capsaicin: impact of pre-existing pain in the QUEPP-studyEur J Pain201418567167924259265
- MaihofnerCHeskampMLProspective, non-interventional study on the tolerability and analgesic effectiveness over 12 weeks after a single application of capsaicin 8% cutaneous patch in 1044 patients with peripheral neuropathic pain: first results of the QUEPP studyCurr Med Res Opin201329667368323551064
- AnandPBleyKTopical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patchBr J Anaesth2011107449050221852280
- KernKUBaustHHofmannWHolzmüllerRMaihöfnerCHeskampMLCapsaicin 8 % cutaneous patches for phantom limb pain. Results from everyday practice (non-interventional study)Schmerz2014284374383 German24939242
- MelzackRThe short-form McGill Pain QuestionnairePain19873021911973670870
- FergusonLSchemanJPatient global impression of change scores within the context of a chronic pain rehabilitation programJ Pain2009104 SupplS73
- HurstHBoltonJAssessing the clinical significance of change scores recorded on subjective outcome measuresJ Manipulative Physiol Ther2004271263514739871
- AthertonDDFacerPRobertsKMUse of the novel Contact Heat Evoked Potential Stimulator (CHEPS) for the assessment of small fibre neuropathy: correlations with skin flare responses and intra-epidermal nerve fibre countsBMC Neurol200772117683543
- CoppiniDVWellmerAWengCYoungPJAnandPSönksenPHThe natural history of diabetic peripheral neuropathy determined by a 12 year prospective study using vibration perception thresholdsJ Clin Neurosci20018652052411683597
- KlothLCFeedarJAAcceleration of wound healing with high voltage, monophasic, pulsed currentPhys Ther19886845035383258429
- BohannonRWPfallerBADocumentation of wound surface area from tracings of wound perimeters. Clinical report on three techniquesPhys Ther19836310162216246622538
- WellmerAMisraVPShariefMKKopelmanPGAnandPA double-blind placebo-controlled clinical trial of recombinant human brain-derived neurotrophic factor (rhBDNF) in diabetic polyneuropathyJ Peripher Nerv Syst20016420421011800042
- AnandPTerenghiGWarnerGKopelmanPWilliams-ChestnutRESinicropiDVThe role of endogenous nerve growth factor in human diabetic neuropathyNat Med1996267037078640566
- HunterJPKatzJDavisKDDissociation of phantom limb phenomena from stump tactile spatial acuity and sensory thresholdsBrain2005128Pt 230832015634736
- HardenRNGagnonCMKhanAWallachGZereshkiAHypoesthesia in the distal residual limb of amputeesPM R20102760761120659715
- JenkinsonMBeckmannCFBehrensTEWoolrichMWSmithSMFSLNeuroimage201262278279021979382
- SmithSMFast robust automated brain extractionHum Brain Mapp200217314315512391568
- JenkinsonMBannisterPBradyMSmithSImproved optimization for the robust and accurate linear registration and motion correction of brain imagesNeuroimage200217282584112377157
- WiechKKieferRTTöpfnerSA placebo-controlled randomized crossover trial of the N-methyl-D-aspartic acid receptor antagonist, memantine, in patients with chronic phantom limb painAnesth Analg200498240841314742379
- WuCLAgarwalSTellaPKMorphine versus mexiletine for treatment of postamputation pain: a randomized, placebo-controlled, crossover trialAnesthesiology2008109228929618648238
- ShuklaGDSahuSCTripathiRPGuptaDKPhantom limb: a phenomenological studyBr J Psychiatry1982141154587116073
- SchleyMTWilmsPToepfnerSPainful and nonpainful phantom and stump sensations in acute traumatic amputeesJ Trauma200865485886418849803
- HunterJPKatzJDavisKDStability of phantom limb phenomena after upper limb amputation: a longitudinal studyNeuroscience2008156493994918755249
- LiSMeltonDHLiSTactile, thermal, and electrical thresholds in patients with and without phantom limb pain after traumatic lower limb amputationJ Pain Res2015816917425945065
- WahrenLKChanges in thermal and mechanical pain thresholds in hand amputees. A clinical and physiological long-term follow-upPain19904232692772250918
- MaclverKLloydDMKellySRobertsNNurmikkoTPhantom limb pain, cortical reorganization and the therapeutic effect of mental imageryBrain2008131Pt 82181219118567624
- RaffinEMattoutJReillyKTGirauxPDisentangling motor execution from motor imagery with the phantom limbBrain2012135Pt 258259522345089
- RaffinERichardNGirauxPReillyKTPrimary motor cortex changes after amputation correlate with phantom limb pain and the ability to move the phantom limbNeuroimage201613013414426854561
- MakinTRFilippiniNDuffEPHenderson SlaterDTraceyIJohansen-BergHNetwork-level reorganisation of functional connectivity following arm amputationNeuroimage201511421722525776216
- MakinTRScholzJFilippiniNHenderson SlaterDTraceyIJohansen-BergHPhantom pain is associated with preserved structure and function in the former hand areaNat Commun20134157023463013
- MakinTRWilfMSchwartzIZoharyEAmputees “neglect” the space near their missing handPsychol Sci2010211555720424023
- MakinTRScholzJHenderson SlaterDJohansen-BergHTraceyIReassessing cortical reorganization in the primary sensorimotor cortex following arm amputationBrain2015138Pt 82140214626072517
- BoströmKJde LussanetMHWeissTPutaCWagnerHA computational model unifies apparently contradictory findings concerning phantom painSci Rep20144529824931344
- KunerRFlorHStructural plasticity and reorganisation in chronic painNat Rev Neurosci2016181203027974843
- MakinTRBensmaiaSJStability of sensory topographies in adult cortexTrends Cogn Sci201721319520428214130
- BarbinJSeethaVCasillasJMPaysantJPerennouDThe effects of mirror therapy on pain and motor control of phantom limb in amputees: a systematic reviewAnn Phys Rehabil Med201659Se149
- DiersMChristmannCKoeppeCRufMFlorHMirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb painPain2010149229630420359825