Abstract
Now that the vascular hypothesis of migraine is no longer the prevailing theory of migraine pathogenesis, there is interest in developing acute migraine treatments that act exclusively on non-vascular targets. There is a large percentage of non-responders to current acute migraine treatments and the vasoconstriction associated with triptans limit their use in patients with pre-existing cardiovascular risk factors. Preferential 5-HT1F agonists have shown promising results in in vitro and early proof-of-concept trials. Lasmiditan, a highly selective 5-HT1F agonist, has completed two Phase III randomized, double blind, placebo-controlled clinical trials, with a third – a long-term, open-label safety study – still underway. Research to date suggests lasmiditan lacks vasoconstrictive properties and may be a safe and effective treatment option in patients refractory to current acute migraine medications or who have cardiovascular risk factors.
Brief introduction on 5-HT1F receptors as novel targets in the treatment of migraine
Migraine is a pathophysiologically complex disease. Current evidence suggests that migraine is a neuronal process involving activation and sensitization of the trigeminal nociceptors and the trigeminocervical complex, as well as cortical spreading depression and abnormal brainstem activity.Citation1,Citation2 During a migraine, there is meningeal vessel dilatation, an observation that was once believed to be the cause of migraineCitation3 but is now thought to be an epiphenomenon.Citation3–Citation5
Triptans, selective 5-HT1B/D agonists, were initially developed when migraine was believed to be a “vascular headache” and triptans’ ability to activate the 5-HT1B and 5-HT1D receptors on cerebral vessels and induce cerebral vessel vasoconstriction was desired.Citation3 Triptans are currently a first-line treatment for acute migraine. Their activity works not only on cerebral vessels but also on cardiac endothelial cells, causing cardiac vasoconstriction.Citation3,Citation6 It is reported that 76% of patients taking oral sumatriptan experience heavy arms and 50% experience chest pressure,Citation7 but this chest pain is non-ischemic.Citation8 While ischemic events attributable to triptans are uncommon,Citation8 there are case reports of myocardial infarctions and strokes after initiation of sumatriptan.Citation9–Citation11 Non-steroidals, which are also often used for acute migraine treatment, have been associated with increased risk of myocardial infarction.Citation12
Triptans are contraindicated in patients with cardiovascular disease, cerebrovascular disease, uncontrolled hypertension, and hemiplegic migraine.Citation6,Citation12 With emerging evidence that cerebral vasodilation is not the nociceptive stimulus in migraine, attention has turned to developing new acute migraine treatments targeting the trigeminal pathways while avoiding the vasoactive 5-HT1B and 5-HT1D receptors. The 5-HT1F receptor has become a receptor of interest as a putative migraine treatment target. Lasmiditan, a 5-HT1F agonist, has successfully completed Phase III clinical trials and holds promise as an emerging acute migraine treatment.
There are existing summaries on lasmiditan,Citation13–Citation16 however no prior review includes results from the Phase III GLADIATOR trial. This paper summarizes all results and information on lasmiditan available as of July 2018.
Review of pharmacology, mode of action, pharmacokinetics, and administration of lasmiditan
Pharmacology
Lasmiditan, previously known as COL-144 and LY573144, is a highly selective 5-HT1F agonist. Structurally different than triptans, this compound constitutes a new class of drugs, “ditans”. Whereas triptans possess an indole structure that closely resembles the 5-HT receptor, ditans replace this indole group with a pyridine-piperidine scaffold. Triptans non-specifically bind to the 5-HT1B and 5-HT1D receptors and with varying affinity bind the 5-HT1F receptors, causing direct vascular vasoconstriction.Citation17 In contrast, ditans are selective for the 5-HT1F receptor and its mechanism of action is neuronal without evidence of vasoactive effects. In pre-clinical and clinical models believed to mimic human cerebral and cardiovascular contractility,Citation18 5-HT1F agonists have not been associated with rabbit saphenous veinCitation17,Citation19 or cat carotid arteryCitation18 vasoconstriction.
Mode of action
In the current prevailing theory of migraine pain, trigeminal activation triggers the release of signaling proteins including CGRP, causing secondary cerebral vessel dilation, plasma protein extravasation, and mast cell degranulation.Citation4,Citation20–Citation22 While triptans are potent 5-HT1B/D agonists, they also exhibit partial 5-HT1F activity and act upon neuronal pathways.Citation17 Some researchers suggest that the efficacies of triptans are in part due to their activity on the 5-HT1F receptor.Citation20 Using established animal models of migraine, 5-HT1F receptor agonists showed decreased plasma protein extravasationCitation17 as well as c-fos expression,Citation20 and suppressed neuronal firing within the trigeminal nucleus caudalis.Citation23
Lasmiditan is a highly selective 5-HT1F agonist, having a greater than 450-fold increased affinity for 5-HT1F over 5-HT1A, 5-HT1B and 5-HT1D receptors. Moreover, lasmiditan has been shown to have low potency for the vasoactive 5-HT1B and 5-HT1D receptors.Citation17 Importantly, the 5-HT1F receptor is not found on vascular structures and lasmiditan has not been shown to have vasoconstrictive effects.Citation17
Plasma protein extravasation
Neurogenic inflammation of the dura is an important cause of migraine pain.Citation24 The 5-HT1F receptors are found in high density in the neurons of the trigeminal ganglion. The release of pain-provoking stimuli (including calcitonin gene-related peptide and substance P) from the presynaptic membrane of the trigeminal system can be indirectly measured by quantifying protein extravasation from dura mater following painful stimulation.Citation25 Johnson et al showed that extravasation of dura plasma proteins in guinea pigs was unaffected by the 5-HT1B/D receptors but significantly decreased with increasing 5-HT1F receptor affinity.Citation20 Lasmiditan has been shown to block plasma protein extravasation.Citation17
C-fos
C-fos is a protein that regulates the transcription rate of genes and is used as an indicator of the level of synaptic activation. After painful stimuli, c-fos is measured in high levels within the trigeminal nerve complex.Citation17 With repetitive noxious stimuli, it is believed that the persistently elevated levels of c-fos trigger long-lasting transcription changes.Citation26 5-HT1F agonists have been shown to decrease c-fos activity within the trigeminal nucleus.Citation27 Rats pretreated with lasmiditan have shown decreased c-fos expression in the nucleus caudalis.Citation17
Suppression of neuronal firing in the trigeminal nucleus caudalis
Shepheard et al observed a 5-HT1F dose response decrease in action potential generation in response to electrical dural stimulation, suggesting that 5-HT1F agonists inhibit second order neurons in the trigeminal nucleus caudalis.Citation23
Pharmacokinetics
There are limited data on the pharmacokinetics of lasmiditan. During Phase I clinical trials, the peak drug concentrations (Cmax), time at peak concentration (Tmax), area under the curve from administration to time 30 hours (AUC[0-t]) and area under the curve from pre-dose to infinity (AUC[0-inf]) were measured in fed and fasted states. The Cmax (ng/mL) and Tmax (hours) with 200 mg of lasmiditan were 394.7 ng/mL and 2.5 hours in the fed state and 322.8 ng/mL and 1.5 hours in the fasted state. The AUC(0-t) and AUC(0-inf) were higher in the fed (2,244 ng.h/mL, 2,265 ng.h/mL) than the fasted states (1,892 ng.h/mL, 1,906 ng.h/mL) respectively.Citation15 It is not known whether the differences observed in the fed and fasted states have clinical significance.
Early Phase II clinical trials evaluated intravenous (IV) formulations of lasmiditan but there are currently no Phase III trials for IV lasmiditan underway. Three doses of oral lasmiditan have been studied in Phase III clinical trials: 50, 100, and 200 mg.
Efficacy studies, including any comparative studies and relevant case reports
LY334370
Early studies evaluating the effectiveness of preferential agonists of the 5-HT1F receptor were favorable. A randomized, placebo-controlled, double blind, parallel-design migraine study evaluating LY334370, a strong agonist of 5-HT1F, showed a clinically significant dose response. Single oral doses of 20, 60, or 200 mg were used and end points were 2-hour response, 2-hour pain free, sustained response and sustained pain free response. Dosages of 60 and 200 mg were better than placebo on all measured outcomes, and the 200 mg dose was more effective than the 60 mg dose. Patients who took 60 and 200 mg had improved 2-hour headache response rates (50% vs 71%), were more likely to be pain free after 2 hours (27% vs 38%), and achieved higher sustained headache response rates (37% vs 52%). These findings were statistically significant (P<0.05) when compared to placebo’s 2-hour headache response rate (19%), pain free rate (4%), and sustained headache response rate (8%). Adverse reactions (parenthesis, somnolence, dizziness, and asthenia) were more likely in the therapeutic dosages (60 and 200 mg) than in the placebo and 20 mg groups.Citation28
While LY334370 showed promising results, it exhibited partial 5-HT1A affinity.Citation17 Efforts were made to develop an agent with even more preferential affinity for the 5-HT1F receptor.
Preclinical pharmacological profile of lasmiditan
Receptor affinity
Following the positive Phase II clinical trial of LY334370, lasmiditan was developed as an even more selective and structurally different 5-HT1F receptor agonist. Early preclinical pharmacologic in vitro studies showed that lasmiditan and LY334370 were potent 5-HT1F agonists, however the preference for the 5HT1F receptor compared to other 5-HT receptors was greatest with lasmiditan. While LY334370 had over eight times the affinity for the 5HT1F compared to the 5-HT1A receptor, lasmiditan’s preferential affinity for the 5-HT1F receptor was 475 times that for the 5-HT1A and 5-HT1B receptors and over 270-fold higher than for all 5-HT receptors evaluated (5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT2A, 5-HT2B, 5HT2C, 5-HT6, and 5-HT7).Citation17
Vasoconstriction
Lasmiditan showed no binding affinity across receptors known to regulate vasoconstriction. In a pre-clinical study using rabbit saphenous veins as a model for human coronary arteries, no contraction was identified with 100 μmol of lasmiditan whereas 50% contractility was noted with the equivalent dose of sumatriptan.
In vitro migraine models
Using the in vitro migraine models described in the “Mode of action” section, oral administration of lasmiditan showed decreased plasma protein extravasation and decreased c-fos levels in rat brains and yielded similar results when compared to a triptan (rizatriptan).Citation17
Phase I clinical trials
Between 2003 and 2018, 14 completed Phase I clinical trials have been conducted, however there are currently no peer-reviewed publications. Presented results of a Phase I clinical trial comparing QT/QTc of two doses of lasmiditan to moxifloxacin showed that neither the therapeutic (100 mg) nor supratherapeutic dose (400 mg) had QT/QTc prolonging effects, whereas moxifloxacin exhibited the expected prolonged QT/QTc. Comparison of EKGs with oral doses of 100 mg and 400 mg of lasmiditan did not show arrhythmias.Citation29
Phase II clinical trials
Two Phase II clinical trials were performed in 2006 and 2009, each with an associated peer-reviewed publication. The first study (ID NCT00384774, COL MIG-201) aimed to test the efficacy of IV lasmiditan and evaluate the effective dose range. Using a prospective, randomized, double blind, placebo-controlled design, 130 healthy study participants between the ages of 18–65 with moderate to severe migraines were given adjusted doses of IV lasmiditan or placebo. The doses ranged form 2.5 mg to 45 mg and were given to small cohorts (five or six people) and adjusted based on the response rates or adverse events experienced with the preceding group. The primary end point was the reduction of a moderate to severe headache to mild or none after 2 hours of initiation of study dose. The most effective dose based on the primary end point was 20 mg of IV lasmiditan. There was a statistically significant 2-hour headache response rate with 20 mg (P=0.048) and 30 mg (P=0.017) of lasmiditan when compared to placebo, whereas the 2-hour headache response rate for the 10 mg dose was not statistically significant compared to that of placebo (). The linear trend of 2-hour headache response observed with escalating doses of IV lasmiditan 10 mg (54%), 20 mg (64%), 30 mg (69%), and 45 mg (75%) when compared to placebo (45%) () was statistically significant (P=0.01). There were no serious adverse events reported. Subjects did not experience common triptan side effects such as chest pain or chest pressure. The most common side effects were parasthesias and dizziness with no clear dose-related response.Citation30
Figure 1 Proportion of patients with headache relief 2 hours after lasmiditan infusion.
Abbreviation: PBO, placebo.
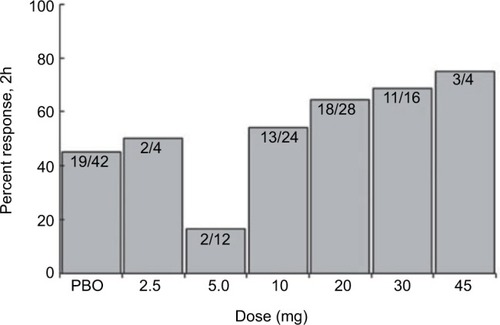
Table 1 Summary of results from COL MIG-201
The second Phase II clinical trial (ID NCT00883051, COL MIG-202) evaluated the safety and efficacy of oral lasmiditan in acute migraine. In this randomized, double blind, placebo-controlled, dose ranging study, otherwise healthy patients between the ages of 18–65 were randomized to either oral lasmiditan (50, 100, 200 or 400 mg) or placebo in a 1:1:1:1:1 ratio (). Of the 378 participants included in the study, 297 received lasmiditan. The percentage of headache responders measured by improvement from moderate or severe to mild or none after 2 hours was statistically significant when compared to placebo (26%) for the 50 mg (43%, P=0.022), 100 mg (64%, P=0.0001), 200 mg (51%, P=0.0018), and 400 mg (65%, P=0.0001) doses of lasmiditan. The percentage of patients who were pain free at 2 hours was not statistically significant when compared to placebo (7.4%) at the lower oral doses of 50 mg (14%, P=0.18) and 100 mg (14%, P=0.19) but was significant with the higher 200 mg (19%, P=0.032) and 400 mg doses (28%, P=0.0007).Citation31
Table 2 Results summary of COL MIG-202, SAMURAI, and SPARTAN
Phase III trials
Lasmiditan has been studied in three Phase III clinical trials. The two double blind, placebo-controlled, randomized controlled trials that have been completed have presented results. The one ongoing study, an open-label, long-term safety study, has reported interim results. These three studies have not yet been published in the peer-reviewed literature.
SAMURAI (ID NCT02439320, COL MIG-301)
The first Phase III clinical trial is SAMURAI. This prospective randomized, double blind, placebo-controlled, parallel-group study aimed to evaluate the efficacy of two doses of lasmiditan compared to placebo for acute migraine. Primary end points were headache freedom at 2 hours post-dose and secondary end points were headache relief, use of rescue medication, headache recurrence, relief of the most bothersome symptom (nausea, photophobia, phonophobia), and safety. The study enrolled its last of 1,856 patients in July 2016.
While not yet published in the peer-reviewed literature, early, partial results have been presented. The percentages of patients with 2-hour headache relief were statistically significant (P<0.05) when compared to placebo (43%) for 100 (59%) and 200 mg doses of lasmiditan (59%). Similarly (), the 2-hour headache-free rates were statistically significant (P<0.05) for the 100 mg (28.2%) and 200 mg (32.2%) doses when compared to placebo (15.3%). Approximately 41% of patients had relief of the most bothersome symptom in both 100 and 200 mg groups compared to 30% with placebo (P<0.05).
One patient who took two doses of 100 mg lasmiditan reported palpitations and another who took 100 mg of study drug followed by placebo reported tachycardia. Details of these events were not reported. Consistent with the Phase II trials, the most common side effect was dizziness, reported in 12.5% and 16.3% of those in the 100 and 200 mg group, respectively.Citation32
SPARTAN (ID NCT02605174, COL MIG-302)
With a similar study design as well as primary and secondary outcomes as SAMURAI, SPARTAN evaluated three doses of lasmiditan (50, 100, and 200 mg) compared to placebo in the treatment of acute migraine. In contrast to SAMURAI’s participants who were healthy, SPARTAN did not exclude patients with coronary artery disease, cardiac arrhythmias or uncontrolled hypertension.
In 2017, SPARTAN reached its primary and secondary end points in all three doses evaluated (). The percentage of patients who were pain free 2 hours after administration of 50 mg (28.6%), 100 mg (31.4%), and 200 mg (38.8%) of lasmiditan was statistically significantly different (P<0.005) when compared to placebo (21.3%). Improvements of the most bothersome symptom 2 hours post-treatment were statistically significant when compared to placebo (33.5%) with 50 mg (40.8%, P=0.003), 100 mg (44.2%, P<0.001), and 200 mg (48.7%, P<0.001) doses of lasmiditan.Citation33 A limitation of SPARTAN for demonstrating safety in people with cardiovascular risk factors is that subjects only used a single dose of lasmiditan; the study does not demonstrate cardiovascular safety with repeated doses.
GLADIATOR (ID NCT02565186, COL MIG-305)
A prospective, open-label study evaluating the safety and tolerability of lasmiditan, GLADIATOR, enrolled participants who completed the SAMURAI or SPARTAN trials. The study started in October 2015 and was expected to continue through May 2018. Subjects were randomized to receive either 100 mg or 200 mg of oral lasmiditan.
The primary end points are the proportion of patients who experienced adverse events and the proportion of migraine attacks associated with adverse events. Secondarily, researchers aim to evaluate the proportion of attacks treated with study drug 2 hours post-treatment.
In 2016, preliminary GLADIATOR results were presented at the fifth European Headache and Migraine Trust International Congress. At that time, approximately 1,100 of the anticipated 2,500 participants were enrolled. Approximately 20% of patients taking both 100 and 200 mg of lasmiditan experienced side effects. Consistent with prior studies, dizziness was the most commonly reported side effect. No cardiovascular events or side effects have been observed or reported.Citation34
Safety profile and patient selection
The safety profile of lasmiditan was published in Phase II clinical trials. In COL MIG-202, study participants were monitored for adverse events and safety. Vitals signs, laboratory studies, and EKGs did not show any clinically significant drug-related morbidities. Most of the adverse events were mild or moderate in intensity, the percentage of severe adverse events increased with dose 50 (20%), 100 (28%), 200 (39%), and 400 mg (44%), respectively. Dizziness was the most frequently reported severe adverse eventCitation31 and is consistent with the preliminary findings of the Phase III trials ().Citation32–Citation34 It is likely that the side effect of dizziness will be dose-limiting in some patients and lead to discontinuation of lasmiditan in other patients. No chest pain or chest symptoms were reported in the Phase II clinical trials.Citation31
Table 3 Summary of side effects
While in the Phase II clinical trials and the Phase III SAMURAI study, participants were healthy without cardiovascular risk factors, the Phase III SPARTAN clinical trial enrolled patients with pre-existing cardiovascular disease. Peer-reviewed results of the Phase III studies have not yet been published, but preliminary data report no chest pain or cardiovascular side effects.Citation32–Citation34 This is in contrast to the 76% of patients taking oral sumatriptan who experience heavy arms and the 50% with chest pressure.Citation7 The long-term tolerability remains to be seen but preliminary results suggest lasmiditan holds promise for patients with pre-existing cardiovascular disease or who do not tolerate triptans due to side effects.
Patient focused perspectives such as quality of life, patient satisfaction/acceptability, adherence, and uptake
According to the American Migraine Prevalence and Prevention Study, 40% of episodic migraine patients identified themselves as having at least one of the following: severe headache-related disability (47%), dissatisfaction with current treatment regimens (32%), excessive opioid use (32%) or history of cardiovascular events (26.2%).Citation35 Moreover, 35% of participants in clinical trials do not benefit from oral triptans.Citation35 There is a need for additional acute migraine treatment options. Preliminary results suggest that lasmiditan may be a new safe and effective option for acute migraine treatment, especially for patients refractory to or unable to tolerate triptans, and/or for patients with pre-existing cardiovascular disease.
Conclusions, place in therapy
Triptans are the current standard-of-care treatment for acute migraines not responsive to over-the-counter medications. Unfortunately, there are a high percentage of patients who do not respond to triptans, do not tolerate triptans, or have contraindications to triptans. In a meta-analysis of 133 randomized controlled trials of triptans, standard dose triptans provided 42%–76% 2-hour pain relief and 18%–50% pain freedom.Citation36 As such, there is a need for additional safe and effective acute migraine treatments. Clinical trials for lasmiditan to date have been promising, and lasmiditan appears to avoid typical triptan side effects. While preclinical work suggests that lasmiditan does not cause vasoconstriction, clinical evidence to date is insufficient to conclude the safety of lasmiditan with long-term use in patients with cardiovascular risk factors, and especially not in patients with a history of coronary artery disease or stroke. While lasmiditan’s Phase III studies are not yet published, the results reported to date suggest that lasmiditan is more effective than placebo in the treatment of acute migraine with 2-hour pain freedom and 2-hour pain relief rates similar to those seen in oral triptan studies. With Eli Lilly & Co. having announced plans to apply for US FDA approval in early 2018, lasmiditan may soon be a new addition to the expanding headache armamentarium.
Disclosure
Nathaniel M Schuster has a research collaboration with Eli Lilly & Co. not related to lasmiditan. The authors have not received funding for the generation of this manuscript. The authors report no conflicts of interest in this work.
References
- SchulteLHMayAThe migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacksBrain2016139Pt 71987199327190019
- MayASchulteLHChronic migraine: risk factors, mechanisms and treatmentNat Rev Neurol201612845546427389092
- HumphreyPPFeniukWPerrenMJBeresfordIJSkingleMWhalleyETSerotonin and migraineAnn N Y Acad Sci1990600600587598 discussion 598–6002252337
- PietrobonDMoskowitzMAPathophysiology of migraineAnnu Rev Physiol20137536539123190076
- AkermanSHollandPRGoadsbyPJDiencephalic and brainstem mechanisms in migraineNat Rev Neurosci2011121057058421931334
- MaassenvandenbrinkAReekersMBaxWAFerrariMDSaxenaPRCoronary side-effect potential of current and prospective antimigraine drugsCirculation199898125309665056
- VisserWHJaspersNMde VriendRHFerrariMDChest symptoms after sumatriptan: a two-year clinical practice review in 735 consecutive migraine patientsCephalalgia19961685545598980858
- DodickDLiptonRBMartinVConsensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraineHeadache200444541442515147249
- O’ConnorPGladstonePOral sumatriptan-associated transmural myocardial infarctionNeurology19954512227422768848207
- JayamahaJEStreetMKFatal cerebellar infarction in a migraine sufferer whilst receiving sumatriptanIntensive Care Med199521182837560482
- AbbresciaVDPearlsteinLKotlerMSumatriptan-associated myocardial infarction: report of case with attention to potential risk factorsJ Am Osteopath Assoc19979731621649107127
- BallyMDendukuriNRichBRisk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient dataBMJ19092017(357)j
- RaffaelliBIsraelHNeebLReuterUThe safety and efficacy of the 5-HT 1F receptor agonist lasmiditan in the acute treatment of migraineExpert Opin Pharmacother201718131409141528749698
- CapiMde AndrésFLionettoLLasmiditan for the treatment of migraineExpert Opin Investig Drugs2017262227234
- NegroAKoverechAMartellettiPSerotonin receptor agonists in the acute treatment of migraine: a review on their therapeutic potentialJ Pain Res20181151552629563831
- Vila-PueyoMTargeted 5-HT1F Therapies for MigraineNeurotherapeutics201815229130329488143
- NelsonDLPhebusLAJohnsonKWPreclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditanCephalalgia201030101159116920855361
- GoadsbyPJClasseyJDEvidence for serotonin (5-HT)1B, 5-HT1D and 5-HT1F receptor inhibitory effects on trigeminal neurons with craniovascular inputNeuroscience2003122249149814614913
- CohenMLSchenckK5-Hydroxytryptamine(1F) receptors do not participate in vasoconstriction: lack of vasoconstriction to LY344864, a selective serotonin(1F) receptor agonist in rabbit saphenous veinJ Pharmacol Exp Ther1999290393593910454462
- JohnsonKWSchausJMDurkinMM5-HT1F receptor agonists inhibit neurogenic dural inflammation in guinea pigsNeuroreport199789-10223722399243618
- CharlesAAdvances in the basic and clinical science of migraineAnn Neurol200965549149819479724
- GoadsbyPJLiptonRBFerrariMDMigraine--current understanding and treatmentN Engl J Med2002346425727011807151
- ShepheardSEdvinssonLCumberbatchMPossible antimigraine mechanisms of action of the 5HT1F receptor agonist LY334370Cephalalgia1999191085185810668103
- MarkowitzSSaitoKMoskowitzMANeurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brainJ Neurosci1987712412941363694267
- AdhamNBardJAZgombickJMCloning and characterization of the guinea pig 5-HT1F receptor subtype: a comparison of the pharmacological profile to the human species homologNeuropharmacology1997364-55695769225282
- AntonFHerdegenTPeppelPLeahJDc-FOS-like immunoreactivity in rat brainstem neurons following noxious chemical stimulation of the nasal mucosaNeuroscience1991412-36296411908066
- HuntSPPiniAEvanGInduction of c-fos-like protein in spinal cord neurons following sensory stimulationNature198732861316326343112583
- GoldsteinDJRoonKIOffenWWSelective seratonin 1F (5-HT(1F)) receptor agonist LY334370 for acute migraine: a randomised controlled trialLancet200135892891230123411675061
- CoLucid Pharmaceuticals Details Phase 3 Development Strategy for Lasmiditan to Address Major Unmet Needs in Acute Migraine Therapy [webpage on the Internet]Durham NCPappas Ventures2018 Available from: http://www.pappasventures.com/2012/09/18/colucid-pharmaceuticals-details-phase-3-development-strategy-lasmiditan-address-major-unmet-needs-acute-migraine-therapy/Accessed August 10, 2018
- FerrariMDFärkkiläMReuterUAcute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan--a randomised proof-of-concept trialCephalalgia201030101170117820855362
- FärkkiläMDienerHCGéraudGEfficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging studyLancet Neurol201211540541322459549
- SAMURAI: Lasmiditan Reduces Pain in Acute Migraine2018 Available from: https://globenewswire.com/news-release/2016/09/06/869611/0/en/CoLucid-Pharmaceuticals-Announces-Achievement-of-Both-Primary-and-Key-Secondary-Endpoints-in-the-SAMURAI-Phase-3-Pivotal-Trial-of-Lasmiditan-in-Migraine.htmlAccessed March 19, 2018
- Lilly Announces Positive Results for Second Phase 3 Study of Lasmidi-tan for the Acute Treatment of Migraine (NYSE:LLY); 2018 Available from: https://investor.lilly.com/static-files/15cf1efc-da8f-485c-9001-6ff3b432b129Accessed March 19, 2018
- CoLucid Pharmaceuticals Provides Interim Update on GLADIATOR [webpage on the Internet]GlobeNewswire, Inc.2016 Available from: https://globenewswire.com/news-release/2016/09/19/872772/0/en/CoLucid-Pharmaceuticals-Provides-Interim-Update-on-GLADIATOR.htmlAccessed August 10, 2018
- LiptonRBBuseDCSerranoDHollandSReedMLExamination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) StudyHeadache20135381300131123879870
- CameronCKellySHsiehSCTriptans in the Acute Treatment of Migraine: A Systematic Review and Network Meta-AnalysisHeadache201555Suppl 422123526178694
