Abstract
We report a complication related to epidural analgesia for delivery in a 24- year-old woman who was admitted with mild pre-eclampsia and for induction of labor. At the first postpartum day she developed a postdural puncture headache, which was unresponsive to conservative measures. On the fifth day an epidural blood patch was done, and her headache subsided. Sixteen hours later she developed paralysis of the right facial nerve, which was treated with prednisone. Seven days later she complained of pain in the left arm and the posterior region of the shoulder. She was later admitted and diagnosed with partial brachial plexopathy.
Case report
A 24-year-old woman with mild pre-eclampsia was admitted for induction of labor under normal-term labor after 40 weeks’ gestation. Ten days before her admission she was admitted for 6 days for mild hypertension and moderate edema of her legs. The patient was afebrile and her general examination was normal. Neurological examination showed a fully conscious patient. Her blood pressure at the time of admission was 152/82 mm Hg and her heart rate was 86 and regular. Blood analyses disclosed normal hepatic and renal function. Electrolyte and hematological and coagulation tests were normal. Electrocardiogram and chest X-ray were also normal.
Labor was induced with intravaginal prostaglandin on the second day of admission. The patient requested epidural analgesia, and an epidural catheter was inserted successfully at the L3-4 interspace through an 18-gauge Tuohy needle. Sensory anesthesia was established with 8 mL of 0.25% bupivacaine and 0.1 mL of fentanyl. The patient underwent vaginal delivery of a 3365 g female infant with an Apgar score of 9. After delivery, the epidural anesthesia was stopped by the anesthesiologist, who removed the epidural catheter from the patient’s back.
One day after the delivery the patient developed a postdural puncture headache (PDPH), which was managed by conservative measures: bed rest (patient’s position of choice), increased hydration (normal saline 3 L per day intravenously), and metamizole sodium (Dipyrone®; Garan S.K. Ltd, Ramat Gan, Israel) 500 mg three times per day, which is commonly used in many countries as a powerful analgesic and antipyretic. Despite the conservative treatment, the patient’s condition did not improve. Her headache worsened when she was in an upright position and was relieved when she was lying flat. On the fifth postpartum day, an epidural blood patch (EBP) was recommended. This was performed at one level above the epidural anesthesia, with 18 mL of autologous blood taken from the antecubbital vein. The headache improved immediately.
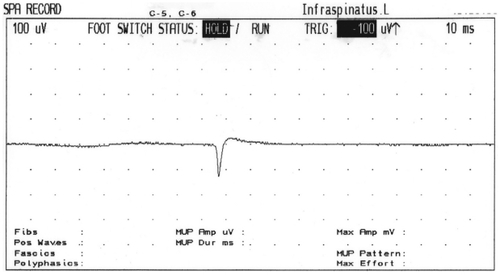
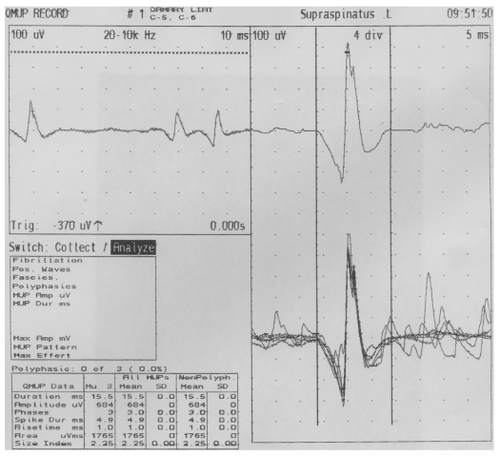
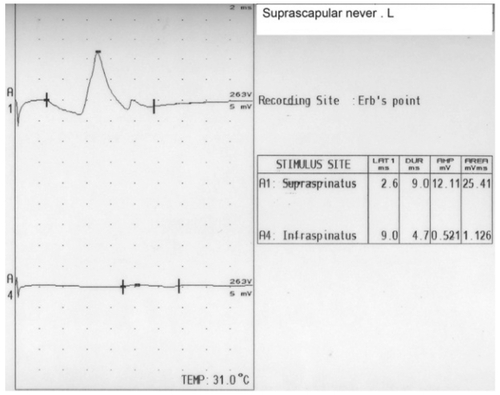
Sixteen hours later she developed paralysis of lower motor neuron type of her right facial nerve, which was treated with prednisone at a dosage of 50 mg daily for 5 days, tapering off by 10 mg/day for an additional 5 days. Resolution of the patient’s symptoms and complete recovery of the seventh nerve was observed after 9 days. Six days after application of the EBP, a nerve conduction study (NCS) of the seventh cranial nerve and blink reflexes was performed; these tests showed normal findings. Seven days after the application of the EBP, the patient suffered pain in the posterior shoulder and in the left arm mainly posteriorly, which was mildly burning and increased gradually over several days. Three weeks after the EBP she was admitted to the neurological department after complaining of continuous pain. Her neurological examination revealed a severe weakness with moderate atrophy of the left infraspinatous muscle (). One day after admission (22 days after application of the EBP), a magnetic resonance imaging (MRI) scan of the brain and cervical spine region showed normal findings of the brain but a spread of the EBP (trace amounts of blood) in the cervical spine region. An electromyography performed 23 days after showed spontaneous activity (positive sharp waves) and active denervation in the left infraspinatus, and a mild neurogenic pattern in the supraspinatus on the same side ( and ). NCS of the suprascapular nerve, which arises from the trunk and is formed by the union of the fifth and sixth cervical nerves and innervates the supraspinatus and infraspinatus muscles, revealed no response in the infraspinatus division (). An NCS of the bilateral median and ulnar nerves as well as the right peroneal, right tibial, and right surral nerves was normal. Physiotherapy of the affected muscle was recommended, and this resulted in mild improvement over 3 months.
Introduction
Dural puncture is a commonly performed invasive procedure for various medical indications like diagnostic lumbar puncture, spinal anesthesia, myelography, and intrathecal chemotherapy. However, in anesthesiology, apart from intentional dural puncture as in spinal anesthesia, unintentional dural puncture can also occur while performing epidural anesthesia or analgesia for various indications, including postoperative and labor pain relief.
EBP is a treatment procedure for PDPH and refers to the injection of 15–20 mL of a patient’s autologous blood into the epidural space of the vertebral column at or near the location of a dural puncture. The first report of blood patchCitation1 used only 2–3 mL. Using this small volume, had the blood clot formed in a position that did not seal the dural tear, the benefits of blood patching may not have been evident. Since that time, the need for adequate volumes of blood has been emphasized. CrawfordCitation2 recommended the injection of 20 mL of blood (but less if there is discomfort). Lower volumes were found to have a higher failure rate. Ostheimer et alCitation3 and Abouleish et al,Citation4 however, found that volumes of less than 10 mL were associated with higher initial failure or recurrence of PDPH after initial apparent success.
The volume injected displaces cerebrospinal fluid (CSF) from the lumbar CSF space into the area surrounding the brain, often yielding immediate headache relief. When the blood clots it seals the dural puncture, prohibiting further leakage of CSF from the subarachnoid space.
When headache appears in the postoperative or postpartum period after regional anesthesia, it can have many causes, apart from a complication of dural puncture during regional anesthesia. However, the most common cause of an anesthesia-induced headache is PDPH. Historically, PDPH was described firstly by Karl August Bier in 1899,Citation5 when he gave the first spinal anesthetic, injecting 10–15 mg of cocaine to seven patients, himself, and his assistant.Citation5 Dr Bier described the headache as a feeling of very high pressure in the head, accompanied by light dizziness when rising quickly from the chair. He also described the most important sign of PDPH as follows: “all symptoms disappeared immediately when I laid horizontally but came back when I got upright”.Citation5 Dr Biers suggested that CSF loss caused the symptoms he experienced, and he recommended preventing the loss of CSF as much as possible. He lost excessive CSF while receiving the experimental spinal block from his assistant, who was unable to fit the syringe to the needle during the procedure.Citation5 Indeed, PDPH typically occurs hours to days after puncture and presents with symptoms such as headache and nausea that typically worsen when the patient assumes an upright posture. It is thought to result from a loss of CSF into the epidural space.Citation6–Citation9 Decreased hydrostatic pressure in the subarachnoid space then leads to traction to the meninges with associated symptoms.
Diagnosis of PDPH depends on its association with body position; the pain is aggravated by sitting or standing and relieved or decreased by lying down flat.Citation10,Citation11 Headache after dural puncture is a complication of spinal anesthesia and is believed to result from leakage of CSF at the time of dural puncture and, probably more importantly, continued leaking afterwards.Citation12,Citation13 Criteria of PDPHCitation14 are summarized by the following characteristics: occurred after mobilization; aggravated by an erect or a sitting position and coughing, sneezing, or straining; and relieved by lying flat. PDPH is mostly localized and classified as occipital, frontal, or generalized.
Epidemiologically, PDPH occurs in 10%–40% of patients who have a lumbar puncture. The overall incidence of PDPH after intentional dural puncture varies from 0.1% to 36%. The highest incidence of 36% was reported after ambulatory diagnostic lumbar puncture using a 20- or 22-gauge standard Quincke spinal needle.Citation15,Citation16 Unintentional dural puncture with a large Tuohy needle (16 and 18 gauge) is associated with a high incidence of 70%–80% PDPH.
In an obstetric population, unintentional dural puncture is one of the most common major complications. Indeed, there is considerable variability in the incidence of PDPH, which is affected by many factors, including age, gender, needle size, and needle type. A prospective review of 100 parturients from Australia who experienced accidental dural puncture with a Tuohy needle had a PDPH rate of 81%.Citation17 The diagnosis of dural puncture was delayed until presentation of headache in 27% of these cases. A similar incidence of PDPH has been found by other investigators,Citation18–Citation20 but Choi et alCitation10 performed a meta-analysis of obstetrical studies and reported that the pooled risk for accidental dural puncture for all epidural needles was 1.5%. Once dural puncture occurred, the risk of PDPH was 52.1%. The risk of PDPH varied amongst spinal needles and ranged from 1.5% to 11.2%.Citation10 The effectiveness and early reports of the EBP procedure demonstrated that immediate and permanent cure rates approached 100%. In this study, we describe an unfamiliar neurological complication of epidural analgesia during labor, the development of facial nerve palsy and partial brachial plexopathy, which, to our knowledge, has not been previously reported. We received a written consent form from our patient.
Discussion
This case report is a distinctive neurological complication of epidural analgesia during labor. We could not find a similar case of brachial plexopathy reported in the literature as a complication of EBP. Here, we report a unique case of facial paralysis and neuralgic amyotrophy of the suprascapular nerve suspected to be secondary to the EBP.
Various neurological consequences following dural puncture are well recognized.Citation5 The most serious rare complication is the occurrence of transient cranial nerves palsy, and almost all cranial nerves have been implicated.Citation20 Usually, single nerve palsy has been reported, with the nerves affected being the third, fourth, sixth, seventh, and eighth.Citation20 A reported incidence of cranial nerve palsies is 1:100,000 to 3.7:100,000.Citation20 The sixth nerve is said to be most susceptible, but length alone is not the sole factor, as the fourth cranial nerve is longer than the sixth cranial nerve but is rarely affected.Citation1 The abducent nerve is suggested to be vulnerable because it is relatively fixed at its entry into the cavernous sinus and at its attachment to the pons. This nerve is most likely to be stretched due to sagging of the brain because of a CSF leak.Citation1
Facial nerve palsy associated with EBP was first reported by Abouleish et al in 1975.Citation4 The paralysis in their patient occurred 4 days after EBP was performed and was considered a coincidence.Citation2,Citation21 Fang et al,Citation22 in 2010, presented a case of a 29-year-old woman who underwent an emergency cesarean section due to a prolonged second stage of labor and suffered trigeminal nerve and facial nerve palsy after combined spinal–epidural anesthesia.Citation22
Population-based epidemiologic studies have demonstrated an increased incidence more than three-fold that of of Bell’s palsy during pregnancy, especially in the third trimester and the early postpartum period.Citation4,Citation23–Citation25 The etiology of Bell’s palsy is unknown,Citation13,Citation24 although two theories exist. The speculated causes are either edema or viral manifestation.Citation25,Citation26 The edema hypothesis postulates that fluid retention and generalized edema of pregnancy result in a compression of the facial nerve with resulting facial paralysis.Citation13,Citation26 This view is supported by the high incidence of Bell’s palsy during the time of maximal fluid retention in the third trimester. There seems to be an association between Bell’s palsy and hypertensive disorders during pregnancy that often leads to increased fluid retention and the higher frequency of other nerve compression manifestations such as carpel tunnel syndrome.Citation15 Support for the viral causation includes the fact that patients with Bell’s palsy have evidence of previous herpes virus infection; the presence of pregnancy-induced immunosuppressant, which may predispose to reactivation of a latent herpes virus within the nerve ganglia; the fact that Bell’s palsy syndrome is sometimes part of a complex cranial polyneuritis,Citation16 with the presence of lymphocytosis and increased protein in the CSF of the affected patients; and occurrence of clusters or epidemics of Bell’s palsy. There is controversy concerning the relationship between Bell’s palsy and pre-eclampsia.Citation17 Notwithstanding the different neurological complications known in the pre-eclampsia period as PDPH and cranial subdural haematoma associated with dural puncture in labor, peripartum cardiomyopathy, anesthesia after cesarean delivery, and seizures, our patient did not demonstrate any facial weakness after the PDPH period. The paralysis was noticed just after the EBP and cessation of the PDPH. In addition, blood injected into the epidural space ascends in the spinal column, and trace amounts get to the cervical spine region, as shown by MRI scans post-EBP. Certainly, some inflammation often occurs in the epidural space, but typically this is manifested in lumbar/sacral symptoms and not cervical, although Horner’s syndrome does occasionally occur with large epidural top-ups.Citation27 This suggests a pathophysiological cause different from the loss of CSF, inflammation, or Horner’s syndrome. Previous reports have postulated that a sudden increase in intracranial pressure caused by a blood patch may compromise the blood supply to the seventh nerve within the facial canal.Citation21,Citation22 An increase in the epidural and subarachnoid pressure secondary to injection of blood volume in the epidural space has been described.Citation18
In a recent study, BoezaartCitation28 proposed that PDPH is probably a vascular-type headache and that an EBP relieves the headache by its vasoconstrictive action.Citation29 This cerebral vasoconstriction may be caused by subarachnoid spread of the injected blood. Indeed, low CSF pressure results in cerebrovascular vasodilatation and headache that is similar in mechanism to other vascular headaches such as migraine.Citation30 In addition, the possible role that the rich innervations of the dura matter with adrenergic, cholinergic, and peptidergic fibers may play in PDPH and its management with an EBP require further research to know the exact mechanism of PDPH. Other hypotheses include the delay in EBP performance that may have led to the patient’s symptoms. In other words, rather than the EBP causing the facial and brachial symptoms, perhaps an earlier EBP may have prevented its occurrence.Citation31
The possibility of accidental epidural injection of anesthetic should be considered. Accidental production of spinal anesthesia has been reported as a complication of attempted brachial plexus blockade using the posterior approach of brachial plexus blockade, which may indicate that there is a possible pathway that fluid (either blood or local anesthetic) can traverse between the epidural space and the brachial plexus roots or trunks. In fact, the tip of the block needle may have entered a dural cuff that can run with a nerve root for some distance from the intervertebral foramen, in the end causing brachial plexus palsy.Citation32–Citation34
The course and succession of events in our case study have special characteristics, such as the rapid resolution of the patient’s symptoms and the complete recovery of the seventh nerve, which was observed after only 9 days. This speedy recovery has not been encountered among patients suffering from Bell’s palsy, where improvement is gradual and recovery times vary. With or without treatment, most individuals begin to get better within 2 weeks after the initial onset of symptoms and most recover completely, returning to normal function within 3–6 months. Also, we do not exclude the hypothesis of irritation of the nerve roots due to the spread of blood as an etiology of our case.
The suprascapular nerve derived from the upper trunk of the brachial plexus, typically receiving fibers from C5 and C6, contains both motor and sensory components and sends sensory branches to both the glenohumeral and acromioclavicular joints but does not innervate the skin. It passes downward laterally (deeply to the omohyoid and trapezius and then posteriorly to run under cover of trapezius) along with the suprascapular vein and artery. It reaches the suprascapular notch, then the nerve travels beneath the suprascapular notch, whereas the vessels travel above the notch; after giving off two branches to supraspinatus, it passes around the lateral border of the scapular spine (spinoglenoid notch) and ends in the infraspinatus fossa to supply infraspinatus. Peripheral nerves are highly susceptible to injury from stretching and compression. Both of these mechanisms result in nerve ischemia, edema, microenvironmental changes, and conduction impairment. These changes are proportional to the magnitude and duration of the insult and eventually lead to irreversible damage.Citation35,Citation36 Indeed, the suprascapular nerve may be injured as a result of trauma, repetitive overuse, a mass lesion, or iatrogenic causes. Traumatic causes of suprascapular nerve injury include scapular fractures,Citation37 clavicular fractures,Citation38 shoulder dislocations,Citation39 and penetrating trauma.Citation40 Also, iatrogenic injury to the suprascapular nerve may occur during operative procedures.Citation41,Citation42 A common location for injury to the suprascapular nerve is the suprascapular notch. The mechanism by which injury occurs at the suprascapular notch has been termed “the sling effect” by Rengachary et al.Citation43 They evaluated the motion of the suprascapular nerve relative to the suprascapular notch with various movements of the arm and shoulder. They noted that the nerve was often opposed to the sharp inferior margin of the superior transverse scapular ligament and that the contact was accentuated with depression and retraction, or hyperabduction, of the shoulder.
The spinoglenoid notch is another site where the suprascapular nerve is frequently injured.Citation44 Several hypotheses have been proposed regarding the etiology of this injury. One theory is that the nerve is compressed in the fibro-osseous tunnel formed by the spine of the scapula and a hypertrophied spinoglenoid ligament. Other hypotheses have expressed the belief that the nerve is injured as a result of the anatomy of the spinoglenoid notch and stress that is placed on the nerve by repetitive overhead activities. This theory is supported by clinical data that show that the majority of these injuries occur in athletes who repetitively stress their shoulder. A large percentage of the cases that have been reported were in professional volleyball players.Citation45 Another hypothesis is that the nerve is compressed between the spine of the scapula and the medial tendinous margin of the infraspinatus and supraspinatus muscles during extreme abduction of the shoulder with full external rotation.Citation46,Citation47 It has been proposed that stretching of the suprascapular artery during pitching results in intimal damage to the suprascapular artery. Microthrombi form and embolize to the vasa nervorum of the suprascapular nerve with resultant ischemic damage. However, to our knowledge there are no scientific data to support this theory.
Another mechanism by which the suprascapular nerve may be injured is compression by a mass, most commonly a ganglion cyst. Other masses that have been described include synovial sarcoma, Ewing’s sarcoma, chondrosarcoma, metastatic renal cell carcinoma, and a bone cyst.Citation48
Disclosure
The authors report no conflicts of interest in this work.
References
- GormleyJBTreatment of post-spinal headacheAnesthesiology196021565566
- CrawfordJSExperiences with epidural blood patchAnaesthesia1980355135157396157
- OstheimerGWPalahniukRJSchniderSMEpidural blood patch for post-lumbar puncture headacheAnesthesiology1974413073084852778
- AbouleishEDela VegaSBleningerITiong-OenTLong-term follow- up of epidural blood patchAnesth Analg197554459463125053
- VersucheBAUber cocainisierung des rucken markesDeutsch Zeitschrift fur Chirurgie189951361369
- BezovDLiptonRBAshinaSPost-dural puncture headache: Part I diagnosis, epidemiology, etiology, and pathophysiologyHeadache2010501144115220533959
- GrantRCondonBHartIChanges in intracranial CSF volume after lumbar puncture and their relationship to post-LP headacheJ Neurol Neurosurg Psychiatry1991544404421865208
- SerpellMGRawalNHeadache after diagnostic dural punctures (editorial)BMJ20003219731974
- KunkleECRayBSWolffHGExperimental studies on headache: analysis of the headache associated with changes in intracranial pressureArch Neurol Psychiatry194349323358
- ChoiPTGalinskiSETakeuchiLPDPH rates onset and durationCan J Anaesth20035046046912734154
- GarryMDaviesSFailure of regional blockade for Caesarean sectionInt J Obstet Anesth20021191215321571
- KleinmanWMikhailMSpinal, epidural and caudal blocksMorganGEMikhailMSMurrayMJClinical Anesthesiology4th ed2006319
- VandamLDDrippsRDLong-term follow-up of patients who received 10,098 spinal anaesthetics. Syndrome of decreased intracranial pressure (headache and ocular and auditory difficulties)JAMA1956161586591
- ShahABhatiaPKTulsianiKLPost dural puncture headach in caesarean section: a comparative study using 25 G Quincke, 27 G Quincke and 27 G Whitacre needleIndian J Anaesth200246373377
- Mac ArthurCLewisMKnoxEGAccidental dural puncture in obstetric patients and long-term symptomsBMJ19933068838858490410
- KuntzKMKohmenEStevenJCPost lumbar puncture headache: experience in 501 consecutive procedureNeurology199242188418871407567
- TurnbillDKShepherdDBPostdural puncture headache: pathogenesis, prevention and treatmentBr J Anaesth20039171872914570796
- CruickshankRHHopkinsJMFluid flow through dural puncture sites. An in vitro comparison of needle point typesAnaesthesia1989444154182742103
- CostiganSNSpriggeJSDural puncture: the patients perspective. A patient survey of cases at a DGH maternity unit 1983–1993Acta Anaesthesiol Scand1996407107148836266
- ReynoldsFDural puncture and headacheBMJ19933068748768490408
- LoweDMMcculloughAM7th nerve palsy after extradural blood patchBr J Anaesth1990657217222248852
- FangJYLinJWLiQTrigeminal nerve and facial nerve palsy after combined spinal-epidural anesthesia for cesarean sectionJ Clin Anesth201022565820206854
- CooperGEpidural blood patchEur J Anaesth199916211215
- StridePCCooperGMDural taps re-visited. A 20 year survey from Birmingham Maternity HospitalAnaesthesia1993482472558460807
- CostigenSNSpriggeJSDural puncture: the patient’s perspective. A patient survey of cases at a DGH Maternity Unit (1983–1993)Acta Anaesthesiol Scand1996407107148836266
- LanceJWBranchGBPersistent headache after lumbar punctureLancet19943434147905563
- PawHGWHorner’s syndrome following low-dose epidural infusion for labour: a cautionary taleEur J Anaesth199815110111
- BoezaartAPEffects of cerebrospinal fluid loss and epidural blood patch on cerebral blood flow in swineReg Anesth Pain Med20012640140611561258
- DigiovanniAJGilbertMWWahleWMEpidural injection of autologous blood for post-lumbar puncture headache II. Additional clinical experience and laboratory investigationAnesth Analg1972512262325062124
- VernieriFMoroLAltamuraCPatients with migraine with aura have increased flow mediated dilationBMC Neurology201010101820109231
- DunbarSAKatzNPFailure of delayed epidural blood patching to correct persistent cranial nerve palsiesAnesth Analg1994798068077943797
- AramidehMvan Der OeverHLWalstraGJDzoljicMSpinal anesthesia as a complication of brachial plexus using the posterior approachAnesth Analg2002941338133911973216
- GomezRSMendesTCBSEpidural anaesthesia complication of brachial plexus blockAnaesthesia20066159159216704597
- RettigHCGielenMJMJackNTMA comparison of the lateral and posterior approach of the brachial plexusReg Anesth Pain Med20063111912616543097
- LundborgGRydevikBEffects of stretching the tibial nerve of the rabbit. A preliminary study of the intraneural circulation and the barrier function of the perineuriumJ Bone and Joint Surg197355390340
- LundborgGStructure and function of the intraneural microvessels as related to trauma, edema formation and nerve functionJ Bone and Joint Surg197557938948171272
- EdelandHGZachrissonBEFracture of the scapular notch associated with lesion of the suprascapular nerveActa Orthop Scandinavica197546758763
- BerryHKongKHudsonARMoultonRIsolated suprascapular nerve palsy: a review of nine casesCanadian J Neurol Sci199522301304
- TravlosJGoldbergIBoomeRSBrachial plexus lesions associated with dislocated shouldersJ Bone and Joint Surg19907268712295675
- WeaverHLIsolated suprascapular nerve lesionsInjury1983151171266629486
- De MulderKMarynissenHvan LaereCArthroscopic transglenoid suture of Bankart lesionsActa Orthop Belgica199864160166
- BiglianiLUDalseyRMMcCannPDAprilEWAn anatomical study of the suprascapular nerveArthroscopy199063013052264898
- RengacharySSNeffJPSingerPABrackettCESuprascapular entrapment neuropathy: a clinical, anatomical, and comparative study. Part 1: clinical studyNeurosurgery19795441446534047
- AielloISerraGTrainaGCTugnoliVEntrapment of the suprascapular nerve at the spinoglenoid notchAnn Neurol1982123143167137969
- CumminsCABowenMAndersonKMesserTSuprascapular nerve entrapment at the spinoglenoid notch in a professional baseball pitcherAm J Sports Med19992781081210569371
- SandowMJIlicJSuprascapular nerve rotator cuff compression syndrome in volleyball playersJ Shoulder and Elbow Surg199875165219814933
- RingelSPTreihaftMCarryMSuprascapular neuropathy in pitchersAm J Sports Med19901880862154138
- FritzRCHelmsCASteinbachLSGenantHKSuprascapular nerve entrapment: evaluation with MR imagingRadiology19921824374441732962