Abstract
Background
Cognitive impairment is a common complication in patients with chronic neuropathic pain, without effective therapy. Recent works have indicated that curcumin (Cur) possesses antinociceptive and neuroprotective potentials, suggesting its possible effectiveness for the treatment of this complication.
Objective
The aim of this study was to explore the effects of Cur on pain behaviors and cognitive impairment in rats with cobra venom-induced trigeminal neuralgia (TN).
Design
This is a randomized, controlled experiment.
Setting
This study was conducted at the Experimental Animal Center, Beijing Friendship Hospital, Capital Medical University.
Subjects
A total of 40 adult male Sprague Dawley rats were used in this study.
Methods
A cobra venom solution was injected into the sheath of infraorbital nerve. Cur was administered intragastrically at 45 mg/kg twice daily for 28 successive days from postoperative day 15. Mechanical allodynia was evaluated using von Frey filaments. Free behaviors were observed using video recording. Cognitive capacity was tested using the Morris water maze. Both morphology and ultrastructure of the CA1 hippocampal region were visualized using hematoxylin and eosin (HE) staining and transmission electron microscopy, respectively.
Results
Cur treatment reduced mechanical allodynia and face-grooming activities but increased exploratory activities and improved spatial learning and memory deficits. Microscopic examination revealed nucleus pyknosis, swollen organelles, and decreased synapse density in the CA1 hippocampal region after cobra venom injection. However, chronic Cur treatment reversed damage to hippocampal neurons and synapses.
Conclusion
Cur can alleviate pain, improve spatial learning and memory deficits, and restore the damage to hippocampal neurons and synapses in cobra venom-induced TN rats. Cur may be useful as an adjuvant to treat chronic neuropathic pain-induced cognitive deficits.
Introduction
Cognitive impairment is a common complication in patients with chronic neuropathic pain, and memory deterioration is the primary clinical manifestation.Citation1 Chronic pain predisposes patients to cognitive impairment, which affects daily activities and deteriorates the quality of life.Citation2 Cognitive deficits in chronic pain patients restrict the effectiveness of communicating their pain symptoms, which further constrains the application of cognitive-based therapy and may exacerbate preexisting pain.Citation3 Thus, the identification of effective strategies for this complication is imperative. In available literatures, numerous studies have been performed to elucidate the mechanisms of neuropathic pain and investigate the relationship between neuropathic pain and cognitive impairment.Citation4–Citation6 Few studies are focused on the treatment of pain-induced cognitive impairment.
Current strategies for the management of pain-induced cognitive impairment primarily concentrated on the amelioration of neuropathic pain and improvement in cognitive deficits induced by chronic pain, but the results are contradictory and inconclusive. Some studies show that pain relief by analgesic drugs can reverse the cognitive deficits observed in chronic pain models.Citation7 However, other studies find that neuropathic pain is a chronic intractable pain with a poor response to opioids and nonsteroidal anti-inflammatory drugs.Citation8,Citation9 Antiepileptic drugs (AED) are the first-line drugs commonly used for the treatment of neuropathic pain, but the prolonged use of AED itself may lead to cognitive deficits.Citation10,Citation11 Thus, studies on novel therapeutic agents using dietary or phytomedicine should be performed to alleviate neuropathic pain and improve pain-induced cognitive deficits.
Curcumin (Cur), a yellow pigment derived from the rhizome of Curcuma longa,Citation12 has been widely studied for its pleiotropic properties, including anti-inflammatory, antinociceptive, and neuroprotective activities.Citation13–Citation15 It has been shown that Cur provides analgesic activity against various neuropathic pains.Citation14,Citation16 Furthermore, Cur possesses neuroprotective property and improves learning and memory performances in different experimental models.Citation15,Citation17 The concurrent antinociceptive and neuroprotective properties of Cur suggest its potential as a treatment for cognitive impairment induced by chronic neuropathic pain. Thus, this study was designed to determine whether Cur could improve pain behaviors and cognitive impairments in cobra venom-induced trigeminal neuralgia (TN) rats and elucidate the underlying mechanisms.
Methods
Animals
After experimental plan was approved by the Animal Ethical Committee of Beijing Friendship Hospital, Capital Medical University (China), all procedures were performed according to the guidelines of the International Association for the Study of Pain. Male Sprague Dawley rats (200–250 g) were used in this study. Rats were housed under controlled temperature (22–25°C), humidity (60–80%), and a 12-hour light/dark cycle (lights on for 07:00 h). By a random table, 40 rats were assigned into the following four groups (n=10 each group): sham + vehicle group, sham + Cur group, TN + vehicle group, and TN + Cur group.
Surgery procedure
The TN rat model was established as previously described.Citation18,Citation19 Briefly, animal was anesthetized using sodium pentobarbital (50 mg/kg intraperitoneally). After the left infraorbital nerve (ION) trunk was exposed, 4 µL of a cobra venom solution (containing 0.4 mg of lyophilized cobra venom) was injected into the ION sheath. Then, the incision was closed. The sham group underwent the same procedure with an injection of saline. One rat died accidentally, and nine rats remained in the TN + vehicle group.
Drug preparation and treatment
Lyophilized cobra venom (Formosan cobra; Sigma-Aldrich Co., St Louis, MO, USA) was dissolved in 0.9% saline for injection. Cur (Sigma-Aldrich Co.) was dissolved in peanut oil for oral administration (via gavage, po). A dose of 45 mg/kg Cur was administered intragastrically twice daily (morning and evening) according to the previous report.Citation20 The treatment was started on the 15th day after surgery, when the neuropathic TN was well established, and continued for 28 consecutive days.
Behavioral testing
Both mechanical allodynia and free behavioral tests were used to determine whether the TN model was well established.
Animals were tested 3 days before surgery and 4, 7, 14, 21, 28, 35, and 42 days after surgery. They were placed individually in a transparent plastic cage on an elevated mesh (1.0 cm ×1.0 cm perforations) and allowed to acclimatize for 20 min. A set of von Frey hairs (Stoelting, Chicago, IL, USA) with calibrated bending forces (0.41–15.1 g) was used to measure mechanical sensitivity. Each filament was applied to the ipsilateral ION territory near the center of the vibris-sal pad and held for ~5 s. Mechanical withdraw thresholds (MWTs) were calculated using the up–down method as described previously.Citation21
Free behaviors of rats were recorded in a transparent plastic cage (24 cm ×35 cm ×18 cm) using a video camera placed 1 m in front of the cage. Animals were placed in the cage for 15 min, and a 7-minute video was recorded. An experimenter who was blinded to the experimental schemes analyzed the videos. Face-grooming (movement patterns in which paws contact facial areas) and exploratory (walking, running, climbing, or rearing) behaviors were observed to assess changes in spontaneous activity. Behavior tests were performed 1 h before each Cur administration in the morning.
Morris water maze (MWM) task
Cur was continuously administered for 28 days, and both spatial learning and memory abilities were evaluated using an MWM as described previously.Citation19 The maze was modified slightly, which consists of a circular pool (180 cm diameter and 60 cm high) filled with 19–22°C opaque water and a black escape platform (10 cm diameter and 2 cm above the water level). The pool was divided into four equivalent quadrants and surrounded by external cues that were visible in the wall. Rats underwent four acquisition trials separated by 20-minute intervals daily for 4 consecutive days. Animals were placed into the water facing the pool at different predetermined positions (E, S, W, and N) on each trial, while the platform remained in the same position. Swimming speed and escape latency were video recorded. On day 5 (24 h following the last acquisition trial), a probe trial was performed, in which the hidden platform was removed. Rats were placed in the pool at the location opposite to the platform position and allowed to swim freely for 90 s. The percentage of time spent in the target quadrant and the number of crossings over the platform location were collected.
HE staining
After the MWM test, rats in the sham + vehicle, TN + vehicle, and TN + Cur groups (n=3 each group) were deeply anesthetized and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Brain slices obtained at coronal level (bregma −3 to −4 mm) were immediately removed, fixed in 10% neutral buffered formalin, and embedded in paraffin. Coronal sections (6 µm thick) of hippocampus were cut, and the sections were stained with HE. The morphology of the CA1 region of hippocampus in different groups was observed by a light microscope (XI-70; Olympus Corporation, Tokyo, Japan). Dense and irregular-shaped pyknotic cells were identified as degenerated neurons.
Electron microscopy
Three rats were taken from the sham + vehicle, TN + vehicle, and TN + Cur groups for ultrastructure analysis on day 48 after surgery. Animals were deeply anesthetized and perfused via the left ventricle with warm saline followed by the mixed fixation of 2% glutaraldehyde and 4% paraformaldehyde (Sigma-Aldrich Co.) under anesthesia. The CA1 region of hippocampus was dissected out, washed in 3% glutaraldehyde for 2 h, and then rinsed with 0.1 M phosphate buffer for three times. Subsequently, the hippocampus specimens were fixed in 1% osmium tetroxide (Sigma-Aldrich Co.) for 2 h, dehydrated in graded ethanol, embedded using araldite, polymerized for 2 days, and cut into 50–70 nm ultrathin sections. Finally, sections were stained with uranyl acetate and lead citrate. Ultrastructural changes in neurons, organelles, and synapses in the CA1 region of hippocampus were observed using the transmission electron microscopy (TEM; H-9000NARI baraki; Hitachi Ltd., Tokyo, Japan). Electron microscopic photographs were analyzed using the Image-Pro Plus 6.0 software (Media Cybemetics, Silver Spring, MD, USA). The number of synapses, the synaptic clefts, and the area of the postsynaptic density (PSD) were calculated as described previously.Citation22
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Repeated measures two-way ANOVA followed by Bonferroni post hoc test were used to compare intergroup differences in the behavior activities and latencies to reach the platform in the MWM task. Data on swimming speeds, time spent in target quadrant, and platform crossings were compared using one-way ANOVA. The Student–Newman–Keuls test was performed for multiple comparisons, followed by Student’s t-test to evaluate the difference between groups at the same time points. All data were analyzed by SPSS 16.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Effect of Cur on mechanical allodynia
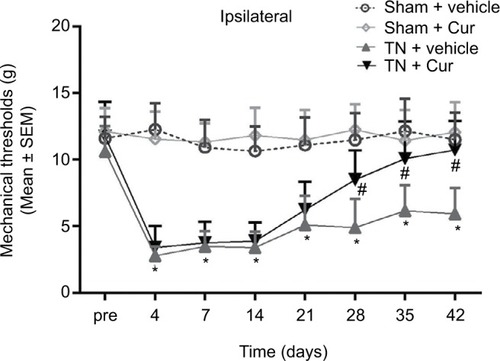
There was no significant difference in the mechanical thresholds between the sham + vehicle and sham + Cur groups. Compared to rats in the sham + vehicle and sham + Cur groups, animals in the TN + vehicle group exhibited a significant decrease in the MWT after cobra venom injection, which lasted for the 42-day observation period (P<0.001; ). A 28-day Cur treatment markedly attenuated the mechanical allodynia in cobra venom-induced TN rats (P<0.001; ). The ipsilateral MWT displayed a significant increase at the time points on days 28 (P<0.001), 35 (P<0.001), and 42 (P<0.001) after surgery compared to the TN + vehicle group. Chronic Cur treatment resulted in a recuperating effect on the mechanical allodynia in the TN rats but not any influence on the mechanical allodynia in the sham + Cur group.
Figure 1 Effect of Cur on mechanical allodynia in rats.
Abbreviations: Cur, curcumin; MWTs, mechanical withdraw thresholds; SEM, standard error of the mean; TN, trigeminal neuralgia.
Effect of Cur on spontaneous behaviors
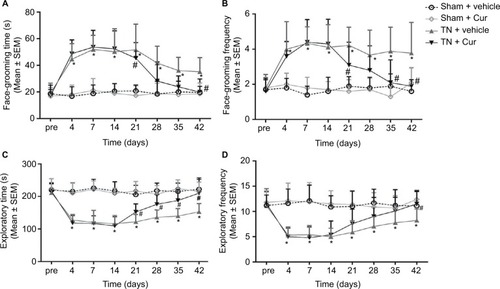
Video recordings of the free behaviors revealed that rats injected with cobra venom present a significant increase in time (P<0.001; ) and frequency (P<0.001; ) of face-grooming activities as well as a significant decrease in time (P<0.001; ) and frequency (P<0.001; ) of exploratory behaviors from day 4 after surgery. Rats in the sham + vehicle and sham + Cur groups did not display any significant change in behavior activities after surgery. However, chronic Cur treatment significantly reversed abnormal alterations in spontaneous behaviors.
Figure 2 Alterations of spontaneous behaviors in rats.
Abbreviations: Cur, curcumin; SEM, standard error of the mean; TN, trigeminal neuralgia.
Effect of Cur on spatial learning and memory function
The spatial learning abilities in the MWM task were impaired in TN rats treated with vehicle compared to sham (sham + vehicle and sham + Cur) animals (). However, the Cur-treated rats exhibited a significant decrease in the latency to platform. There was a significant difference in the latency to platform between groups (P<0.001; ). Post hoc analyses indicated that latency curve to reach the platform was significantly longer in the TN + vehicle group compared to the sham group (P<0.01). The latency time was significantly shorter in the TN + Cur group than in the TN + vehicle group (P<0.01). There was no significant difference in swimming speed among all groups ().
Figure 3 Learning curve and swimming speeds in the Morris water maze task in the experimental groups.
Abbreviations: Cur, curcumin; SEM, standard error of the mean; TN, trigeminal neuralgia.
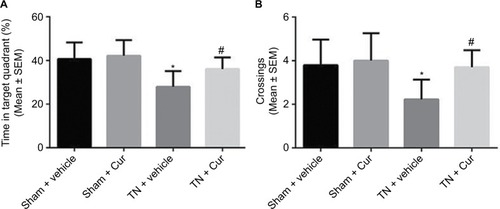
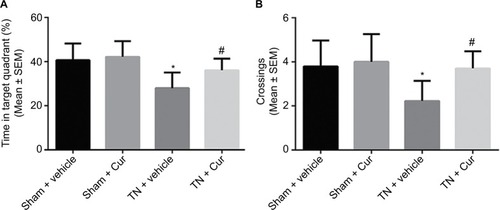
The TN rats exhibited a decreased time in the target quadrant and a reduced number of platform site crossings compared to the sham rats during the probe trials. Chronic Cur treatment reversed this effect (). Memory retention was aggravated in the TN rats treated with vehicle, shown by a decreased time in the target quadrant (P<0.01; ). Cur treatment attenuated the cognitive deficits induced by TN. Moreover, the number of platform site crossings was significantly reduced in the TN rats treated with vehicle compared with sham groups (P<0.05; ). However, Cur-treated rats did not exhibit a significant decrease in the number of platform crossings.
Figure 4 Results of probe trials in Morris water maze.
Abbreviations: Cur, curcumin; SEM, standard error of the mean; TN, trigeminal neuralgia.
Effect of Cur on morphological alterations in the CA1 region of hippocampus
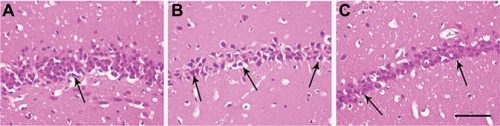
Most hippocampal neurons in the sham group were normal and arranged in alignment with obvious nucleolus (). In contrast, the number of pyknotic neurons significantly increased in the TN group, with the loss of neurons, cytoplasm shrinkage, and nucleus pycnosis. Neurons were arranged loosely (). The density of pyknotic neurons markedly decreased in the Cur-treated group compared to the TN group. Moreover, neurons were less damaged and arranged closer than those in the TN group ().
Figure 5 Representative photomicrographs of morphological changes in the hippocampal CA1 region.
Abbreviations: Cur, curcumin; HE, hematoxylin and eosin; TN, trigeminal neuralgia.
Effect of Cur on ultrastructure changes in the CA1 region of the hippocampus
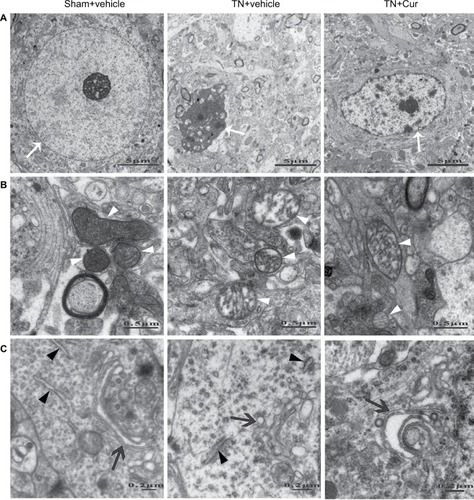
The ultrastructure changes in the hippocampal CA1 area by TEM are shown in . In the sham + vehicle group, large and round nuclei containing a uniform density of chromatin were observed and organelles were clear with neatly arranged mitochondria cristae, a regular Golgi apparatus, and numerous dispersed rough endoplasmic reticulum in the cytoplasm. However, in the TN rats, CA1 neurons exhibited an irregular clumping of chromatin, swollen mitochondria with disordered and reduced numbers of cristae, an enlarged Golgi apparatus, and a dilated rough endoplasmic reticulum. Chronic Cur treatment attenuated these aberrant alterations and neurons displayed more regular nuclei and clearer organelles.
Figure 6 Representative photomicrographs showing the ultrastructures of neurons in the hippocampal CA1 region.
Abbreviations: Cur, curcumin; TN, trigeminal neuralgia.
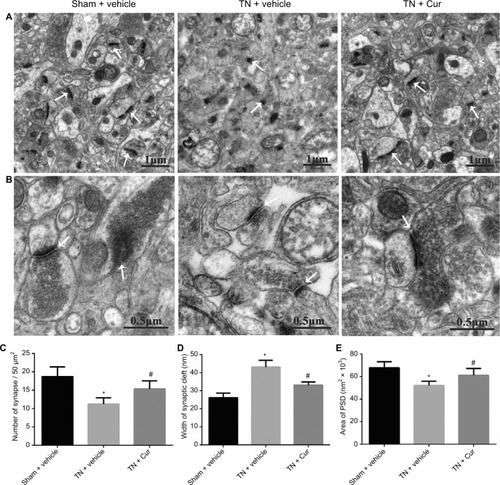
In the sham + vehicle group, the outline of synaptic membranes was clear with abundant vesicles distributed in the presynaptic membrane and the PSD was thick. Compared to sham rats, the TN rats exhibited a decreased synapse density with obscure synaptic outlines, fewer presynaptic vesicles, and thinner PSD. Chronic Cur treatment restored the synaptic structure with increased synapse density, distinct outline, more presynaptic vesicles, and thicker PSD in TN rats ().
Figure 7 Representative photomicrographs showing the ultrastructures of synapses in the hippocampal CA1 region.
Abbreviations: Cur, curcumin; PSD, postsynaptic density; SEM, standard error of the mean; TN, trigeminal neuralgia.
Discussion
The present study demonstrated that Cur treatment ameliorated pain and improved cognition deficits in a cobra venom-induced TN rat model. Furthermore, Cur provided a protection against neuropathic pain-induced neuron degeneration and synapse damage in hippocampus.
Cognitive deficits, especially memory deterioration, are the most common complaints in patients with neuropathic pain, without effective therapy. Patients with long-term moderate-to-severe pain predispose to cognitive impairment.Citation23 Some analgesics may be associated with cognitive deficits de novo, or even can further exacerbate preexisting cognitive deficits. Thus, novel strategies for the treatment of cognitive impairment induced by chronic neuropathic pain deserve extensive attentions from health care professionals. However, knowledge of the pathology and molecular mechanisms of pain-induced cognitive deficits is limited and effective drugs remain scarce, despite ever-increasing researches.
In this study, the cobra venom-induced TN model was used to induce persistent neuropathic pain as it can reliably mimic signs and symptoms of neuropathic patients.Citation18 Moreover, our previous work showed that both spatial learning and memory abilities were impaired in cobra venom-induced TN rats.Citation19 Thus, the cobra venom-induced TN model is suitable to mimic the cognitive impairments caused by chronic neuropathic pain.
Cur attenuated mechanical allodynia and abnormal changes in free behaviors
This study showed that chronic Cur treatment alleviated pain-related behaviors, increased PWTs and explorative activities, and decreased face-grooming behaviors. Furthermore, these effects were clearly time dependent. The alterations in pain behaviors diminished over time and approximately returned to basal levels after 28 days of Cur treatment. These results are consistent with findings of previous studies, in which chronic Cur treatment markedly facilitates recovery from surgery in a rat model of postoperative painCitation24 and alleviates complete Freund’s adjuvant-induced inflammatory hyperalgesia by decreasing oxidative stress and downregulating proinflammatory cytokines.Citation25 In a chronic constriction injury model of neuropathic pain, moreover, Zhao et alCitation20 demonstrated that Cur ameliorates mechanical and thermal hyperalgesia by modulation of the descending monoamine system and opioid receptors. These findings indicate that Cur provides antinociceptive potential and attenuates cobra venom-induced TN. However, the mechanisms underlying the antinociceptive action of Cur in TN are poorly understood and future research is still needed.
Cur improved spatial learning and memory
Our results showed that chronic Cur treatment shortened escape latencies and increased time in the target quadrant and platform site crossings in the water maze task, indicating that Cur treatment improves spatial learning and memory deficits, similar to the findings of previous studies.Citation26–Citation28 It has been shown that Cur ameliorates memory deficits in senescence-accelerated mice.Citation26 Moreover, Cur can provide a protection against chronic unpredictable stress-induced cognitive deficits in mice.Citation27 Additionally, Cur improves Alzheimer’s disease-related cognitive deficits by the BDNF–ERK signaling pathway.Citation28 These findings suggest that Cur has a potential neuroprotection and can improve the function of spatial learning and memory in rats with TN. To exclude the possible effect of partial vision loss in the eye on the lesion side of the rats,Citation19 visible platform training was adopted to assess sensorimotor function in our study. Thus, our results exclude the possibility that sensorimotor deficits are the cause of cognitive deficits in the MWM task.
Cur ameliorated hippocampal neuron and synapse damage
It has been shown that persistent alterations in neuronal development and synaptic plasticity in cognition-related brain areas underlie the impairments in neurobehavioral performance, especially for hippocampus-dependent water maze tasks.Citation29 The hippocampus is a critical brain region responsible for learning and memory, and it is likely involved in various cognitive deficits.Citation30,Citation31 Thus, abnormalities in neurons or synapses in the hippocampus may be involved in the mechanisms of pain-induced cognitive deficits. In this study, TEM showed that chronic Cur treatment alleviated nucleus shrinkage, mitochondria swell and vacuolation, Golgi apparatus enlargement, and endoplasmic expansion in the TN rats. Furthermore, increased number of synapses and presynaptic vesicles, narrower synapse clefts, and thicker PSD were observed in Cur-treated TN rats. It is well believed that the number of presynaptic vesicles represents the quantity of neurotransmitters released into the synaptic cleftCitation32 and the thickness of PSD reflects synaptic efficacy.Citation33 Subtle changes in the synaptic structure can lead to significant alterations in the biophysical properties of synapse,Citation33 resulting in variations of memory abilities. In our study, Cur improved neuronal activity or synaptic efficacy and eventually ameliorated learning and memory deterioration. These beneficial effects of Cur on the neuronal activity and synaptic plasticity are consistent with the findings of previous studies.Citation34,Citation35 A recent study demonstrates that long-term Cur administration prompts hippocampal neurogenesis and improves memory in senescent-age rats.Citation34 Additionally, Hoppe et alCitation35 show that Cur modulates the synaptic-related proteins, such as CaMKII and synapsin1, to prevent the deleterious effects of Aβ on synaptic activity in hippocampus. Consequently, the amelioration of neuronal and synapse damages following Cur administration suggests the possible involvement of neurons and synapses in the beneficial effect of Cur on pain-induced cognitive deficits.
It has been demonstrated that chronic pain can produce alterations of neuronal and synaptic plasticities in the central nervous system.Citation36 Secondary to altered nociceptive signaling in peripheral neuropathic pain, both the neural circuit remodeling and structural synaptic plasticity occur in the “pain matrix” cortices.Citation37 Given that Cur possesses antinociceptive and neuroprotective properties, the improvement in pain-induced cognitive deficits by Cur in the TN rats may be attributable to these properties. On one hand, the neuroprotective potential exerted by Cur can directly improve the hippocampal neuronal and synaptic plasticity. On the other hand, the antinociceptive potential exhibited by Cur may attenuate cobra venom-induced TN and further prevent the structural plasticity alterations of neuronal and synaptic connections under chronic pain conditions, which indirectly ameliorates pain-induced cognitive deficits. Thus, Cur may be a potentially therapeutic alternative for the treatment of pain-induced cognitive deficits.
There are some limitations in our design that deserve attention. First, this study was focused on the pathophysiological mechanisms of Cur affecting cognitive function. In fact, specific molecular mechanisms of Cur in improving cobra venom-induced TN and pain-induced cognitive deficits remain elusive. Second, this study only examined hippocampal ultrastructural alterations. Thus, it is unclear whether similar changes occur in other brain regions associated with learning and memory. Further experiments are needed to clarify the molecular mechanisms and alterations in other pain-related brain regions. Third, as cobra venom is a complex molecular compound with several biological and physiological effects,Citation19 it is uncertain whether it has a direct and/or indirect neurotoxic effect on the hippocampus, after retrogradely transported along the intraorbital nerve into the central nervous system (CNS) and/or diffusion into the systemic circulation and subsequent transportation to the CNS. Perhaps, contralateral facial von Frey fiber analysis should be performed as a control measure to account for the systemic effects of the venom. In the future study, moreover, unilateral chronic constriction injury of the ION, as previously described,Citation38 may be adopted to exclude the neurotoxic effect on the CNS, produced by the agent being used to induce neuropathic pain. Fourth, the contribution of chronic pain to exploratory behavior may be considered as a possible confounder. Other cognitive tests, such as radial arm maze and T maze, should be carried out to clarify the relationship between pain and cognition.
Conclusion
The results of this study confirm that in cobra venom-induced TN rat model, chronic Cur treatment can alleviate pain-related behaviors (mechanical allodynia and free behavioral alterations) and improve spatial learning and memory deficits. These results suggest that Cur may be a therapeutic strategy of cognitive impairment induced by chronic neuropathic pain. The improvement in cognitive impairment by Cur may be associated with the structural alterations of neurons and synapses in the hippocampal CA1 region.
Acknowledgments
We are grateful to Dr Fushan Xue for advice in revising this paper. This work was supported by the National Natural Science Foundation of China (81401860) and the Research Foundation of Beijing Friendship Hospital, Capital Medical University (yyqdkt2016-7).
Disclosure
The authors report no conflicts of interest in this work.
References
- GraceGMNielsonWRHopkinsMBergMAConcentration and memory deficits in patients with fibromyalgia syndromeJ Clin Exp Neuropsychol199921447748710550807
- NivDKreitlerSPain and quality of lifePain Pract20011215016117129291
- KreitlerSNivDCognitive impairment in chronic painPain Clin Update20071514
- YalcinIBarthasFBarrotMEmotional consequences of neuropathic pain: insight from preclinical studiesNeurosci Biobehav Rev20144715416425148733
- BerrymanCStantonTRBoweringKJTaborAMcFarlaneAMoseleyGLDo people with chronic pain have impaired executive function? A meta-analytical reviewClin Psychol Rev201434756357925265056
- HartRPMartelliMFZaslerNDChronic pain and neuropsychological functioningNeuropsychol Rev200010313114910983898
- HuYYangJHuYWangYLiWAmitriptyline rather than lornoxicam ameliorates neuropathic pain-induced deficits in abilities of spatial learning and memoryEur J Anaesthesiol201027216216819915478
- ArnérSMeyersonBALack of analgesic effect of opioids on neuropathic and idiopathic forms of painPain198833111232454440
- VoTRiceASDworkinRHNon-steroidal anti-inflammatory drugs for neuropathic pain: how do we explain continued widespread use?Pain2009143316917119362418
- BesiEBonifaceDRCreggRZakrzewskaJMComparison of tolerability and adverse symptoms in oxcarbazepine and carbamazepine in the treatment of trigeminal neuralgia and neuralgiform headaches using the Liverpool Adverse Events Profile (AEP)J Headache Pain20151656326335440
- SchiltenwolfMAkbarMHugAEvidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioidsPain Physician201417191924452649
- NafisiSAdelzadehMNorouziZSarboloukiMNCurcumin binding to DNA and RNADNA Cell Biol200928420120819364279
- MotterliniRForestiRBassiRGreenCJCurcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stressFree Radic Biol Med20002881303131210889462
- DiYXHongCJunLRenshanGQinquanLCurcumin attenuates mechanical and thermal hyperalgesia in chronic constrictive injury model of neuropathic painPain Ther201431596925135388
- ThiyagarajanMSharmaSSNeuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in ratsLife Sci200474896998514672754
- BabuAPrasanthKGBalajiBEffect of curcumin in mice model of vincristine-induced neuropathyPharm Biol201553683884825429779
- KumarADograSPrakashAProtective effect of curcumin (Curcuma longa), against aluminium toxicity: possible behavioral and biochemical alterations in ratsBehav Brain Res2009205238439019616038
- AnJXHeYQianXYA new animal model of trigeminal neuralgia produced by administration of cobra venom to the infraorbital nerve in the ratAnesth Analg2011113365265621778333
- WuZQianXYAnJXTrigeminal neuralgia induced by cobra venom in the rat leads to deficits in abilities of spatial learning and memoryPain Physician2015182E207E21625794221
- ZhaoXWangCZhangJFChronic curcumin treatment normalizes depression-like behaviors in mice with mononeuropathy: involvement of supraspinal serotonergic system and GABAA receptorPsychopharmacology (Berl)2014231102171218724297305
- DixonWJEfficient analysis of experimental observationsAnnu Rev Pharmacol Toxicol1980204414627387124
- WuJDongLZhangMClass I histone deacetylase inhibitor valproic acid reverses cognitive deficits in a mouse model of septic encephalopathyNeurochem Res201338112440244924072674
- GlassJMReview of cognitive dysfunction in fibromyalgia: a convergence on working memory and attentional control impairmentsRheum Dis Clin North Am200935229931119647144
- ZhuQSunYYunXOuYZhangWLiJXAntinociceptive effects of curcumin in a rat model of postoperative painSci Rep20144493224816565
- SinghAKVinayakMCurcumin attenuates CFA induced thermal hyperalgesia by modulation of antioxidant enzymes and down regulation of TNF-a, IL-1b and IL-6Neurochem Res201540346347225479948
- SunCYQiSSZhouPNeurobiological and pharmacological validity of curcumin in ameliorating memory performance of senescence-accelerated micePharmacol Biochem Behav2013105768223402943
- RinwaPKumarAPiperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in miceBrain Res20121488385023099054
- ZhangLFangYXuYCurcumin improves amyloid β-peptide (1–42) induced spatial memory deficits through BDNF–ERK signaling pathwayPLoS One2015106e013152526114940
- AnLZhangTVitamins C and E reverse melamine-induced deficits in spatial cognition and hippocampal synaptic plasticity in ratsNeurotoxicology20144413213924960222
- JiSTianYLuYIrradiation-induced hippocampal neurogenesis impairment is associated with epigenetic regulation of bdnf gene transcriptionBrain Res20141577778825020123
- AguiarASJrCastroAAMoreiraELShort bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signalingMech Ageing Dev201113211–1256056721983475
- Ahnert-HilgerGMünster-WandowskiAHöltjeMSynaptic vesicle proteins: targets and routes for botulinum neurotoxinsCurr Top Microbiol Immunol201336415917723239353
- JingYWangZSongYQuantitative study of aluminum-induced changes in synaptic ultrastructure in ratsSynapse200452429229815103695
- DongSZengQMitchellESCurcumin enhances neurogenesis and cognition in aged rats: implications for transcriptional interactions related to growth and synaptic plasticityPLoS One201272e3121122359574
- HoppeJBHaagMWhalleyBJSalbegoCGCimarostiHCurcumin protects organotypic hippocampal slice cultures from Abeta1–42-induced synaptic toxicityToxicol In Vitro20132782325233024134851
- MayAChronic pain may change the structure of the brainPain2008137171518410991
- KimWKimSKNeural circuit remodeling and structural plasticity in the cortex during chronic painKorean J Physiol Pharmacol20162011826807017
- VosBPStrassmanAMMaciewiczRJBehavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerveJ Neurosci1994145 pt 1270827238182437
