Abstract
Introduction
High comorbidity of osteoarthritis (OA) and neuropathic pain has been reported in aged patients. Evidence shows that central sensitization of pain processing occurs in late-phase OA and may facilitate the development of neuropathic pain. Few studies reveal whether acute monoarthritis (MA) aggravates neuropathic pain on the opposite side of the body from the injury.
Methods
To address whether neuropathic pain is affected by contralateral MA through distinct inflammatory pathway, MA was induced by intra-articular injection of complete Freund’s adjuvant (CFA) into the right tibiotarsal joint, and neuropathic pain was established by chronic constriction injury (CCI) of the left sciatic nerve.
Results
We observed that MA aggravated mechanical allodynia and thermal hyperalgesia in CCI rats. Furthermore, MA affected the other side of the spinal cord in multiple aspects, including the upregulation of iNOS mRNA and the enhancement of forskolin-induced facilitation of excitatory synaptic transmission in the spinal cord dorsal horn substantia gelatinosa neurons.
Discussion
Interestingly, intrathecal injection of 1400W, an antagonist of iNOS, attenuated intensity of pain behaviors in CCI rats with contralateral MA to similar levels in CCI rats without MA, and also normalized the facilitatory effect of forskolin on excitatory synaptic transmission in the spinal cord dorsal horn neurons in contralateral MA rats. Therefore, contralateral MA worsened CCI-induced pain hypersensitivity probably through upregulating iNOS and enhancing the facilitation of synaptic transmission following CCI.
Conclusion
Inhibiting the iNOS might be a potential therapeutic strategy for concurrent OA and neuropathic pain.
Introduction
Osteoarthritis (OA) is a common disease in aging population.Citation1,Citation2 As a pain disease, OA significantly impairs physical function and reduces quality of life.Citation3 Neuropathic pain following a lesion/injury or disease of the somatosensory systemCitation4 is also very common in aging patients,Citation5 especially in those with diabetes mellitus (diabetic neuropathy), herpes zoster (postherpetic neuralgia), low back pain (such as lumbar spinal stenosis), cancers, and stroke.Citation6 Both OA and neuropathic pain show pain sensitization,Citation6,Citation7 and their comorbidity in aged patients is not rare.Citation8 In patients with concurrent OA and neuropathic pain, the medication for either disease may not adequately relieve the symptoms. To improve the treatment of the two concurrent diseases, it is necessary to elucidate the pathophysiological relationship between these conditions.
Chronic pain after arthritis is associated with chronic neuroinflammation in the peripheral or central nervous system.Citation9,Citation10 Levels of many proinflammatory cytokines (IL-1β, TNFα, IL-6, etc.) and chemokines (iNOS, eNOS, COX-2) are known to be increased in the primary afferent and dorsal horn neurons innervating arthritic joints.Citation9 These elevated inflammatory mediators subsequently enhance synaptic transmission at the spinal cord level referred to as “central sensitization” and facilitate the development of neuropathic pain.Citation11 Many studies dealing with OA use contralateral noninjured side as a control and focus on alterations in local or ipsilateral neuroinflammatory reactions. However, there is conflicting evidence, showing that inflammatory mediators in the contralateral side of arthritis rats are elevated in OA in some studiesCitation12–Citation14 but remain unchanged in others.Citation12,Citation15,Citation16 The discrepancy may come from the difference in animal models, the injection site of complete Freund’s adjuvant (CFA), the concentration and dose of CFA, and the time line of the experiment. Furthermore, it is unclear about the roles of the inflammatory reactions following contralateral OA in the development of neuropathic pain.
The present study confirms that monoarthritis (MA) did affect the contralateral spinal cord and exacerbated pain hypersensitivity in chronic constriction injury (CCI). Additionally, the potential strategy that could reverse the exacerbation was also explored.
Methods
All animal care and experimental protocols follow the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996, Washington, DC) and were approved by the Institute of Animal Care and Use Committee and the Office of Laboratory Animal Resources in the Second Hospital of Shandong University. Male Sprague Dawley rats (200–250 g) were used in this study. All rats were cage housed in an environment with a temperature of 22±2°C, relative humidity between 45% and 75%, and 12 h light–dark cycle. Water and food were provided ad libitum.
The rats were randomly divided into five groups: sham group (Sham, n=8), received intra-articular injection of 50 μL sterile saline; MA group (n=8), received intra-articular injection of 50 μL CFA; CCI group (n=8); and CCI + MA group (n=16), having two subgroups: MA induction was performed either 2 days before (n=8) or 2 days after (n=8) CCI.
Drug treatment
Forskolin (Sigma-Aldrich Co., St Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO) and applied to spinal cord slices in artificial cerebrospinal fluid (ACSF) at a final concentration of 20 μM. 1400W (Selleck Chemicals, Houston, TX, USA) was dissolved in DMSO and intrathecally administrated to each animal (10 μg in 20 μL) right after CFA injection.
Induction of MA
MA was induced by injecting 50 μL CFA (Sigma-Aldrich Co.) into the right tibiotarsal joint. This procedure was performed under isoflurane inhalation for anesthesia, as described previously.Citation17 Briefly, the fossa of the lateral malleolus of the fibula was located, and a 28-gage needle was inserted from the gap between the tibiofibular bone and tarsus bone into the articular cavity. CFA (50 μL) was injected into the articular cavity. The Sham rats were intra-articularly injected with sterile saline. The rats after CFA injection showed pronounced swelling in the right ankle measured using a plethysmometer (Ugo Basile, Milan, Italy). Animals without or with minimal swelling in the right ankle were discarded.
Chronic constriction injury (CCI)
CCI was performed as described previously.Citation18,Citation19 Briefly, a rat was anesthetized with intraperitoneal injection of sodium pentobarbital (40–60 mg/kg); the sciatic nerve on the left side was exposed by making a skin incision and cutting through the connective tissue between the gluteus superficialis and biceps femoris muscles. Four chromic gut ligatures were tied loosely around the sciatic nerve with 1 mm intervals between two neighboring ligatures to just occlude but not arrest epineural blood flow. Muscle and skin layers were sutured separately. The rat was allowed to recover from surgery for 24 h before pain hypersensitivity tests began.
Histology
Hematoxylin–eosin (HE) staining was performed using 5 μm sections of the right tibiotarsal joints of the Sham and MA rats. Briefly, the extremity of the right hindlimb containing tibiotarsal joints was dissociated and fixed in 10% neutral buffered formalin. For decalcification of tibia and tarsus, the specimen was immersed in 10% nitric acid and 1% ethylenediaminetetraacetic acid (EDTA) in PBS. Then, the sample was subjected to dehydration in a series of ethanol solutions with gradually increasing concentrations from 50% to 100%. The dehydrated sample was embedded in paraffin and was sliced with a sliding microtome into 5 μm thick sections, which were mounted onto glass slides. After dewaxing and washing, the sections were stained with HE and toluidine blue. The stained sections were examined using an upright microscope, equipped with a 4× objective. As illustrated in , MA rats showed pathological damages in tibiotarsal joint, including deformation in tibia and tarsus; enhancement of mononuclear infiltration; and hyperplasia of synovial cells.
Examination of pain behaviors
Mechanical paw withdrawal threshold (PWT)
Mechanical PWT was calculated as described previously.Citation20 Rats were individually placed in a box without bottom on an elevated metal mesh floor and were allowed to habituate for ≥30 min before examination. A series of calibrated von Frey hairs (Stoelting, Wood Dale, IL, USA) were applied one by one to the central region of the plantar surface of the left hind paw in an ascending order (1.4, 2, 4, 6, 8, 10, 15, and 26 g). The 50% PWT was determined using Dixon’s up–down method.
Thermal withdrawal latency
Thermal withdrawal latency was measured using the thermal plantar analgesia instruments (IITC, Woodland Hills, CA, USA) as follows. Rats were placed on the glass floored testing cages for at least 30 min until the exploratory behavior ceased. The heat source was placed directly beneath the mid-plantar surface of the hind paw through a transparent glass surface. The delay of the hind paw withdrawal from the heat source, relative to the onset of thermal stimulus, was recorded. A cutoff time of 20 s was used to avoid tissue damage. The heat stimulation was repeated at least three times with an interstimulus interval of 5 min, and then the mean of paw withdrawal latencies of individual rats was calculated.
Quantitative polymerase chain reaction (qPCR)
The left and right lumber segments (L3–L6) of the spinal cord from each rat were harvested separately 48 h after the injection of CFA for the induction of MA and immediately stored at −80°C. The samples were lysed in 500 μL of TRIzol® reagent (Thermo Fisher Scientific, Waltham, MA, USA), and total RNA was isolated according to the manufacturer’s instruction. Integrity of RNA was confirmed in a denaturing agarose gel, and no contaminating DNA or RNA degradation was observed. Afterward, the RNA was reverse transcribed using 2 μg total RNA as the template using a reverse transcription polymerase chain reaction (RT-PCR) kit (Promega Corporation, Fitchburg, WI, USA). Reverse transcription was performed at 45°C for 45 min, followed by 5 min heating at 92°C. The real-time PCR was performed in Roche LightCycler 480 (Hoffman-La Roche Ltd., Basel, Switzerland) with Maxima SYBR Green/ROX qPCR Master Mix (2×) (Thermo Fisher Scientific) for detection. Samples were amplified in 25 μL reaction mixtures, containing 0.3 μM forward and reverse primers, 12.5 μL Maxima SYBR Green/ROX qPCR Master Mix (2×), template DNA ≤ 500 ng/reaction, and nuclease-free water. All primers were designed using Primer-BLAST design software (NIH, Bethesda, MD, USA) and were synthesized by Sangon Biotech (Shanghai, People’s Republic of China). The primers we used are listed in . All PCRs were performed with the following parameters: 10 min at 95°C for initial denaturation, 35 cycles of sequential steps (15 s at 95°C for denaturation, 1 min at 55°C–64°C for annealing, 30 s at 72°C for extension), and then a 7 min final incubation at 72°C. Each sample was tested in triplicates. The relative expression was calculated using the ΔΔCq methodCitation21 and optimized with a standard curve to confirm the high specificity.
Table 1 Sequences of primers used in RT-PCR
Patch-clamp recording
Whole-cell patch-clamp recordings were made from substantia gelatinosa (SG) neurons in lumbar spinal cord slices as described previously.Citation22
In brief, 48 h after the injection of CFA, the rats were suffocated to death with CO2 and were decapitated. After laminectomy, the lumber spinal cord was removed and sliced (300 μm) with a vibratome (VT-1200S; Leica Inc., Wetzlar, Germany) in ice-cold-modified, sucrose-based artificial cerebral spinal fluid (SACSF) saturated with 95% O2/5% CO2 containing (mM) 250 sucrose, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 26 NaHCO3, and 11 glucose. Slices were first recovered at 32°C for at least 1 h in SACSF and then kept at room temperature (22–24°C) in ACSF bubbled with 95% O2/5% CO2 containing (mM) 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 26 NaHCO3, and 11 glucose for at least 30 min. Then, a slice was placed in a recording chamber and perfused (1.5–2.0 mL/min) with ACSF, which was constantly bubbled with 95% O2/5% CO2 continually. In all, 1 μM tetrodotoxin (TTX) and 10 μM bicuculline were applied continually with perfusate when miniature excitatory postsynaptic currents (mEPSCs) were recorded. Drugs were applied with the perfusate as needed.
The neurons in spinal cord slices were visualized with an upright microscope (Eclipse FN1; Nikon Instruments, Mel-ville, NY, USA) and near-infrared illumination. Whole-cell patch-clamp techniques were used to record electrophysiological signals with a MultiClamp 700B amplifier (Axon Instruments, Molecular Devices LLC, Sunnyvale, CA, USA), Digidata 1440 analog-to-digital converters (Axon Instruments), and pClamp 10.2 software (Axon Instruments). Data were sampled at 10 kHz and filtered at 2 kHz. The patch electrode had a resistance of 5–7 MΩ when filled with pipette solution containing (mM) 135 potassium gluconate, 5 KCl, 5 ethylene glycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA), 0.5 CaCl2, 10 HEPES, 2 Mg-ATP, and 0.1 GTP. The pH of these solutions was adjusted to 7.2 with Tris-base, and the osmolarity was adjusted to 300 mOsm with sucrose. The junction potential between the patch pipette and bath solution was nulled before gigaseal formation. Series resistance was monitored without compensation throughout the experiment. All recordings were done at a temperature of 32 ± 0.5°C.
mEPSCs were counted and analyzed using Clampfit 10.3 (Molecular Devices LLC) after the traces were low-pass filtered at 2 kHz. Waveform templates for mEPSCs were defined according to the rise and decay times. The events that match the waveform and exceed the amplitude threshold were counted. The frequency and amplitude of mEPSCs before and after drug application were compared.
Statistical analysis
Data are presented as mean ± standard error of measurement (SEM). Statistical significance was determined using parametric or nonparametric one-way or two-way analysis of variance (ANOVA) with Bonferroni test for pairwise comparison, using GraphPad Prism® 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Difference was considered as statistically significant when p value was <0.05.
Results
Effect of contralateral MA on CCI-induced hypersensitivity to pain
First, we tested whether CCI-induced ipsilateral nociceptive hypersensitivity in rats can be facilitated by contralateral MA. In this set of experiments, we established contralateral MA 2 days before or after CCI to clarify whether MA affects the development or maintenance of CCI-induced hypersensitivity to pain.
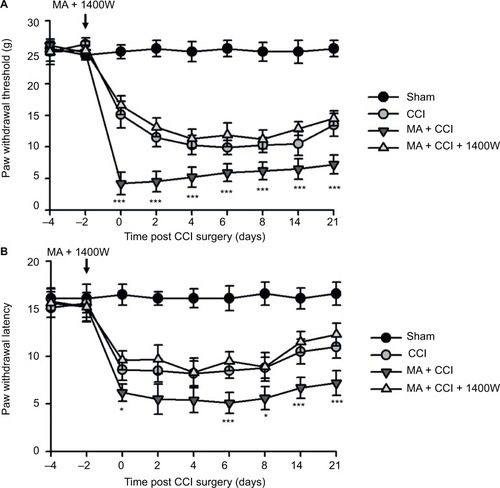
In our observation, there was no significant difference in baseline mechanical PWT and thermal paw withdrawal latency among the groups that received different treatments ( and ). In comparison with the sham group, CCI groups showed decreased mechanical PWT ( and ) and thermal paw withdrawal latency on the ipsilateral side ( and ) that were maintained up to 3 weeks, confirming the successful establishment of CCI-neuropathic pain models, displaying mechanical allodynia and thermal hyperalgesia.
Figure 1 Contralateral MA induced 2 days after CCI exacerbated pain behaviors.
Abbreviations: ANOVA, analysis of variance; CCI, chronic constriction injury; CFA, complete Freund’s adjuvant; MA, monoarthritis; SEM, standard error of measurement.
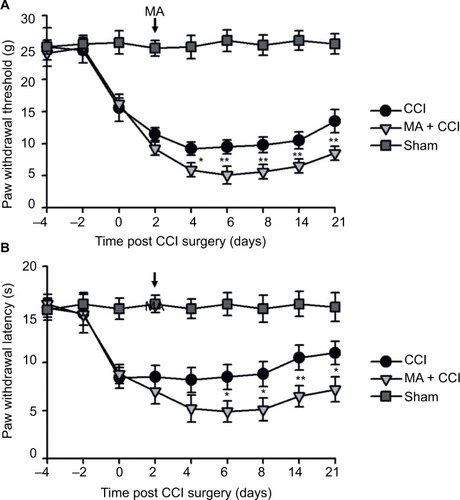
Figure 2 Contralateral MA 2 days before CCI exacerbated pain behaviors in CCI rats.
Abbreviations: ANOVA, analysis of variance; CCI, chronic constriction injury; CFA, complete Freund’s adjuvant; MA, monoarthritis; SEM, standard error of measurement.
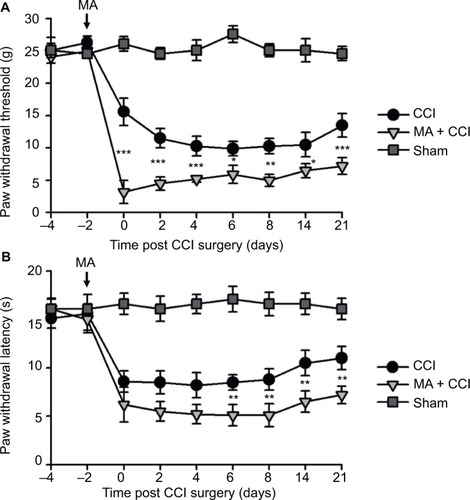
Interestingly, although MA alone did not affect pain sensation of the paw on the other side (), contralateral MA performed 2 days after CCI worsened CCI-induced mechanical allodynia in 2 days () and thermal hyperalgesia in 4 days (), and the deterioration of pain hypersensitivity persisted up to 3 weeks. Similarly, applying contralateral MA 2 days before CCI exacerbated CCI-induced ipsilateral pain hypersensitivity, and the deterioration remained up to 3 weeks after CCI (). Note that contralateral MA before CCI reduced mechanical PWT immediately after CCI and shortened thermal paw withdrawal latency 6 days after CCI.
Taken together, our data suggest that contralateral MA before and after CCI both exacerbated CCI-induced mechanical allodynia and thermal hyperalgesia.
mRNA levels of inflammatory mediators in both sides of the spinal cord after MA
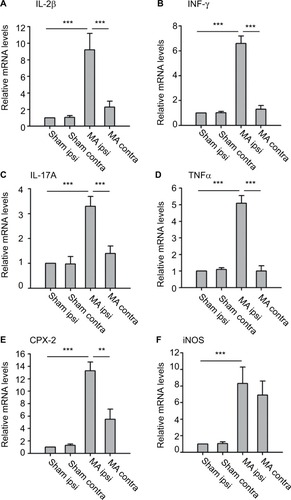
We examined mRNA levels of cytokines and chemokines in the lumber spinal cord ipsilateral and contralateral to the side that MA was induced. We observed that MA significantly increased mRNA levels of IL-1β, INF-γ, IL-17A, TNFα, and COX-2 in the ipsilateral but not in the contralateral lumber spinal cord of MA rats compared to Sham rats (). Interestingly, mRNA levels of iNOS in MA rats were increased bilaterally in the spinal cord in comparison with those of Sham rats. The levels of iNOS mRNA increased by sevenfold in the ipsilateral spinal cord and by sixfold in the contralateral side (). There was no statistically significant difference between two sides. Thus, following MA, elevation in iNOS mRNA levels was similar in two sides of the spinal cords.
Figure 3 Effects of MA on mRNA levels of several inflammatory mediators in either side of the lumbar segments of the spinal cord.
Abbreviations: ANOVA, analysis of variance; contra, contralateral; ipsi, ipsilateral; MA, monoarthritis; qPCR, quantitative polymerase chain reaction; SEM, standard error of measurement.
iNOS enhances forskolin-induced facilitation of synaptic transmission
Since increased levels of iNOS in the spinal cord dorsal horn have been reported to be associated with neuronal excitability and long-term potentiation,Citation23,Citation24 we next studied whether MA-induced upregulation of iNOS affects synaptic transmission in the opposite side of the spinal cord.
First, mEPSCs were recorded using the whole-cell patch-clamp technique from the SG neurons in one side of spinal cord opposite to the side sham or MA operation was performed. The frequency and amplitude of mEPSCs were at the same levels in Sham and MA rats (). Forskolin (50 μM, 5 min), an activator of adenylyl cyclase, when bath-applied with perfusate, induced a sustained increase in mEPSC frequency and amplitude in both Sham and MA rats, and the effects reached maximal levels 20 min after the initiation of forskolin perfusion. Interestingly, the enhancement of mEPSCs was stronger in MA rats than in Sham rats (). The results suggest that synaptic facilitation by forskolin is significantly greater in contralateral spinal cord SG neurons of MA rats.
Figure 4 Effects of MA on excitatory synaptic transmission in the contralateral side of the spinal cord.
Abbreviations: ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; MA, monoarthritis; mEPSC, miniature excitatory postsynaptic current; SEM, standard error of measurement; SG, substantia gelatinosa; TTX, tetrodotoxin.
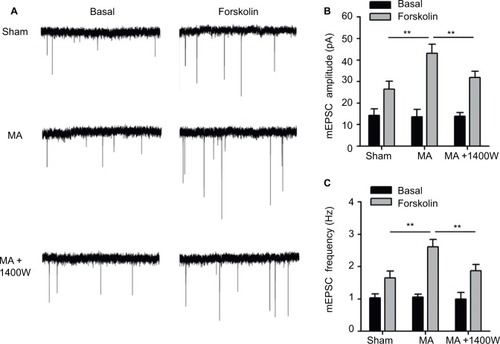
As we found that MA led to elevated iNOS mRNA in the contralateral side of the spinal cord, we tested whether blocking iNOS eliminates the enhanced effects of forskolin on mEPSCs in MA rats. To address this question, we intrathecally administered 1400W (10 μg), an iNOS antagonist, right after intra-articular injection of CFA. In MA rats, 1400W pretreatment attenuated forskolin-induced enhancement of mEPSC frequency and amplitude in contralateral SG neurons to the level similar to Sham rats (). These data suggest that upregulation of iNOS in the contralateral side of the spinal cord in MA rats contributes to enhanced forskolin-induced facilitation of synaptic transmission.
Effect of 1400W on MA-sensitized pain in contralateral CCI models
We observed that MA led to multiple levels of alterations in the contralateral side of the spinal cord, including elevated expression of iNOS and worsened pain hypersensitivity. We were wondering whether elevated iNOS expression could contribute to pain hypersensitivity. To answer this question, we tested the effect of 1400W on exacerbation of contralateral CCI-induced pain hypersensitivity following MA. As showed in , in comparison with Sham rats, all rats in CCI, MA + CCI, and MA + CCI + 1400W groups developed mechanical allodynia and thermal hyperalgesia, which remained stable throughout the experimental period. Additionally, 1400W pretreatment significantly attenuated mechanical allodynia and thermal hyperalgesia in the MA + CCI group to a level comparable to the CCI group. These data suggest that inhibition of iNOS can reverse MA-induced deterioration of contralateral neuropathic pain.
Figure 5 Effects of iNOS inhibition on MA facilitation of contralateral neuropathic pain.
Abbreviations: ANOVA, analysis of variance; CCI, chronic constriction injury; CFA, complete Freund’s adjuvant; MA, monoarthritis; SEM, standard error of measurement.
Discussion
The aim of this study is to evaluate the effect of contralateral MA on CCI-induced neuropathic pain. We observed that in CCI models, pre- or post-induction of contralateral MA enhanced CCI-induced mechanical allodynia and thermal hyperalgesia. We also found that contralateral MA similarly elevated the levels of iNOS mRNA in both sides of the spinal cord. Although MA had no effect on synaptic transmission on the opposite side, it enhanced forskolin-induced facilitation of synaptic transmission, and this enhancement was eliminated by intrathecal administration of iNOS inhibitor. Furthermore, contralateral MA-induced aggravation of mechanical allodynia and thermal hyperalgesia in CCI was attenuated by 1400W pretreatment to the similar levels in CCI rats without MA. Overall, upregulation of iNOS mRNA following contralateral MA might be a mechanism underlying exacerbation of pain hypersensitivity in neuropathic pain.
Although peripheral nerve injury is directly associated with neuronal dysfunction (hyperexcitability) in spinal cord dorsal horn, inflammatory changes play critical roles in worsening the neuronal dysfunction.Citation25 Especially, widespread inflammatory reaction usually exceeds the borders of tissue damage. Our study specifically evaluated mRNA levels of several proinflammatory cytokines and chemokines in the lumber spinal cord following MA. Although the upregulation of IL-1β, INF-γ, IL-17A, TNF-α, and COX-2 in the spinal cord remained restricted on the side of MA, iNOS mRNA in the spinal cord was bilaterally elevated by MA. These results suggest that inflammatory mediators in the spinal cord were differentially regulated by MA with diverse patterns.
It is noteworthy that the magnitudes of iNOS upregulation on both sides were comparable. Increased expression of contralateral iNOS has been shown in the lumbar spinal cord of arthritis rats by other research groups.Citation14,Citation16,Citation26 The differences in iNOS expression levels found in these studies could result from diverse paradigms for pain induction and/or the timing and methods for iNOS examination.
The iNOS is not constitutively expressed but must be induced to be synthesized and become abundant in a variety of cell types, including neurons and glial cells.Citation27 Previous studies demonstrated that iNOS is involved in the development of inflammatory neuropathic pain.Citation14,Citation28 The expression of iNOS in lamina II neurons has been shown to contribute to spinal nociceptive processing in several pain models.Citation24,Citation29–Citation31 However, glial activation could account, at least in part, for the observed increases in iNOS levels in the spinal cord dorsal horn following MA. Investigations are warranted to determine to what extent the increased iNOS depends on the altered neuronal and/or glial function in the spinal cord dorsal horn.
It is noteworthy that, in addition to MA-induced iNOS, 1400W may directly affect neuropathic pain. A previous study has reported that 1400W might exert its analgesic effects by reducing iNOS and altering the balance between the proinflammatory (IL-1α and IL-1β) and anti-inflammatory (IL-10) cytokines.Citation32 In our study, 1400W partly reversed the pain behavior from the animal receiving MA + CCI. It is possible that part of this rescue effect can be attributed to the ameliorated CCI pain. In order to directly test the role of MA-induced iNOS in the facilitation of pain development, future study needs to specifically and precisely inhibit contralateral iNOS expression induced by MA, e.g., viral vector-mediated knockdown of iNOS mRNA. Facilitation of excitatory synaptic transmission in the spinal cord dorsal horn is thought to contribute to persistence of clinical pain and is fundamental for the central nervous system to generate pain hypersensitivity.Citation22 Few electrophysiological studies have been carried out to directly study the functional plasticity that occurs in contralateral spinal cord following the induction of MA. We showed here that excitatory synapses on contralateral SG neurons of MA rats had normal baseline activity, consistent with our data () and a previous study, showing that unilateral arthritis rats did not show pain hypersensitivity in the contralateral side.Citation17 We argue that in our experimental condition, MA-induced upregulation of iNOS in the contralateral dorsal horn, by itself, may not be enough to enhance excitatory synaptic transmission and induce hyperalgesia. As activation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), a transcription factor, parallels with the appearance and maintenance of pain hypersensitivity in CCI rats,Citation33 to mimic this pathophysiological condition, we tested the effect of forskolin on mEPSCs, because, as an agonist of adenosine monophosphate (AMP) cyclase, forskolin leads to accumulation of cAMP, which binds CREB and triggers its activation. Intriguingly, stronger forskolin-induced potentiation of excitatory synaptic transmission in the spinal cord was observed in the side contralateral to MA (). Interestingly, this facilitation of synaptic transmission was abolished by a specific iNOS blocker, 1400W (), suggesting an involvement of NO-cGMP cascade, downstream of iNOS activation.
Our behavioral data show that mechanical allodynia and thermal hyperalgesia in CCI rats were strengthened by contralateral MA. This phenomenon occurred 2 days after MA induction and persisted up to 3 weeks, indicating that MA can induce rapid and persistent changes in the contralateral side of the spinal cord. Upregulation of iNOS induced by contralateral MA possibly participated in the persistently enhanced nociceptive responses, because intrathecal injection of 1400W attenuated pain behaviors in CCI rats with MA to the levels similar to those in CCI rats without MA. These data suggest that contralateral MA-induced potentiation of pain behaviors in the CCI model possibly depends on the elevated expression of iNOS. This notion was supported by previous reports showing that an increased expression of iNOS in the spinal cord dorsal horn was associated with neuron excitability and long-term potentiation that contribute to central hyperalgesia.Citation23,Citation24,Citation34
Taken together, we provide evidence to support that an initially localized arthritis is able to elevate the levels of iNOS in the contralateral side of the spinal cord. This may in turn mediate facilitation of central sensitization (hyperactivity in excitatory synaptic transmission), and exacerbate symptoms of neuropathic pain. This study suggests that patients with neuropathic pain may have higher intensity of pain when they have comorbid OA. We provide evidence for the rationale that iNOS inhibitors might have therapeutic potential in the treatment of neuropathic pain comorbid with arthritis.
Acknowledgments
This study was supported by the Key Research and Development Plan of Shandong Province (number 2015GSF118107).
Supplementary materials
Figure S1 CFA-induced histopathological alterations in the tibiotarsal joint.
Notes: Two days after saline or CFA injection in the right tibiotarsal joint, the rats were sacrificed and the right tibiotarsal joints were fixed, sectioned, and subjected to HE staining. A representative histological image of a section of tibiotarsal joint from a sham rat (A) and a CFA rat (B) (40× magnification).
Abbreviations: CFA, complete Freund’s adjuvant; HE, hematoxylin–eosin; MCI, mononuclear cell infiltration; Sh, synovial cell lining hyperplasia.
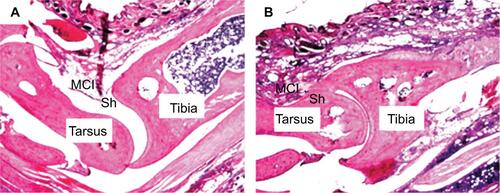
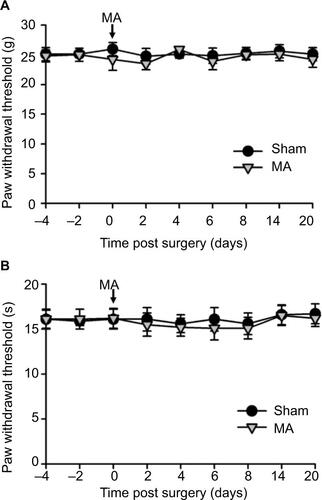
Figure S2 Contralateral MA alone did not induce pain behaviors.
Notes: CFA was injected into the right tibiotarsal joint to induce MA. Saline was injected into the right tibiotarsal joint of the rat in the sham group. Von Frey filament tests and thermal plantar tests were respectively applied to assess mechanical allodynia (A) and heat hyperalgesia (B). Data are shown as mean ± SEM. n=6 in each group. Statistical analysis showed no difference between these two groups.
Abbreviations: CFA, complete Freund’s adjuvant; MA, monoarthritis; SEM, standard error of measurement.
Disclosure
The authors report no conflicts of interest in this work.
References
- NeogiTThe epidemiology and impact of pain in osteoarthritisOsteoarthritis Cartilage20132191145115323973124
- FelsonDTNaimarkAAndersonJKazisLCastelliWMeenanRFThe prevalence of knee osteoarthritis in the elderly. The Framingham osteoarthritis studyArthritis Rheum19873089149183632732
- SalaffiFCarottiMStancatiAGrassiWHealth-related quality of life in older adults with symptomatic hip and knee osteoarthritis: a comparison with matched healthy controlsAging Clin Exp Res200517425526316285189
- OchoaJLNeuropathic pain: redefinition and a grading system for clinical and research purposesNeurology2009721412821283
- HaslamCNurmikkoTPharmacological treatment of neuropathic pain in older personsClin Interv Aging20083111112018488882
- CollocaLLudmanTBouhassiraDNeuropathic painNat Rev Dis Primers201731700228205574
- FingletonCSmartKMoloneyNFullenBMDoodyCPain sensitization in people with knee osteoarthritis: a systematic review and meta-analysisOsteoarthritis Cartilage20152371043105625749012
- PowerJDPerruccioAVGandhiRNeuropathic pain in end-stage hip and knee osteoarthritis: differential associations with patient-reported pain at rest and pain on activityOsteoarthritis Cartilage201826336336929326061
- LeeASEllmanMBYanDA current review of molecular mechanisms regarding osteoarthritis and painGene2013527244044723830938
- MillerREMillerRJMalfaitAMOsteoarthritis joint pain: the cytokine connectionCytokine201470218519325066335
- LluchETorresRNijsJVan OosterwijckJEvidence for central sensitization in patients with osteoarthritis pain: a systematic literature reviewEur J Pain201418101367137524700605
- DonaldsonLFSecklJRMcQueenDSA discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant doseJ Neurosci Methods1993491–25108271831
- BeicheFScheuererSBruneKGeisslingerGGoppelt-StruebeMUp-regulation of cyclooxygenase-2 mRNA in the rat spinal cord following peripheral inflammationFEBS Lett199639021651698706851
- DolanSFieldLCNolanAMThe role of nitric oxide and prostaglandin signaling pathways in spinal nociceptive processing in chronic inflammationPain200086331132010812261
- PaquetJGoebelJCDelaunayCCytokines profiling by multiplex analysis in experimental arthritis: which pathophysiological relevance for articular versus systemic mediators?Arthritis Res Ther2012142R6022414623
- InfanteCDíazMHernándezAConstandilLPelissierTExpression of nitric oxide synthase isoforms in the dorsal horn of monoarthritic rats: effects of competitive and uncompetitive N-methyl-D-aspartate antagonistsArthritis Res Ther200793R5317521446
- ButlerSHGodefroyFBessonJMWeil-FugazzaJA limited arthritic model for chronic pain studies in the ratPain199248173811738577
- BennettGJXieYKA peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in manPain1988331871072837713
- YangJXHuaLLiYQCaveolin-1 in the anterior cingulate cortex modulates chronic neuropathic pain via regulation of NMDA receptor 2B subunitJ Neurosci2015351365225568101
- DixonWJStaircase bioassay: the up-and-down methodNeurosci Biobehav Rev199115147502052197
- LivakKJSchmittgenTDAnalysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) methodMethods200125440240811846609
- ZhouCLuoZDElectrophysiological characterization of spinal neuron sensitization by elevated calcium channel alpha-2-delta-1 subunit proteinEur J Pain201418564965824151064
- JinXGChenSRCaoXHLiLPanHLNitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neuronsJ Biol Chem201128638331903320221813646
- PedersenLMJacobsenLMMollerupSGjerstadJSpinal cord long-term potentiation (LTP) is associated with increased dorsal horn gene expression of IL-1beta, GDNF and iNOSEur J Pain201014325526019596210
- EllisABennettDLNeuroinflammation and the generation of neuropathic painBr J Anaesth20131111263723794642
- HsiehYLEffects of ultrasound and diclofenac phonophoresis on inflammatory pain relief: suppression of inducible nitric oxide synthase in arthritic ratsPhys Ther2006861394916386061
- HancockCMRiegger-KrughCModulation of pain in osteoarthritis: the role of nitric oxideClin J Pain200824435336518427234
- MukherjeePCinelliMAKangSSilvermanRBDevelopment of nitric oxide synthase inhibitors for neurodegeneration and neuropathic painChem Soc Rev201443196814683824549364
- LeeJSZhangYRoJYInvolvement of neuronal, inducible and endothelial nitric oxide synthases in capsaicin-induced muscle hypersensitivityEur J Pain200913992492819084437
- TanabeMNagataniYSaitohKTakasuKOnoHPharmacological assessments of nitric oxide synthase isoforms and downstream diversity of NO signaling in the maintenance of thermal and mechanical hypersensitivity after peripheral nerve injury in miceNeuropharmacology200956370270819111753
- De AlbaJClaytonNMCollinsSDColthupPChessellIKnowlesRGGW274150, a novel and highly selective inhibitor of the inducible isoform of nitric oxide synthase (iNOS), shows analgesic effects in rat models of inflammatory and neuropathic painPain20061201–217018116360270
- StauntonCABarrett-JolleyRDjouhriLThippeswamyTInducible nitric oxide synthase inhibition by 1400W limits pain hypersensitivity in a neuropathic pain rat modelExp Physiol2018103453554429441689
- MileticGPankratzMTMileticVIncreases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany thermal hyperalgesia following chronic constriction injury in ratsPain200299349350012406525
- D’MelloRDickensonAHSpinal cord mechanisms of painBr J Anaesth2008101181618417503
