Abstract
Purpose
To review the recent literature on opioid rotation (ie, switching from one opioid drug to another or changing an opioid’s administration route) in cancer patients experiencing severe pain and to develop a novel equianalgesia table for use in routine clinical practice.
Methods
The MEDLINE database was searched with terms “cancer pain,” “opioid rotation,” “opioid switching,” “opioid ratio,” “opioid conversion ratio,” and “opioid equianalgesia” for the major opioids (morphine, oxycodone, fentanyl, and hydromorphone) and the intravenous, subcutaneous, oral, and transdermal administration routes. Selected articles were assessed for the calculated or cited opioid dose ratio, bidirectionality, and use of the oral morphine equivalent daily dose or a direct drug-to-drug ratio.
Results
Twenty publications met our selection criteria and were analyzed in detail. We did not find any large-scale, prospective, double-blind randomized controlled trial with robust design, and most of the studies assessed relatively small numbers of patients. Bidirectionality was investigated in seven studies only.
Conclusion
The updated equianalgesic table presented here incorporates the latest data and provides information on bidirectionality. Despite the daily use of equianalgesic tables, they are not based on high-level scientific evidence. More clinical research is needed on this topic.
Introduction
Over the last 20 years, opioid rotation, sometimes referred to as opioid switching, has become a common practice for optimizing pain management in many fields of medicine including oncology, postsurgical care, and palliative care.Citation1 Opioid rotation is defined as switching from one opioid drug to another or changing an opioid’s administration route. This approach may be of value if the patient starts suffering from some of the well-known adverse drug reactions associated with opioids,Citation2 such as nausea, vomiting, constipation,Citation3,Citation4 acute urinary retention, myoclonus,Citation5 respiratory depression, sedation, and cognitive impairment (including hallucinations, nightmares, agitation).Citation6–Citation9 These side effects can persist despite symptomatic treatment and may thus limit opioid use. Opioid rotation is also particularly useful if a patient becomes tolerant to a given opioid drug, develops hyperalgesia,Citation7,Citation8,Citation10–Citation12 or can no longer achieve sufficient pain control. Rotation may also be indicated by a change in the patient’s clinical state, such as kidney failure, liver failure, fever, and other disorders that may impair the pharmacokinetics or metabolism of opioid drugs. By way of an example, an opioid patch is contraindicated if a patient becomes feverish. Lastly, rotation may be required if oral administration is no longer feasible, such as in terminally ill patients, patients undergoing head and neck surgery, those with gastrostomies or nasogastric tubes, or those with incompatible gastrointestinal symptoms (eg, irrepressible vomiting).Citation10,Citation13–Citation22
Opioid rotation is particularly relevant for patients with cancer pain: Recent advances in cancer treatment increase the survival of patients with advanced cancer.Citation23 They require longer periods of opioid treatment and are therefore more exposed to opioid rotation.
Opioid rotation is closely linked to – but distinct from – the pharmacological concept of equianalgesia. Given that opioid drugs differ in their analgesic potency, changing from one to another will generally require re-evaluation of the dose if, for the patient’s comfort, equipotency is to be achieved. Equivalence ratios can be calculated from the difference between the two drugs’ respective potency curves.Citation24 In routine clinical practice, opioid rotation relies on expert-validated equianalgesia tables that are available in a variety of formats: printed tables, stand-alone computer software, Internet-based converters, etc.Citation25 However, the equivalence ratios are primarily based on data from the pharmaceutical industry or on old data that need updating or adjustment for specific disease settings (eg, for cancer pain, neurological pain). Lastly, equianalgesic ratios are not always bidirectional (a bidirectional ratio has the same value for the switch A to B as for B to A) and clinicians need to be aware that there are directional differences in opioid equivalents and that some ratios may not be “reversible” in direction.Citation26 The mechanisms underlying this phenomenon are unclear but could be related to the generation of active metabolites.Citation27
Although opioid rotation has been recommended by international expertsCitation8,Citation10,Citation13,Citation15,Citation19,Citation20,Citation24,Citation28–Citation35 and endorsed by a Cochrane Collaboration review published in 2010,Citation28 these recommendations have also been criticized by pain specialists, pointing that some conversion tables do not accurately reflect the dose ratios for which evidence is available.Citation8,Citation24,Citation25,Citation29–Citation31 Importantly, there are few publications on well-designed, randomized controlled trials (RCTs) in “ideal” study populations (ie, large number of patients, clinically stable, with stable pain).Citation36–Citation43 In a 2011 review of conversion ratios, Mercadante and CaraceniCitation44 found 31 prospective papers on this topic, but the majority of these studies had methodological flaws and were not designed to explore or demonstrate equianalgesic dose data. In fact, the primary criterion for evaluation in most of opioid studies is safety or efficacy rather than equianalgesia. Given this context, we decided to review the literature on opioid rotation with a view to provide practical suggestions based on the published evidence.
Methods
The MEDLINE (via PubMed) database was searched up until April 30, 2018.
Given the lack of MESH terms for opioid rotation, opioid switching, or the opioid ratio, we used the search terms “cancer pain,” “opioid rotation,” “opioid switching,” “opioid ratio,” “opioid conversion ratio,” and “opioid equianalgesia.” The search was limited to studies of human subjects: published systematic reviews of RCTs, RCTs, other clinical trials, meta-analyses, and case reports. There were no restrictions on year, language, minimum number of study participants, or length of follow-up.
We selected articles for further analysis if at least one of the keywords was mentioned in the title or abstract. We also searched the reference lists of articles identified using this search strategy and selected those judged to be relevant. Given that our ultimate objective is to make an inventory of ratios, which could serve as a basis for devising proposals for routine clinical practice, we focused on the major opioids (morphine [M], oxycodone [OX], fentanyl [F], and hydromorphone [HM]) and the most common administration routes (oral [po], intravenous [iv], subcutaneous [sc], intramuscular [im], transdermal [td], and suppository [su]). Studies were eligible for inclusion if they involved adult patients with chronic cancer pain and contained clear data on opioid conversion ratios and equianalgesia. Evaluation of articles was performed by two pain specialists (ET and YH). Both reviewers agreed on inclusion of the specific articles. In case of disagreement, a third reviewer (SL) decided regarding the inclusion.
After reading the full text of each selected article, we noted i) the calculated opioid ratio (when this was a trial end point) or the cited ratio (when used in the study design), ii) whether dose dependency (ie, whether the ratio differed according to the dose of the first opioid) or directionality (ie, whether the ratio differed according to the direction of the switch) had been investigated, and iii) whether the ratios were based on the oral morphine equivalent daily dose (MEDD) or on direct (drug-to-drug) ratios.
Results
Twenty publications met our selection criteria () and were analyzed in detail. Our search did not identify any meta-analyses or large RCTs. The main reasons for excluding studies were methodological limitations (ie, studies not designed to explore or demonstrate equianalgesic dose data), mixed or unclear pain etiology (cancer and noncancer pain, ie, nociceptive, neuropathic, and postsurgical pain), and rotational studies to or from molecules not included in our scope (ie, methadone, oxymorphone, tapentadol). Most of the selected studies had been published within the past 20 years.
Morphine
In most patients requiring an opioid for moderate to severe pain, morphine is both efficacious and acceptable and is the drug of choice according to the World Health Organization guidelinesCitation45 and the opioid against which all others are measured. The effective analgesic dose of morphine varies considerably partly because of individual variations in systemic bioavailability. In addition to its pharmacologically active parent compound, morphine is glucuronidated to two metabolites with potentially important differences in efficacy: morphine-6-glucuronide, with potent analgesic activity, and morphine-3-glucuronide, which lacks analgesic activity.
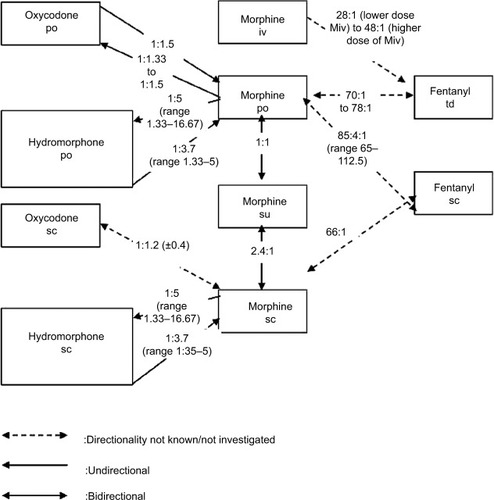
Six randomized controlled, double-blind, crossover studies, three uncontrolled prospective, and five retrospective studies reported data on conversion ratios between morphine (po, sc, su, iv) and other opioids targeted in our search ().
Table 1 Equianalgesic dose ratios for morphine vs other opioids and routes in cancer pain
Babul et al compared the efficacy and safety of a controlled-release suppository of morphine (Msu) and controlled-release morphine tablets (Mpo) in a randomized double-blind crossover study, in 27 patients with cancer pain.Citation46 There were no significant differences between treatments in overall scores for pain intensity, visual analog scale, ordinal pain intensity, sedation, and mean daily rescue analgesic use. The author concluded that Msu provides pain control comparable with that provided by Mpo when given every 12 hours at a 1:1 dose ratio.
Bruera et al compared the clinical efficacy and safety of Msu every 12 hours and subcutaneous morphine (Msc) every 4 hours for 4 days each, in a randomized double-blind crossover study using a 2.5:1 analgesic equivalence ratio.Citation47 Twenty-three patients with cancer pain completed the study, and the results showed that the mean calculated ratio of rectal to sc morphine dose was 2.4:1 (mean daily morphine dose 326+69 mg [range, 60–1,200] for Msu and 138±28 mg [range, 24–480] for Msc).
Kalso and Vainio switched 20 patients with uncontrolled cancer pain who were taking “weak” opioids (codeine, dextropropoxyphene, etc) to intravenous morphine or oxycodone in a randomized, double-blinded, crossover design.Citation48 Patients first titrated themselves pain-free using IV patient-controlled analgesia for 48 hours. After 48 hours, they were switched to the oral form of either morphine or oxycodone. Following the switch in route, patients were able to adjust their oral doses to improve pain control during 48 hours. This phase was followed by the crossover phase to the alternative opioid. The median calculated oral:IV potency ratios (giving comparable analgesia) were 0.31 for morphine and 0.70 for oxycodone.
Five studies compared morphine administered orally,Citation49–Citation51 subcutaneously,Citation36 or intravenouslyCitation52 vs subcutaneous (Fsc) or transdermal fentanyl (Ftd). Two crossover studies compared Mpo with oxycodone per os (OXpo)Citation38,Citation53 and one compared Msc with OXsc.Citation32 Three studies assessed the analgesic equivalence ratio between morphine (po or sc) and hydromorphone (po, sc, or iv).Citation34,Citation54,Citation55
These studies are detailed below, and the results are summarized in and .
Fentanyl
Fentanyl is a phenylpiperidine opioid agonist. Its low molecular weight is low and high lipid solubility provides excellent bio-availability (90%) via the transdermal or transmucosal routes, although the oral bioavailability is low (<30%). Fentanyl is predominantly converted by CYP3A4-mediated N-dealkylation to norfentanyl, a nontoxic and inactive metabolite. The mean elimination half-life is approximately 17 hours. According to the summary of product characteristics (fentanyl 50 μg/mL solution for injection), a dose of 100 μg (2 mL) per day transdermal has an analgesic action that is comparable with 10 mg oral morphine.
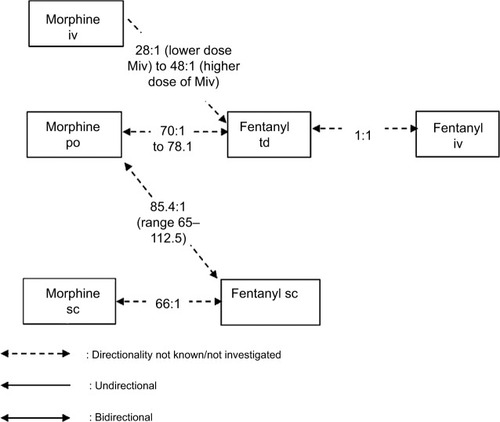
One randomized controlled, double-blind, crossover study, three prospective studies, and three retrospective studies reported data on conversion ratios between fentanyl (td, sc, iv) and other opioids targeted in our search (). These studies demonstrate considerable variability in conversion ratios, both within and across studies, and underscore the need for caution in applying ratios during opioid rotation from and to fentanyl.
Table 2 Equianalgesic dose ratios for fentanyl vs other opioids or routes in cancer pain
Two published studies on the dose conversion ratio between the transdermal fentanyl patch (Ftd) and morphine preparations have been identified. Kato et al published in 2004 the results of their retrospective observational study of 144 patients with chronic pain of cancer origin and with difficulty tolerating oral morphine, undergoing rotation to fentanyl patch.Citation49 The authors calculated the conversion ratio from the regression equation obtained from the daily oral morphine dose prior to rotation and daily fentanyl patch delivered dose in subjects undergoing opioid rotation from oral morphine to fentanyl patch (R2=0.68), reporting a daily oral morphine dose/daily fentanyl patch delivered dose ratio of 78:1.
Donner et al performed a multicenter study with 98 patients (38 patients were treated according to protocol and their data were analyzed) with controlled cancer pain with the aim of studying the most convenient conversion ratio when switching from morphine to transdermal fentanyl.Citation51 Initial doses were calculated with the Mpo:Ftd ratio of 100:1. The regression analysis comparing fentanyl dosages at the end of the study (day 15) with the morphine dosages at day 1 demonstrated a mean Mpo:Ftd ratio of 70:1 (R2=0.82). This result implicates that a calculation table with a ratio 100:1 is by 30% subequianalgesic but may be appropriate with allowance of a safety margin.
Watanabe et al reviewed the charts of 22 patients with cancer-related pain who were switched from a variety of opioids to subcutaneous fentanyl by continuous infusion.Citation50 In 13 patients who were switched from morphine or hydromorphone and reached dose stability (defined as no dose changes and no more than two rescue doses per day for 48 hours), the median relative potency Mpo:Fsc ratio (n=4 patients who stabilized) was 85.4:1 (range 65:1–112.5:1). The authors noted a wide range of dose ratios and suggested that a more cautious morphine to fentanyl potency ratio of 100:1 may be advisable and acknowledged that the small size of the study was a major limitation.
In a prospective study of 23 hospice cancer patients (published in 1999), Hunt et al compared subcutaneous morphine to subcutaneous fentanyl in a trial designed to evaluate pain control and side effects.Citation36 In this study, they used and then confirmed a conversion ratio of 10 mg Msc:150 μg Fsc. This equates to an Msc:Fsc conversion ratio of 66:1. Although this was a crossover double-blind trial, bidirectionality was not investigated. Furthermore, the crossover was performed early (on day 3), which complicated the interpretation of adverse events, and the small number of subjects in this report limits the conclusions that can be made.
Kawano et al examined dose conversion ratios in patients undergoing opioid rotation from morphine injection (continuous IV administration [Miv]) to transdermal fentanyl patches in a prospective observational study of 45 patients with chronic pain of cancer origin.Citation52 The results supported the feasibility of rotation from continuous endovenous morphine to fentanyl patches, with a conversion ratio of 50:1, but also showed that fentanyl doses decreased with the equivalent target morphine dose, ratio ranging from 28:1 (lower dose Miv) to 48:1 (higher dose Miv). These results suggest that in opioid rotation from low dose, 50:1 is not enough for the fentanyl patch. Similarly, Matsumura et al reported retrospective data from 122 patients, suggesting that the typical conversion OXpo:Ftd ratio was 95:1 but was significantly reduced in patients taking a daily oral morphine equivalent dose of <45 mg/d and in patients with poor pain control to 52:1 and 64:1, respectively (study using MEDD for conversion, not selected in our analysis).Citation56
In 2016, Reddy et al reported a ratio of 1:100 for Fsc mg/d to MEDD (n=47 cancer patients)Citation57 and 100:1 MEDD to Fsc mg/d (n=129) in two retrospective studies.Citation58 As these ratios had been calculated using the MEDD conversion method, we did not retain these results for our analysis.
Two prospective studies evaluated the conversion from IV to transdermal fentanyl. Results demonstrated that this conversion can be accomplished safely and effectively using a 1:1 (Fiv:Ftd) conversion ratio. Two different conversion strategies are proposed. Kornick et al used a 12-hour conversion method in 15 patients with cancer pain: the fentanyl infusion dose was decreased in half 6 hours after patch application, then completely stopped after 12 hours.Citation59 Samala et al proposed a continuous 6-hour overlap method as a safe and effective alternative strategy, which may be simpler and may eliminate Fiv dose adjustments during conversion to Ftd.Citation60
The above findings are summarized in and .
Oxycodone
Oxycodone is a synthetic derivative of thebaine and is structurally related to codeine. Oxycodone is well absorbed when orally administered, and compared with morphine, it has a higher oral bioavailability (up to 87%). The central opioid effects of oxycodone are governed primarily by the parent drug, with a negligible contribution from its circulating oxidative and reductive metabolites. The relative oral bioavailability of controlled-release to immediate-release oral dosage forms is 100%.Citation61 According to the summary of product characteristics (oxycodone prolonged release tablets), patients receiving oral morphine before oxycodone therapy should have their daily dose based on the following ratio: 10 mg of oral oxycodone is equivalent to 20 mg of oral morphine.
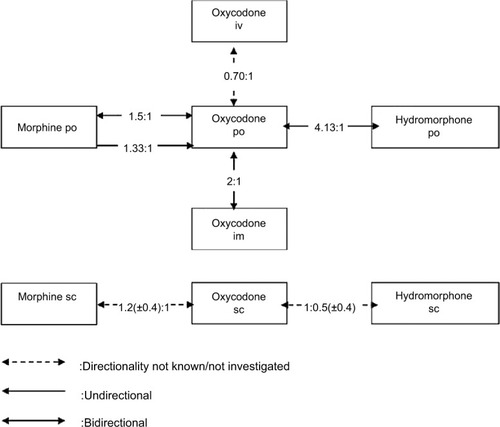
We identified five double-blind crossover studies and one prospective observational study with relevant data regarding equianalgesic conversion ratios to and from oxycodone in cancer pain ().
Table 3 Equianalgesic dose ratios for oxycodone vs other opioids or routes in cancer pain
Two double-blind, randomized, crossover studies have attempted to measure the relative potency between oral oxycodone and oral morphine, using an initial 1:1.5 ratio. Heiskanen and Kalso confirmed that the total opioid consumption ratio of oxycodone to morphine was 1:1.5 when oxycodone was administered first but found that the appropriate ratio was 1.33:1 when oxycodone was administered after morphine.Citation53 One year later, Bruera et al tested and confirmed a 1:1.5 conversion ratio between controlled-release oxycodone and controlled-release morphine but did not find oxycodone-morphine’s directionality established by Heiskanen and Kalso.Citation38
Beaver et al published in 1978 the results of a double-blind crossover comparison of oral with intramuscular oxycodone.Citation62 In total, the data showed that oral oxycodone is half as potent as intramuscular oxycodone.
Our selection also included Kalso and Vainio studyCitation48 (presented above, in the “Morphine” section), and two other trialsCitation32,Citation37 comparing oxycodone and hydromorphone, which are discussed below.
The above findings are summarized in and .
Hydromorphone
Hydromorphone is a hydrogenated ketone analogue of morphine. As with morphine, there is great interindividual variation in oral bioavailability (10%–65%). Some metabolites may have greater analgesic activity than hydromorphone itself but are unlikely to contribute to the pharmacological activity of hydromorphone.Citation63 According to the summary of product characteristics (hydromorphone hydrochloride 1.3 mg and 2.6 mg capsules), 1.3 mg of hydromorphone has an efficacy approximately equivalent to 10 mg of morphine given orally.
We identified one double-blind crossover study, three prospective studies, and three retrospective studies with relevant data regarding equianalgesic conversion ratios to and from hydromorphone (po, sc) in cancer pain (). In a study by Wallace et al, patients with chronic cancer pain were enrolled in an open-label conversion trial, from various opioids to HMpo, using an oral M:HM ratio of 5:1 for a MEDD conversion.Citation40 Of the 127 patients who received HMpo, 85 (67%) completed the study. The majority of patients who achieved stabilization did so without titration or with just one or two titration steps. The conclusion was that the 5:1 ratio was safe and effective when converting from morphine to hydromorphone. Wallace et al did not evaluate converting patients back from hydromorphone to oral morphine.
Table 4 Equianalgesic dose ratios for hydromorphone vs other opioids or routes in cancer pain
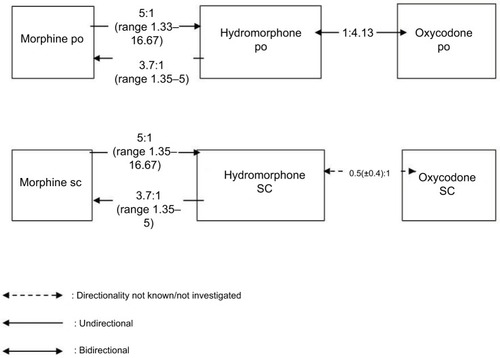
Two retrospective studies (published in 1996 by Bruera et al and in 1997 by Lawlor et al) investigated the ratios for HMpo, Mpo, and Msc and their directionality.Citation27,Citation34 Both studies found that the po ratio was not bidirectional. According to Bruera et al, the ratio for Mpo:HMpo was 5.33:1 and the ratio for HMpo:Mpo was 1:3.57. The results of Lawlor et al were similar: Expressing all ratios as M:HM, the median dose ratios (lower-upper quartiles) for sc and po rotations were 4.92 (4.1–5.9) vs 5.76 (4.9–5.8) for M to HM (P=0.28, NS) and 4.0 (3.1–4.8) vs 3.45 (2.8–4.2) for HM to M (P=0.4, NS), respectively. These data suggest that HM is five times more potent than M when given second (M to HM) but is only 3.7 times more potent when given first (HM to M). The authors therefore recommended a ratio of 5 for M:HM in rotating from M to HM and ratio of 3.7 for M:HM when rotating from HM to M in patients exposed to chronic intake of these opioids. This directional difference in potency between M and HM is accepted by expert groups and may apply to both oral and parenteral dosing and may be independent of prior opioid exposure.Citation8,Citation24,Citation35,Citation64,Citation65
Hagen and Babul compared the clinical efficacy and safety of an OXpo with that of HMpo in patients with stable cancer pain in a double-blind crossover study (31 patients completed the study).Citation37 Based on their results, the calculated ratio for HMpo:OXpo is 1:4.13 and is bidirectional.
Gagnon et al prospectively collected the data of cancer patients who were rotated from another strong opioid to OXsc to establish conversion ratios.Citation32 For the 11 patients who were switched from HMsc to OXsc, the authors reported an equianalgesic ratio of 0.5 (±0.4):1.
Inoue et al investigated the efficacy and safety of switching from oral morphine to hydromorphone immediate-release tablets at the HM to M conversion ratio of 1:5 or 1:8 in cancer patients with adequate pain control with oral morphine (60 or 90 mg/d).Citation54 This multicenter, active-controlled, randomized, double-blind, parallel-group, comparative study enrolled 30 adults patients in conversion ratio 1:5 group and 40 patients in conversion ratio 1:8 group. The results showed no statistical difference in pain control between the conversion ratio groups (P=0.1298), and no intergroup difference was observed in the incidence of adverse events or serious adverse events.
The authors concluded that a conversion ratio between 1:5 and 1:8 is considered clinically appropriate for a switch from oral morphine to oral hydromorphone for pain control in cancer patients.
Reddy et al retrospectively reviewed the charts of consecutive inpatient palliative care consultations (2010–2014), in order to determine the conversion ratio from intravenous hydromorphone to oral hydromorphone and other oral opioids.Citation55 From that cohort, they reviewed patients who were subsequently converted to oral hydromorphone (extended or immediate release or both) or to oral formulations of morphine and oxycodone and successfully discharged home without readmission within 1 week for uncontrolled pain. Among 394 patients on HMiv, 147 underwent conversion to HMpo and 247 underwent rotation to Mpo (163) or OXpo (84). Conversion ratio was defined as 24-hour oral opioid dose before discharge/net IV hydromorphone dose for each patient.
They found that the median (interquartile range) conversion ratio from HMiv to HMpo was 2.5 (2.14–2.75) with correlation of 0.95 (P<0.0001). They also found that a dose-dependency as the conversion ratio from HMiv to HMpo was significantly lower in patients receiving ≤30 mg of hydromorphone/d (2.07 vs 2.5).
When patients were rotated to other opioids, the observed equianalgesic ratio from HMiv to OXpo was 1:8.06 and the ratio from HMiv to Mpo was 1:11. The ratios were significantly lower for doses of HMiv ≥30 mg/d. This finding may suggest possible hyperalgesia with high doses of hydromorphone, wherein a lower conversion ratio to other opioids would be required.
The above findings are summarized in and .
Other opioids
The equivalence ratios for methadone will not be discussed here, as they have already been extensively studied. It has been found that the ratio varies markedly as a function of the initial opioid dose (expressed as the oral MEDD) and the reason for rotation (side effects, uncontrolled pain, tolerance, cost, etc).Citation21,Citation31,Citation66 Other drugs (such as buprenorphine, hydrocodone, tapentadol, meperidine, and step 2 analgesics of the World Health Organization analgesic ladder) are not reviewed here because they are not part of the usual armamentarium or are too recent.
The use of short-acting transmucosal fentanyl warrants comment. In France, the indication for this formulation is restricted to breakthrough cancer pain.Citation67 In fact, the dose required to achieve complete analgesia is obtained by titration, which has to be performed iteratively and is therefore not taken into account in the estimation of the equivalent dose. This methodology is subject to debate and illustrates the difficulty of performing well-designed, scientifically robust studies in the field of pain.
Discussion
Obstacles to clinical and pharmacological research on pain and analgesics
Although many clinical trials have been published, clinicians should be aware that research in this field faces a large number of obstacles. We have classified these limitations into three groups: limitations related to pharmacological studies of equivalence ratios, those related to the design of clinical trials with rotation, and those related to the characteristics of the patients and their pain.
Limitations related to the assessment of pharmacological ratios
The most reliable method for ratio calculation is based on the evaluation of pain after a single injection of an opioid.Citation68 In general, this ratio is calculated in a postsurgical setting, where patients are usually opioid-naïve. However, this situation does not match everyday practice in cancer pain management, where the patients are rarely opioid-naïve and long-term treatment is necessary.Citation24,Citation36,Citation69
Some researchers consider that the ratio for a given opioid may change over time (ie, during long-term treatment).Citation70 This constitutes a further argument against the “single injection” method of ratio calculation.
The equianalgesic ratio for hydromorphone is unidirectional.Citation34 It is well-known that the ratio for the switch from hydromorphone to morphine is lower than for the switch in the opposition direction. However, directionality has rarely been investigated for other opioids.
In general, the equianalgesic ratios proposed in the literature are median values that do not always confer reliable equivalence; for example, the ratio for switching from methadone to oral morphine depends on the initial opioid dose. In such a case, using a median ratio would be a mistake. The dose-dependency may apply to trans-dermal fentanyl and perhaps other opioids, as this has been recently reported for intravenous hydromorphone at doses ≥30 mg/d.Citation55 This must be tested.
The published equianalgesic ratios are often derived from pharmaceutical industry data and have not been confirmed by clinical trials or studies of routine clinical practice.
MEDD is often used as the common denominator for opioid rotation, rather than direct drug-to-drug ratios, but this method may be less safe in patients receiving high doses of opioids. For example, patients switching from high-dose oxycodone to transdermal fentanyl will need two approximations (to MEDD and from MEDD) to calculate the final dose.
Limitations related to trial design
We did not find any large-scale, prospective, double-blind RCT with robust design. Most of the trials in this field studied small numbers of patients and/or were retrospective.
A crossover design has occasionally been used. Unfortunately, the crossover was usually performed early (eg, on day 3),Citation36 which complicates the interpretation of side effects.
The presence or absence of concomitant nonopioid analgesic use is not always mentioned in study publications, even though an impact on pain control is highly probable.
The assessment of a small number of patients may lead to extremely wide dosage ranges (eg, a mean [range] level of 96 mg [4.5–660] for oxycodone).Citation32 This may constitute a source of bias when calculating an equianalgesic ratio.
Limitations related to the characteristics of the patients and their pain
Very few studies have examined primary care for cancer patients, despite the fact that primary care physicians also prescribe opioids.Citation71,Citation72
Many variability factors complicate the interpretation of equianalgesic ratios, and not all of these factors can be accounted for in a pain analysis. Gender, ethnic origin,Citation73 clinical history, changes over time in pain,Citation74 psychological factors,Citation75,Citation76 opioid-naïve status, inter-and intrasubject variability in pharmacodynamics and pharmacokinetics,Citation21,Citation22 drug–drug interactions,Citation8,Citation24,Citation29 and genetic factorsCitation21 all influence pain perception.Citation77
The pain profile may depend on the disease, so appropriate postsurgical pain relief may not be relevant for cancer pain.
Increased pain levels due to disease progression also complicate the calculation of equianalgesic ratios because the latter requires stable pain. The few studies that attempted to tackle this limitation have seen their patient population collapse.Citation51
A number of factors seem to be significantly associated with interindividual variability in the conversion ratios of fentanyl in opioid switching: For instance, higher modified Glasgow Prognostic Score, breast cancer, total protein, alanine aminotransferase, advanced age, and male sex have been identified as significant predictors of a need for higher dose transdermal fentanyl.Citation78,Citation79
Expert guidelines on opioid rotation
In view of the abovementioned limitations, the pain specialist may have trouble deciding on the best choice for clinical practice. Hence, a number of general rules and recommendations have been suggested by expert panels, with a view to improve overall safety and effectiveness. However, these recommendations are not supported by high levels of scientific evidence. Equianalgesia tables are nevertheless useful in routine practice and physicians must continue to use them, while bearing in mind the inherent limitations of these tables.Citation10,Citation24,Citation29,Citation80 Fine and PortenoyCitation8 proposed guidelines for safe, two-step rotations with the available ratios:
Step 1: A 25% to 50% dose decrease in the new opioid (relative to the dose suggested by the equianalgesic table), except when the latter is methadone (with a decrease of 75%–90%), transdermal fentanyl (no decrease), or short-acting transmucosal fentanyl (for which titration is essential).
Step 2: Apply an additional increase or decrease of 15%–30%, depending on the clinical context (hyperalgesia, sedation, age, etc).
In 2012, Webster and FineCitation81 suggested a potentially safer method of opioid rotation that obviates the need to use a conversion table: The dose of the original opioid is slowly decreased (by about 10%–25% per week), while the new opioid dose is slowly titrated beginning at a dose that would normally be used in an opioid-naïve patient or at the lowest available dose for the formulation. Webster and Fine recommend providing sufficient immediate-release opioid throughout the rotation to prevent withdrawal and/or increased pain if the dosing changes prove insufficient. In most instances, the complete switch can occur within 3–4 weeks.
A practical tool for applying clinically observed ratios
In order to take the abovementioned limitations into account, we propose the use of a table that takes into consideration the direction of the switching (ie, from the initial opioid to the target opioid) and calculating the direct dose equivalence without having to link to the MEDD (). The table is based on the clinical studies reviewed here. The principle is the same as a “distance chart”. The reader should note the absence of published data for many pairs of opioids.
Table 5 Ratios for use in routine clinical practice
Conclusion
Although most experts recommend the use of equianalgesia tables (due to their ease of use, clear clinical value, and good long-term safety record), they also acknowledge that currently available tools are not ideal. Hence, we consider that this aspect of pain medicine warrants a solid scientific basis. Although the table presented in this article is far from perfect, it does attempt to incorporate scientific uncertainties and data ranges so that the practitioner is aware of the limitations of this approach and can decide on possible alternatives (based on the latest data). The table also provides information on directionality (when available), which has rarely been investigated and may not apply to all opioids. Completion and optimization of the table will be a difficult but worthwhile task.
Acknowledgments
The authors acknowledge the assistance of Pascal Fangio (Poissy Hospital, Poissy, France) and Anna Patrikidou (Institut Gustave Roussy, Villejuif, France) in the development of the present review’s search strategy. The authors thank Philippe Mayran of PM Sante (Garches, France) for providing medical writing support, which was funded by Mundipharma, Paris, France.
Disclosure
Yacine Hadjiat is an employee of Mundipharma and reports no other conflicts of interest in this work. Erwan Treillet and Sophie Laurent report no conflicts of interest in this work.
References
- MercadanteSBrueraEOpioid switching in cancer pain: From the beginning to nowadaysCrit Rev Oncol Hematol20169924124826806145
- LawlorPGBrueraESide-effects of opioids in chronic pain treatmentCurr Opin Anaesthesiol199811553954517013271
- WaltersJBMontagniniMCurrent concepts in the management of opioid-induced constipationJ Opioid Manag20106643544421269005
- ReimerKHoppMZenzMMeeting the challenges of opioid-induced constipation in chronic pain management - a novel approachPharmacology2009831101718957874
- MccannSYakshTLvon GuntenCFCorrelation between myoclonus and the 3-glucuronide metabolites in patients treated with morphine or hydromorphone: A pilot studyJ Opioid Manag201062879420481173
- de SchepperHUCremoniniFParkMICamilleriMOpioids and the gut: pharmacology and current clinical experienceNeurogastroenterol Motil200416438339415305992
- InturrisiCEClinical pharmacology of opioids for painClin J Pain200218SupplementS3S1312479250
- FinePGPortenoyRKEstablishing “Best Practices” for opioid rotation: conclusions of an expert panelJ Pain Symptom Manage200938341842519735902
- KuritaGPLundorffLPimentaCASjøgrenPThe cognitive effects of opioids in cancer: a systematic reviewSupport Care Cancer2009171112118762994
- MercadanteSBrueraEOpioid switching: A systematic and critical reviewCancer Treat Rev200632430431516624490
- SilvermanSMOpioid induced hyperalgesia: clinical implications for the pain practitionerPain Physician200912367968419461836
- MitraSOpioid-induced hyperalgesia: pathophysiology and clinical implicationsJ Opioid Manag20084312313018717507
- Müller-BuschHCLindenaGTietzeKWoskanjanSOpioid switch in palliative care, opioid choice by clinical need and opioid availabilityEur J Pain20059557157916139186
- de Leon-CasasolaOACurrent developments in opioid therapy for management of cancer painClin J Pain200824Supplement 10S3S718418225
- MercadanteSOpioid rotation for cancer pain: rationale and clinical aspectsCancer19998691856186610547561
- ChernyNJChangVFragerGOpioid pharmacotherapy in the management of cancer pain: a survey of strategies used by pain physicians for the selection of analgesic drugs and routes of administrationCancer1995767128312938630910
- LevyMHPharmacologic treatment of cancer painN Engl J Med Overseas Ed19963351511241132
- MaddocksISomogyiAAbbottFHayballPParkerDAttenuation of morphine-induced delirium in palliative care by substitution with infusion of oxycodoneJ Pain Symptom Manage19961231821898803381
- MercadanteSFerreraPVillariPCasuccioAIntravaiaGMangioneSFrequency, indications, outcomes, and predictive factors of opioid switching in an acute palliative care unitJ Pain Symptom Manage200937463264119345298
- RipamontiCDickersonEDStrategies for the treatment of cancer pain in the new millenniumDrugs200161795597711434451
- SmithHSVariations in opioid responsivenessPain Physician200811223724818354715
- SlatkinNEOpioid switching and rotation in primary care: implementation and clinical utilityCurr Med Res Opin20092592133215019601703
- MillerKDSiegelRLLinCCCancer treatment and survivorship statistics, 2016CA Cancer J Clin201666427128927253694
- KnotkovaHFinePGPortenoyRKOpioid rotation: the science and the limitations of the equianalgesic dose tableJ Pain Symptom Manage200938342643919735903
- BerdineHJNesbitSAEquianalgesic dosing of opioidsJ Pain Palliat Care Pharmacother20062047984
- DavisMPCancer painOlverINThe MASCC Textbook of Cancer Supportive Care and SurvivorshipBoston, MASpringer US20111122
- LawlorPTurnerKHansonJBrueraEDose ratio between morphine and hydromorphone in patients with cancer pain: a retrospective studyPain1997721–279859272790
- QuigleyCOpioid switching to improve pain relief and drug tolerabilityCochrane Database Syst Rev20043CD004847
- AndersonRSaiersJHAbramSSchlichtCAccuracy in equi-analgesic dosing. conversion dilemmasJ Pain Symptom Manage200121539740611369161
- PatanwalaAEDubyJWatersDErstadBLOpioid conversions in acute careAnn Pharmacother200741225526717299011
- VissersKCPBesseKHansGDevulderJMorlionBOpioid rotation in the management of chronic pain: where is the evidence?Pain Practice2010102859320070552
- GagnonBBielechMWatanabeSWalkerPHansonJBrueraEThe use of intermittent subcutaneous injections of oxycodone for opioid rotation in patients with cancer painSupport Care Cancer19997426527010423053
- NarabayashiMSaijoYTakenoshitaSOpioid rotation from oral morphine to oral oxycodone in cancer patients with intolerable adverse effects: an open-label trialJpn J Clin Oncol200838429630418326541
- BrueraEPereiraJWatanabeSBelzileMKuehnNHansonJOpioid rotation in patients with cancer pain. A retrospective comparison of dose ratios between methadone, hydromorphone, and morphineCancer19967848528578756381
- de StoutzNDBrueraESuarez-AlmazorMOpioid rotation for toxicity reduction in terminal cancer patientsJ Pain Symptom Manage19951053783847673770
- HuntRFazekasBThorneDBrooksbankMA comparison of subcutaneous morphine and fentanyl in hospice cancer patientsJ Pain Symptom Manage199918211111910484858
- HagenNABabulNComparative clinical efficacy and safety of a novel controlled-release oxycodone formulation and controlled-release hydromorphone in the treatment of cancer painCancer1997797142814379083166
- BrueraEBelzileMPituskinERandomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer painJ Clin Oncol19981610322232299779695
- WirzSWartenbergHCElsenCWittmannMDiederichsMNadstawekJManaging cancer pain and symptoms of outpatients by rotation to sustained-release hydromorphone: a prospective clinical trialClin J Pain200622977077517057558
- WallaceMRauckRLMoulinDThipphawongJKhannaSTudorICConversion from standard opioid therapy to once-daily oral extended-release hydromorphone in patients with chronic cancer painJ Int Med Res200836234335218380946
- AhmedzaiSBrooksDTransdermal fentanyl versus sustained-release oral morphine in cancer pain: preference, efficacy, and quality of life. The TTS-Fentanyl Comparative Trial GroupJ Pain Symptom Manage19971352542619185430
- MoritaTTakigawaCOnishiHOpioid rotation from morphine to fentanyl in delirious cancer patients: an open-label trialJ Pain Symptom Manage20053019610316043013
- ClemensKEKlaschikEClinical experience with transdermal and orally administered opioids in palliative care patients-a retrospective studyJpn J Clin Oncol200737430230917519302
- MercadanteSCaraceniAConversion ratios for opioid switching in the treatment of cancer pain: a systematic reviewPalliat Med201125550451521708857
- World Health OrganizationCancer Pain Relief: With a Guide to Opioid Availability2nd edGeneva, SwitzerlandWorld Health Organization2009
- BabulNProvencherLLabergeFHarsanyiZMoulinDComparative efficacy and safety of controlled-release morphine suppositories and tablets in cancer painJ Clin Pharmacol199838174819597563
- BrueraEFainsingerRSpachynskiKBabulNHarsanyiZDarkeACClinical efficacy and safety of a novel controlled-release morphine suppository and subcutaneous morphine in cancer pain: a randomized evaluationJ Clin Oncol1995136152015277751901
- KalsoEVainioAMorphine and oxycodone hydrochloride in the management of cancer painClin Pharmacol Ther19904756396462188774
- KatoKMizakiTYamazakiSA study of transdermal fentanyl in cancer pain at Aichi-Cancer CenterYakugaku Zasshi2004124528729115118241
- WatanabeSPereiraJHansonJBrueraEFentanyl by continuous subcutaneous infusion for the management of cancer pain: a retrospective studyJ Pain Symptom Manage19981653233269846027
- DonnerBZenzMTrybaMStrumpfMDirect conversion from oral morphine to transdermal fentanyl: a multicenter study in patients with cancer painPain19966435275348783318
- KawanoCHirayamaTKuroyamaMDose conversion in opioid rotation from continuous intravenous infusion of morphine hydrochloride injection to fentanyl patch in the management of cancer painYakugaku Zasshi2011131346346721372544
- HeiskanenTKalsoEControlled-release oxycodone and morphine in cancer related painPain199773137459414055
- InoueSSaitoYTsunetoSArugaEOgataTUemoriMA double-blind, randomized comparative study to investigate the morphine to hydromorphone conversion ratio in Japanese cancer patientsJpn J Clin Oncol201848544244929635632
- ReddyAVidalMStephenSThe conversion ratio from intravenous hydromorphone to oral opioids in cancer patientsJ Pain Symptom Manage201754328028828711751
- MatsumuraCYamadaMFujiharaSChisakiYTakahashiKYanoYIndication of adequate transdermal fentanyl dose in opioid switching from oral oxycodone in patients with cancerAm J Hosp Palliat Med2016332109114
- ReddyAYennurajalingamSReddySThe opioid rotation ratio from transdermal fentanyl to “Strong” opioids in patients with cancer painJ Pain Symptom Manage20165161040104526826675
- ReddyATayjasanantSHaiderAThe opioid rotation ratio of strong opioids to transdermal fentanyl in cancer patientsCancer2016122114915626451687
- KornickCASantiago-PalmaJKhojainovaNPrimaveraLHPayneRManfrediPLA safe and effective method for converting cancer patients from intravenous to transdermal fentanylCancer200192123056306111753984
- SamalaRVBloiseRDavisMPEfficacy and safety of a six-hour continuous overlap method for converting intravenous to transdermal fentanyl in cancer painJ Pain Symptom Manage201448113213624291296
- ColuzziFMattiaCOxycodone. Pharmacological profile and clinical data in chronic pain managementMinerva Anestesiol2005717-845146016012419
- BeaverWTWallensteinSLRogersAHoudeRWAnalgesic studies of codeine and oxycodone in patients with cancer. I. Comparisons of oral with intramuscular codeine and of oral with intramuscular oxycodoneJ Pharmacol Exp Ther1978207192100359779
- SarhillNWalshDNelsonKAHydromorphone: pharmacology and clinical applications in cancer patientsSupport Care Cancer200192849611305075
- ChouRFanciulloGJFinePGClinical guidelines for the use of chronic opioid therapy in chronic noncancer painJ Pain200910211313019187889
- CaraceniAHanksGKaasaSUse of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPCLancet Oncol2012132e58e6822300860
- WeschulesDJBainKTRicheimerSActual and potential drug interactions associated with methadonePain Medicine20089331534418386306
- ZeppetellaGO’DohertyCACollinsSPrevalence and characteristics of breakthrough pain in cancer patients admitted to a hospiceJ Pain Symptom Manage2000202879210989246
- EddyNBLeeLEJrThe analgesic equivalence to morphine and relative side action liability of oxymorphone (14-hydroxydihydro morphinone)J Pharmacol Exp Ther1959125211612113631610
- WoodhouseAHobbesAFTMatherLEGibsonMA comparison of morphine, pethidine and fentanyl in the postsurgical patient-controlled analgesia environmentPain19966411151218867253
- DunbarPJChapmanRCBuckleyPFGavrinJRClinical analgesic equivalence for morphine and hydromorphone with prolonged PCAPain19966822652709121813
- ReidMCHendersonCRPapaleontiouMCharacteristics of older adults receiving opioids in primary care: treatment duration and outcomesPain Medicine20101171063107120642732
- BuckleyDICalvertJFLapidusJAMorrisCDChronic opioid therapy and preventive services in rural primary care: an Oregon rural practice-based research network studyAnn Fam Med20108323724420458107
- VallerandAHHasenauSTemplinTCollins-BohlerDDisparities between black and white patients with cancer pain: the effect of perception of control over painPain Medicine20056324225015972088
- WoolMSMorVA multidimensional model for understanding cancer painCancer Invest200523872773416377591
- SyrjalaKLChapkoMEEvidence for a biopsychosocial model of cancer treatment-related painPain199561169797644251
- KeefeFJAbernethyAPC CampbellLPsychological approaches to understanding and treating disease-related painAnnu Rev Psychol200556160163015709948
- HanksGWReidCContribution to variability in response to opioidsSupport Care Cancer200513314515215761703
- JiaSSShangLMeLZhaoDMWhXWangYQModified Glasgow prognostic score predicting high conversion ratio in opioid switching from oral oxycodone to transdermal fentanyl in patients with cancer painInt J Clin Exp Med2015857606761226221306
- KanbayashiYHosokawaTOkamotoKFactors predicting requirement of high-dose transdermal fentanyl in opioid switching from oral morphine or oxycodone in patients with cancer painClin J Pain201127866466721471811
- PereiraJLawlorPViganoADorganMBrueraEEquianalgesic dose ratios for opioids. a critical review and proposals for long-term dosingJ Pain Symptom Manage200122267268711495714
- WebsterLRFinePGOverdose deaths demand a new paradigm for opioid rotationPain Med201213457157422458858