Abstract
Purpose
Generalized pain hypersensitivity is frequently observed in chronic pain conditions. Currently, identification is based on expert clinical opinion, and in very few cases combined with quantitative sensory testing. The objectives of this study were to develop and evaluate a short self-report measure of generalized pain hypersensitivity: a generalized pain questionnaire (GPQ).
Methods
Items for the GPQ were developed based on a literature review, followed by an interview study with ten rheumatic patients with suspected pain hypersensitivity. We examined the psychometric properties of the preliminary items in a sample of 212 outpatients suffering from either fibromyalgia (FM; n=98) or rheumatoid arthritis (n=114). Additionally, self-reported data were gathered on sociodemographics, fibromyalgia-survey criteria, health status, and neuropathic-like pain features.
Results
Mokken-scale analyses demonstrated a unidimensional seven-item scale with strong homogeneity (H=0.65) and high reliability (r=0.90). Correlations between total GPQ scores and relevant external measures, such as the FM-survey criteria and neuropathic-like pain features, were consistent with a priori expectations, supporting its external construct validity. Furthermore, the GPQ had good accuracy in distinguishing between patients with FM (generally assumed to be the result of central nervous system hypersensitization) and patients with RA (assumed to result mostly in local nociceptive or inflammatory pain), with an area under the receiver-operating characteristic curve of 0.89. A cutoff value >10 had the highest combination of sensitivity (82.7%) and specificity (77.2%).
Conclusion
The GPQ is psychometrically sound and appears promising for measuring the presence and severity of generalized pain hypersensitivity in chronic pain patients.
Introduction
Chronic pain is a major health problem with an estimated prevalence of 17%–31% in developed countries.Citation1–Citation3 Traditionally, chronic pain has been classified into two broad categories: pain in response to peripheral tissue damage or inflammation, and pain associated with damage to or a lesion of the nervous system (neuropathic pain).Citation4,Citation5 Both are characterized by pain hypersensitivity at the injury site and in adjacent tissue as a manifestation of neuronal plasticity.Citation6–Citation8
Many patients with chronic pain, however, show symptoms of generalized pain hypersensitivity. Using quantitative sensory testing (QST), Schliessbach et alCitation9 and Curatolo et alCitation10 found that generalized pain hypersensitivity is common in patients with various chronic pain conditions. It is generally thought that generalized pain hypersensitivity is a manifestation of central sensitization.Citation11,Citation12 Central sensitization is an increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input.Citation6,Citation13 It is characterized by enhanced spontaneous neuronal activity, enlarged receptive-field areas, and an increase in responses evoked by primary afferent fibers.Citation14 Central sensitization is an adaptive physiological process that occurs in everybody, and normally it stops after termination of the nociceptive afferent input and returns to baseline. It is hypothesized that in chronic pain patients, this adaptive process persists, despite elimination of most nociceptive input.Citation15 Additionally, endogenous descending pain facilitatory modulatory systems from the brain contribute to pain hypersensitivity.Citation16 These mechanisms are some of the most important currently known; however, knowledge about pain mechanisms is constantly evolving. Generalized pain hypersensitivity typically manifests as allodynia, (secondary) hyperalgesia, and aftersensations,Citation11,Citation12 and is a characteristic feature of fibromyalgia (FM).Citation12,Citation17–Citation23
Accurate pain measurement and classification of clinical pain phenotypes is essential for adequate pain treatment. In clinical practice, the recognition of generalized pain hypersensitivity is generally based on the clinician’s evaluation of symptoms and medical history, occasionally combined with QST using thermal, electrical, and/or mechanical stimuli.Citation22–Citation25 QST methods, however, are complex and time-consuming, and thus often impractical in daily clinical care or research settings.Citation26 Consequently, a self-report measure of generalized pain hypersensitivity could provide a time-efficient method for the initial identification of patients with elevated pain sensitivity. Moreover, such a measure could be useful for evaluating efficacy of treatments specifically targeting generalized hypersensitivity to pain.
The objectives of this study were to develop and evaluate a short and easy-to-use self-report instrument for identifying the presence and intensity of generalized pain hypersensitivity. We subsequently explored the psychometric performance of this generalized pain questionnaire (GPQ) in patients with FM and rheumatoid arthritis (RA).
Methods
Development of items
An initial set of symptoms characteristic of or associated with generalized pain hypersensitivity was derived from a literature review and reviewed by the project team. A pragmatic interview study was then performed on patients with suspected pain hypersensitivity in September 2014. Patients were recruited from participants in a previous survey in which the PainDetect questionnaire (PDQ) was administered.Citation27 We expected that ten patients would be necessary to obtain appropriate patient descriptions and examples of these symptoms. Patients with high PDQ total scores and divergent scores on items suggestive of allodynia and hyperalgesia were contacted by phone to ask if they were interested in participating in an interview study about their pain symptoms.
After 23 patients had been contacted, 10 agreed to participate in the study, of which 7 were female. Interviews were held at the participants’ homes. Using a semistructured interview scheme, participants were asked if they recognized the symptoms of pain hypersensitivity identified in the literature, and if so, to describe their personal experience with these symptoms in their own words.
As the aim of the interviews was to develop understandable items and recognizable examples of manifestations of symptoms identified in the literature, no formal inductive coding or thematic analyses of the data were undertaken. Instead, patients’ comments, descriptions, and examples were categorized by symptoms. Based on this, preliminary items were developed by one of the authors (MOV), who also performed the interviews, and discussion with two of the other authors (PvB, PtK) for item clarity and relevance. This resulted in seven items covering typical symptoms of generalized pain hypersensitivity described in the patient’s language and with common examples that patients mentioned during the interviews. To measure the severity of the symptoms, all items were provided with a 5-point Likert-type rating scale (0= never, 1= hardly noticed, 2= moderately, 3= strongly, 4= very strongly), based on the response options of the PDQ.Citation28 Like the PDQ, no recall period was added.
Psychometric evaluation
Study population and data collection
The preliminary items were administered to two cross-sectional samples of patients with clinically established FM or RA from the rheumatology outpatient department of the Medisch Spectrum Twente hospital in Enschede, the Netherlands. The FM patients were identified using the ICD10 code M79.7. In December 2016, all FM patients who had visited the hospital for consultation with a rheumatologist or rheumatology nurse between November 2015 and October 2016 were invited by postal mail to participate in this survey study. In total, 99 of 196 invited patients (51%) returned the paper-and-pencil questionnaire. All FM patients received one reminder. RA patients completed the same questionnaires as the FM patients. Data for RA patients were collected within the Dutch Rheumatoid Arthritis Monitoring (DREAM) RA registry. The DREAM-RA is a quality registry in which clinical and patient-reported data from patients with a clinical diagnosis of RA are prospectively registered. Between January 2017 and March 2017, RA patients were invited to participate after logging in to their online patient portal. In total, 114 RA patients completed the preliminary items.
According to the Dutch Medical Research Involving Human Subjects Act, the interview and survey study did not need formal approval of a medical ethical review board, as they were observational nonintervention studies, without a high burden to patients. Therefore, written informed consent was not obtained for the interview or survey study. However, in both studies, all patients were fully informed about the goal of the study and the voluntary nature of participation.
Other measures
Besides the seven preliminary generalized pain items, all patients were asked to complete several other standardized patient-reported outcome measures (PROMs). All PROMs were administered in Dutch, and (except for the FM-survey questionnaire), had previously been validated for use in Dutch patient populations. Additional self-reported information was obtained on sociodemographics, the use of conventional (eg, paracetamol, nonsteroidal anti-inflammatory drugs, or opioids) and neuropathic (central nervous acting drugs such as amitriptyline, nortriptyline, gabapentin) pain killers, and on disease duration.
The Short Form health survey (SF36 version 2) was used to measure health-related quality of life using 36 questions assessing eight aspects of health: physical functioning, role – physical, bodily pain, general health, vitality, social functioning, role – emotional, and mental health.Citation29,Citation30 These dimensions can be summarized into a physical component summary and a mental component summary. These summaries are standardized using normative data from the US population with a mean of 50 and a SD of 10.
Physical disability was measured with the Health Assessment Questionnaire – disability index (HAQ-DI).Citation31,Citation32 The HAQ-DI consists of 20 questions divided over eight categories (dressing, arising, eating, walking, hygiene, reach, grip, and usual activities). The highest score in each of the categories are added up and divided by eight, which results in a total score between 0 and 3. The HAQ-DI is particularly, but not exclusively, used in rheumatic disease studies.
The FM-survey questionnaire was used to assess whether patients met the modified American College of Rheumatology 2010 classification criteria for FM.Citation33 These criteria provide a severity scale for symptoms characteristic of FM, consisting of the widespread pain index (WPI) and symptom-severity (SS) score. The WPI consists of 19 possible painful body areas, and the SS score includes items about 6 symptoms. Patients satisfy the FM-survey criteria if the following conditions are met: WPI ≥7 and SS ≥5 or WPI 3–6 and SS ≥9. The Dutch FM-survey questionnaire has not undergone formal psychometric testing, but both the WPI and SS are already frequently used in Dutch patient populations.Citation34,Citation35
Neuropathic-like pain features were assessed with the PDQ, a screening tool that was initially validated in patients with low-back pain.Citation28,Citation36 This questionnaire consists of seven questions evaluating pain qualities, one item about the course pattern of pain, and one item about pain radiation. An overall score of 1–38 is generated. A score ≥19 indicates likely neuropathic pain, 13–18 indicates possible neuropathic pain, and ≤12 unlikely neuropathic pain. Additionally, the PDQ contains three 0–10 numeric rating scales for current, strongest, and average pain severity.
Statistical analysis
Descriptive statistics are presented as mean ± SD when continuous and normally distributed and medians with IQR when continuous and non-normally distributed. Categorical variables are presented as numbers with percentages. Differences between patients with FM and RA were tested using independent t-tests or Mann–Whitney U tests for non-normally distributed variables and χ2 tests for categorical variables. P<0.05 (two-tailed) was chosen to define statistical significance. Analyses were performed with IBM SPSS version 23.
Scaling properties of the seven preliminary items were examined using the models of monotone and double homogeneityCitation37 with the Mokken package in R. The model of monotone homogeneity applies if all items measure the same latent variable and the expected item scores are monotonically non-decreasing throughout the observed score continuum. The first assumption was evaluated using the automatic item-selection procedure with the item-selection algorithm using different lower-bound values for the Loevinger coefficient of scalability (Hi), as suggested by Hemker et al.Citation38 The second assumption was evaluated using the check.monotonicity function. The minimum size of rest-score groups was set at n=30.
The more restrictive model of double homogeneity applies if in addition to the assumptions already tested, the same ordering of items by their expected scores applies to all patients, irrespective of their total score. This is a desirable property for a scale, because it supports inferring from a patient’s total score which specific symptom intensities are likely to be present. We used the backward-item-elimination procedure for polytomous responses proposed by Ligtvoet et al.Citation39 The minimum size of rest-score groups was again set at n=30. Measurement properties of the resulting scale were examined. First, we obtained Hi coefficients for each item. If all items have high Hi values, this suggests that they discriminate well between people with different symptom-intensity levels and/or that items are well spread out across the latent variable. When aggregated over all items in a scale, values >0.5 are considered to indicate a strong scale. The overall accuracy of invariant item ordering was examined using the HT coefficient. Finally, the Molenaar–Sijtsma method was used to estimate reliability of the total score. Coefficients >0.70 and >0.90 are generally taken to be sufficient for group- and individual-level use, respectively.Citation37 Items that satisfied the criteria of Mokken-scale analysis were included in the final GPQ.
Next, we investigated associations of total GPQ scores with scores on the FM-survey questionnaire, PDQ, numeric rating-scale pain severity, SF36, and HAQ-DI to examine external construct validity. Associations were analyzed using Spearman correlations. Since FM is a condition characterized by widespread pain and hyperalgesia, we hypothesized that GPQ scores should correlate strongly (r≥0.7) with scores on the FM-survey questionnaire.Citation12,Citation18–Citation23,Citation25,Citation40 We also hypothesized that GPQ scores should correlate strongly with PDQ scores, because patients with generalized pain hypersensitivity and patients with neuropathic pain report very similar pain qualities (eg, allodynia and hyperalgesia).Citation41–Citation43 Furthermore, we hypothesized that GPQ scores should correlate strongly with pain-intensity scores.Citation11,Citation12,Citation44 We also expected that GPQ scores should correlate moderately (r≥0.50) with health-related quality of life and more strongly with physical component scores than with mental component scores of the SF36, because we specifically referred to pain in the GPQ items. Finally, we hypothesized that GPQ scores should correlate at least moderately with scores on the HAQ-DI as well.
To examine the discriminative ability of GPQ scores, we compared total scores between patients with FM, which is generally considered a central sensitization-driven pain syndrome,Citation12,Citation17–Citation23 and patients with RA, where pain is mainly attributed to peripheral inflammation of the joints.Citation45 Additionally, receiver-operating characteristic (ROC) curve analyses were used to determine the accuracy and optimal cutoff value for the GPQ score for classifying patients with FM (vs RA). The area under the ROC curve (AUC) was used as an overall measure of classification accuracy of the GPQ. As a general rule of thumb, an AUC of 0.7–0.8 is considered to indicate adequate discrimination and between 0.8 and 0.9 excellent discrimination.Citation46 Optimal cutoff values were determined using Youden’s index, which identifies the score with the highest combination of sensitivity and specificity.Citation47
Using this cutoff value, the incremental validity of the GPQ in predicting FM above and beyond female sex, age, and pain severity as known predictors of FM was determined using a series of univariate and multivariate logistic regression analyses with associated ORs with 95% CIs and Wald statistics. Pain severity was dichotomized to allow better interpretation and comparison of ORs of the different independent variables. According to Tubach et al,Citation48 pain becomes clinically significant when it is ≥4 out of 10. Multivariable models were tested for goodness of fit with the Hosmer– Lemeshow test, where small P-values indicate a lack of fit of the model. The overall goodness of fit was evaluated using Nagelkerke’s pseudo-R2.
Results
Patient characteristics
Although 99 FM patients returned the questionnaire (), 1 FM patient was excluded for not completing the GPQ, resulting in 98 evaluable FM patients. A total of 114 RA patients were included. Patients with FM were significantly more often women and were also ~15 years younger than RA patients on average. FM and RA patients did not differ significantly in other sociodemographic factors. Patients with FM reported more intense pain and used painkillers (both for conventional and for neuropathic pain) more often. Finally, FM patients reported worse health status in terms of more physical disabilities and a lower physical and mental quality of life.
Table 1 Patient characteristics
Psychometric evaluation of the GPQ
The item-selection algorithm consistently yielded a single scale in which all seven items were included when lower-bound values for the Hi coefficient of up to <0.6 were used, supporting the unidimensionality of the GPQ items. In several iterations with a higher lower bound, either item 1 (light touch) or item 2 (rubbing skin) was omitted from the scales, but no secondary dimensions emerged. Although some violations of monotonicity were observed, none was statistically significant (). The results of these analyses support using the summed score for ordering patients along a single continuum of generalized pain hypersensitivity. No items showed violations of invariant item ordering, and thus no items were eliminated by the backward-elimination procedure. The overall conclusion of the Mokken analysis was that both the models of monotone and double homogeneity applied to the seven GPQ items.
Table 2 Item scores and Mokken-scale analysis
Hi coefficients for individual items were all large, suggesting that each item usefully contributed to the ordering of patients on the latent metric (). The overall H coefficient was 0.65, which constitutes a strong scale (>0.50), and HT was 0.40, which suggests medium accuracy of invariant item ordering according to Ligtvoet et al.Citation39 Scores were highly reliable according to the SM coefficient (r=0.90).
Construct validity
As expected, meeting FM-survey criteria, PDQ, pain-intensity, and HAQ-DI scores all correlated strongly (≥0.7) with total GPQ scores (). In contrast to hypothesis, the GPQ correlated slightly more strongly with mental than with physical health-related quality of life.
Table 3 Spearman correlations with GPQ total score
Discriminant validity
As expected, patients with FM scored significantly higher on the GPQ compared to patients with RA. The mean total score for FM patients was 15.8±5.1 compared to 6.6±5.1 for RA patients (P<0.001). The ROC curve of the GPQ score for classifying patients with FM is presented in . The GPQ had excellent accuracy in predicting FM, with an AUC of 0.89 (95% CI 0.85–0.93). The optimal cutoff value for the GPQ in classifying FM was >10, with a sensitivity of 82.7%, specificity of 77.2%, positive predicted value of 75.7%, negative predicted value of 83.8%, and 79.7% correctly classified in total.
Figure 1 ROC curve of the GPQ for classifying patients with FM vs RA.
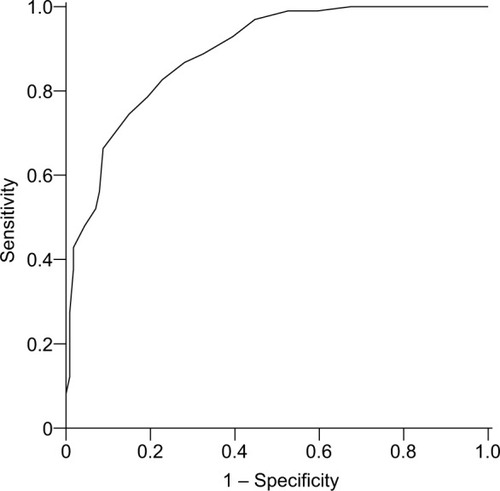
Univariate associations of generalized pain, pain intensity, female sex, and younger age with FM are presented in . A cutoff value >10 for the GPQ turned out to be one of the strongest predictors (OR 13.74) for FM. Female sex and age <50 years were both able independently to predict FM in a multivariate model (, step 1). Female sex was no longer an independent predictor of FM when the GPQ cutoff value of >10 was added to the model (step 2). A GPQ score >10 was the strongest predictor of FM in this model. Clinically relevant current pain severity was included last, since it was most strongly intercorrelated (r=0.63) with a GPQ score >10, which could result in multicollinearity. Even after controlling for clinically relevant pain, a GPQ score >10 had strong incremental value in predicting FM. The final model had a good fit to the data, and together these four variables explained around 60% of the variance.
Table 4 Univariate and multivariate associations with FM
Discussion
In the present study, we introduced a short self-report instrument for assessing the presence and severity of various symptoms commonly associated with generalized pain hypersensitivity and provided an initial evaluation of its scaling properties and discriminative performance in patients with RA or FM. The findings showed that the seven-item GPQ has strong scaling properties and excellent accuracy in distinguishing between RA and FM pain phenotypes.
The results of psychometric evaluation using Mokken-scale analysis showed that a simple summed score can be used to measure patients’ overall level of generalized pain hypersensitivity. Reliability of the scale was high (r=0.90), suggesting that these summed scores can be used to monitor individuals or groups of patients over time and should be useful for discriminating between groups of patients with different levels of generalized pain hypersensitivity.Citation49 Correlations with validated measures of similar concepts, such as FM-survey criteria and neuropathic-like pain features, were of the expected magnitude, supporting the validity of the scores.
This study additionally showed that the GPQ was useful for discriminating between patients with RA, characterized by localized pain in response to inflamed or damaged tissue, and patients with FM, characterized by widespread pain hyperresponsiveness. As expected, given these different pain phenotypes, FM patients reported a higher number and intensity of pain-hypersensitivity symptoms, and a GPQ score >10 was a predictor of FM that had strong incremental value in predicting FM beyond other known predictors, such as female sex, age, and clinically relevant pain severity. FM patients in the current study scored significantly worse than RA patients on other aspects of self-reported health measured for instance by the SF36 as well. However, as pain is an established determinant of such aspects of self-reported health,Citation50 these were not included as covariates in the final multivariate model to avoid possible multicollinearity problems. Based on the current findings, a cutoff score >10 is suggested for identifying possible generalized pain hypersensitivity. However, more studies in different chronic pain populations are needed to confirm this value. Moreover, the current cutoff was determined based on the optimal combination of sensitivity and specificity only, so users specifically needing either high sensitivity or high specificity may also consider using a lower or higher cutoff score.
Despite recently increased interest in identifying the presence or assessing the severity of generalized pain hypersensitivity, the GPQ is the first dedicated self-report measure that specifically assesses commonly associated symptoms. Most studies to date have used short screeners originally developed for assessing neuropathic pain symptoms instead of assessing generalized pain hypersensitivity. For instance, several recent studies have suggested that the PDQ, a short neuropathic pain screener, may also be useful for identifying individuals with abnormal central pain processing.Citation41,Citation51–Citation53 The GPQ arguably has higher content validity for assessing widespread pain hypersensitivity, since the questionnaire was developed based on typical symptoms for generalized pain hypersensitivity described in the literature, followed by a qualitative study with rheumatic patients with atypical pain symptoms suggestive of generalized pain hypersensitivity.
The Pain Sensitivity QuestionnaireCitation26 also measures self-reported pain sensitivity, but includes only items measuring intensity ratings of imagined painful situations, as opposed to actual complaints, such as those measured by the GPQ. Moreover, the Pain Sensitivity Questionnaire includes only items related to allodynia (eg, walking across a cool tiled floor with bare feet) and hyperalgesia (eg, trapping your finger in a drawer), and does not include other important signs of generalized pain hypersensitivity, such as aftersensations and spreading of pain. Finally, another frequently used instrument is the Central Sensitization Inventory.Citation54 This assesses a long list of 25 health-related symptoms that are common to central sensitization syndromes in general. However, only five of these items focus on pain-related symptoms, whereas the other items assess a wide range of aspecific symptoms, such as difficulty concentrating and sensitivity to light. Consequently, its length and content also limit is usefulness for those interested in measuring generalized pain-hypersensitivity symptoms per se.
In the current study, FM patients completed the GPQ and other PROMs on paper, while RA patients completed these in a web-based questionnaire. In recent years, many studies have examined the equivalence of electronic and paper-and-pencil administration of PROMs. In general, these studies show that PROMs administered on paper produce practically interchangeable scores compared with the same measures administered on an electronic device.Citation55,Citation56
No recall period is used for the items of the GPQ. Although this is quite common for similar questionnaires, such as those assessing neuropathic pain symtoms,Citation28,Citation57,Citation58 there is considerable debate about the importance of using recall periods and selecting appropriate recall periods. For instance, the Patient-Reported Outcome Measurement Information System network explicitly advocates the use of recall periods that should be short enough to reduce recall errors and biases, but sufficiently long to capture a period of experience considered clinically relevant for outcome research.Citation59 However, they also noted that there is currently little research available to inform the selection of an optimal recall period.Citation59 The US Food and Drug Administration indicates that when using a recall period, the appropriateness of this period depends on the instrument’s purpose and intended use. Also, the choice of a recall period should consider the variability, duration, frequency, and intensity of the concept measured, the disease or condition’s characteristics, and the tested treatment.Citation60 Because the GPQ was not intended to measure the exact frequency of symptoms in a certain period, but the severity of the presence of typical pain symptoms in general or in specific example situations (such as pain from cold water or holding cold objects), we preferred to avoid the use of any specific reference period. Additionally, there was no information available about how often these specific symptoms or situations typically occur in patients’ daily lives that could be used to guide the selection of an appropriate recall period for all items. Although this is not expected to affect the discriminative ability of the GPQ negatively, it may reduce its sensitivity to change when used as an outcome measure. Future studies using the instrument in longitudinal observational or intervention studies are needed to examine this issue.
A limitation of this study is that we relied on a diagnosis of FM as a surrogate criterion for the presence of generalized pain hypersensitivity, since no true gold standard for diagnosing generalized pain hypersensitivity currently exists. Interestingly, only about 88% of the FM patients fulfilled the new American College of Rheumatology classification criteria for FM.Citation33 One possible explanation is that in the current study, FM patients were identified using the ICD10 coding system. These patients were clinically diagnosed and not by means of the FM-survey criteria, which were developed for epidemiologic and clinical studies. For comparison, in the development study of the FM-survey criteria, only 60% of patients who had been previously (clinically) diagnosed with FM satisfied the criteria.Citation33
Additionally, we used self-reported external instruments only to examine the construct validity of the scale. Future studies should further examine the criterion and construct validity of the GPQ by validating it against different methods of measuring pain, such as QST or imaging-based techniques, such as functional magnetic resonance imaging of the brain. Furthermore, the GPQ should be validated in other chronic pain populations, such as those in pain-rehabilitation settings. Finally, the results of the psychometric analysis of GPQ in the present study are encouraging for its use in the evaluation of the efficacy of therapies targeting generalized pain hypersensitivity. However, future studies are needed to confirm its responsiveness to change for such applications.
Conclusion
This study shows that the newly developed GPQ is psycho-metrically sound and has excellent accuracy in differentiating between chronic pain patients with pain presumably due to central nervous system hypersensitization (FM) and patients with pain primarily due to local nociception or inflammation (RA). Future validation studies are needed to examine its validity for identifying the presence of generalized pain hypersensitivity in other chronic pain populations and for monitoring changes in the extent of these pain symptoms over time or after treatment.
Author contributions
MAHOV, PMTK, HEV and MAFJVDL conceptualized and designed the study. PFVB organized and supervised data collection and management. PFVB, MAHOV and PMTK analyzed the data. PFVB drafted the initial manuscript. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by Lilly Netherlands, without restriction on publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- BlythFMMarchLMBrnabicAJJormLRWilliamsonMCousinsMJChronic pain in Australia: a prevalence studyPain2001892–312713411166468
- BreivikHCollettBVentafriddaVCohenRGallacherDSurvey of chronic pain in Europe: prevalence, impact on daily life, and treatmentEur J Pain200610428733316095934
- JohannesCBLeTKZhouXJohnstonJADworkinRHThe prevalence of chronic pain in United States adults: results of an Internet-based surveyJ Pain201011111230123920797916
- BessonJMThe neurobiology of painLancet199935391641610161510334274
- FornasariDPain mechanisms in patients with chronic painClin Drug Investig20123236994552
- WoolfCJSalterMWNeuronal plasticity: increasing the gain in painScience200028854721765176810846153
- JiRRWoolfCJNeuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological painNeurobiol Dis20018111011162235
- JiRRKohnoTMooreKAWoolfCJCentral sensitization and LTP: do pain and memory share similar mechanisms?Trends Neurosci2003261269670514624855
- SchliessbachJSiegenthalerAStreitbergerKThe prevalence of widespread central hypersensitivity in chronic pain patientsEur J Pain2013171012023233315
- CuratoloMUllerMAshrafAPain hypersensitivity and spinal nociceptive hypersensitivity in chronic pain: prevalence and associated factorsPain201515611125599292
- LatremoliereAWoolfCJCentral sensitization: a generator of pain hypersensitivity by central neural plasticityJ Pain200910989592619712899
- WoolfCJCentral sensitization: implications for the diagnosis and treatment of painPain20111523 SupplS2S1520961685
- International Association for the Study of Pain Taxonomy Available from: https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698Accessed December 28, 2018
- LiJSimoneDALarsonAAWindup leads to characteristics of central sensitizationPain199979175829928779
- StaudREvidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgiaCurr Rheumatol Rep201113651352021833699
- RenKDubnerRDescending modulation in persistent pain: an updatePain20021001–21612435453
- WolfeFRaskerJJFibromyalgiaFiresteinGSBuddRCGabrielEMcInnesIBO’DellJRKelley’s Textbook of Rheumatology9th edPhiladelphia, PASaunders/Elsevier2013733751
- VierckCJMechanisms underlying development of spatially distributed chronic pain (fibromyalgia)Pain2006124324226316842915
- PetzkeFClauwDJAmbroseKKhineAGracelyRHIncreased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentationPain2003105340341314527701
- McDermidAJRollmanGBMcCainGAGeneralized hypervigilance in fibromyalgia: evidence of perceptual amplificationPain1996662–31331448880834
- StaudREvidence of involvement of central neural mechanisms in generating fibromyalgia painCurr Rheumatol Rep20024429930512126581
- StaudRVierckCJCannonRLMauderliAPPriceDDAbnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndromePain2001911–216517511240089
- StaudRRobinsonMEPriceDDTemporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patientsJ Pain200781189390117681887
- HurtigIMRaakRIKendallSAGerdleBWahrenLKQuantitative sensory testing in fibromyalgia patients and in healthy subjects: identification of subgroupsClin J Pain200117431632211783811
- DesmeulesJACedraschiCRapitiENeurophysiologic evidence for a central sensitization in patients with fibromyalgiaArthritis Rheum20034851420142912746916
- RuscheweyhRMarziniakMStumpenhorstFReinholzJKnechtSPain sensitivity can be assessed by self-rating: development and validation of the Pain Sensitivity QuestionnairePain20091461–2657419665301
- KoopSMTen KloosterPMVonkemanHESteunebrinkLMvan de LaarMANeuropathic-like pain features and cross-sectional associations in rheumatoid arthritisArthritis Res Ther201517123726335941
- FreynhagenRBaronRGockelUTölleTRpainDETECT: a new screening questionnaire to identify neuropathic components in patients with back painCurr Med Res Opin200622101911192017022849
- WareJEJeWJSF36 health survey updateSpine200025243130313911124729
- ten KloosterPMVonkemanHETaalEPerformance of the Dutch SF36 version 2 as a measure of health-related quality of life in patients with rheumatoid arthritisHealth Qual Life Outcomes20131117723651685
- FriesJFSpitzPWYoungDYThe dimensions of health outcomes: the health assessment questionnaire, disability and pain scalesJ Rheumatol1982957897937175852
- ten KloosterPMTaalEvan de LaarMARasch analysis of the Dutch Health Assessment Questionnaire disability index and the Health Assessment Questionnaire II in patients with rheumatoid arthritisArthritis Rheum200859121721172819035413
- WolfeFClauwDJFitzcharlesMAFibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgiaJ Rheumatol20113861113112221285161
- OudejansLHeXNiestersMDahanABrinesMvan VelzenMCornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgiaSci Rep2016612357327006259
- van WilgenCPVuijkPJKregelJPsychological distress and widespread pain contribute to the variance of the central sensitization inventory: a cross-sectional study in patients with chronic painPain Pract201818223924628449376
- TimmermanHWolffAPSchreyerTCross-cultural adaptation to the Dutch language of the PainDETECT-QuestionnairePain Pract201313320621422776283
- van SchuurWHOrdinal Item Response Theory: Mokken Scale AnalysisThousand Oaks, CASAGE Publications2011
- HemkerBTSijtsmaKMolenaarIWSelection of unidimensional scales from a multidimensional item bank in the polytomous Mokken I RT modelAppl Psychol Meas1995194337352
- LigtvoetRvan der ArkLATe MarveldeJMSijtsmaKInvestigating an invariant item ordering for polytomously scored itemsEduc Psychol Meas2010704578595
- StaudRAbnormal pain modulation in patients with spatially distributed chronic pain: fibromyalgiaRheum Dis Clin North Am200935226327419647141
- HochmanJRDavisAMElkayamJGaglieseLHawkerGANeuropathic pain symptoms on the modified PainDetect correlate with signs of central sensitization in knee osteoarthritisOsteoarthritis Cartilage20132191236124223973136
- Rifbjerg-MadsenSChristensenAWBoesenMFRI0099 indications of reversibility of central sensitization according to the paindetect questionnaire in patients with rheumatoid arthritis: results from The Prospective Frame-Cohort StudyAnn Rheum Dis201675Suppl 2463.246464
- AhmedSMaganTVargasMHarrisonASofatNUse of the PainDetect tool in rheumatoid arthritis suggests neuropathic and sensitization components in pain reportingJ Pain Res2014757958825378947
- MeeusMNijsJCentral sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndromeClin Rheumatol200726446547317115100
- LeeYCEffect and treatment of chronic pain in inflammatory arthritisCurr Rheumatol Rep201315130023292816
- HosmerDWLemeshowSApplied Logistic Regression2nd edNew York, NYJohn Wiley & Sons, Inc2000
- YoudenWJIndex for rating diagnostic testsCancer195031323515405679
- TubachFRavaudPMartin-MolaEMinimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinaArthritis Care Res2012641116991707
- AaronsonNAlonsoJBurnamAAssessing health status and quality-of-life instruments: attributes and review criteriaQual Life Res200211319320512074258
- RuppIBoshuizenHCDinantHJJacobiCEvan den BosGADisability and health-related quality of life among patients with rheumatoid arthritis: association with radiographic joint damage, disease activity, pain, and depressive symptomsScand J Rheumatol200635317518116766363
- AmrisKJespersenABliddalHSelf-reported somatosensory symptoms of neuropathic pain in fibromyalgia and chronic widespread pain correlate with tender point count and pressure-pain thresholdsPain2010151366466920832941
- MoretonBJTewVdas NairRWheelerMWalshDALincolnNBPain phenotype in people with knee osteoarthritis; classification and measurement properties of PainDetect and S-LANSS in a cross-sectional studyArthritis Care Res2015674519528
- ChristensenAWRifbjerg-MadsenSChristensenRNon-nociceptive pain in rheumatoid arthritis is frequent and affects disease activity estimation: cross-sectional data from the FRAME studyScand J Rheumatol201645646146926987470
- MayerTGNeblettRCohenHThe development and psychometric validation of the central sensitization inventoryPain Pract201212427628521951710
- GwaltneyCJShieldsALShiffmanSEquivalence of electronic and paper-and-pencil administration of patient-reported outcome measures: a meta-analytic reviewValue Health200811232233318380645
- MuehlhausenWDollHQuadriNEquivalence of electronic and paper administration of patient-reported outcome measures: a systematic review and meta-analysis of studies conducted between 2007 and 2013Health Qual Life Outcomes201513116726446159
- BennettMISmithBHTorranceNPotterJThe S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal researchJ Pain20056314915815772908
- BouhassiraDAttalNAlchaarHComparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4)Pain20051141–2293615733628
- DeWaltDARothrockNYountSStoneAAPROMIS Cooperative GroupEvaluation of item candidates: the PROMIS qualitative item reviewMed Care2007455 Suppl 1S12S2117443114
- FoodUSAdministrationDrugGuidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims2009 Available from: https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdfAccessed December 28, 2018
