Abstract
Introduction
The undertreatment of acute pain presents a significant challenge in the Emergency Department. This post hoc subgroup analysis of a previously reported randomized controlled UK study reports the efficacy and safety of low-dose methoxyflurane analgesia in treating adolescent patients with moderate-to-severe trauma pain.
Patients and methods
Three hundred patients (96 in the adolescent subgroup) aged ≥12 years requiring analgesia for acute trauma pain (pain score of 4–7 on the Numerical Rating Scale) at triage were randomized 1:1 to methoxyflurane (up to 6 mL) or placebo (normal saline), both administered using a Penthrox® inhaler. The patient could request rescue medication (paracetamol/opioids) at any time. The primary endpoint was the change from baseline in visual analog scale (VAS) pain intensity.
Results
Mean VAS pain score for the adolescent subgroup at baseline was ~ 61 mm. Adjusted mean change in VAS pain intensity from baseline to 5, 10, 15, and 20 minutes was −24.5, −28.1, −31.6, and −31.7 mm for methoxyflurane and −14.6, −18.8, −19.2, and −23.7 mm for placebo, with a statistically significant treatment effect in favor of methoxyflurane overall across all four time points (−9.9 mm; 95% CI: −17.4, −2.4 mm; P=0.0104). Median time to first pain relief was significantly shorter with methoxyflurane (1 minute) than placebo (3 minutes, P<0.0001). Pain relief was reported within 1–10 inhalations in 95.7% of methoxyflurane-treated patients and 64.6% of placebo-treated patients. Rescue medication was requested by two (4.3%) methoxyflurane-treated patients and three (6.3%) placebo-treated patients. Over 95% of patients, physicians, and nurses rated methoxyflurane treatment as “Excellent”, “Very Good” or “Good” compared with between 64% and 68% for placebo. The incidence of adverse events was higher with methoxyflurane (51%) than placebo (42%), mostly comprising mild/transient dizziness and headache.
Conclusion
This subgroup analysis shows that low-dose inhaled methoxyflurane is a rapid-acting and effective analgesic in adolescent patients presenting with moderate-to-severe trauma pain.
Trial registration
Clinicaltrials.gov identifier: NCT01420159, EudraCT number: 2011-000338-12.
Introduction
Pain is the most frequent complaint in the emergency setting,Citation1,Citation2 yet suboptimal evaluation and treatment of acute pain are still common.Citation2–Citation4 This may be due to underassessment of pain, especially if pain is evaluated based on visible signs rather than on patient reports of pain.Citation5 It has been demonstrated that nurses significantly underestimate acute musculoskeletal pain in adult patients in the emergency department (ED).Citation6 Along with underassessment of pain, other factors such as lack of formal training or practice variations in pain management, time constraints, opiophobia, and access to medications increase the likelihood of oligoanalgesia.Citation4,Citation7,Citation8
Low frequency of pain severity assessment (~18%–32%) has also been reported for children with acute pain and traumatic injuries in the prehospital setting.Citation9–Citation11 Fewer than half of such patients receive analgesia from paramedicsCitation11–Citation13 or within 1 hour of arrival at the ED.Citation14 Younger patients are less likely to have a documented assessment of pain intensity or receive adequate pain medication.Citation11,Citation12,Citation14 Even when analgesia is provided, pediatric patients are frequently under-dosedCitation15 and only a small proportion achieve adequate pain relief.Citation14 Consequently, assessment and management of pediatric pain in emergency care is a research priority.Citation16,Citation17 Intranasal (IN) or inhaled delivery of analgesic agents allow rapid and simple drug administration without the distress of intravenous (IV) placement or delay while oral or topical analgesics take effect, and may provide another option for the management of acute moderate-to-severe pain in children both in the prehospital and ED settings.
Methoxyflurane has well documented analgesic properties at low doses.Citation18 Since discontinuation of its use as an anesthetic in the late 1970s due to reports of nephrotoxicityCitation19–Citation21 and the introduction of newer anesthetic agents, methoxyflurane has continued to be used in Australia and New Zealand (3 mL dose in a handheld inhaler; Penthrox®, Medical Developments International, Scoresby, VIC Australia) as a self-administered, rapid-acting analgesic agent for short-term relief of acute pain in adults and children in emergency medicine and for minor surgical and dental procedures.Citation22,Citation23 More recently, methoxyflurane has been licensed in Europe for the emergency relief of moderate-to-severe pain in conscious adults with trauma and associated pain,Citation24 as well as in other territories including Asia, Eastern Europe, Latin America, South Africa, and the Gulf Area. Methoxyflurane is administered via a lightweight, handheld, green pen-shaped plastic disposable inhaler, which in Europe is supplied with an activated carbon chamber to reduce environmental exposure.
Studies of low-dose methoxyflurane indicate that it is an efficacious analgesic in the ED and prehospital settingsCitation25–Citation27 and for procedural analgesia.Citation28 The physiochemical characteristics of methoxyflurane mean that absorption is rapid, hence it has a fast onset of analgesia (usually within 6–10 inhalations).Citation24,Citation29 Two observational case series,Citation30,Citation31 a retrospective comparative study vs IV morphine and IN fentanylCitation32 and a pilot randomized controlled trial (RCT)Citation33 have also demonstrated rapid efficacy and high patient/health care provider (HCP) satisfaction with low-dose methoxyflurane analgesia in children with acute trauma pain; however, there is a paucity of data for pediatric patients from larger RCTs or outside of Australia.
The STOP! study was a UK-based RCT that investigated the short-term efficacy and safety of low-dose methoxyflurane analgesia (3 mL dose) for the treatment of moderate-to-severe pain in 300 patients aged ≥12 years presenting to the ED with moderate-to-severe trauma-related pain. The results for the full study populationCitation34 and adult subgroup aged ≥18 yearsCitation35 have previously been reported. The focus of this secondary paper is a post hoc subgroup analysis that evaluated efficacy and safety data for adolescent patients aged 12–17 years inclusive.
Material and methods
Study design
STOP! was a randomized, double-blind, multicenter, placebo-controlled study conducted at six EDs in the UK between August 05, 2011 and July 26, 2012. Patients presenting to the ED with moderate-to-severe acute trauma-related pain were randomized 1:1 to treatment with methoxyflurane (up to 2×3 mL) or placebo, each administered via a Penthrox inhaler, as required while in the ED. Assessments including visual analog scale (VAS) pain intensity, rescue medication use, medication performance, and adverse events were performed by a blinded research nurse in the ED, and a safety follow-up visit was conducted 14±2 days after ED discharge. The randomized study population (N=300) included 96 adolescent patients (aged 12–17 years) whose data are presented in this report. The full methodology for this study has been previously described in the primary publication.Citation34 The study was conducted in accordance with the Declaration of Helsinki, International Council on Harmonization Good Clinical Practice, and local guidelines. The protocol and amendments were reviewed and approved by each participating National Health Service ethics committee and each study site’s research and development department. Favorable opinion for the study was received from the National Research Ethics Service (REC reference 11/YH/0116). All patients (or the patient’s parent or legal representative, if the patient was under 16 years of age) provided written informed consent before study enrollment.
Study participants
Patient eligibility for the study was established at triage after presentation to the ED. Patients aged ≥12 years with moderate-to-severe pain (score of 4–7 on the 11-point Numerical Rating Scale [NRS]) due to minor trauma and requiring analgesia were enrolled. In this study, trauma referred to a physical wound or injury, such as dislocations, contusions, fractures, lacerations, burns or injury due to a foreign body. For this adolescent subgroup analysis, all patients were aged 12–17 years.
Patients were excluded from the study if they met any of the following criteria: analgesic use within 5 hours before presentation to the ED (8 hours for diclofenac sodium); ongoing analgesic use for chronic pain; methoxyflurane use within 4 weeks before enrollment; history of hypersensitivity to fluorinated anesthetics; clinically significant respiratory depression, cardiovascular instability, renal or hepatic impairment; acute intoxication with alcohol or drugs; a life-threatening condition requiring immediate admission to the operating room or intensive care unit.
Treatments
Patients were randomized in a 1:1 ratio to treatment with methoxyflurane (up to 2×3 mL) or placebo (sterile normal saline), self-administered as required by the patient via a Penthrox inhaler, assisted where required by the research nurse. The Penthrox inhaler is a lightweight, handheld, single-use green cylindrical device with a whistle-like mouthpiece at one end. Methoxyflurane is absorbed by an internal polypropylene wick and vaporizes within the inhaler, and is inhaled by the patient through the mouthpiece. Patients are instructed to also exhale through the inhaler so that unmetabolized methoxyflurane can be absorbed by the activated carbon chamber attached to the device, avoiding occupational exposure. The amount of methoxyflurane (and thus the degree of analgesia) is controlled by the patient by inhaling more or less frequently from the device. Initially, the patient was provided with one inhaler; a second inhaler was available upon request of the patient. One inhaler was predicted to provide up to 1 hour’s pain relief with intermittent use. The patient could inhale a higher concentration of study medication, if required, by covering the diluter hole at the mouthpiece end of the inhaler with their index finger. The Penthrox inhaler provides a methoxyflurane concentration of 0.1%–0.2% with the diluter hole open, which increases to 0.3%–0.4% with diluter hole occlusion.Citation36 Since this was a placebo-controlled study, rescue medication (IV, IN or oral opioids or paracetamol) was available at any time upon request of the patient, as recommended in the CHMP guideline CPMP/EWP/612/00Citation37 and guidance from the Declaration of Helsinki. Patients were provided with 16×500 mg paracetamol tablets at ED discharge for the treatment of pain, as needed, during the 14±2 day safety follow-up period.
Randomization and blinding
Randomization was performed by an independent statistician using permuted blocks and was stratified by center and age group (adolescent/adult). Patients were assigned the next randomization number in the appropriate stratum at enrollment. Study medication was assembled and dispensed in a sealed plastic bag by an unblinded research team member. Prior to sealing the bag, a drop of methoxyflurane was placed on the outside of each inhaler, so that all inhalers had the characteristic fruity odor of methoxyflurane and the smell between active and placebo inhalers was indistinguishable upon opening the bag. To maintain the blind with regard to inhaler weight, 5 mL of saline solution was used in the placebo inhalers compared with 3 mL of methoxyflurane in the active inhalers, since the relative density of methoxyflurane (1.42) is greater than that of normal saline (1). The investigator, patient, and all site personnel (except the staff member responsible for dispensing study medication) were blinded to the treatment assignment.
Efficacy assessments
Study assessments were performed by a blinded research nurse, who remained with the patient in the ED while they were receiving care. Pain intensity was assessed using the Painlog™100 mm VAS (Schlenker Enterprises, Ltd., Lombard, IL, USA) at the following time points: baseline, 5, 10, 15, 20, and 30 minutes after the start of study treatment, then every 30 minutes until administration of rescue medication or discharge from the ED, whichever occurred first. The pain VAS is easy to use, does not require verbal or reading skills, and is commonly used in pain studies.Citation38,Citation39
The time from the start of treatment to first pain relief (subjectively reported by the patient), the number of inhalations taken before pain relief was reported by the patient, and use of the diluter hole during inhalation were recorded. Any rescue medication use was recorded, including the time of the request for rescue relative to the start of study treatment. A global assessment of medication performance (GMP) assessed using a 5-point Likert scale (“Poor”, “Fair”, “Good”, “Very Good”, or “Excellent”) was completed separately by the patient, the treating physician, and the research nurse before ED discharge.
Safety assessments
Patients were observed by a research nurse throughout their stay in the ED. All adverse events (except those relating to the trauma presentation) were recorded from enrollment until ED discharge, with any subsequent adverse events being recorded at the safety follow-up visit on Day 14±2. For each adverse event, the investigator assessed the relationship to study treatment and evaluated its severity. Vital signs, including blood pressure, heart rate and rhythm, and respiratory rate, were measured at the same time points as pain intensity. Blood samples were taken for safety laboratory tests (full blood count and clinical chemistry) at the start of study treatment and at the follow-up visit. The 15-point Glasgow coma score was used to assessed the patient’s level of consciousness at 10, 20, and 30 minutes after the start of study medication inhalation and prior to ED discharge.
Statistical analyses
The modified intention-to-treat (ITT) population (including all randomized patients who used the study medication and had at least one on-treatment efficacy assessment) was used for all efficacy analyses. The primary efficacy variable was the change from baseline in VAS pain intensity at 5, 10, 15, and 20 minutes after the start of study treatment. The change at each time point was analyzed using a repeated-measures analysis of covariance adjusted for baseline VAS score, and time–treatment interaction. Treatment effects (methoxyflurane vs placebo) were estimated as least squares mean differences. The primary analysis was the overall test for treatment effect across all four time points. Time to first pain relief and time to request for rescue medication were compared between the treatments using a Cox proportional hazards model adjusted for baseline VAS score. Time was censored at 2 hours after the start of treatment, physician-initiated rescue medication, start of treatment for the injury, or early withdrawal, whichever occurred first. GMP ratings by the patient, research nurse, and treating physician at discharge were each compared between the treatments using ordinal logistic regression with proportional odds assumption adjusted for baseline VAS. Baseline was defined as the last recorded value before the start of study treatment; if the baseline value was missing, change from baseline was not calculated (ie, no imputation was performed). All hypothesis testing was performed at the 5% (two-sided) significance level. The analyses performed for the adolescent subgroup mirrored the original study analysis methods,Citation34 with the exception of removing the redundant term for age group (adult/adolescent). Other efficacy endpoints were summarized descriptively.
Adverse events were summarized for the safety population, defined as all randomized patients who used the study medication. Adverse events were classified as treatment-emergent if they started or increased in severity after the start of study treatment. Adverse events were coded using the Medical Dictionary for Regulatory Activities (Med-DRA version 14.0) coding system. Statistical analyses were performed using SAS® version 9.4.
Sample size
The original sample size calculation estimated that 150 patients per treatment group in the whole study (adult and adolescent patients) would provide at least 94.5% power to detect a treatment difference of 13 mmCitation40 in the overall change from baseline of VAS pain score over the first 20 minutes of treatment. The drop-out rate was expected to be minimal in this setting, therefore a sample size of 150 patients per arm was considered adequate, and was achieved in the study (300 patients randomized). However, the study sample size was not intended to provide sufficient power to demonstrate a statistically significant treatment difference in the adolescent-only subgroup presented in this report (96 patients randomized).
Results
Study patients
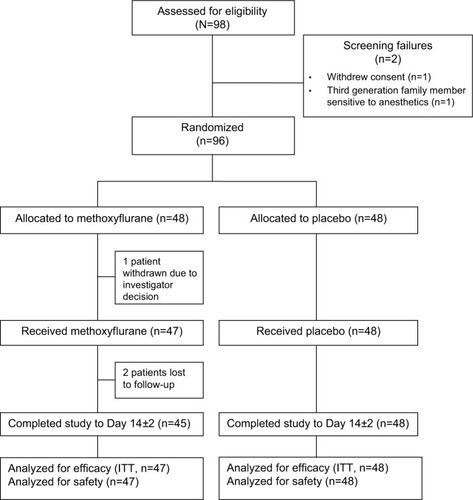
Participant flow for the adolescent subgroup is presented in . Ninety-eight adolescent patients were screened; 96 of these patients were randomized to double-blind treatment (48 to each treatment group) and two patients were ineligible. One patient randomized to the methoxyflurane group was withdrawn before receiving study treatment due to the decision of the investigator, thus 95 adolescent patients were treated and included in the modified ITT and safety populations. All except two of the treated patients (both lost to follow-up) completed the study to Day 14±2.
Patient demographic characteristics, baseline pain severity, and injury type were similar for both treatment groups (). The mean age of patients in the adolescent subgroup was 14 years and the majority of patients (85.3%) were White. More male (70.5%) than female (29.5%) adolescent patients were enrolled. More than half of the patients (55.8%) had an injury type of “other” (generally soft tissue injuries, sprains, and muscular pain), 23.2% had fractures, 15.8% had contusions, while lacerations, dislocations, and injuries due to foreign body were reported for one or two patients each. Only one patient had more than one injury (contusion and abrasion to shoulder, knee, and finger). Patients enrolled in the study were required to have moderate-to-severe pain (NRS score of 4–7) at baseline; accordingly, the mean (SD) baseline VAS pain intensity score was 61.7 (16.56) mm in the methoxyflurane group and 61.0 (13.33) mm in the placebo group.Citation41
Table 1 Demographic and baseline characteristics (modified intention-to-treat population)
Pain relief
The reduction in pain intensity was significantly greater with methoxyflurane than placebo in adolescent patients. The adjusted mean change from baseline to 5, 10, 15, and 20 minutes for VAS pain intensity was −24.5, −28.1, −31.6, and −31.7 mm, respectively, for methoxyflurane and −14.6, −18.8, −19.2, and −23.7 mm, respectively, for placebo, with a statistically significant treatment difference over the first 20 minutes of −9.9 mm (95% CI: −17.4 to −2.4 mm; P=0.0104; ). The largest treatment difference was at 15 minutes after the start of inhalation (estimated treatment effect: −12.4 mm; 95% CI: −21.9 to −2.9 mm). Further reductions in pain intensity were observed beyond 20 minutes after the start of methoxyflurane inhalation, with mean decreases from baseline in VAS pain of −38.7, −37.8, and −42.5 mm at 30 (n=32), 60 (n=5), and 90 minutes (n=2). In the placebo group, mean decreases of −30.9, −26.9, and −31.0 mm were observed at 30 (n=26), 60 (n=7), and 90 minutes (n=2). Fewer patients had available data at later time points due to discharge or undergoing further interventions.
Table 2 Analysis of visual analog scale (VAS) pain intensity score (modified intention-to-treat population)
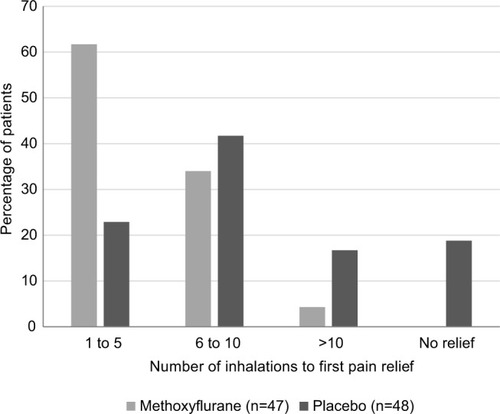
Pain relief was reported by 46 of 47 methoxyflurane-treated patients (97.9%), with a median time to pain relief of 1 minute (95% CI: 1–2 minutes). In the placebo group, pain relief was reported by 39 of 48 patients (81.3%), with a median time to pain relief of 3 minutes (95% CI: 2–5 minutes). The time to first pain relief was significantly shorter in the methoxyflurane group compared with the placebo group (HR: 2.35; 95% CI: 1.48, 3.76; P=0.0003). Pain relief was experienced within 1–5 inhalations for 61.7% of patients in the methoxyflurane group and 22.9% of patients in the placebo group, and within 1–10 inhalations for 95.7% and 64.6% of patients, respectively ().
Inhaler and rescue medication use
Most patients required only one inhaler. A second inhaler was used by six (12.8%) methoxyflurane-treated patients and nine (18.8%) placebo-treated patients, with a shorter time between dispensing the first and second inhalers for the placebo patients (median: 30 minutes; range: 1–63 minutes) compared with the methoxyflurane patients (median 58 minutes; range: 43–65 minutes). The diluter hole was covered during use by slightly more patients using the placebo inhalers (31 patients [64.6%]) compared with the methoxyflurane inhalers (25 patients [53.2%]).
Only two patients (4.3%) in the methoxyflurane group and three patients (6.3%) in the placebo group requested rescue medication (prior to censoring), and therefore no significant difference between the treatment groups in the time to request for rescue medication was identifiable (HR: 0.31; 95% CI: 0.03, 2.97; P=0.3085). The median time to request for rescue medication could not be estimated due to the low number of patients requesting rescue medication.
Satisfaction with treatment
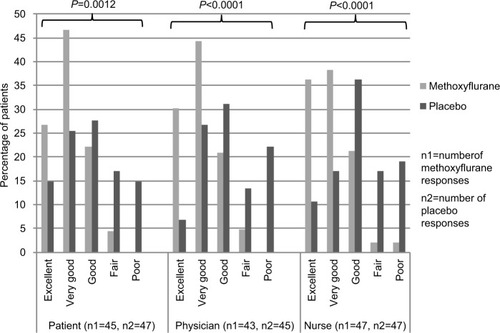
Satisfaction with study treatment was significantly higher in the methoxyflurane group compared with the placebo group across all GMP ratings (P≤0.0012, ). Over 95% of treating physicians, research nurses, and patients rated methoxyflurane treatment as “Excellent”, “Very Good” or “Good”, compared with between 64% and 68% for placebo.
Safety
Treatment-emergent adverse events (TEAEs) are presented in . Fifty-five TEAEs were reported by 24 patients (51.1%) in the methoxyflurane group and 35 TEAEs were reported by 20 patients (41.7%) in the placebo group. Fifteen TEAEs in eleven (23.4%) methoxyflurane-treated patients and six TEAEs in five (10.4%) placebo-treated patients were considered to be related to study treatment according to the investigator’s causality assessment. The most commonly reported TEAEs (reported by ≥3 patients in either treatment group) were headache, dizziness, somnolence, and influenza-like illness. Dizziness and somnolence were more common in methoxyflurane-treated patients, influenza-like illness was more common in placebo-treated patients, and headache was reported at a similar frequency in both groups. The majority of TEAEs were mild and self-limiting; no patients experienced a severe or serious adverse event. In methoxyflurane-treated patients, most cases of dizziness commenced within 5 minutes of the start of dosing and resolved within 1 minute to 3 hours, somnolence events started within 2 hours of the start of dosing and resolved within 2 hours, while headache was generally reported in the period between ED discharge and follow-up. Four patients withdrew due to a TEAE: two patients (4.3%) in the methoxyflurane group (one patient due to feeling dizzy and one patient due to light-headedness, bad taste and burning in his mouth) and two patients (4.2%) in the placebo group (one patient due to light-headedness and nausea and one patient due to loss of consciousness lasting for ~1 minute).
Table 3 Treatment-emergent adverse events (safety population)
Inspection of individual patient vital signs showed no observable effects of low-dose methoxyflurane on cardiovascular or respiratory parameters. Glasgow coma score was 15 across all patients and observations. Although clinical laboratory sampling was limited, examination of clinical chemistry results did not identify any renal or hepatic damage in the methoxyflurane group.
Discussion
This subgroup analysis shows that methoxyflurane provided rapid and effective pain relief in adolescent patients with acute trauma pain, with both patients and HCPs expressing a high level of treatment satisfaction. The treatment difference (methoxyflurane-placebo) in the primary analysis of the VAS pain intensity score over the first 20 minutes of treatment was smaller for adolescent patients (−9.9 mm [95% CI: −17.4, −2.4 mm]) compared with adult patients (−17.4 mm [95% CI: −22.3, −12.5 mm]).Citation35 This appears to be due to a much larger “placebo-effect” in the adolescent population; while the overall adjusted mean change from baseline in VAS pain intensity with methoxyflurane was the same for adult and adolescent patients (−29.0 mm); it was noticeably different for adult (−11.6 mm) and adolescent (−19.1 mm) patients in the placebo group. Whilst the difference was statistically significant, this phenomenon resulted in the treatment difference for the adolescent subgroup being less than the minimum clinically significant change in acute pain of 13 mm on a 100 mm VAS scale previously suggested by Todd et al in a hypothesis-generating study in trauma patients.Citation42 For secondary pain relief and satisfaction (GMP) endpoints, the results for the adolescent subgroup were generally positive and continued to show a larger placebo effect compared with the adult subgroup.Citation35 In the adult subgroup, 82.4% of methoxyflurane-treated patients and 52.5% of placebo-treated patients experienced pain relief within a median of 5 and 20 minutes, respectively,Citation35 compared with 97.9% of methoxyflurane-treated patients and 81.3% of placebo-treated patients within a median of 1 and 3 minutes, respectively for adolescent patients. GMP of methoxyflurane was rated as “Excellent”, “Very good” or “Good” for approximately 75% of adult patients and >95% of adolescent patients, while placebo treatment received the same ratings for 26%–31% of adult patients and 64%–68% of adolescent patients. Despite the strong placebo effect in adolescent patients, a significant treatment difference in favor of methoxyflurane was shown for all secondary efficacy endpoints, with the exception of time to request for rescue medication (only five patients in total requested rescue medication). Although few placebo-treated patients requested rescue medication, the shorter time to request for a second inhaler and higher number of patients covering the diluter hole in the placebo group are suggestive of additional need for analgesia in this group.
The placebo effect is well recognized in adults but is less well studied in children.Citation43 However, it is likely that in our adolescent subgroup, verbally-induced expectations of potential benefit and physician/nurse-child-parent interactions had an influence on perception of pain and perceived benefit of treatment. Approximately 80% of children are considered suggestible compared to 15% in the adult population,Citation44 and work on the impact of suggestions on pain perception and placebo analgesia has shown a 5-fold (heat pain tolerance) and 3-fold (heat pain threshold) greater effect in childrenCitation45 compared with adults.Citation46
The rapid onset of analgesia with methoxyflurane and the degree of reduction in VAS pain intensity in this adolescent subgroup is comparable to that described in previous observational case series of its use in children (reported on 11-point pain scales). Babl et al reported a drop in mean (95% CI) paramedic numerical pain scores from 7.9 (7.5–8.3) at baseline to 4.5 (3.9–5.0) at 2–5 minutes and 3.2 (2.8–3.7) at 10 minutes in 105 children mostly with extremity injuries.Citation30 Gillis et al found a similar decrease in average pain score from 7.6±1.7 on arrival to 5.3±2.1 and 4.3±2.3 after 15 and 30 minutes’ treatment in the ED in a mixed population of 59 pediatric and adult patients with trauma pain.Citation31 Another observational case series of 14 children investigating methoxyflurane for procedural analgesia or as a bridging agent in the ED reported onset of analgesia within 2–5 minutes, adequate analgesia in 71% of patients, and high patient/parent/HCP satisfaction with analgesia.Citation47 These studies were observational and uncontrolled; however, an earlier pilot randomized, double-blind, placebo-controlled trial in 41 children with upper limb fractures by Chin et al showed a 2.7 point greater reduction in pain score at 10 minutes for methoxyflurane (4.0) compared with placebo (1.3; P<0.05).Citation33 Interestingly, this study by Chin et al did not show such a strong placebo effect as our adolescent subgroup analysis. Although these studies used 11-point pain scales rather than the 100 mm VAS used in the STOP! study, the degree of pain relief reported with methoxyflurane is similar to our findings in the adolescent subgroup analysis of the STOP! study.
The reduction in pain intensity observed with methoxyflurane in this adolescent subgroup is comparable with that observed for IV morphine and IN fentanyl in a similar study by Borland et al that enrolled children with acute long-bone fractures.Citation48 Borland et al reported mean changes from baseline in VAS pain at 5, 10, and 20 minutes of −25 mm, −26 mm, and −32 mm for morphine and −13 mm, −22 mm, and −31 mm for fentanyl (from a baseline mean of 67 mm and 68 mm, respectively); similar to results for methoxyflurane of −25 mm, −28 mm, and −32 mm from a baseline of 62 mm in this adolescent subgroup analysis. This is in contrast with findings of a large (N=3,312), retrospective comparative study of IV morphine, IN fentanyl, and methoxyflurane in the prehospital treatment of children with moderate-to-severe acute pain by Bendall et al, which found that methoxyflurane was significantly less effective than morphine and fentanyl, providing effective analgesia for 78.3% of patients, compared with 87.5% for morphine and 89.5% for fentanyl.Citation32 While methoxyflurane was found to be significantly less effective than IV morphine and IN fentanyl in Bendall et al’s study, methoxyflurane still provided effective analgesia for a large majority of children (78.3%) and may be a preferred treatment option given its ease of use and concerns regarding opioid-related side effects.Citation49,Citation50 Furthermore, clinical studies of methoxyflurane as procedural analgesia have suggested that it has anxiolytic properties, which may also be of benefit in distressed children with trauma injuries.Citation51,Citation52
Although the STOP! study is one of very few prospective studies of methoxyflurane, a weakness of the study is that it was placebo-controlled rather than using an active comparator.Citation53 This approach was taken due to the difficulties in double-blinding a comparator study, due to the distinctive mode of administration and odor of methoxyflurane, equipment needed for administration of nitrous oxide, and the slow onset of action of possible oral comparators such as acetaminophen or non-steroidal anti-inflammatories, as previously discussed by Coffey et al.Citation35 Another placebo-controlled trial investigating the efficacy and safety of methoxyflurane for acute, trauma-related pain in 220 children aged 6–17 years is ongoing in the UK, which will provide further data on pediatric use.Citation54
To our knowledge, no prospective studies comparing methoxyflurane with other analgesics for acute pediatric trauma pain have previously been reported. However, in a prospective cohort study, methoxyflurane had a more rapid onset of analgesic action than tramadol in 40 adult patients with ankle injuries in the ED.Citation55 A further prospective randomized study of methoxyflurane vs IV midazolam and fentanyl for procedural analgesia during colonoscopy by Nguyen et al found methoxyflurane to be equally effective with a shorter recovery time.Citation52 Further prospective studies are needed to provide direct evidence of the effectiveness of methoxyflurane analgesia compared with other analgesic agents, and a prospective open randomized study comparing pain relief between methoxyflurane and standard of care for treating patients with trauma pain in Spanish EDs (InMEDIATE) has recently completed.Citation56
Deep sedation has occasionally been reported with methoxyflurane use in younger children under 5,Citation30 but this has not been observed in studies enrolling older childrenCitation47 and was not reported in our adolescent subgroup. The most common adverse events in the methoxyflurane group were headache (25.5%), dizziness (14.9%), and somnolence (6.4%), which are all expected eventsCitation24 and were generally brief and self-limiting, resolving soon after cessation of therapy. Two patients discontinued use of methoxyflurane due to adverse events (one patient due to feeling dizzy and one patient due to light-headedness, bad taste and burning in his mouth), but the majority of patients, research nurses, and physicians rated their satisfaction with methoxyflurane treatment highly (>95% assessing treatment as “Excellent”, “Very Good” or “Good”). We found no detectable effect of methoxyflurane on the cardiovascular system or respiratory system in this adolescent subgroup, which is in agreement with the results of two earlier retrospective observational studiesCitation26,Citation57 using data from Australian ambulance services. In addition, there was no indication of any renal or hepatic effects of methoxyflurane in this subgroup analysis, or in a previous large controlled observational study of 135,770 patients receiving emergency prehospital analgesia in Australia (13% of whom received methoxyflurane).Citation58
Conclusion
The results of this adolescent subgroup analysis of the STOP! study show that low-dose inhaled methoxyflurane is an effective and well tolerated analgesic with fast onset of action in children aged 12–17 years with moderate-to-severe acute trauma pain, with no evidence that the efficacy or safety profile for such children differs to that reported in adults. Further data from prospective randomized studies with active comparators are needed to help inform optimal treatment strategies, but with its simple non-invasive method of administration and rapid onset of action, and favorable safety profile, methoxyflurane may have the potential to overcome some of the barriers to effective analgesia in adolescent patients.
Data sharing statement
Medical Developments International Limited are currently finalizing our Data Sharing Policy. The Data Sharing Policy will adhere to all ICMJE recommendations. If you have any questions or would like to request data please get in touch via the following e-mail address: [email protected].
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Penthrox® is a registered trade mark of Medical Developments International (MDI) Limited and used under license. The study was funded by MDI Limited. This article was supported by Mundipharma Research Limited.
Editorial assistance in the preparation of this manuscript was provided by Karen Mower of Scientific Editorial and funded by Mundipharma Research Limited.
Disclosure
Stuart Hartshorn reports a Chief Investigator grant and personal fees from MDI Limited outside of the submitted work. Patrick Dissmann reports personal fees from Mundipharma International, outside the submitted work. Frank Coffey reports grants from Nottingham University Hospital Trust, during the conduct of the study. Mark Lomax is an employee of Mundipharma Research Limited. The authors report no other conflicts of interest in this work.
References
- CordellWHKeeneKKGilesBKJonesJBJonesJHBrizendineEJThe high prevalence of pain in emergency medical careAm J Emerg Med200220316516911992334
- van WoerdenGvan den BrandCLden HartogCFIdenburgFJGrootendorstDCvan der LindenMCIncreased analgesia administration in emergency medicine after implementation of revised guidelinesInt J Emerg Med201691426860533
- PierikJGIjzermanMJGaakeerMIPain management in the emergency chain: the use and effectiveness of pain management in patients with acute musculoskeletal painPain Med201516597098425546003
- MotovSMKhanANProblems and barriers of pain management in the emergency department: are we ever going to get better?J Pain Res2008251121197290
- CarterDSendziukPEliottJABraunack-MayerAWhy is pain still Under-Treated in the emergency department? Two new hypothesesBioethics201630319520226104124
- PierikJGJIjzermanMJGaakeerMIVollenbroek-HuttenMMRDoggenCJMPainful discrimination in the emergency department: risk factors for Underassessment of patients’ pain by nursesJ Emerg Nurs201743322823828359711
- AlbrechtETaffePYersinBSchoettkerPDecosterdIHugliOUnder-treatment of acute pain (oligoanalgesia) and medical practice variation in prehospital analgesia of adult trauma patients: a 10 yr retrospective studyBr J Anaesth201311019610623059961
- ThomasDKircherJPlintACPediatric pain management in the emergency department: the triage nurses’ perspectiveJ Emerg Nurs201541540741325837698
- BrowneLRStudnekJRShahMIBrousseauDCGuseCELernerEBPrehospital opioid administration in the emergency care of injured childrenPrehosp Emerg Care2016201596526727339
- BrowneLRShahMIStudnekJRMulticenter evaluation of prehospital opioid pain management in injured childrenPrehosp Emerg Care201620675976727411064
- MurphyAMccoySO’ReillyKA prevalence and management study of acute pain in children attending emergency departments by ambulancePrehosp Emerg Care2016201525826024309
- LordBJenningsPASmithKThe epidemiology of pain in children treated by paramedicsEmerg Med Australas201628331932427147481
- RutkowskaASkotnicka-KlonowiczGPrehospital pain management in children with traumatic injuriesPediatr Emerg Care201531531732025651478
- DongLDonaldsonAMetzgerRKeenanHAnalgesic administration in the emergency department for children requiring hospitalization for long-bone fracturePediatr Emerg Care2012282111422193690
- MilaniGPBeniniFDell’eraLAcute pain management: acetaminophen and ibuprofen are often under-dosedEur J Pediatr2017176797998228600631
- HartshornSO’SullivanRMaconochieIKBevanCCleughFLyttleMPaediatric emergency Researcg in the UK and Ireland (PERUKI). establishing the research priorities of paediatric emergency medicine clinicians in the UK and irelandEmerg Med J2015321186486825678575
- BrowneLRShahMIStudnekJR2015 pediatric research priorities in prehospital carePrehosp Emerg Care201620331131626808233
- TomiKMashimoTTashiroCAlterations in pain threshold and psychomotor response associated with subanaesthetic concentrations of inhalation anaesthetics in humansBr J Anaesth19937066846868329263
- CrandellWBPappasSGMacdonaldANephrotoxicity associated with methoxyflurane anesthesiaAnesthesiology19662755916075918999
- MazzeRIShueGLJacksonSHRenal dysfunction associated with methoxyflurane anesthesia. A randomized, prospective clinical evaluationJAMA197121622782885107910
- MazzeRIMethoxyflurane revisited: tale of an anesthetic from cradle to GraveAnesthesiology2006105484384617006084
- Australian Therapeutic Goods administration Approved product information for Penthrox2016 Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=pi&q=methoxyfluraneAccessed September 03, 2018
- New Zealand Datasheet for Penthrox2017 Available from: http://www.medsafe.govt.nz/profs/datasheet/p/penthroxinh.pdfAccessed September 03, 2018
- Penthrox® summary of product characteristics Available from: https://www.medicines.org.uk/emc/medicine/31391Accessed September 03, 2018
- GrindlayJBablFEReview article: efficacy and safety of methoxyflurane analgesia in the emergency department and prehospital settingEmerg Med Australas200921141119254307
- JohnstonSWilkesGJThompsonJAZimanMBrightwellRInhaled methoxyflurane and intranasal fentanyl for prehospital management of visceral pain in an Australian ambulance serviceEmerg Med J2011281576320466829
- BuntinePThomOBablFBaileyMBernardSPrehospital analgesia in adults using inhaled methoxyfluraneEmerg Med Australas200719650951418021102
- GaskellALJephcottCGSmithellsJRSleighJWSelf-administered methoxyflurane for procedural analgesia: experience in a tertiary Australasian centreAnaesthesia201671441742326877169
- DayanADAnalgesic use of inhaled methoxyflurane: evaluation of its potential nephrotoxicityHum Exp Toxicol20163519110025926525
- BablFEJamisonSRSpicerMBernardSInhaled methoxyflurane as a prehospital analgesic in childrenEmerg Med Australas200618440441016842312
- GillisMKeirensASteinkammCVerbelenJMuysomsWReyndersNThe use of methoxyflurane (penthrox) in the emergency departmentReg Anesth Pain Med200833Sup 1e247
- BendallJCSimpsonPMMiddletonPMEffectiveness of prehospital morphine, fentanyl, and methoxyflurane in pediatric patientsPrehosp Emerg Care201115215816521294628
- ChinRMccaskillMBrowneGLamLA randomized controlled trial of inhaled methoxyflurane pain relief in children with upper limb fractureJ Paediatr Child Health200238A1314
- CoffeyFWrightJHartshornSStop!: a randomised, double-blind, placebo-controlled study of the efficacy and safety of methoxyflurane for the treatment of acute painEmerg Med J201431861361824743584
- CoffeyFDissmannPMirzaKLomaxMMethoxyflurane analgesia in adult patients in the emergency department: a subgroup analysis of a randomized, double-blind, placebo-controlled study (stop!)Adv Ther201633112012203127567918
- CrankshawDPEagleAKomesaroffDMethoxyflurane dosage with the Penthrox inhaler for analgesia during short painful procedures [abstract]Anaesth Intensive Care2004323428
- CHMP guideline CPMP/EWP/612/00. Note for guidance on clinical investigations of medicinal products for treatment of nociceptive pain2002 Available from: https://www.ema.europa.eu/documents/scientific-guideline/note-guidance-clinical-investigation-medicinal-products-treatment-nociceptive-pain_en.pdfAccessed February 07, 2019
- JensenMPKarolyPBraverSThe measurement of clinical pain intensity: a comparison of six methodsPain19862711171263785962
- HoKSpenceJMurphyMFReview of pain-measurement toolsAnn Emerg Med19962744274328604852
- GallagherEJLiebmanMBijurPEProspective validation of clinically important changes in pain severity measured on a visual analog scaleAnn Emerg Med200138663363811719741
- CollinsSLMooreRAMcquayHJThe visual analogue pain intensity scale: what is moderate pain in millimetres?Pain1997721–295979272792
- ToddKHFunkKGFunkJPBonacciRClinical significance of reported changes in pain severityAnn Emerg Med19962744854898604867
- SimmonsKOrtizRKossowskyJPain and placebo in pediatrics: a comprehensive review of laboratory and clinical findingsPain2014155112229223525180010
- ParelladaMMorenoCMorenoMEspliegoAde PortugalEArangoCPlacebo effect in child and adolescent psychiatric trialsEur Neuropsychopharmacol2012221178779922030230
- KrummenacherPKossowskyJSchwarzCExpectancy-induced placebo analgesia in children and the role of magical thinkingJ Pain201415121282129325261340
- KrummenacherPCandiaVFolkersGSchedlowskiMSchönbächlerGPrefrontal cortex modulates placebo analgesiaPain2010148336837419875233
- BablFBarnettPPalmerGOakleyEDavidsonAA pilot study of inhaled methoxyflurane for procedural analgesia in childrenPaediatr Anaesth200717214815317238886
- BorlandMJacobsIKingBO’BrienDA randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency departmentAnn Emerg Med200749333534017067720
- HartlingLAliSDrydenDMHow safe are common analgesics for the treatment of acute pain for children? A systematic reviewPain Res Manag201620162115
- KendallJMaconochieIWongICHowardRStudyDDIASAFE studyA novel multipatient intranasal diamorphine spray for use in acute pain in children: pharmacovigilance data from an observational studyEmerg Med J201532426927324406329
- NguyenNToscanoLLawrenceMPortable inhaled methoxyflurane is feasible and safe for colonoscopy in subjects with morbid obesity and/or obstructive sleep apneaEndosc Int Open20150305E487E493
- NguyenNQToscanoLLawrenceMPatient-controlled analgesia with inhaled methoxyflurane versus conventional endoscopist-provided sedation for colonoscopy: a randomized multicenter trialGastrointest Endosc201378689290123810328
- CarleySBodyRMethoxyflurane is a better painkiller than placebo: but do we want to know more?Emerg Med J201431861024743586
- Medical Developments International LimitedA randomised, double-blind, multicentre, placebo controlled study to evaluate the safety and efficacy of methoxyflurane (PENTHROX®) for the treatment of acute pain in children and adolescents from 6 to less than 18 years of AGE (presenting to an emergency department with minor trauma) Available from: https://clinicaltrials.gov/ct2/show/NCT03215056?term=NCT03215056&rank=1.ClinicalTrials.govIdentifierNCT03215056Accessed September 03, 2018
- KonkayevAKBaymagambetovSSainovMEvaluation of clinical effectiveness of inhalatory analgesic ‘Penthrox’ for pain relief in ankle injuriesArch Balk Med Union201348239243
- Borobia PérezAMCapilla PueyoRCasal CodesidoJRInMEDIATE groupPhase IIIB, open label randomised clinical trial to compare pain relief between methoxyflurane and standard of care for treating patients with trauma pain in Spanish emergency units (InMEDIATE): study protocolIBJ Clin Pharmacol20171e0008
- OxerHFEffects of Penthrox® (methoxyflurane) as an analgesic on cardiovascular and respiratory functions in the pre-hospital settingJ Mil Veterans Health20162421420
- IgJJacobsIGHealth effects of patient given methoxyflurane in the pre-hospital setting: a data linkage studyOpen Emerg Med J20103713