Abstract
Background
The purpose of this study was to determine the effectiveness and safety of morphine sulfate extended-release capsules among primary care patients with chronic, moderate-to-severe pain using a universal precautions approach that assessed and monitored risk for opioid misuse and abuse.
Methods
This open-label, uncontrolled, multicenter, prospective study was conducted in primary care centers (n = 281) and included opioid-naïve and opioid-experienced patients with either a pain score ≥4 (0 = no pain, 10 = pain as bad as you can imagine), or with unacceptable side effects while taking opioids. The patients were treated with morphine sulfate extendedrelease capsules for up to four months. Patient-rated pain intensity (worst, least, average) over the past 24 hours (0–10 scale), pain interference with seven activities of daily living (0 = no interference, 10 = completely interferes), and adverse events were recorded.
Results
Of 1487 patients who filled at least one prescription, 561 (38%) completed the study. Patients were primarily white (87%) and female (57%); 92% had pain for more than one year; and 79% were opioid-experienced. Median age was 52 years. Decreases in mean (± standard deviation) average pain scores (baseline 6.2 ± 2.3) were −0.8 ± 2.2 at visit 2 (5–14 days later), and −1.6 ± 2.3 and −1.7 ± 2.2 at visits 3 and 4 (spaced 3–4 weeks apart), respectively, and −1.1 ± 2.4 at visit 5 (included patients withdrawn from the study who were no longer taking the study drug). A similar trend was observed for worst pain and least pain scores and for pain interference with activities. Fifty-one percent of the safety population patients and 81% in the completer population reported being satisfied or very satisfied with the study treatment. Most common adverse events were typical of opioids, ie, constipation (14%), nausea (11%), vomiting (5%), and somnolence (5%).
Conclusion
The results suggest that pain outcomes improved in patients with chronic, moderate-to-severe pain receiving morphine sulfate extended-release capsules within the context of a structured universal precautions approach in the primary care setting.
Introduction
One of the challenges associated with using opioid therapy for pain management is achieving benefit in an environment in which the misuse, abuse, and diversion of prescription pain medications have become nearly as common as use of illicit drugs.Citation1–Citation3 Before therapy is initiated, all patients under consideration for management with opioids should receive a thorough diagnostic workup and evaluation, and careful assessment of risk for opioid abuse.Citation4,Citation5 Treatment goals should be established during patient-physician discussions.Citation4 Guidelines recommend stratification of patients according to potential risk of aberrant behavior to aid in the choice of appropriate management and intervention.Citation4,Citation6 Gourlay et al have suggested using a “universal precautions” approach, modeled after that for infectious disease, whereby an appropriate minimum level of precaution is applied to all patients.Citation6 Such an approach for pain management assumes all patients considered for opioid therapy should be screened for potential opioid or other drug misuse/abuse. Once opioid therapy has been initiated, all patients should be carefully monitored, with interventions based on their underlying risk factors and any emergent issues.Citation4,Citation6
Primary care providers are the largest single group of opioid prescribers in the United States.Citation7 In general, primary care providers are the first to see patients with acute and chronic pain.Citation8,Citation9 However, there are few published studies on opioid misuse and abuse among patients on long-term opioid therapy for the management of chronic, moderate-to-severe pain in a primary care setting.Citation10,Citation11 Providers who have little specific training in pain medicine or addiction may be faced with providing pain management while managing the risks of misuse, abuse, and diversion associated with opioid analgesics.Citation8,Citation9,Citation12,Citation13
There are no large-scale studies to date assessing the effectiveness of opioids in pain management (eg, control of pain and/or improvement of function at tolerable doses) while employing a universal precautions approach in the primary care setting. The current multicenter, uncontrolled, open-label study,Citation14 conducted in the primary care setting among a broad geographically distributed population, evaluated the effectiveness and tolerability of morphine sulfate extended-release capsules (Avinza®, King Pharmaceuticals Inc, Bristol, TN, acquired by Pfizer Inc in March 2011) in patients with chronic, moderate-to-severe pain.Citation15 Effectiveness, an evaluation of treatment under real-world conditions,Citation16,Citation17 was based on pain and functional assessments. This study was also designed to assess risk of opioid misuse and abuse, and the utility of a universal precautions approach in the primary care setting.
The universal precautions approach in this study included evaluation, documentation, and monitoring for potential risk of opioid misuse and abuse during treatment, intervention when aberrant drug-related behaviors were identified, and regular assessments to ensure that pain management goals were being met.Citation6 Morphine sulfate extended-release, which contains both immediate-release and extended-release beads of morphine sulfate, is intended for once-daily administration for the relief of moderate-to-severe pain requiring around-the-clock opioid therapy for an extended period of time.Citation15
The primary study objectives were to evaluate the effectiveness and safety of morphine sulfate extended-release in a primary care setting, assess the potential risk of misuse and abuse, and monitor aberrant behaviors and interventions among patients receiving the study drug. Secondary objectives were to determine the level of compliance using a universal precautions approach to chronic pain management during the study and after study completion, and to evaluate the activities of daily living of patients on chronic opioid therapy with morphine sulfate extended-release.
This paper focuses on the effectiveness and safety of morphine sulfate extended-release and the impact of chronic pain management on activities of daily living when pain was managed using a universal precautions approach to assess and monitor pain and risk of opioid abuse; a separate paper will report the results for risk of misuse and abuse and level of compliance in this study.
Methods
Before study initiation, the protocol and informed consent form were approved by an independent central institutional review board. All patients provided signed informed consent before beginning screening/baseline procedures. The investigator was responsible for ensuring that the study was conducted in accordance with the protocol, current International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines on Good Clinical Practice, and regulatory requirements. Study centers were selected from a nationally representative list of primary care physicians with experience in prescribing opioids. Investigators were questioned about their ability to complete the study requirements, and their credentials with respect to the Drug Enforcement Administration were verified. Investigators involved in the study participated in a 1.5-hour training program conducted live or via Webcast that reviewed procedures to be used and counseling to be provided to the patients. Those investigators who completed the training also received an hour-long instructional DVD on study procedures that they could review as needed during the study.
Patients
Patients were adults (aged ≥ 21 years) who had chronic, moderate-to-severe pain for at least three months prior to study entry. They could be opioid-naïve with a pain score ≥ 4 on an 11-point numerical rating scale (0 = no pain, 10 = pain as bad as you can imagine) or opioid-experienced but with suboptimal response (numerical rating scale pain score ≥ 4 or unacceptable side effects). Patients were required to be able to read and understand English and comply with protocol requirements.
Main exclusion criteria were hypersensitivity to morphine, morphine salts, or any components of morphine sulfate extended-release, respiratory depression, acute or severe bronchial asthma or severe chronic obstructive pulmonary disease, currently taking morphine sulfate extended-release or would have required a dose of >1600 mg/day, pregnancy or breast-feeding, residing in a hospital or nursing home, or life expectancy less than two months. Patients could not have had more than two surgeries for lower back pain, or be required to undergo surgery or steroid injections for chronic pain over the next 12 weeks.
Study design
This was an open-label, nonrandomized, uncontrolled, multicenter study that included three periods, ie, screening/baseline, treatment, and end of study (). During each visit, patients underwent a series of evaluations to assess the effectiveness and safety of morphine sulfate extended-release and compliance under the universal precautions approach, to identify aberrant drug use, and to determine level of risk for misuse and abuse. At end of study and post-study, investigators completed assessments to determine their use of risk assessment tools and the universal precautions approach to pain management. Pain-related assessments will be discussed here; assessments of drug behavior and risk for misuse and abuse will be described in more detail in a separate paper.
Figure 1 Study design.
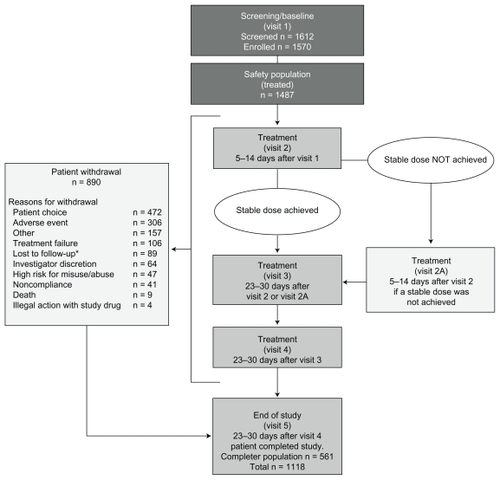
Treatment with morphine sulfate extended-release was initiated without a washout period from prior medication. The initial morphine sulfate extended-release dose was tailored to the patient (per morphine sulfate extended-release conversion tables and investigator discretion).Citation15 In general, the starting dose was to be 30 mg once daily for opioid-naïve patients. Opioid-experienced patients were to be initiated on an equivalent daily dose of morphine sulfate extended-release given once daily and instructed to destroy any remaining prior medication. The investigator was allowed to adjust the dose throughout the study to achieve a stable dose, defined as a dose that provided a pain score <4 on the numerical rating scale, required up to two doses of rescue medication daily, and provided a level of side effects deemed acceptable by patients and investigators. Determination of a stable dose was made at the discretion of the investigator. Patients were provided with a debit card for prescription medication to present to a pharmacy to receive study drugs free of charge. The card also allowed the investigator to obtain information about the date and location where the prescription was filled and the number of pills dispensed. The prescription card was reinitialized at visits 2 to 4.
Ibuprofen 200 mg (not to exceed 1200 mg/day unless directed by the prescriber) or acetaminophen 500 mg (not to exceed 4 g/day) could be taken as rescue medication; aspirin ≤325 mg/day was permitted for cardiovascular prophylaxis; other analgesic medications were not permitted. Concurrent nonanalgesic medications were permitted unless they were contraindicated for use with morphine sulfate extended-release, ibuprofen, or acetaminophen. Investigators were to take appropriate steps to prevent or minimize constipation, including recommending the use of laxatives or stool softeners.
Outcome measures
Pain outcomes were measured using a patient-completed questionnaire that included components of the Brief Pain Inventory (Short Form).Citation18 Questions included indication of any problems experienced since last visit; quantity of daily rescue medication required on average during the previous week (0 to >6 doses); rating of pain intensity (worst, least, and average) over the previous 24 hours, measured using an 11-point numerical rating scale to rate pain intensity from 0 (no pain) to 10 (pain as bad as you can imagine); how much pain relief had been achieved over the previous 24 hours from pain treatment or medications in 10% increments, from 0% (no relief) to 100% (complete relief); an indication (yes or no) whether the pain relief obtained from the current medication was enough to make a difference; and an assessment of how much pain had interfered with seven activities of daily living (general activity, mood, walking ability, normal work, relationships with other people, sleep, and enjoyment of life) during the previous 24 hours using an 11-point numerical rating scale from 0 (does not interfere) to 10 (completely interferes). The scores assessed at visits 2–5 were used to guide investigator decisions about patient pain management at each visit.
At baseline, the investigator determined each patient’s level of risk for misuse and abuse using scores obtained from the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP®-R) questionnaire.Citation19 Risk levels were then further adjusted by increasing the level if aberrant results were detected on urine drug screening and/or aberrant drug behaviors were observed, including purposeful oversedation, frequent requests for early prescription renewals, increased dose without authorization, reports of lost or stolen prescriptions, or abuse of alcohol or illicit drugs. Patients considered to be at low risk for opioid misuse and abuse were treated with morphine sulfate extended-release and monitored; those at moderate risk were treated, provided with additional counseling and reminders of their treatment agreements and responsibilities, and monitored; and those considered at high risk were to be withdrawn from the study and potentially referred to a pain specialist and/or addictionologist.
At visit 5, ie, the end-of-study visit, all patients, including those who withdrew from the study, returned to the clinic for a final evaluation of pain and activity level, adverse events, and signs of aberrant behavior using the universal precautions approach. Patients who withdrew completed this visit at the time of withdrawal if they were at the study center at the time of withdrawal; if they withdrew between visits, they returned to the study center to complete this visit. Patients and investigators evaluated therapeutic response satisfaction with treatment using patient-completed Patient Global Assessment and investigator-completed Clinician Global Assessment tools for each patient. The Patient Global Assessment allowed the patient to compare morphine sulfate extended-release with his or her usual pain medication in the following four areas: pain relief during the whole day, ability to perform daily activities, ability to sleep, and side effects using a five-point rating scale (much better, better, same, worse, much worse); and to rate their satisfaction with the medication and investigator use of universal precautions tools employed in the study using a five-point rating scale (very satisfied, satisfied, neutral, dissatisfied, very dissatisfied). Investigators used a five-point scale on the Clinician Global Assessment tool to rate their satisfaction with study medication, level of improvement, and level of utility of the universal precautions intervention regimen (very satisfied, satisfied, neutral, dissatisfied, very dissatisfied). Safety assessments included vital signs, physical examination, and adverse events, which were categorized according to the Medical Dictionary for Regulatory Activities.
Analysis populations and withdrawal from study
For this study, the safety population included all patients who enrolled and filled a prescription for morphine sulfate extended-release. The intent-to-treat population included all patients with at least one completed visit in the treatment part of the study. The completer population included those patients who completed all treatment visits.
Patients could be withdrawn from the study at any time for reasons including, but not limited to: pregnancy, patient choice, investigator discretion (eg, occurrence of a serious adverse event, changes in patient condition that rendered study participation unacceptable), sponsor termination (eg, noncompliance, administrative reasons), assignment to high-risk level for misuse/abuse of prohibited drugs, or illegal activity involving morphine sulfate extended-release. Patients who had withdrawn from the study were asked to provide a reason for discontinuation, which was recorded by the investigator; more than one reason could be given.
Statistical analysis
In this exploratory study, a sample size of 2000 patients from up to 600 centers was planned to provide 90% power to detect a change from baseline that was at least 7% of the standard deviation (SD) of the change score (one-sample t-test, significance level of 5%, two-sided). Due to later time constraints, 1612 patients were screened.
Analyses were based on all available data for the safety and completer populations. The investigator was to record all patient data and provide a documented explanation for any missing data. Descriptive statistics were used to report continuous variables, categorical variables, between subgroup comparisons, and changes from baseline. A paired t-test was used to compare changes from baseline in average pain scores in the last 24 hours at each visit and changes from baseline in pain relief at each visit. The primary outcome was the change from baseline in average pain score at each study visit. Secondary outcomes included change from baseline in the other pain scores, as well as change in pain interference with activities of daily living. Analyses were based on all available data at a time point. No imputation methods were employed. No multiple comparison adjustments were made for this exploratory study. Summaries of all available data were presented.
Results
Disposition, demographics, and baseline scores
A total of 286 primary care centers entered into the study and 281 (with 281 investigators) contributed data. The investigators were from 34 states in the United States and from Puerto Rico. Of 1612 patients screened, 1570 (97%) were enrolled, 1487 (92%) were enrolled and used the prescription card at least once (safety population), and 561 (561/1487; 38%) completed the study. There were 890 patients who withdrew during the course of the study and for whom a reason was recorded; the most common reasons for discontinuation (more than one could be provided) among these patients were patient choice (53%), adverse events (34%), and treatment failure (12%). Most patients who discontinued due to patient choice also had other reasons identified, most commonly adverse events (28%), treatment failure (16%), and investigator’s decision (4%). Patients rated as having a high risk level of misuse and abuse were required to be withdrawn from the study and accounted for 5% of discontinuations.
Results are reported based on all nonmissing data. Demographics and baseline characteristics of the safety population are shown in . The safety population was primarily female (57%), white (87%), and had chronic pain for more than one year (92%). Median age was 52 years (range 21–92 years). At baseline, 79% of patients were taking an opioid. The most common pain category, reported by 70% of patients, was musculoskeletal. The most common location of pain was the back (73%). Few patients indicated that they had a history of illicit drug use (5%) or had participated in a 12-step drug treatment program (2%). At baseline, mean (±SD) pain intensity scores were 6.2 ± 2.3 for average pain in the last 24 hours, 7.8 ± 2.5 for worst pain in the last 24 hours, and 4.7 ± 2.7 for least pain in the last 24 hours.
Table 1 Demographics and baseline characteristics
Effectiveness of morphine sulfate extended-release
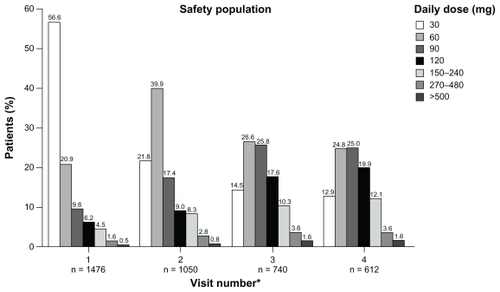
shows the daily doses of morphine sulfate extended-release prescribed at each of the study visits. Total daily doses ranged from 30 to 1440 mg. During visits 1–4 most (≥83%) patients received a total daily dose of 30–120 mg; ≤17% patients received a total daily dose of ≥150 mg and ≤8% received a total daily dose of ≥240 mg.
Figure 2 Daily doses of morphine sulfate extended-release across study visits in safety population.
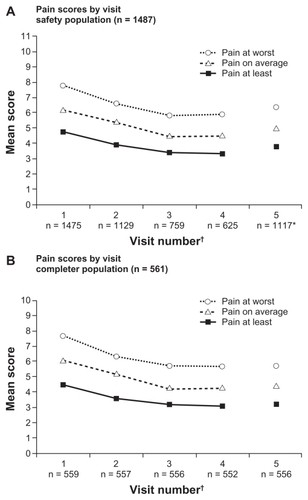
Pain intensity scores were reduced from the baseline value at each visit (P < 0.0001 for all visits). In the safety population, the mean (±SD) change from baseline in average pain intensity in the last 24 hours (baseline 6.2 ± 2.3) at each visit was: visit 2 (−0.8 ± 2.2), visit 3 (−1.6 ± 2.3), and visit 4 (−1.7 ± 2.2, ). At visit 5, which included scores from patients who had discontinued from the study as well as completed patients, the change from baseline was −1.1 ± 2.4. Similar trends were observed for pain at its worst and least in the last 24 hours at each visit. When patients were asked to report percent pain relief, mean values ranged from 46.1% to 55.4% at each visit (P < 0.0001 at visits 3 and 4; not significant at visit 2 and at visit 5, which included those patients who had discontinued).
Figure 3 Pain intensity scores in the last 24 hours by study visit in the safety (A) and completer (B) populations.
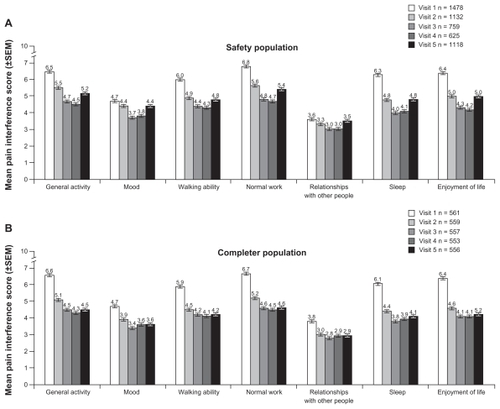
Patients experienced reductions in interference with activities of daily living scores, particularly in the general activity, normal work, sleep, enjoyment of life, and walking ability categories; smaller effects were seen in mood and relationships with other people (). In response to the question “Is the amount of pain relief you are now obtaining from your current pain reliever (morphine sulfate extended-release) enough to make a difference?”, increasing percentages of the safety population between visits 2 and 4 (51.2% at visit 2, 77.3% at visit 3, and 79.4% at visit 4) replied in the affirmative.
Figure 4 Pain interference with activities of daily living by study visit in safety (A) and completer (B) populations.
Average daily use of rescue medication was 1–4 pills for most (60%–70%) patients at each visit. The percentage of patients using more than six pills ranged from 7.3% to 10.5% across visits. The percentage of patients who had taken acetaminophen ranged from 40% to 47%. Percent use of ibuprofen ranged from 37% to 42%.
Effectiveness data were also evaluated for patients in the completer population. In this population, scores for mean (±SD) pain intensity on average also decreased from baseline (average pain in the last 24 hours 6.1 ± 1.7) at each subsequent visit, but the mean decrease from baseline in scores for average pain intensity in the last 24 hours was maintained through visit 5 and not increased from visit 4, as in the safety population (). Mean (±SD) decreases from baseline in average pain intensity scores in the last 24 hours in the completer population were −0.9 ± 2.1 at visit 2, −1.7 ± 2.2 at visit 3, −1.8 ± 2.2 at visit 4, and −1.7 ± 2.3 at visit 5. Similar trends were observed for pain at its worst and least in the last 24 hours at each visit after baseline in the completer population (). For the completer population, mean patient-reported percent relief ranged from 47% to 57%. Reductions in pain interference with activities of daily living were observed for all functions examined in the completer population ().
Global assessments at visit 5
In-clinic Patient Global Assessment values at visit 5 are shown in . Of patients in the safety population, 89% indicated that they were satisfied or very satisfied with the investigator’s use of universal precautions tools (8%, neutral; 2%, dissatisfied or very dissatisfied), and 51% indicated they were satisfied or very satisfied with their morphine sulfate extended-release treatment (18%, neutral; 30%, dissatisfied or very dissatisfied). Pain relief during the whole day was rated as better or much better by 56% (23%, same; 21%, worse or much worse); ability to perform daily activities by 45% (31%, same; 24%, worse or much worse); sleep by 40% (40%, same; 20%, worse or much worse); and side effects by 30% of patients in the safety population (29%, same; 41%, worse or much worse).
Table 2 Patient global assessment at visit 5
Of the completer population, 95% indicated they were satisfied or very satisfied with the investigator’s use of universal precautions tools (5%, neutral; <1% dissatisfied or very dissatisfied), and 81% indicated they were satisfied or very satisfied with their morphine sulfate extended-release treatment (14%, neutral; 5%, dissatisfied or very dissatisfied, ). Pain relief during the whole day was rated as better or much better by 78% (15%, same; 6%, worse or much worse); ability to perform daily activities by 68% (26%, neutral; 7%, worse or much worse); sleep by 52% (40%, same; 8%, worse or much worse); and side effects by 51% of patients (35%, same; 14%, worse or much worse) in the completer population.
Using the Clinician Global Assessment, investigators reported being satisfied or very satisfied with the use of morphine sulfate extended-release for management of moderate-to-severe pain in 63% of patients in the safety population (19%, neutral; 18%, dissatisfied or very dissatisfied) and were satisfied or very satisfied with the level of improvement in chronic pain control in 63% of patients in the safety population (18%, neutral; 19%, dissatisfied or very dissatisfied, ). In addition, investigators were satisfied or very satisfied with the utility of the universal precautions program in 75% of patients (21%, neutral; 4%, dissatisfied or very dissatisfied) in the safety population.
Table 3 Clinician global assessment at visit 5
Investigators reported being satisfied or very satisfied with morphine sulfate extended-release use for management of moderate-to-severe pain in 91% of completed patients (6%, neutral; 3%, dissatisfied or very dissatisfied). Investigators were also satisfied or very satisfied with the improvement of chronic pain control in 90% of completed patients (7%, neutral; 3%, dissatisfied or very dissatisfied) and with the utility of the universal precautions program for 87% of the completed patients (12%, neutral; 1%, dissatisfied or very dissatisfied, ).
Safety
Nearly half (48%) of the patients in the safety population experienced at least one adverse event. The most common adverse events reported were constipation (14%), nausea (11%), vomiting (5%), and somnolence (5%, see ). Adverse events were identified as one reason for withdrawal from the study in 21% of the safety population; an additional 7% of patients reported adverse events leading to withdrawal of study medication on the adverse event case report forms. The most common adverse events (≥1%) resulting in withdrawal from the study were nausea (8%; n = 114), constipation (5%; n = 77), vomiting (4%; n = 53), somnolence (3%; n = 40), fatigue (2%; n = 35), headache (2%; n = 32), dizziness (2%; n = 31), pruritus (2%; n = 26), and abdominal pain (1%; n = 18). Sixty patients (4%) in the safety population reported serious treatment-emergent adverse events, two of which (nausea) were considered treatment-related. The most common serious adverse events were pneumonia (n = 8), congestive cardiac failure (n = 4), gastrointestinal hemorrhage (n = 4), nausea (n = 4), hypoglycemia (n = 4), and acute renal failure (n = 5).
Table 4 Patients with treatment-emergent adverse events ≥1% of population
Ten deaths (0.7%) occurred during the study. Most were due to concomitant medical conditions and none were considered by the investigators to be attributable to the study drug. One death occurred in a 46-year-old man with significant diabetes and hypertension whose concomitant medications included metformin, glipizide, citalopram, and diazepam. At enrollment this patient indicated hydrocodone, nonsteroidal anti-inflammatory drugs, and tramadol as concurrent medications. The patient did not have a history of opioid abuse or recreational drug use. The starting dose of morphine sulfate extended-release was 240 mg/day. The coroner ruled that the death, which occurred five days after enrollment, was accidental secondary to the use of opiate, citalopram, and diazepam. In the investigator’s opinion, the death was not reasonably attributed to the study drug; however, the death was judged by the sponsor as reasonably attributable to the study drug.
Universal precautions
Most patients in the safety population (52%) were identified as being at moderate risk for opioid misuse and abuse at baseline, while 47% were assigned a low risk level, and 1% were assigned a high-risk level. On urine drug screen at baseline, 14% of patients were reported as positive for marijuana and 10% were reported as positive for cocaine. Positive urine drug screen results were also reported for other illicit drugs, such as phencyclidine and 3,4-methylenedioxymethamphetamine, each in 6% of patients. Positive urine drug screen results for illicit drugs were reported throughout all study visits. More detail regarding risks and incidence of misuse and abuse and levels of compliance will be reported in a separate paper.Citation20
Discussion
In this population of primary care patients with chronic, moderate-to-severe pain, in a study assessing a universal precautions approach to determine risk level for opioid misuse and abuse and provide appropriate management, patients receiving morphine sulfate extended-release experienced measurable improvement in pain control as measured by reduced pain intensity scores in both the safety and completer populations. Pain scores fell until visit 3 when the dose of morphine sulfate extended-release was stabilized. Patients in both the safety and completer populations also experienced reduced interference of pain in activities of daily living, in all functions and activities evaluated. Fifty-one percent of patients in the safety population and 81% in the completer population reported being satisfied or very satisfied with morphine sulfate extended-release treatment.
In the current study, a statistical improvement in pain scores was observed. However, it did not reach the two-point criteria for a clinically important difference based on analyses of other studies in patients with various types of chronic painCitation21,Citation22 or other studies using the same morphine sulfate extended-release formulation and a similar pain intensity scale.Citation23,Citation24 This is likely due to differences in methodology. In the current study, patients entered the trial on their pain medications without washout from previous analgesics and were not titrated to a prespecified pain intensity level before effectiveness was evaluated. They could also have been experiencing adequate relief but unsatisfactory side effects at baseline, thus limiting the potential reduction in pain scores. It was noted that patient-reported percent pain relief was numerically higher than that calculated based on decrease in pain intensity scores (46.1% to 55.4% pain relief reported by patients versus 27% calculated using pain intensity scores). The reason(s) for this difference is (are) unclear, but may include differences in patient interpretation of the scales, overestimation of pain scores, or inclusion of other considerations, such as quality of life or physical function, in assessing percent relief. Importantly, 81% of patients who completed the study reported being satisfied or very satisfied with treatment (as did 51% of all patients, including noncompleters), and by visits 3 and 4, 77% and 79% of patients, respectively, reported that pain relief was enough to make a difference. Results suggest that assessment of treatment success in future trials and in the clinical setting should include a focus on functional improvement.
The withdrawal rate in this study was 62%; the most common reasons (more than one could be provided) were patient choice, adverse events, and treatment failure. The overall rates appear toward the higher range of discontinuation rates of 38%–63% observed in other studies using opioid therapy over a 2–6-month period for management of chronic, moderate-to-severe pain,Citation16,Citation23,Citation25–Citation28 possibly due to the amount and types of monitoring required of patients. The discontinuation rates due to adverse events and treatment failure were similar to those reported in other studies.Citation16,Citation23,Citation25–Citation28 Unlike other studies, this study required that patients identified at high risk for opioid abuse be withdrawn. Although a small percentage (5%), this did contribute to the withdrawal rate observed. Other possible explanations for the high rate of withdrawal from the study included enrollment of sites that had not previously participated in a research study, patients with fear of exposure about misuse/abuse of illicit/nonprescribed drugs or who did not wish to be monitored for aberrant drug behavior, or patients who felt worse on morphine sulfate extended-release than while not taking the medication. While most patients reported being satisfied with investigator use of the universal precautions tools, it is possible that because this assessment was conducted during clinic visits, patients may not have been fully candid during their assessments. Nonetheless, the withdrawal rate observed in this study did not appear to affect assessments of the effectiveness of morphine sulfate extended-release because statistically significant improvements on pain intensity scores were seen in both the safety population as well as the completers.
As with any open-label study, the design of the study limits generalization of the conclusions. It was not possible to exclude bias. Further, although the intention was to provide a broad sampling of patients and investigators across the United States, patient demographics and investigator selection may not be representative of a “real-world” population. The recruitment of investigators who had experience in prescribing opioids may have yielded a higher proportion of patients who had previously used opioids.
Study entry criteria were based on the presence of chronic, moderate-to-severe pain. Most patients were reported to have musculoskeletal, osteoarthritic, and/or neuropathic pain, although the study protocol did not include a specific workup to establish a differential diagnosis. As in clinical practice, patients were allowed to continue taking other medications, unless they were primarily indicated for analgesia. Given that a substantial proportion of patients also had depression and/or anxiety during the study, it is possible that some of the medications they were taking for these conditions functioned as adjunctive analgesics, impacting the results. While having the potential to impact the study results, this limitation might more closely represent the situation in a real-world population of patients on a variety of medications.
The current study is the first large-scale study to assess the utility of the universal precautions approach, including risk assessment and stratification, as well as the use of morphine sulfate extended-release in the primary care setting. Future studies can build upon the knowledge by including a means of identifying how investigators make treatment and risk assessment decisions using the available information, and how these decisions impact patient outcomes.
Conclusion
In this primary care population of patients with chronic, moderate-to-severe pain, using a universal precautions approach to pain management and treatment with morphine sulfate extended-release, patients experienced decreased pain intensity scores (average, least, and worst) and reduced pain interference with activities of daily living from baseline values. The most common adverse events were those commonly seen with opioid therapy, ie, constipation, nausea, vomiting, and somnolence. Most investigators and patients were satisfied or very satisfied with the use of morphine sulfate extended-release for treatment of moderate-to-severe pain and the level of improvement in pain control attained with this therapy.
While pain relief was achieved in this study, the proportion of patients rated at moderate or greater risk for opioid misuse and abuse and the identification of illicit drug use suggest a need for continuous patient monitoring. Further education of primary care providers, and development of better strategies to aid in the identification of patients with chronic pain receiving long-term opioid therapy who may be at risk for drug misuse and abuse, may also be warranted.
Acknowledgments
The authors gratefully acknowledge the scientific contributions of Sherry Siegel and Nathaniel Katz to the study design and manuscript, Rick Slattery and the team at Clinical Marketing Consortium, Christopher Neumann, Michael Toscani, and the team at KOL, and Philip D’Alessandro and the team at YNF for coordinating the execution of the trial, Mavis Waller and the team at REGISTRAT®-MAPI Inc for carrying out the trial, including monitoring of sites and collection of study data, and Inflexxion Inc for use of the SOAPP-R. of Pfizer Inc and former employees of King Pharmaceuticals Inc, and hold stock and/or stock options in the company. L Wase is a former employee of King Pharmaceuticals Inc and holds stock and/or stock options in the company. This study was sponsored by King Pharmaceuticals Inc, which was acquired by Pfizer Inc in March 2011. Editorial/writing support was provided by Carol Berry and Julie Gerke of Quintiles Medical Communications, Parsippany, NJ, and was funded by King Pharmaceuticals Inc, which was acquired by Pfizer Inc in March 2011.
Disclosure
JB was an investigator on the trial presented here and has previously received honoraria from Abbott, AstraZeneca, Daiichi Sankyo, Forest Laboratories, GlaxoSmithKline, Novartis, and Pfizer Inc. L Webster has performed research, acted as a consultant, and served on an advisory board for King Pharmaceuticals® Inc. In addition, L Webster has performed research for Adolor Corporation, Alkermes Inc, Allergan Inc, Astellas Pharma, AstraZeneca, Bayer HealthCare, BioDelivery Sciences International, Boston Scientific, Cephalon Inc, Collegium Pharmaceutical, Covidien, Eisai Co, Ltd, Elan Corporation, Gilead Sciences, GlaxoSmithKline, Meagan Medical, Medtronic, Nektar Therapeutics, NeurogesX Inc, Shionogi USA Inc, St Renatus LLC, Sucampo Pharma Americas, Teva Pharmaceuticals, Theravance Inc, Vertex Pharmaceuticals, and Xanodyne Pharmaceuticals, and has acted as a consultant, received honoraria, and served on an advisory board for the American Board of Pain Medicine, AstraZeneca, BioDelivery Sciences International, Boston Scientific, Cephalon Inc, Covidien Mallinckrodt, Janssen Pharmaceutical KK, Nevro Corporation, PharmacoFore Inc, Purdue Pharma, and Theravance Inc. KL was an investigator on this trial for King Pharmaceuticals Inc. BS, CLR, and JMC are current employees
References
- KuehnBMOpioid prescriptions soar: increase in legitimate use as well as abuseJAMA200729724925117227967
- Substance Abuse and Mental Health Services AdministrationResults from the 2008 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-36, HHS Publication No SMA 09-4434)Rockville, MD2009 Available at: http://www.oas.samhsa.gov/nsduh/2k8nsduh/2k8results.cfmAccessed October 2, 2009
- GagnonAMKahanMSrivastavaAOpioid use and abuse: is there a problem?Clin J Pain20072366166217885343
- ChouRFanciulloGJFinePGOpioid treatment guidelines. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer painJ Pain20091011313019187889
- WasanADWoottonJJamisonRNDealing with difficult patients in your pain practiceReg Anesth Pain Med20053018419215765460
- GourlayDLHeitHAAlmahreziAUniversal precautions in pain medicine: a rational approach to the treatment of chronic painPain Med2005610711215773874
- PassikSDKirshKLForget the recipes: let’s talk dataPain Med20099261265
- OlsenYDaumitGLFordDEOpioid prescriptions by US primary care physicians from 1992 to 2001J Pain2006722523516618466
- KatzNPAdamsEHBenneyanJCFoundations of opioid risk managementClin J Pain20072310311817237659
- IvesTJChelminskiPRHammett-StablerCAPredictors of opioid misuse in patients with chronic pain: a prospective cohort studyBMC Health Serv Res200664616595013
- ReidMCEngles-HortonLLWeberMBUse of opioid medications for chronic noncancer pain syndromes in primary careJ Gen Intern Med20021717317911929502
- WiedemerNLHardenPSArndtIOGallagherRMThe opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abusePain Med2007857358417883742
- OlsenYDaumitGLOpioid prescribing for chronic nonmalignant pain in primary care: challenges and solutionsAdv Psychosom Med20042513815015248372
- King Pharmaceuticals IncEvaluation of Risk Minimization, Assessment and Outcomes in Patients With Chronic Pain Taking Avinza (ACCESS 2008)ClinicalTrialsgov [Internet]Bethesda (MD)National Library of Medicine (US)2000 [April 26, 2010]. Available from: http://clinicaltrials.gov/ct2/show/NCT00640042 NLM Identifier: NCT00640042Accessed December 8, 2010
- Avinza [package insert]Bristol, TNKing Pharmaceuticals Inc42008
- CaldwellJRRapoportRJDavisJCEfficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: results from a randomized, placebo-controlled, double-blind trial and an open-label extension trialJ Pain Symptom Manage20022327829111997197
- PortenoyRKSciberrasAEliotLSteady-state pharmacokinetic comparison of a new, extended-release, once-daily morphine formulation, Avinza, and a twice-daily controlled-release morphine formulation in patients with chronic moderate-to-severe painJ Pain Symptom Manage20022329230011997198
- CleelandCSRyanKMPain assessment: global use of the Brief Pain InventoryAnn Acad Med Singapore1994231291388080219
- ButlerSFFernandezKBenoitCBudmanSHJamisonRNValidation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R)J Pain2008936037218203666
- BrownJSetnikBLeeKAssessment, stratification, and monitoring of the risk for prescription opioid misuse and abuse in the primary care settingJ Opioid Manag
- FarrarJTYoungJPJrLaMoreauxLWerthJLPooleRMClinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scalePain20019414915811690728
- FarrarJTPritchettYLRobinsonMPrakashAChappellAThe clinical importance of changes in the 0–10 numeric rating scale for worst, least, and average pain intensity: analysis of data from clinical trials of duloxetine in pain disordersJ Pain20101110911819665938
- RauckRBookbinderSBunkerTThe ACTION study: a randomized, open label, multicenter trial comparing once-a-day extended-release morphine sulfate capsules (Avinza) to twice-a-day controlled-release oxycodone hydrochloride tablets (OxyContin) for the treatment of chronic, moderate to severe low back painJ Opioid Manage20062155166
- AdamsEHChwieckoPAce-WagonerYA study of Avinza (morphine sulfate extended-release capsules) for chronic moderate- to- severe noncancer pain conducted under real-world treatment conditions – the ACCPT StudyPain Pract2006625426417129306
- RothSHFleischmannRMBurchFXAround-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: placebo-controlled trial and long-term evaluationArch Intern Med200016085386010737286
- NicholsonBRossESasakiJWeilARandomized trial comparing polymer-coated extended-release morphine sulfate to controlled-release oxycodone HCl in moderate to severe nonmalignant painCurr Med Res Opin2006221503151416870075
- WebsterLRButeraPGMoranLVOxytrex minimizes physical dependence while providing effective analgesia: a randomized controlled trial in low back painJ Pain2006793794617157780
- KatzNRauckRAhdiehHA 12-week, randomized, placebo-controlled trial assessing the safety and efficacy of oxymorphone extended release for opioid-naive patients with chronic low back painCurr Med Res Opin20072311712817257473