Abstract
Background
Studies have shown that N-methyl-D-aspartate (NMDA) receptors play a critical role in pain processing at different levels of the central nervous system.
Methods
In this study, we used adult Wistar rats to examine gender differences in the effects of NR2B NMDA antagonism at the level of the anterior cingulate cortex in phasic pain, and in the first and second phases of a formalin test. Rats underwent stereotactic surgery for cannula implantation in the anterior cingulate cortex. After recovery, paw withdrawal latency to a noxious thermal stimulus was assessed. Rats were also subjected to a formalin pain test whereby 60 μL of 5% formalin was injected into the right hind paw.
Results
Female and male rats that received Ro 25-6981, an NR2B antagonist, before formalin injection showed significantly reduced pain responses to the formalin test compared with saline-injected control rats (P < 0.05). No gender differences in phasic pain responses were found in rats treated with Ro 25-6981.
Conclusion
These results suggest that cortical antagonism of the NR2B subunit reduces inflammatory pain levels in both genders of rat.
Introduction
N-methyl-D-aspartate (NMDA) receptors from different areas in the central nervous system, including the anterior cingulate cortex, are fundamental in the generation of inflammatory pain responses.Citation1 Tissues examined in previous studies have included the spinal cord, periaqueductal gray matter, and thalamus, and have concluded that NMDA receptor agonism generates an increase in the inflammatory pain response.Citation2–Citation6 In addition, one study has found that a decrease in the activation of NMDA receptors by means of genetic or pharmacologic manipulation generates a subsequent decrease in the inflammatory pain response.Citation7
Functional NMDA receptors are typically composed of heterogenic NR1, NR2A, NR2B, NR2C, NR2D, NR3A, and NR3B subunits.Citation8,Citation9 The NR2A and NR2B subunits are highly expressed in the frontal areas of the brain (including the anterior cingulate cortex) in rodents and humans.Citation10 One previous study reported that NR2B subunit overexpression in the insular and anterior cingulate cortices tended to increase inflammatory pain without affecting phasic pain responses. These results suggest the potential role of the NR2B subunit at the forebrain level, especially the anterior cingulate cortex, as a possible strategy for the management of persistent pain conditions.Citation10 Moreover, a study by Wu et al reported that peripheral inflammatory pain induces upregulation of NR2B receptors at the level of the anterior cingulate cortex, and antagonism of these receptors reduces behavioral sensitization related to inflammation in a Freund’s complete adjuvant model in male rodents.Citation11
Participation of the anterior cingulate cortex and other cortical areas in persistent pain responses has been described before. In particular, the anterior cingulate cortex has been related to affective components of pain as well as the sensory part of pain and integrative features of pain (affective-cognition selection of response).Citation12–Citation17
The long-term potentiation process in the anterior cingulate cortex is proposed as the molecular mechanism underlying the sensitization of chronic pain conditions. The long-term potentiation mechanism involves mainly the excitatory neurotransmitter, glutamate, and its postsynaptic receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA.Citation1,Citation17–Citation19 In addition, some authors have proposed specific exploration of NR2B subunit antagonism at the level of the anterior cingulate cortex as a strategy for reducing persistent pain and side effects in contrast with other NMDA antagonists.Citation10,Citation20 Further studies have reported gender differences in mechanisms of pain and analgesia involving NMDA receptors.Citation21–Citation23 In general, the importance of including female groups and their reproductive cycle phases in pain research and in other fields of neurobiological research has been recognized.Citation24–Citation26
A previous study has demonstrated that Ro 25-6981 is a very selective antagonist and activity-dependent blocker of NMDA receptors containing the NR2B subunit.Citation27 However, there have been few studies exploring gender differences in the role of the cortex in pain responses. The objective of the present study was to evaluate gender differences in the effects of anterior cingulate cortex NR2B receptor antagonism on phasic and tonic pain responses.
Materials and methods
Subjects
A total of 86 adult male and female Wistar rats were used. The female rats weighed 200–225 g and the male rats weighed 275–300 g. Female rats performed their behavioral tests in the estrus phase of the estrous cycle. Breeding pairs of rats were obtained from Harlan Company (Mexico City, Mexico) and bred, housed, and maintained in the animal care facilities of the Instituto de Investigaciones Científicas Avanzadas y Servicios de Alta Tecnología, Asociación de Interés Público (INDICASAT-AIP) according to Public Health Service procedures. Animals were housed in light/ dark cycles of 14 hours × 10 hours with water and food ad libitum. All the experiments adhered to the guidelines of the Committee for Research and Ethical Issues of International Association for the Study of Pain.Citation28 Moreover, the scientific work was reviewed by the scientific committee of the INDICASAT-AIP.
Stereotactic surgery and infusion
The animals were anesthetized with an intramuscular injection of ketamine 75 mg/kg + xylazine 10 mg/kg. Surgery was carried out using stereotactic apparatus. For microinfusion experiments, chronic guide cannulae were implanted according to the following procedure: double stainless steel guide cannulae (26GA, Plastics One Inc, Roanoke, VA) were implanted 1 mm over the anterior cingulate cortex injection site (AP + 2.6, D/V −1.6, M/L ± 0.6, Bregma). After chronic guide cannula implantation, the holes were closed with dummy cannulae of the same extension (0.2 mm, Plastics One Inc). One week later, the rats were administered either Ro 25-6981 (Sigma, St Louis, MO) or saline infusions through injector cannulae (33GA, Plastics One Inc) connected to tubing (PE 20, Plastics One Inc) and a Hamilton syringe (Model 7001 Gas Tight, Harvard Apparatus, Holliston, MA). All animals included in the study recovered adequately from surgery, as verified by post-surgery weight measurements.
Drug preparation
Ro 25-6981, an antagonist of the NR2B subunit of the NMDA receptor, was prepared in sterilized isotonic saline solution (0.9% NaCl). The drug was prepared at a 1 μg/0.5 μL concentration and administered at a rate of 0.5 μL/90 seconds for a total volume of 0.5 μL per side.Citation29 The drug or vehicle (isotonic saline) was administered through a cannula system connected to PE 20 tubing and a Hamilton syringe.
Behavioral testing and drug delivery
All the behavioral testing was performed by evaluators blinded to the drug treatment administered. In each test, the rats had been habituated beforehand to the behavioral apparatus (Hargreaves or formalin cages) for 40 minutes on three consecutive days.Citation7 Rats were handled by the investigator before the test on the same days of apparatus habituation. Behavioral experiments were performed during light-cycle hours; furthermore, all the animals were behaviorally assessed in groups of at least 2–4 rats to avoid any effects of isolation analgesia. The injector cannulae were left for a period of 20–30 seconds before injection of the drug or the saline control, and were left for a period of two minutes after injection of the drug. For the Hargreaves experiments, behavioral measurements (latencies) were recorded at five minutes for the control and experimental groups and at 30 minutes for the experimental groups after drug or saline injection. For the formalin test, drug or saline infusion was performed 15 minutes before and 25 minutes after formalin injection.
Hargreaves test
For the Hargreaves test, the experimental groups used were: male controls without surgery (n = 8), female controls without surgery (n = 8), males that received saline infusion (n = 7), females that received saline infusion (n = 6), males that received Ro 25-6981 infusion (n = 7), and females that received Ro 25-6981 infusion (n = 7). After cerebral infusion of the drug or vehicle, phasic pain responses were assessed using the Hargreaves test at five and 30 minutes after infusion. Citation30 Rats were placed in the Plexiglas compartments (12 cm height × 21 cm length × 10 cm width) of an analgesiometer apparatus (IITC Life Sciences, Woodland Hills, CA). A heat source was applied to the plantar surface, and the paw withdrawal latency of each hind paw was measured at 2–5 minute intervals (one latency for each hind paw). A cutoff time of 15 seconds was established for avoiding tissue damage. An average value was calculated for each rat.
Formalin test
The experimental groups used for the formalin test comprised males that received saline infusion before formalin injection (n = 10), females that received saline infusion before formalin injection (n = 6), males that received Ro 25-6981 infusion before formalin injection (n = 7), females that received Ro 25-6981 infusion before formalin injection (n = 7), males that received Ro 25-6981 infusion after formalin injection (n = 8), and females that received Ro 25-6981 infusion after formalin injection (n = 5). The formalin test was used as a model of the tonic response to inflammatory pain.Citation31–Citation34 This test was performed 72 hours after the Hargreaves test. Rats were injected subcutaneously into the plantar surface of the right hind paw with 60 μL of 5% formalin. Cerebral infusions of the drug or saline were carried out 15 minutes before and 25 minutes after formalin injection. Following formalin injection, the behavior of the rats was recorded using a digital video camera for a period of 60 minutes. Licking and/or biting of the injected hind paw was considered as an index of pain-related behavior; this behavior was recorded and quantified in seconds during time blocks of five minutes by two independent and blinded evaluators. Later, for each block of five minutes, the average score of the two evaluators was calculated.
Cannula verification
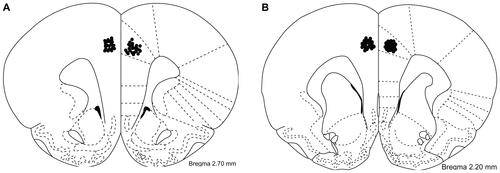
After finishing the behavioral experiments, rats were administered a lethal dose of ketamine/xylazine and received a bilateral infusion of 0.025% methylene blue in the anterior cingulate cortex. Immediately after this, the rats were perfused with isotonic saline solution (0.9% NaCl) via cardiac puncture followed by 4% formaldehyde solution. The brains were then extracted and processed as described elsewhereCitation29 and analyzed further under a light microscope. Only rats with exact cannula location in the anterior cingulate cortex were included in this investigation. The samples included in the accompanying figures describe the number of animals that passed the cannula verification procedure satisfactorily. Drawings of the histology are presented in the additional material in .
Statistical analysis
Statistical analysis was performed using analysis of variance with post hoc tests. Statistical significance was set at P < 0.05. SPSS version 19 for Windows (SSPS Inc, Chicago, IL) was used for the statistical analysis.
Results
No effects of Ro 25-6981 on phasic pain responses
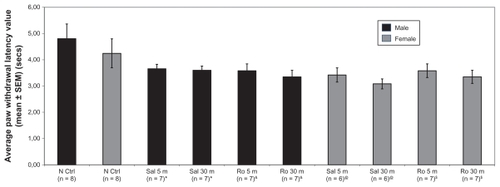
The Hargreaves test was used to determine if infusion of Ro 25-6981 into the anterior cingulate cortex could reduce phasic pain levels in rats of both genders and to exclude possible motor deficits due to stereotactic surgery. There were no significant differences between the experimental groups for mean paw withdrawal latency based on repeated-measures analysis of variance, ie, time effect (Wilk’s λ = 0.97, F[1,23] = 0.82, P = 0.38), gender effect (Wilk’s λ = 1, F[1,23] = 0.02, P = 0.96), treatment effect (Wilk’s λ = 1, F[1,23] = 0.08, P = 0.79), or interaction effect, ie, time × gender × treatment (Wilk’s λ = 0.97, F[1,23] = 0.63, P = 0.43). Moreover, comparing rats that received saline using analysis of variance did not find any gender or treatment effects (see ). This lack of difference suggests that stereotactic surgery did not induce any secondary or side effects in motor response (that otherwise could have been manifested as differences in latency).
Figure 1 Chart showing mean values of paw withdrawal latency in different groups of rats in the Hargreaves test. The paw withdrawal latency to thermal stimulus was measured in normal control rats, rats that received saline (vehicle) and rats that received Ro 25-6981.
Abbreviations: N Ctrl, naive control; Sal 5 m, saline applied at five minutes; Sal 30 m, saline applied at 30 minutes; Ro 5 m, Ro 25-6981 applied at five minutes; Ro 30 m, Ro 25-6981 applied at 30 minutes; SEM, standard error of the mean; PWL, paw withdrawal latency
Effect of Ro 25-6981 on first and second phases of the formalin test
A formalin test was used to evaluate possible gender differences in the effects of Ro 25-6981 on the first and second phases of the formalin test at different time points (infused before or after formalin injection). Gender comparisons of tonic pain responses when the drug was infused before formalin injection (0–10 minutes and 10–20 minutes in ) did not show any differences between males and females (Wilk’s λ = 0.998, F[1,31] = 0.055, P > 0.05). However, a gender difference in tonic pain response was seen when the drug was infused (30–40 minutes and 40–50 minutes in ) after formalin injection (Wilk’s λ = 0.868, F[1,33] = 5.037, P < 0.05). Specifically, male rats that received the drug displayed fewer pain-related behaviors than female rats that received drug.
Figure 2 Average paw biting/licking values of different rat groups across different treatments in the formalin test. Data are expressed as mean (±standard error of the mean) time in seconds spent licking/biting the hind paw after injection of 5% formalin 60 μL. Significant differences were found between male rats that received Ro 25-6981 before formalin injection and male rats that received saline (A) at 0–10 minutes and 10–20 minutes (P < 0.05). Moreover, significant differences were also found between female rats that received Ro 25-6981 before formalin injection and female rats that received saline (B) at 0–10 minutes (P < 0.05). Significant differences were also found between male rats that received Ro 25-6981 before formalin injection and male rats that received saline (C) at 30–40 minutes (P < 0.05), and between male rats that received Ro 25-6981 after formalin injection and male rats that received saline (D) at 30–40 minutes (P < 0.05). All analyses were performed using analysis of variance tests.
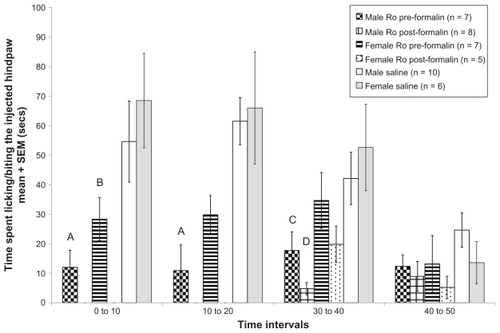
On the other hand, male rats that received the drug before formalin injection showed significantly fewer pain-related behaviors compared with male rats that received saline at 0–10 minutes and 10–20 minutes after formalin injection, according to analysis of variance (Wilk’s λ = 0.39, F[2,11] = 8.63, P < 0.05). Female rats that received the drug before formalin injection also showed fewer pain-related behaviors compared with female rats that received saline at 0–10 minutes, according to analysis of variance (Wilk’s λ = 0.431, F[2,7] = 4.62, P < 0.05, see ).
Male rats that received the drug infusion after the formalin injection also displayed fewer pain-related behaviors compared with male rats that received the saline infusion at 30–40 minutes, based on analysis of variance (Wilk’s λ = 0.527, F[4,38] = 3.59, P < 0.05; post hoc test, P < 0.05). Moreover, male rats that received the drug before the formalin injection still displayed fewer pain-related behaviors compared with rats that received saline at 30–40 minutes (Wilk’s λ = 0.527, F[4,38] = 3.59, P < 0.05; post hoc test, P < 0.05, see ). Female rats that received the drug after the formalin injection also showed fewer pain-related behaviors compared with rats that received saline, but this difference did not reach statistical significance. In general, both male and female rats that received the drug before the formalin injection showed lower levels of inflammatory pain compared with those that received saline. Moreover, male rats that received the drug after the formalin injection showed fewer pain-related behaviors compared with their saline-treated counterparts; however, female rats that received the drug after formalin injection showed fewer pain-related behaviors compared with female rats that received saline, but this tendency was not statistically significant.
Discussion
In this study, we found that Ro 25-6981 infusion was able to reduce formalin-induced pain-related behavior in both genders without affecting phasic pain responses. This study demonstrates that the analgesic action of NR2B antagonism (Ro 25-6981) applied at the level of the anterior cingulate cortex was able to reduce inflammatory pain levels in both male and female rats. A previous study by Wu et al reported a relationship between upregulation of NR2B receptors at the level of the anterior cingulate cortex and behavioral sensitization to inflammation in rodents, and that antagonism of NR2B in the anterior cingulate cortex reduced this sensitization.Citation11 However, gender differences have not been explored in the context of antagonism of NR2B at the level of the anterior cingulate cortex. In the present study, a significant reduction in inflammatory pain was found if the drug was applied before formalin injection in both males and females, indicating no gender differences in the effects of the drug on the inflammatory pain response when Ro 25-6981 is applied before formalin injection. However, the drug had a stronger analgesic effect in males than in females when administered after formalin injection. A possible explanation for this difference could be the estrogen hormone levels in female rodents; we used female rats in the estrus phase with a relatively high level of estrogen, and it has been shown that levels of estrogen and estrogen receptors (ERα and ERβ) can modulate pain-related behavior in female rodents in response to the formalin test.Citation35–Citation37
Other studies by Bereiter et al and Takeshita et al have shown that estrogen levels can modulate temporomandibular joint pain in animals and humans at the lower brainstem level, and also that estrogen levels affect morphine modulation of temporomandibular joint unit activity.Citation38,Citation39 Another study by Bereiter et al has shown that estrogen status could affect glutamatergic neurotransmission in the temporomandibular joint region by modulation of glutamate reuptake, and this could contribute to differences in pain response between males and females.Citation40
We did not find any treatment or gender difference among the experimental groups tested with the phasic pain model (Hargreaves test), suggesting that NMDA antagonism at the anterior cingulate cortex level does not influence phasic responses in either gender. Our results also suggest that there were no adverse effects of stereotactic surgery on motor coordination, because rats that received saline did not differ from the control rats in their latency response to thermal stimulation. The phasic test for exploring side effects on motor coordination processes has also been used previously.Citation3
The results of the present study are consistent with those of previous studies, ie, infusion of Ro 25-6981 in the anterior cingulate cortex is able to reduce tonic pain levels in male rodents. Wu et al have previously shown this in a complete adjuvant pain model, but add that antagonism of the anterior cingulate cortex NR2B receptors is also able to reduce inflammatory pain in female rodents.Citation11 Moreover, like our present results, the research by Wu et al reported a lack of effect of Ro 25-6981 infusion in the anterior cingulate cortex on male phasic responses. Our study further demonstrated an absence of effect of Ro 25-6981 on phasic pain responses in females.
Our results are also in accord with other research using intracisternal administration of Ro 25-6981 in an orofacial formalin pain model (injection of formalin in the vibrissae),Citation41 which essentially showed reduction of tonic pain without side effects.Citation41 The present results are also in agreement with the reduction of inflammatory pain levels and intact phasic pain response in neuropathic pain seen after electrolytic lesion of the anterior cingulate cortex.Citation42
Furthermore, our study did not find marked gender differences in the effects of cortical administration of Ro 25-6981 in inflammatory pain if applied prior to formalin injection. However, when Ro 25-6981 was applied after formalin injection, it had a stronger analgesic effect in males than in females.
The present results differ from those of another study showing, in particular, an absence of effects of another NMDA antagonist (AP5) on inflammatory pain levels after administration in the anterior cingulate cortex.Citation43 In contrast, we found that administration of a NR2B antagonist before formalin injection reduced pain intensity. Differences in our results from those in the study of AP5 cortical administration could be explained by differences in the selectivity of the NMDA antagonist used, the study samples used, and the method used for behavior analysis (eg, automated recording versus scoring of licking/biting).Citation43
The present study did not find any gender differences in the effects of Ro 25-6981 on phasic pain responses at the cortical level. Other studies that have explored the effects of Ro 25-6981 on phasic pain responses in male (but not female) rats have not found effects of Ro 25-6981 on the hot plate test (another test of phasic pain).Citation44 Moreover, a further study found that Ro 25-6981 potentiated morphine analgesia in mice of both genders in a phasic pain model (tail flick test).Citation45 It seems that Ro 25-6981 does not induce gender differences in phasic pain responses even when combined with other drugs.
In terms of connectivity, the possible circuit involved in the inhibitory effects of Ro 25-6981 on tonic pain found in this study could lie in the corticoreticular and corticoventromedullar pathways that have synapses at the dorsal horn levels of the spinal cord, and reduce pain input from the spinal cord.Citation46,Citation47 Indeed, studies on anterior cingulate cortex inhibition of visceral pain suggest that the anterior cingulate cortex could mediate inhibition or enhancement of spinal nociception via the dorsal reticular nucleus and rostral ventral medulla.Citation48
Some investigators consider phosphorylation of the NR2B subunit to be responsible for induction and continuation of the sensitization process seen in neuropathic pain conditions, so future studies could explore antagonism of the NR2B subunit at the anterior cingulate cortex level in other models of chronic pain, such as cancer pain, low back pain, and other newer models like chronic inflammatory joint pain.Citation1,Citation49,Citation50 The novel contribution of this work is that it demonstrates the lack of a gender difference in antagonism of the NR2B subunit at the level of the anterior cingulate cortex with regard to the response to phasic pain, but does uncover some gender differences in the tonic inflammatory pain response. A limitation of this study was the use of females in the estrus phase only. In conclusion, our study found that antagonism of the NR2B subunit at the level of the anterior cingulate cortex can reduce inflammatory pain levels without affecting phasic pain responses or motor responses in both female and male rats.
Acknowledgments
This study was supported by SENACYT (Secretaría Nacional de Ciencia, Tecnología e Innovación de Panamá), PRB08-002 awarded to GQ. Also, grant INF08-017 from SENACYT was awarded to G Britton and G Quintero helping to buy equipment. We thank Dr KS Jagannatha Rao and Dr Gabrielle Britton for revision of the text. We also thank Dr Robert Beatty and his team from Sustainable Sciences Institute (SSI) for their revision and input to the manuscript, and INDICASAT (Instituto de Investigaciones Científicas y Servicios de Alta Tecnología) for use of its animal colony facilities.
Supplementary figure
Disclosure
The authors report no conflicts of interest in this work.
References
- WuLJZhuoMTargeting the NMDA receptor subunit NR2B for the treatment of neuropathic painNeurotherapeutics20096469370219789073
- CoderreTJMelzackRThe role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injuryJ Neurosci1992129367136751326611
- VaccarinoALClemmonsHRMaderGJJrMagnussonJEA role of periaqueductal grey NMDA receptors in mediating formalin-induced pain in the ratNeurosci Lett199723621171199404825
- SouthSMKohnoTKasparBKA conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced painJ Neurosci200323125031504012832526
- EatonSASaltTEThalamic NMDA receptors and nociceptive sensory synaptic transmissionNeurosci Lett199011032973021970146
- KolhekarRMurphySGebhartGFThalamic NMDA receptors modulate inflammation-produced hyperalgesia in the ratPain199771131409200171
- QuinteroGCErzurumluRSVaccarinoALDecreased pain response in mice following cortex-specific knockout of the N-methyl-D-aspartate NR1 subunitNeurosci Lett20074252899317822844
- HollmannMHeinemannSCloned glutamate receptorsAnnu Rev Neurosci199417311088210177
- DingledineRBorgesKBowieDTraynelisSFThe glutamate receptor ion channelsPharmacol Rev199951176110049997
- WeiFWangGDKerchnerGAGenetic enhancement of inflammatory pain by forebrain NR2B overexpressionNat Neurosci20014216416911175877
- WuLJToyodaHZhaoMGUpregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammationJ Neurosci20052548111071111616319310
- CorkinSHNSubjective estimates of chronic pain before and after psychosurgery treatment in a pain unitPain19811150
- de LeeuwRAlbuquerqueROkesonJCarlsonCThe contribution of neuroimaging techniques to the understanding of supraspinal pain circuits: implications for orofacial painOral Surg Oral Med Oral Pathol Oral Radiol Endod2005100330831416122658
- PlonerMSchnitzlerACortical representation of painNervenarzt20047510962969 German15184984
- ShibasakiHCentral mechanisms of pain perceptionSuppl Clin Neurophysiol200457394916106604
- TalbotJDVillemureJGBushnellMCDuncanGHEvaluation of pain perception after anterior capsulotomy: a case reportSomatosens Mot Res19951221151267502602
- ZhuoMA synaptic model for pain: long-term potentiation in the anterior cingulate cortexMol Cells200723325927117646700
- ZhuoMMolecular mechanisms of pain in the anterior cingulate cortexJ Neurosci Res200684592793316862566
- Lujan-MirasRMetabotropic glutamate receptors: new molecular targets in the treatment of neurological and psychiatric diseasesRev Neurol20054014353 Spanish15696426
- BoyceSWyattAWebbJKSelective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal hornNeuropharmacology199938561162310340299
- KavaliersMCholerisESex differences in N-methyl-D-aspartate involvement in kappa opioid and non-opioid predator-induced analgesia in miceBrain Res19977681–230369369297
- VaccarinoALMarekPSternbergWLiebeskindJCNMDA receptor antagonist MK-801 blocks non-opioid stress-induced analgesia in the formalin testPain19925011191231387468
- MogilJSSternbergWFKestBMarekPLiebeskindJCSex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacementPain199353117258316385
- BeckerJBArnoldAPBerkleyKJStrategies and methods for research on gender differences in brain and behaviorEndocrinology200514641650167315618360
- MogilJSChandaMLThe case for the inclusion of female subjects in basic science studies of painPain20051171–21516098670
- MogilJSDavisKDDerbyshireSWThe necessity of animal models in pain researchPain20101511121720696526
- FischerGMutelVTrubeGRo 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitroJ Pharmacol Exp Ther19972833128512929400004
- ZimmermannMEthical guidelines for investigations of experimental pain in conscious animalsPain19831621091106877845
- JohansenJPFieldsHLGlutamatergic activation of anterior cingulate cortex produces an aversive teaching signalNat Neurosci20047439840315004562
- HargreavesKDubnerRBrownFFloresCJorisJA new and sensitive method for measuring thermal nociception in cutaneous hyperalgesiaPain198832177883340425
- AlrejaMMutalikPNayarUManchandaSKThe formalin test: a tonic pain model in the primatePain1984201971056493793
- DubuissonDDennisSGThe formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and catsPain197742161174564014
- HunskaarSFasmerOBHoleKFormalin test in mice, a useful technique for evaluating mild analgesicsJ Neurosci Methods198514169764033190
- LeeIOJeongYSEffects of different concentrations of formalin on paw edema and pain behaviors in ratsJ Korean Med Sci2002171818511850594
- KubaTWuHBNazarianAEstradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female ratsHorm Behav200649444144916257405
- ManninoCASouthSMQuinones-JenabVInturrisiCEEstradiol replacement in ovariectomized rats is antihyperalgesic in the formalin testJ Pain20078433434217140856
- CoulombeMASpoonerMFGaumondICarrierJCMarchandSEstrogen receptors beta and alpha have specific pro- and anti-nociceptive actionsNeuroscience201118417218221377511
- BereiterDAOkamotoKNeurobiology of estrogen status in deep craniofacial painInt Rev Neurobiol20119725128421708314
- TakeshitaSHirataHBereiterDAIntensity coding by TMJ-responsive neurons in superficial laminae of caudal medullary dorsal horn of the ratJ Neurophysiol20018652393240411698529
- BereiterDABenettiAPAmino acid release at the spinomedullary junction after inflammation of the TMJ region in male and female ratsPain20061261–317518316901647
- YangGYWooYWParkMKBaeYCAhnDKBonfaEIntracisternal administration of NR2 antagonists attenuates facial formalin-induced nociceptive behavior in ratsJ Orofac Pain201024220321120401359
- DonahueRRLaGraizeSCFuchsPNElectrolytic lesion of the anterior cingulate cortex decreases inflammatory, but not neuropathic nociceptive behavior in ratsBrain Res20018971–213113811282366
- LeiLGSunSGaoYJZhaoZQZhangYQNMDA receptors in the anterior cingulate cortex mediate pain-related aversionExp Neurol2004189241342115380491
- MathurPGraybealCFeyderMDavisMIHolmesAFear memory impairing effects of systemic treatment with the NMDA NR2B subunit antagonist, Ro 25-6981, in mice: attenuation with ageingPharmacol Biochem Behav200991345346018809426
- NemmaniKVGriselJEStoweJRSmith-CarlissRMogilJSModulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: dependence on gender, site of antagonism, morphine dose, and timePain2004109327428315157688
- CalejesanAAKimSJZhuoMDescending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortexEur J Pain200041839610833558
- ZhangLZhangYZhaoZQAnterior cingulate cortex contributes to the descending facilitatory modulation of pain via dorsal reticular nucleusEur J Neurosci20052251141114816176356
- WuXGaoJYanJFanJOwyangCLiYRole for NMDA receptors in visceral nociceptive transmission in the anterior cingulate cortex of viscerally hypersensitive ratsAm J Physiol Gastrointest Liver Physiol20082944G918G92718258793
- GogasKRGlutamate-based therapeutic approaches: NR2B receptor antagonistsCurr Opin Pharmacol200661687416376149
- WilsonAWMedhurstSJDixonCIAn animal model of chronic inflammatory pain: pharmacological and temporal differentiation from acute modelsEur J Pain200610653754916199187