Abstract
Cyclo-oxygenase (COX)-2 selective and nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) are important in managing acute and chronic pain secondary to inflammation. As a greater understanding of the risks of gastrointestinal (GI), cardiovascular (CV) and renal events with NSAIDs use has emerged, guidelines have evolved to reflect differences in risks among NSAIDs. Updated guidelines have yet to reflect new evidence from recent trials which showed similar CV event rates with celecoxib compared to naproxen and ibuprofen, and significantly better GI tolerability for celecoxib. This practice advisory paper aims to present consensus statements and associated guidance regarding appropriate NSAID use based on a review of current evidence by a multidisciplinary group of expert clinicians. This paper is especially intended to guide primary care practitioners within Asia in the appropriate use of NSAIDs in primary care. Following a literature review, group members used a modified Delphi consensus process to determine agreement with selected recommendations. Agreement with a statement by 75% of total voting members was defined a priori as consensus. For low GI risk patients, any nonselective NSAID plus proton pump inhibitor (PPI) or celecoxib alone is acceptable treatment when CV risk is low; for high CV risk patients, low-dose celecoxib or naproxen plus PPI is appropriate. For high GI risk patients, celecoxib plus PPI is acceptable for low CV risk patients; low-dose celecoxib plus PPI is appropriate for high CV risk patients, with the alternative to avoid NSAIDs and consider opioids instead. Appropriate NSAID prescription assumes that the patient has normal renal function at commencement, with ongoing monitoring recommended. In conclusion, appropriate NSAID use requires consideration of all risks.
Introduction
Chronic pain is one of the most common causes of disability worldwide and is routinely observed in the primary care setting.Citation1 The presence of inflammation is a key underlying mechanism of chronic pain and is a key contributor to the pathophysiology of rheumatic conditions, including rheumatoid arthritis, spondyloarthropathies and osteoarthritis (OA).Citation2,Citation3 These conditions have a major impact in terms of health burden and adverse effects on quality of life in affected people throughout the world, and especially in developing countries including those in Asia.Citation4 Moreover, with an aging population, the prevalence of chronic pain will continue to rise and the role of the primary care practitioner (PCP) as care providers and prescribers of analgesic medications will become more important.
Consistent with an inflammatory mechanism, common analgesics used in the management of chronic pain include paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs). Although considered a first-line analgesic, paracetamol provides limited short-term clinical benefit and is associated with side effects of hepatotoxicity and hypertension.Citation5–Citation8 Opioids are also prescribed in cases requiring step-up pain relief and when pain is thought to have a non-inflammatory etiology. In addition to side effects of nausea, vomiting and constipation, the over-prescription of opioids has led to a sharp increase in the prevalence of opioid addiction and epidemic levels of associated morbidity and mortality.Citation9 NSAIDs, which in terms of prescribing patterns are often the bridge between paracetamol and opioids, are commonly used to treat inflammation through their actions on the cyclo-oxygenase (COX) enzyme, which is found in two distinct isoforms, COX-1 and COX-2.Citation10,Citation11 Whereas inhibition of COX-2 confers relief from inflammation and pain, COX-1 inhibition commonly leads to gastrointestinal (GI) and renal side effects.Citation12,Citation13
NSAIDs may be either nonselective in that they inhibit both COX-1 and COX-2, or selective in that they only inhibit COX-2 (coxibs).Citation13 The COX-2 selective NSAIDs, celecoxib and rofecoxib, were the first members of the new class to be introduced in the 1990s in an attempt to reduce GI side effects associated with NSAID use. However, rofecoxib was withdrawn from the market in 2004 due to its association with an increased incidence of cardiovascular (CV) adverse events.Citation14,Citation15 Research published in the decade after rofecoxib’s market withdrawal attempted to further elucidate the safety of NSAIDs, with an emphasis on determining whether the CV concerns associated with rofecoxib use were a class effect of coxibs. CV risk not only varied among different COX-2 selective NSAIDs (the CV risk was higher for rofecoxib than celecoxib), but when considered overall, serious CV events for nonselective NSAIDs compared with COX-2 selective NSAIDs occurred at approximately equal rates.Citation16 In some studies, elevated risk was associated with certain nonselective NSAIDs, such as out-of-hospital cardiac arrest and major adverse cardiac events (MACE) in the case of diclofenac.Citation17,Citation18 Thus, COX-selectivity is not binary, with the COX-2 isoform alone not defining the CV risk of an NSAID.Citation19 Moreover, NSAIDs have been shown to have differential effects on blood pressure (BP), with rofecoxib, etoricoxib and the nonselective NSAID ibuprofen demonstrating greater increases in systolic BP than celecoxib.Citation20–Citation22 This has obvious implications because hypertension is a known risk factor for CV adverse events. Finally, recent data from the PRECISION trial have shown the noninferiority of celecoxib when compared to ibuprofen and naproxen with regard to CV safety.Citation23 Ibuprofen and naproxen, in contrast, had greater GI and renal toxicity.
Among PCPs, there is often a lack of awareness of the CV, GI and renal risks associated with the use of NSAIDs.Citation24 As a result, PCPs may not routinely identify patient risk factors before prescribing NSAIDs. Conversely, there may be an overestimation of NSAID-associated risk, leading to prescription of suboptimal doses for pain relief. In addition, patients are largely unaware of the potential harms associated with nonselective NSAID use when taking over-the-counter products.Citation25 Moreover, updated practice recommendations are needed to reflect data from recent trials. At present, the only available COX-2 selective inhibitors are celecoxib, etoricoxib and parecoxib. Celecoxib and etoricoxib are available as oral preparations, whereas parecoxib is the injectable prodrug of valdecoxib. Rofecoxib and valdecoxib have been withdrawn from market due to concerns over CV safety and serious dermatological reactions, respectively; lumiracoxib has been withdrawn because of risk of liver toxicity.Citation26,Citation27 The aim of this practice advisory is to summarize the current evidence regarding NSAID use and provide updated guidance to PCPs on prescription of oral NSAIDs, with an emphasis on CV, GI and renal safety.
Materials and Methods
In November 2018, an expert meeting was convened in Kuala Lumpur, Malaysia involving a multidisciplinary group of clinicians to discuss the appropriate use of NSAIDs. The objectives of the meeting were to review current clinical data for NSAIDs including data for PRECISION and other international studies on NSAIDs, and identify knowledge gaps regarding NSAID use in Asia. Members of the group included specialists in pain management, orthopaedic surgery, neurology, cardiology, gastroenterology, nephrology and rheumatology from Indonesia (Rizaldy Pinzon, Sumariyono Sarmidi), Japan (Ken Nakata, Shuichi Tsuruoka), Korea (Ji Hyeon Ju), Malaysia (Mary Cardosa, Ozlan Kamil, Sabarul Mokhtar), Philippines (Sandra Navarra), Singapore (Kok Yuen Ho, Heng Boon Yim), Thailand (Sumapa Chaiamnuay), Vietnam (Ho Huynh Quang Tri, Nguyen Van Hung), and the United Kingdom (Ernest Choy).
At the meeting, the group agreed to develop a practice advisory document to guide Asian PCPs in the appropriate use of NSAIDs in the primary care setting. The group selected pertinent topics to include in two succeeding online meetings. Members of the group were assigned to individual topics, with group representatives conducting a MEDLINE search for relevant articles dated from January 1, 2000, and limited to English language articles. Relevant articles were selected and reviewed, and assigned members subsequently developed proposals for ten clinically relevant consensus statements relating to NSAID use to represent the group’s clinical practice recommendations for Asian PCPs. The consensus process was a modification of the Delphi method, with members of the voting group asked to rate their agreement with each recommendation on a 5-point Likert scale (ie, 5, strongly agree; 4, agree; 3, neither agree nor disagree; 2, disagree; 1, strongly disagree). Agreement by 75% of total voting members based on the proportion of members who either strongly agreed or agreed with a statement was defined a priori as consensus achieved for a statement. Consensus was achieved for all statements in the first voting round. Consequently, members of the group were not required to reconvene as originally planned to discuss modifications of the consensus statements based on feedback from the first voting round.
Current Evidence
Beyond COX selectivity, there are marked differences in the molecular and chemical properties of individual drugs even when comparing within the respective subclasses ().Citation28–Citation31 For example, ibuprofen and naproxen are both derivatives of propionic acid, whereas diclofenac is a benzeneacetic derivative; celecoxib and valdecoxib both have a sulfonamide group, whereas etoricoxib and rofecoxib have a sulfonyl group.Citation32 Although all NSAIDs are acidic compounds, the acid dissociation constant (pKa) varies from 9.7 for celecoxib to 4.0 for diclofenac.Citation31 Compared with COX-2 selective NSAIDs, the nonselective NSAIDs are weak acids. Among the COX-2 selective NSAIDs, selectivity for the COX-2 enzyme varies considerably, with greater selectivity for the discontinued drugs lumiracoxib, rofecoxib and valdecoxib, as well as etoricoxib, and lower selectivity for celecoxib.Citation19
Table 1 Molecular and Chemical Properties of Cyclo-Oxygenase (COX)-2 Selective and Nonselective Nonsteroidal Anti-Inflammatory Drugs
These differences in molecular structure and chemistry naturally confer different pharmacologic properties on the individual drugs. The weak acidity of nonselective NSAIDs confers detergent properties on account of their lipophilicity. This allows interactions with phospholipids of the brush border, increasing cell permeability, and promoting damage to the epithelial lining of the gut.Citation31,Citation33 Weaker acidity may also be associated with the loss of cellular integrity due to pH-dependent effects that involve NSAID-mediated uncoupling of oxidative phosphorylation and reduced intracellular ATP production.Citation31 The sulfonyl group associated with rofecoxib, but not the sulfonamide group of celecoxib or the chemical structures of other nonselective NSAIDs, has been shown to increase the susceptibility of low-density lipoprotein (LDL)-cholesterol and related lipids to oxidative modification independent of COX-2 inhibition.Citation34 This nonenzymatic oxidation of LDL-cholesterol contributes to atherogenesis and CV disease.
Gastrointestinal Risks
Endoscopic evidence of mucosal injury in the upper GI tract is common with chronic use of NSAIDs, affecting as many as 70% of chronic users compared with 10% of people not taking NSAIDs.Citation35 In one meta-analysis, all NSAID regimens including nonselective and COX-2 selective agents were shown to increase the risk of upper GI complications.Citation36 Although ulceration and related bleeding are much less common events, the mechanism of NSAID-mediated GI injury is the same. Previously, this was explained in terms of COX-1-dependent depletion of prostaglandins and the subsequent impairment of the protective role of prostaglandins in stimulating the synthesis and secretion of mucus and bicarbonate, as well as promoting epithelial proliferation.Citation37 However, it is now apparent that inhibition of both COX-1 and COX-2 must occur to spur gastric ulceration, with the reduced impact of COX-2 selective NSAIDs on GI toxicity thus explained by the absence of dual COX inhibition rather than any COX-1 sparing effects.Citation14,Citation37-Citation39
Factors that increase the risk of GI toxicity with NSAID use include older age, history of peptic ulcer disease/complications, Helicobacter pylori infection, high-dose NSAID use, and concomitant use of certain drugs, including corticosteroids, anticoagulants and antiplatelet agents. Age-related risk reflects the tendency of chronic pain medication use among an older age cohort (≥60 years) with an increased likelihood of comorbidities and potential complications associated with polypharmacy.Citation30,Citation31 A pooled analysis of 21 randomized controlled trials (RCTs) showed that among elderly arthritis patients, the use of celecoxib reduced the incidence of GI adverse events including abdominal pain, constipation, diarrhea, dyspepsia, flatulence and nausea compared with naproxen, ibuprofen or diclofenac.Citation40 In another meta-analysis of more than 50,000 patients enrolled in 52 RCTs, when compared with nonselective NSAIDs, celecoxib was associated with a significantly lower risk of all clinically significant GI events throughout the entire GI tract.Citation41 In the MEDAL program, which evaluated the gastrointestinal safety of etoricoxib compared with diclofenac in almost 35,000 patients with OA and rheumatoid arthritis (RA), etoricoxib was associated with significantly fewer upper GI events than diclofenac.Citation42 However, the difference between etoricoxib and diclofenac was explained by a reduction in uncomplicated events in the etoricoxib arm, but not in the more serious complicated events.
In the context of all NSAID therapy, H. pylori infection increases the risk of ulceration and bleeding, and its eradication prior to commencing long-term antiplatelet therapy is recommended to reduce GI risk.Citation31 The risk of GI toxicity increases at high NSAID doses,Citation36 but even at standard doses the risks are not negligible: the CLASS study showed that the risk of upper GI ulceration was higher for standard doses of ibuprofen or diclofenac compared with celecoxib administered at doses greater than those indicated clinically.Citation38 In addition to antiplatelet therapy, GI risk is increased with concomitant use of corticosteroids and selective serotonin reuptake inhibitors (SSRIs).Citation37,Citation43
Appropriate assessment of patient GI risk includes age, prior GI ulceration or bleeding, use of gastroprotective agents, and use of corticosteroids and other medications.Citation44,Citation45 In addition to assessment, the risk of GI toxicity with NSAID use can be mitigated through regular monitoring to facilitate the early detection of injury and appropriate treatment. Hemoglobin levels can be used as an indicator of GI injury, with low hemoglobin and hematocrit attributable to blood loss in the absence of other potential causes.Citation30 A drop in hemoglobin of ≥2 g/dL is a well-recognized surrogate endpoint for investigating NSAID-associated GI toxicity in clinical trials.
One strategy used to minimize the risk of GI complications involves the coadministration of NSAIDs with a proton pump inhibitor (PPI).Citation46 Such coadministration is generally regarded as safe and is recommended in guidelines. However, recent evidence challenging this view suggests that in addition to the adverse effects of PPIs, their coadministration with NSAIDs may potentiate the GI risks of the latter.Citation30 In particular, PPIs have been shown to alter gut microbiome composition leading to the risk of bacterial overgrowth and contributing to a low-grade, chronic inflammation that can exacerbate NSAID-induced mucosal injury of the small bowel.Citation30 In some patients at least, the use of PPIs may increase the risk of bone fractures, Clostridium difficile and other enteric infections, and gastric cancer.Citation47,Citation48 Extrapolation of results of the CONDOR trial and related studies also suggest that PPI prophylaxis may be unnecessary in some long-term NSAID users.Citation39,Citation49,Citation50 The CONDOR trial evaluated celecoxib compared with diclofenac plus omeprazole in patients with OA and RA. The findings showed that the risk of a clinically significant upper or lower GI event was lower in patients treated with a COX-2 selective NSAID compared with a nonselective NSAID plus PPI.Citation50 In patients at high risk of GI bleeding, concomitant PPI use remains an appropriate strategy for managing GI risk, particularly when prescribed in accordance with any risk factors.Citation37 In the MEDAL program, the reduction in uncomplicated GI events with etoricoxib compared with diclofenac was maintained in patients treated with PPIs.Citation42
Preventative strategies for GI toxicity may be used both for primary and secondary prevention, and include the eradication of H. pylori and use of PPIs as already discussed, together with the use of enteric-coated NSAIDs and high-dose H2 receptor antagonists (H2RAs).Citation37,Citation51 Evidence suggests that enteric-coating of NSAIDs does not reduce the incidence of upper GI complications compared with other formulations, but may shift the site of injury to more distal regions of the gut.Citation30 The use of PPI prophylaxis is likely to be more effective than the use of H2RAs, with the latter protective at high doses, but ineffective at reducing the risk of gastric ulcers at lower doses.Citation52 Although the use of PPI prophylaxis can improve GI tolerability during chronic NSAID administration and may also prevent upper GI complications, video endoscopy has shown that the risk of small bowel lesions remains even in healthy subjects.Citation39 Furthermore, the beneficial effects of PPIs on upper GI complications do not extend to the lower GI tract, with PPI use unable to prevent NSAID or aspirin-associated lower GI bleeding.Citation53 Another gastroprotective strategy involves the use of misoprostol, which is effective at preventing upper GI bleeding in high-risk patients and may be appropriate in case of intolerance to PPIs.Citation54 There is also support for H. pylori eradication particularly in Asia where the prevalence is high,Citation55–Citation57 but available data suggest that H. pylori eradication in infected patients is at best equivalent to PPIs in preventing GI complications and may even be inferior.Citation30
Cardiovascular Risks
As the previous experience with rofecoxib demonstrates, there is also the potential for adverse CV events with NSAIDs. Already discussed, the proatherogenic potential of rofecoxib which was shown in the VIGOR trial to manifest as an increased risk of CV events compared with placeboCitation14,Citation15,Citation58,Citation59 may arise as a consequence of nonenzymatic oxidation.Citation34 However, experimental evidence that COX-2 inhibition may contribute to a prothrombotic state has placed suspicion on COX-2 selective inhibitors in general.Citation60 In particular, by suppressing vasodilation and the anti-aggregation effects associated with prostacyclin production, and leaving COX-1-dependent platelet thromboxane (TX) A2 synthesis unopposed, COX-2 selective inhibitors may increase platelet aggregation via prostacyclin blockade and thus promote thrombosis.Citation58,Citation61 COX-dependent inhibition of prostaglandin synthesis may also contribute to sodium and water retention, worsening heart failure, hypertension, and effects on the renal system.Citation21,Citation62 At a mechanistic level, there are differences among NSAIDs. For example, celecoxib but not rofecoxib reduces endothelial tissue-factor expression, a key initiator of the coagulation cascade, and may thus have a lower prothrombotic potential.Citation61,Citation63
Considering CV safety, there is considerable evidence that different COX-2 selective inhibitors and non-selective NSAIDs have different CV safety profiles.Citation18,Citation23,Citation64 For example, the nonselective NSAID diclofenac presents significantly greater CV risk compared with ibuprofen, naproxen, paracetamol, and non-analgesic use.Citation18 In the MEDAL study, the COX-2 selective NSAID etoricoxib was associated with a similar risk of thrombotic events to diclofenac,Citation65 while at moderate doses, celecoxib afforded similar CV safety to ibuprofen and naproxen.Citation23 Naproxen use compared with other NSAIDs has previously been associated with a protective effect against acute myocardial infarction (MI).Citation66 However, in meta-analyses, all NSAIDs including naproxen were associated with an increased risk of acute MI,Citation67 and most increased the risk of heart failure.Citation36 Real-world data also suggest a heightened CV risk with NSAID use.Citation64 Among the discontinued COX-2 selective inhibitors, the TARGET RCT showed in more than 18,000 patients with OA that lumiracoxib had similar CV safety to ibuprofen and naproxen, irrespective of aspirin use.Citation68 However, findings of a post hoc analysis of TARGET subsequently suggested that ibuprofen may confer an increased risk of both thrombotic events and congestive heart failure events compared with lumiracoxib among aspirin users at high CV risk.Citation69 In a subsequent meta-analysis of six trials, there were no significant differences in CV outcomes between lumiracoxib and placebo or other NSAIDs in patients with OA.Citation70 The APPROVe study compared rofecoxib with placebo in patients with a history of colorectal adenomas and showed an increase in the composite endpoint of nonfatal MI, nonfatal stroke and death from CV, hemorrhagic and unknown causes for patients receiving rofecoxib.Citation71
The SCOT and PRECISION studies are useful in framing the evidence related to the CV safety of NSAIDs. SCOT enrolled patients aged 60 years and older with OA or RA and without established CV disease who were taking prescribed chronic nonselective NSAIDs.Citation72 Switching to celecoxib resulted in a similar rate of CV events as continuing on prescribed nonselective NSAIDs; GI safety was improved with celecoxib, though more patients assigned to nonselective NSAIDs remained on treatment. PRECISION assessed the noninferiority of celecoxib compared with ibuprofen and naproxen with respect to the primary composite outcome of CV death, nonfatal MI or nonfatal stroke, also in patients with OA and RA.Citation23 At approved dosages (mean daily dose 209 mg), celecoxib was associated with a significantly lower risk of GI events whereas overall CV safety was similar for the three drugs. However, allocation to ibuprofen compared with celecoxib was associated with a significant increase in systolic BP and a higher rate of new onset hypertension.Citation21 Among patients with symptomatic arthritis who had at least a moderate CV risk, patients using naproxen or ibuprofen had a significantly higher risk of a major toxicity, including time to first occurrence of MACE, important GI events, renal events, and all-cause mortality.Citation73 Among non-selective NSAIDs, naproxen may be preferred over ibuprofen. In one study, ibuprofen and diclofenac were associated with an increased early risk of out-of-hospital cardiac arrest.Citation17 Further, the use of ibuprofen in patients receiving aspirin as secondary prevention of MI may abrogate the benefits of aspirin.Citation74
The MEDAL program similarly showed differences between a nonselective and a COX-2 selective NSAID in terms of BP effects. However, unlike PRECISION whose findings favored celecoxib compared with ibuprofen, the use of etoricoxib in MEDAL was associated with significantly increased systolic BP compared with diclofenac.Citation75 A subsequent study concluded that baseline BP rather than the BP-elevating effects of etoricoxib explained the risk of thrombotic events.Citation76
Celecoxib may thus be associated with increased CV risk, but only at dosages that are substantially higher than recommended.Citation77 Indeed, greater risk of MI and MACE have been documented for higher doses of NSAIDs, with risk in the case of MI also greatest during the first month of use.Citation64,Citation67 Greater CV risk may also be associated with older age, and related concerns regarding comorbidities and polypharmacy.
Renal Risks
In addition to the GI and CV effects of NSAIDs, epidemiological and pathologic data also associate NSAID use with the potential for both acute and chronic kidney disease (CKD).Citation78–Citation84 Renal side effects which include sodium and water retention with edema, hyponatremia, hyperkalemia, and acute kidney injury may precipitate renal failure resulting in acute dialysis.Citation62,Citation85 Risk factors include older age, renal impairment, heart failure, liver disease, diabetes mellitus (DM), and concurrent prescription with antihypertensive drugs (eg, diuretics, renin-angiotensin system inhibitors).Citation62,Citation86,Citation87 Again, mechanisms of NSAID-induced kidney damage relate to inhibition of prostaglandin synthesis and are dose- and duration-dependent.Citation80,Citation81 Low levels of COX-2 are constitutively expressed in the macula densa, with COX-2 inhibition leading to a reduction in renal blood flow and resulting functional impairment. NSAIDs may also accumulate in renal tubular cells during secretion. While NSAID-induced sodium retention is COX-2-mediated, NSAID-induced reductions in glomerular filtration rate are mediated via COX-1.Citation13
Consistent with the dual COX-1/COX-2-dependent mechanisms, which predict the possibility of differences in renal toxicity for different NSAIDs, there are limited data to suggest clinically relevant differences.Citation84,Citation88 In the MEDAL program, etoricoxib had a greater risk of renovascular adverse events than diclofenac.Citation65 In PRECISION, the risk of renal events was significantly lower with celecoxib compared with ibuprofen, and was similar for celecoxib compared with naproxen.Citation23 In a meta-analysis, NSAIDs with high COX-2 selectivity had a lower association with acute kidney injury (AKI) compared to NSAIDs with low COX-2 selectivity.Citation83 Overall, NSAIDs have a low but tangible risk in causing AKI, electrolyte imbalances and increased BP, but their role in progressive kidney disease is associated only with long-term use in high cumulative doses.Citation78 In patients with CKD, withdrawal of NSAID use is recommended by nephrology consensus groups, but initiation of alternatives such as opioids conveys different and no less important drug-related concerns.Citation89,Citation90 In a study conducted in China that included age- and sex-matched controls of NSAID users, long-term (≥48 months) use of NSAIDs was independently associated with reduced renal function.Citation91 It is recommended that patients with risk factors for renal impairment have preventative strategies in place that include using the lowest effective NSAID dose for the shortest possible time, as well as monitoring renal function, fluid retention and electrolyte abnormalities.Citation62,Citation92 The concomitant use of NSAIDs and angiotensin converting enzyme (ACE) inhibitors should be avoided.
Practice Advisory Statement
Screening for CV, GI and Renal Risk Factors Prior to Initiating NSAID Therapy
Statement 1: Prior to initiating NSAID therapy for a patient, the following factors must be taken into consideration. (Level of agreement: strongly agree, 86%; agree, 14%; consensus, 100%).
Age, associated medical comorbidities, previous medical or surgical history, concomitant use of medications (particularly antiplatelet medications, anticoagulants, corticosteroids, ACE inhibitors and SSRIs), H. pylori infection, and BP monitoring should be taken into consideration before initiating NSAID therapy. The use of aspirin increases bleeding risk even at low cardioprotective doses, with bleeding risk also increased when non-aspirin NSAIDs are combined with aspirin.Citation93 Furthermore, coadministration of aspirin and most other NSAIDs can lead to drug–drug interactions, which is based on competition for access to the acetylation site of platelet-expressed COX-1. Non-aspirin NSAIDs drive the reversible, transient inhibition of platelet aggregation, thus blocking aspirin’s irreversible inhibition and potentially allowing clot formation. Although aspirin maintains its cardioprotective benefits in the presence of non-aspirin NSAIDs,Citation94 the combination of aspirin and ibuprofen has been shown to increase the risk of a CV event.Citation95,Citation96
Several meta-analyses have reviewed the potential for SSRIs to cause upper GI bleeding. All have reported an increased risk of such bleeding when SSRIs are used alone and especially in combination with NSAIDs.Citation97–Citation100 Caution is advised when there is a need to administer both medication classes in combination.
As discussed, H. pylori infection has a high prevalence in Asian patients, which has important implications for potential GI risk. NSAID use and H. pylori infection are independent risk factors for GI complications,Citation101,Citation102 with synergism for the development of peptic ulcer and bleeding found when NSAIDs are used in patients with H. pylori infection. Conversely, peptic ulcer disease is rare in people who are negative for H. pylori infection.Citation101 This provides a rationale for eradicating H. pylori in patients requiring chronic NSAID use. Moreover, eradication of H. pylori prior to the start of long-term NSAID use is associated with a reduced risk of ulcers and appears to be especially effective in NSAID drug-naïve patients.Citation103−Citation105 Patients requiring long-term treatment with NSAIDs and with epigastric pain or dyspepsia, or otherwise assessed as high GI risk, should be referred to a gastroenterologist for a detailed evaluation, including H. pylori testing and possible gastroscopy; those testing positive for H. pylori may be offered eradication therapy.
Statement 2: Consider taking a baseline complete blood count and renal function test (if not previously available) in the following patients. (Level of agreement: strongly agree, 64%; agree, 36%; consensus, 100%).
In patients with a history of renal impairment, congestive heart failure, elevated BP and/or type 2 diabetes mellitus, or the presence of unexplained anemia, consider a complete blood count and assessment of renal function prior to initiating an NSAID.
Statement 3: Use NSAIDs with caution in the following high-risk patients. (Level of agreement: strongly agree, 79%; agree, 21%; consensus, 100%).
Patients at high risk of NSAID-associated adverse events may be stratified according to GI, CV and renal risk. Patients considered to be at high GI risk are those with age greater than 65 years, use of high-dose NSAIDs, history of peptic ulcer and related complications, and concurrent use of aspirin, anti-platelet therapy or anti-coagulant therapy (and especially patients receiving double anti-platelet therapy). Patients at high CV risk are those with a history of acute coronary syndrome or percutaneous/surgical coronary revascularization, stable angina and angiographic evidence of significant coronary artery stenosis, a history of stroke/transient ischemic attack, documented significant carotid artery stenosis, or congestive heart failure. Patients at high renal risk are those with age greater than 75 years, impaired renal function based on estimated glomerular filtration rate less than 60 mL/min, and concomitant administration of an antihypertensive from any of the diuretic, angiotensin converting enzyme (ACE) inhibitor, or angiotensin receptor blocker (ARB) classes. Analgesics such as paracetamol, tramadol or codeine may be used in place of NSAIDs if the risks of NSAID treatment outweigh the potential benefits. However, the efficacy, availability and potential adverse effects of these alternatives also need to be considered in any decision-making regarding appropriate analgesia.
Choice of NSAIDs
All oral nonselective NSAIDs and COX-2 selective inhibitors have analgesic effects of a similar magnitude, but differences may exist among individual drugs in terms of potential GI, CV, renal or liver toxicities.Citation106
Statement 4: The choice of NSAID should depend on patient risk profile, pathophysiology of the pain condition, duration of therapy, and efficacy/side effects of the drug. Level of agreement: strongly agree, 93%; agree, 7%; consensus, 100%).
Statement 5: The lowest effective dose and for the shortest duration, consistent with treatment goals, remains the guiding principle. (Level of agreement: strongly agree, 100%; consensus, 100%).
Statement 6: Current GI protective therapies are generally adequate for protection of the upper GI tract of NSAID users. (Level of agreement: strongly agree, 21%; agree, 57%; consensus, 78%).
This statement was a point of contention among the group and represents a compromise from the original statement, which was formulated as: GI protective therapies are generally inadequate or inappropriate in NSAID users. Consensus was not formed for this statement, necessitating rephrasing of the statement to the above wording (Level of agreement: strongly agree, 21%; agree, 43%; consensus, 64% [not reached]). In line with the revision, the group agreed that GI protective therapies (eg, PPIs) benefit patients who require an NSAID and who have a moderate to high risk for upper GI complications.Citation37,Citation46,Citation51,Citation54 There is little or no evidence to support any protection against lower GI side effects.
Statement 7: COX-2 selective NSAIDs are superior to nonselective NSAIDs for preventing both upper and lower GI tract adverse events. (Level of agreement: strongly agree, 43%; agree, 57%; consensus, 100%).
CONDOR, a large RCT that compared upper and lower GI safety of celecoxib with that of diclofenac plus omeprazole in patients with OA and RA, was the first trial to show that GI risk throughout the GI tract was significantly reduced in patients treated with a COX-2 selective inhibitor compared with a nonselective NSAID.Citation41,Citation50 Along with the SUCCESS trial and the MEDAL program, which also showed superior upper GI safety for celecoxib and etoricoxib, respectively, compared with nonselective NSAIDs, these data support the statement that COX-2 selective NSAIDs are superior to nonselective NSAIDs in the prevention of GI adverse events.
Statement 8: Both nonselective NSAIDs and COX-2 selective NSAIDs may increase renal adverse effects. (Level of agreement: strongly agree, 36%; agree, 64%; consensus, 100%).
The findings of a meta-analysis showing that NSAIDs with high COX-2 selectivity (≥5-fold) had a lower association with AKI than NSAIDs with lower (<5-fold) COX-2 selectivity together with findings from PRECISION showing a lower risk of renal events with celecoxib compared with ibuprofen provide some evidence of differences in renal risk based on individual NSAID selection.Citation23,Citation83
Statement 9: The treatment algorithm should consider the renal function, GI risk and CV risk profile of the patient. (Level of agreement: strongly agree, 79%; agree, 21%; consensus, 100%).
All NSAIDs have features that are useful to highlight from a safety perspective. Among the nonselective NSAIDs, although diclofenac has the least risk of GI side effects, it also has the highest risk of CV events while also associated with increased risk of hepatic impairment. Based on data from PRECISION, ibuprofen not only has a higher risk than celecoxib of GI side effects, but is also associated with a higher risk of new-onset hypertension.Citation21,Citation23,Citation73 In addition, ibuprofen and diclofenac use carries a higher risk of cardiac arrest compared with celecoxib.Citation17 Based on findings of the MEDAL study, etoricoxib has a comparable risk to diclofenac of thrombotic CV events but a higher risk of renovascular events.Citation65 Etoricoxib use is also associated with dose-related increases in risk of hypertension, edema and congestive heart failure.Citation65
In patients taking aspirin for secondary stroke or coronary thrombosis prevention, COX-2 selective NSAIDs are the drugs of choice due to the potential for COX-1-associated drug–drug interactions and, in particular, the known risks of combining aspirin with ibuprofen.Citation93 While previous guidelines have recommended that naproxen plus PPI or celecoxib plus PPI can be given in patients with high GI and high CV risk, for patients taking low-dose aspirin the use of celecoxib plus PPI is the better choice. As shown in PRECISION, celecoxib has better overall GI safety than ibuprofen or naproxen despite treatment with low-dose aspirin or corticosteroids.Citation107
Based on data from PRECISION, a toxicity risk score that predicts the one-year risk of major toxicity has been validated among NSAID users.Citation108 Major toxicity included MACE, AKI, significant GI events and mortality. In the derivation cohort from PRECISION, significant variables that predicted increased risk of a major toxicity were age, male sex, history of CV disease, hypertension, DM, tobacco use, statin use, elevated serum creatinine, hematocrit level, and type of arthritis. Based on an individual patient’s calculated risk score, the patient can be classified into one of three categories, including low risk (<1%), moderate risk (1–4%) and high risk (≥4%).
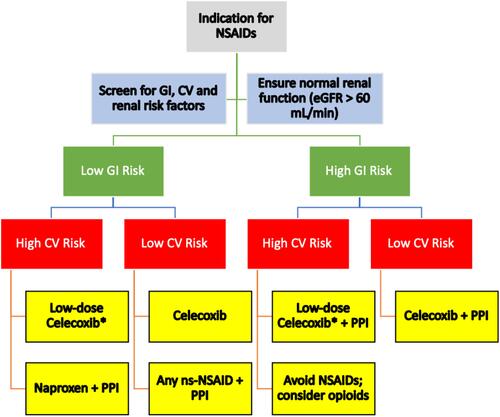
An updated treatment algorithm is provided, which supports recommended NSAID prescribing practices based on GI, CV and renal risk factors ().
Figure 1 Treatment algorithm for choice of NSAID in patients with different risk profiles. *Low-dose celecoxib = 200 mg/day. Data from Scarpignato et al 2015, Ho et al 2018.Citation31,Citation109
Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs; GI, gastrointestinal; CV, cardiovascular; eGFR, estimated glomerular filtration rate; PPI, proton pump inhibitor; ns-NSAID, nonspecific NSAID.
Statement 10: Regularly monitor for drug adverse events, including high blood pressure and signs of GI bleeding. (Level of agreement: strongly agree, 79%; agree, 21%; consensus, 100%).
BP should be measured at each visit, and laboratory tests should be conducted at least once yearly to determine blood counts and renal function.
Conclusion
Inflammation is common in many chronic pain conditions where the burden of disease is high. NSAIDs are an effective therapy for such conditions, but appropriate risk evaluation is important when selecting an NSAID in order to balance efficacy and risk. This practice advisory serves to update previously published guidelines, and in particular offers PCPs a simplified approach to choosing an appropriate NSAID for pain management based on recent evidence and according to the risk profile of individual patients. In this regard, all NSAIDs have a safety profile that requires consideration of GI, CV and renal risk. Whereas GI and CV risk were previously acknowledged according to COX-1 and COX-2 selectivity, respectively, it is apparent that this is too simplistic and appropriate risk management requires consideration of the individual (ie, non-class) effects of each NSAID. Having chosen an appropriate NSAID, there is an ongoing need for patient monitoring and risk assessment.
Acknowledgments
Medical writing support was provided by Howard Christian, PhD, and Jose Miguel (Awi) Curameng, MD, at MIMS (Hong Kong) Limited and was funded by Pfizer.
Disclosure
The meeting during which these recommendations were initially discussed was organized and funded by Pfizer. Pfizer representatives did not participate in discussions. KY Ho has received honoraria from Pfizer, Mundipharma, P&G, Avanos and A. Menarini, and reports non-financial support from MIMS HK (manuscript preparation) and personal fees from Pfizer for attending expert meeting during the conduct of the study, and personal fees from Menarini, Baxter Healthcare, Mundipharma, Johnson & Johnson, and Avanos, outside the submitted work.
M Cardosa has received honoraria for giving lectures and participating in expert group discussions for Pfizer, Mundipharma, A. Menarini and GlaxoSmithKline and reports that IASP received Pfizer Independent Grants for Learning and Change (IGLC) grant in 2017 for the project “A Toolkit for Multidisciplinary Pain Clinics in Southeast Asia”. S Chaiamnuay has received honoraria from Pfizer, Amgen, Janssen-Cilag, Astellas and Novartis.HQT Ho has received honoraria from Pfizer, A. Menarini, Merck and Boehringer-Ingelheim. O Kamil has served on advisory boards for Amgen and Pfizer, and has received honoraria from Amgen, Pfizer, MIMS, Johnson & Johnson and Merck Sharp & Dohme. S Mokhtar has received honoraria from Pfizer, Lilly and Amgen. S Navarra has received honoraria from Pfizer, Abbott, Johnson & Johnson and Novartis. R Pinzon has received honoraria from Pfizer, P&G and Soho Pharma. S Tsuruoka has received honoraria from Pfizer, Astellas, AstraZeneca, Teijin Pharma and Tanabe-Mitsubishi Pharma. HB Yim served as a principal investigator for the CONDOR trial and has served on advisory boards and received honoraria from Takeda. E Choy has received research grants and/or personal fees from, and/or served as member of advisory boards and speaker bureaus of, AbbVie, Allergan, Amgen, AstraZeneca, Bio-Cancer, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Chugai Pharma, Daiichi Sankyo, Eli Lilly, Ferring Pharmaceutical, Gilead, GlaxoSmithKline, Hospira, ISIS, Jazz Pharmaceuticals, Janssen, MedImmune, Merrimack Pharmaceutical, Merck Sharp & Dohme, Napp, Novimmune, Novartis, ObsEva, Pfizer, Regeneron, Roche, R-Pharm, Sanofi, SynAct Pharma, Synovate, Tonix and UCB. All other authors report no conflicts of interest in this work.
References
- Mills S, Torrance N, Smith BH. Identification and management of chronic pain in primary care: a review. Curr Psychiatry Rep. 2016;18(2):22. doi:10.1007/s11920-015-0659-9
- Lipnik-Stangelj M. Mediators of inflammation as targets for chronic pain treatment. Mediators Inflamm. 2013;2013:783235. doi:10.1155/2013/783235
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16–21. doi:10.1016/j.joca.2012.11.012
- Lau CS, Chia F, Dans L, et al. 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int J Rheum Dis. 2019;22(3):357–375. doi:10.1111/1756-185X.13513
- Graham GG, Day RO, Graudins A, Mohamudally A. FDA proposals to limit the hepatotoxicity of paracetamol (acetaminophen): are they reasonable? Inflammopharmacology. 2010;18(2):47–55. doi:10.1007/s10787-010-0036-6
- Sudano I, Flammer AJ, Periat D, et al. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation. 2010;122(18):1789–1796. doi:10.1161/CIRCULATIONAHA.110.956490
- Zhang W, Jones A, Doherty M. Does paracetamol (acetaminophen) reduce the pain of osteoarthritis? A meta-analysis of randomised controlled trials. Ann Rheum Dis. 2004;63(8):901–907. doi:10.1136/ard.2003.018531
- Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi:10.1136/bmj.h1225
- Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. doi:10.1146/annurev-publhealth-031914-122957
- Mitchell JA, Warner TD. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br J Pharmacol. 1999;128(6):1121–1132. doi:10.1038/sj.bjp.0702897
- Fitzpatrick FA. Cyclooxygenase enzymes: regulation and function. Curr Pharm Des. 2004;10(6):577–588. doi:10.2174/1381612043453144
- Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96(13):7563–7568. doi:10.1073/pnas.96.13.7563
- Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. J Pharm Pharm Sci. 2008;11(2):81s–110s. doi:10.18433/J3T886
- Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343(21):1520–1528, 1522 p following 1528. doi:10.1056/NEJM200011233432103
- Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–1102. doi:10.1056/NEJMoa050493
- Moore RA, Derry S, McQuay HJ. Cyclo-oxygenase-2 selective inhibitors and nonsteroidal anti-inflammatory drugs: balancing gastrointestinal and cardiovascular risk. BMC Musculoskelet Disord. 2007;8:73. doi:10.1186/1471-2474-8-73
- Sondergaard KB, Weeke P, Wissenberg M, et al. Non-steroidal anti-inflammatory drug use is associated with increased risk of out-of-hospital cardiac arrest: a nationwide case-time-control study. Eur Heart J Cardiovasc Pharmacother. 2017;3(2):100–107. doi:10.1093/ehjcvp/pvw041
- Schmidt M, Sorensen HT, Pedersen L. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ. 2018;362:k3426. doi:10.1136/bmj.k3426
- Warner TD, Mitchell JA. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet. 2008;371(9608):270–273. doi:10.1016/S0140-6736(08)60137-3
- Sowers JR, White WB, Pitt B, et al. The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med. 2005;165(2):161–168.
- Ruschitzka F, Borer JS, Krum H, et al. Differential blood pressure effects of ibuprofen, naproxen, and celecoxib in patients with arthritis: the PRECISION-ABPM (Prospective randomized evaluation of celecoxib integrated safety versus ibuprofen or naproxen ambulatory blood pressure measurement) trial. Eur Heart J. 2017;38(44):3282–3292. doi:10.1093/eurheartj/ehx508
- Walker C. Are all oral COX-2 selective inhibitors the same? A consideration of celecoxib, etoricoxib, and diclofenac. Int J Rheumatol. 2018;2018:1302835. doi:10.1155/2018/1302835
- Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375(26):2519–2529. doi:10.1056/NEJMoa1611593
- KAJ AK, Veeramuthu S, Isa HA, Sequeira RP. Prescription audit of NSAIDs and gastroprotective strategy in elderly in primary care. Int J Risk Saf Med. 2017;29(1–2):57–68. doi:10.3233/JRS-170742
- Gul SAM. Prevalence of prescribing pattern of more than one NSAID in Pakistan. J Sci Innovat Res. 2014;3(2):148–154.
- Sun SX, Lee KY, Bertram CT, Goldstein JL. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: impact on NSAID and gastroprotective drug prescribing and utilization. Curr Med Res Opin. 2007;23(8):1859–1866. doi:10.1185/030079907X210561
- Geusens P, Lems W. Efficacy and tolerability of lumiracoxib, a highly selective cyclo-oxygenase-2 (COX2) inhibitor, in the management of pain and osteoarthritis. Ther Clin Risk Manag. 2008;4(2):337–344. doi:10.2147/TCRM.S1209
- Boursinos LA, Karachalios T, Poultsides L, Malizos KN. Do steroids, conventional non-steroidal anti-inflammatory drugs and selective Cox-2 inhibitors adversely affect fracture healing? J Musculoskelet Neuronal Interact. 2009;9(1):44–52.
- Capone ML, Tacconelli S, Sciulli MG, Patrignani P. Clinical pharmacology of selective COX-2 inhibitors. Int J Immunopathol Pharmacol. 2003;16(2 Suppl):49–58.
- Gwee KA, Goh V, Lima G, Setia S. Coprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: risks versus benefits. J Pain Res. 2018;11:361–374. doi:10.2147/JPR.S156938
- Ho KY, Gwee KA, Cheng YK, Yoon KH, Hee HT, Omar AR. Nonsteroidal anti-inflammatory drugs in chronic pain: implications of new data for clinical practice. J Pain Res. 2018;11:1937–1948.
- Chang IJ, Harris RC. Are all COX-2 inhibitors created equal? Hypertension. 2005;45(2):178–180. doi:10.1161/01.HYP.0000153049.77150.d7
- Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J Gastroenterol. 2009;44(Suppl 19):23–29. doi:10.1007/s00535-008-2266-6
- Walter MF, Jacob RF, Day CA, Dahlborg R, Weng Y, Mason RP. Sulfone COX-2 inhibitors increase susceptibility of human LDL and plasma to oxidative modification: comparison to sulfonamide COX-2 inhibitors and NSAIDs. Atherosclerosis. 2004;177(2):235–243. doi:10.1016/j.atherosclerosis.2004.10.001
- Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3(1):55–59. doi:10.1016/S1542-3565(04)00603-2
- Bhala N, Emberson J, Merhi A, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–779.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52(18):1502–1517. doi:10.1016/j.jacc.2008.08.002
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284(10):1247–1255. doi:10.1001/jama.284.10.1247
- Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3(2):133–141. doi:10.1016/S1542-3565(04)00619-6
- Mallen SR, Essex MN, Zhang R. Gastrointestinal tolerability of NSAIDs in elderly patients: a pooled analysis of 21 randomized clinical trials with celecoxib and nonselective NSAIDs. Curr Med Res Opin. 2011;27(7):1359–1366. doi:10.1185/03007995.2011.581274
- Moore A, Makinson G, Li C. Patient-level pooled analysis of adjudicated gastrointestinal outcomes in celecoxib clinical trials: meta-analysis of 51,000 patients enrolled in 52 randomized trials. Arthritis Res Ther. 2013;15(1):R6. doi:10.1186/ar4134
- Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP, Committee MS. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2007;369(9560):465–473. doi:10.1016/S0140-6736(07)60234-7
- Lewis JD, Strom BL, Localio AR, et al. Moderate and high affinity serotonin reuptake inhibitors increase the risk of upper gastrointestinal toxicity. Pharmacoepidemiol Drug Saf. 2008;17(4):328–335. doi:10.1002/pds.1546
- Cheetham TC, Levy G, Spence M. Predicting the risk of gastrointestinal bleeding due to nonsteroidal antiinflammatory drugs: NSAID electronic assessment of risk. J Rheumatol. 2003;30(10):2241–2244.
- Lanas A, Tornero J, Zamorano JL. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann Rheum Dis. 2010;69(8):1453–1458. doi:10.1136/ard.2009.123166
- Scheiman JM, Yeomans ND, Talley NJ, et al. Prevention of ulcers by esomeprazole in at-risk patients using non-selective NSAIDs and COX-2 inhibitors. Am J Gastroenterol. 2006;101(4):701–710. doi:10.1111/j.1572-0241.2006.00499.x
- McCarthy DM. Adverse effects of proton pump inhibitor drugs: clues and conclusions. Curr Opin Gastroenterol. 2010;26(6):624–631. doi:10.1097/MOG.0b013e32833ea9d9
- Cheung KS, Leung WK. Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol. 2019;12:1756284819834511. doi:10.1177/1756284819834511
- Goldstein JL, Eisen GM, Lewis B, et al. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment Pharmacol Ther. 2007;25(10):1211–1222. doi:10.1111/j.1365-2036.2007.03312.x
- Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet. 2010;376(9736):173–179. doi:10.1016/S0140-6736(10)60673-3
- Lanza FL, Chan FK, Quigley EM. Practice parameters committee of the American College of G. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–738. doi:10.1038/ajg.2009.115
- Rostom A, Wells G, Tugwell P, Welch V, Dube C, McGowan J. The prevention of chronic NSAID induced upper gastrointestinal toxicity: a Cochrane collaboration metaanalysis of randomized controlled trials. J Rheumatol. 2000;27(9):2203–2214.
- Lue A, Lanas A. Protons pump inhibitor treatment and lower gastrointestinal bleeding: balancing risks and benefits. World J Gastroenterol. 2016;22(48):10477–10481. doi:10.3748/wjg.v22.i48.10477
- Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123(4):241–249. doi:10.7326/0003-4819-123-4-199508150-00001
- Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25(3):479–486. doi:10.1111/j.1440-1746.2009.06188.x
- Goh KL, Parasakthi N. The racial cohort phenomenon: seroepidemiology of Helicobacter pylori infection in a multiracial South-East Asian country. Eur J Gastroenterol Hepatol. 2001;13(2):177–183. doi:10.1097/00042737-200102000-00014
- Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19(Suppl 1):1–5. doi:10.1111/hel.12165
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954–959. doi:10.1001/jama.286.8.954
- Curfman GD, Morrissey S, Drazen JM. Expression of concern: bombardier et al., “Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis” N Engl J Med 2000;343:1520-8. N Engl J Med. 2005;353(26):2813–2814. doi:10.1056/NEJMe058314
- Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071–1080. doi:10.1056/NEJMoa050405
- Steffel J, Luscher TF, Ruschitzka F, Tanner FC. Cyclooxygenase-2 inhibition and coagulation. J Cardiovasc Pharmacol. 2006;47(Suppl 1):S15–20. doi:10.1097/00005344-200605001-00004
- Kovic SV, Vujovic KS, Srebro D, Medic B, Ilic-Mostic T. Prevention of renal complications induced by non-steroidal anti-inflammatory drugs. Curr Med Chem. 2016;23(19):1953–1964. doi:10.2174/0929867323666160210125920
- Steffel J, Akhmedov A, Fahndrich C, Ruschitzka F, Luscher TF, Tanner FC. Differential effect of celecoxib on tissue factor expression in human endothelial and vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;349(2):597–603. doi:10.1016/j.bbrc.2006.08.075
- McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8(9):e1001098. doi:10.1371/journal.pmed.1001098
- Combe B, Swergold G, McLay J, et al. Cardiovascular safety and gastrointestinal tolerability of etoricoxib vs diclofenac in a randomized controlled clinical trial (The MEDAL study). Rheumatology (Oxford). 2009;48(4):425–432. doi:10.1093/rheumatology/kep005
- Rahme E, Pilote L, LeLorier J. Association between naproxen use and protection against acute myocardial infarction. Arch Intern Med. 2002;162(10):1111–1115. doi:10.1001/archinte.162.10.1111
- Bally M, Dendukuri N, Rich B, et al. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ. 2017;357:j1909. doi:10.1136/bmj.j1909
- Farkouh ME, Kirshner H, Harrington RA, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004;364(9435):675–684. doi:10.1016/S0140-6736(04)16894-3
- Farkouh ME, Greenberg JD, Jeger RV, et al. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66(6):764–770. doi:10.1136/ard.2006.066001
- Mackenzie IS, Wei L, Macdonald TM. Cardiovascular safety of lumiracoxib: a meta-analysis of randomised controlled trials in patients with osteoarthritis. Eur J Clin Pharmacol. 2013;69(2):133–141. doi:10.1007/s00228-012-1335-1
- Baron JA, Sandler RS, Bresalier RS, et al. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet. 2008;372(9651):1756–1764. doi:10.1016/S0140-6736(08)61490-7
- MacDonald TM, Hawkey CJ, Ford I, et al. Randomized trial of switching from prescribed non-selective non-steroidal anti-inflammatory drugs to prescribed celecoxib: the Standard care vs. Celecoxib Outcome Trial (SCOT). Eur Heart J. 2017;38(23):1843–1850. doi:10.1093/eurheartj/ehw387
- Solomon DH, Husni ME, Libby PA, et al. The risk of major NSAID toxicity with celecoxib, ibuprofen, or naproxen: a secondary analysis of the PRECISION trial. Am J Med. 2017;130(12):1415–1422. doi:10.1016/j.amjmed.2017.06.028
- Hudson M, Baron M, Rahme E, Pilote L. Ibuprofen may abrogate the benefits of aspirin when used for secondary prevention of myocardial infarction. J Rheumatol. 2005;32(8):1589–1593.
- Krum H, Swergold G, Curtis SP, et al. Factors associated with blood pressure changes in patients receiving diclofenac or etoricoxib: results from the MEDAL study. J Hypertens. 2009;27(4):886–893. doi:10.1097/HJH.0b013e328325d831
- Krum H, Swergold G, Gammaitoni A, et al. Blood pressure and cardiovascular outcomes in patients taking nonsteroidal antiinflammatory drugs. Cardiovasc Ther. 2012;30(6):342–350. doi:10.1111/j.1755-5922.2011.00283.x
- Howes LG. Selective COX-2 inhibitors, NSAIDs and cardiovascular events – is celecoxib the safest choice? Ther Clin Risk Manag. 2007;3(5):831–845.
- Sriperumbuduri S, Hiremath S. The case for cautious consumption: nSAIDs in chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28(2):163–170. doi:10.1097/MNH.0000000000000473
- Singh S, Patel PS, Doley PK, et al. Outcomes of hospital-acquired acute kidney injury in elderly patients: a single-centre study. Int Urol Nephrol. 2019;51(5):875–883. doi:10.1007/s11255-019-02130-4
- Chiasson JM, Fominaya CE, Gebregziabher M, Taber DJ. Long-term assessment of NSAID prescriptions and potential nephrotoxicity risk in adult kidney transplant recipients. Transplantation. 2019;103(12):2675–2681. doi:10.1097/TP.0000000000002689
- Nelson DA, Marks ES, Deuster PA, O’Connor FG, Kurina LM. Association of nonsteroidal anti-inflammatory drug prescriptions with kidney disease among active young and middle-aged adults. JAMA Netw Open. 2019;2(2):e187896. doi:10.1001/jamanetworkopen.2018.7896
- Bell S, Rennie T, Marwick CA, Davey P. Effects of peri-operative nonsteroidal anti-inflammatory drugs on post-operative kidney function for adults with normal kidney function. Cochrane Database Syst Rev. 2018;11:CD011274.
- Zhang X, Donnan PT, Bell S, Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2017;18(1):256. doi:10.1186/s12882-017-0673-8
- Chang YK, Liu JS, Hsu YH, Tarng DC, Hsu CC. Increased risk of End-Stage Renal Disease (ESRD) requiring chronic dialysis is associated with use of nonsteroidal anti-inflammatory drugs (NSAIDs): nationwide case-crossover study. Medicine (Baltimore). 2015;94(38):e1362. doi:10.1097/MD.0000000000001362
- Wehling M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol. 2014;70(10):1159–1172. doi:10.1007/s00228-014-1734-6
- Abd ElHafeez S, Hegazy R, Naga Y, Wahdan I, Sallam S. Non-steroidal anti-inflammatory drugs among chronic kidney disease patients: an epidemiological study. J Egypt Public Health Assoc. 2019;94(1):8. doi:10.1186/s42506-018-0005-2
- Tsai HJ, Hsu YH, Huang YW, Chang YK, Liu JS, Hsu CC. Use of non-steroidal anti-inflammatory drugs and risk of chronic kidney disease in people with Type 2 diabetes mellitus, a nationwide longitudinal cohort study. Diabet Med. 2015;32(3):382–390. doi:10.1111/dme.12610
- Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Eur J Intern Med. 2015;26(4):285–291. doi:10.1016/j.ejim.2015.03.008
- Zhan M, St Peter WL, Doerfler RM, et al. Patterns of NSAIDs use and their association with other analgesic use in CKD. Clin J Am Soc Nephrol. 2017;12(11):1778–1786. doi:10.2215/CJN.12311216
- Keohane DM, Dennehy T, Keohane KP, Shanahan E. Reducing inappropriate non-steroidal anti-inflammatory prescription in primary care patients with chronic kidney disease. Int J Health Care Qual Assur. 2017;30(7):638–644. doi:10.1108/IJHCQA-09-2016-0145
- Pan Y, Zhang L, Wang F, Li X, Wang H. China National Survey of Chronic Kidney Disease Working G. Status of non-steroidal anti-inflammatory drugs use and its association with chronic kidney disease: a cross-sectional survey in China. Nephrology (Carlton). 2014;19(10):655–660. doi:10.1111/nep.12318
- Nderitu P, Doos L, Jones PW, Davies SJ, Kadam UT. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: a systematic review. Fam Pract. 2013;30(3):247–255. doi:10.1093/fampra/cms086
- Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061–1075. doi:10.2147/TCRM.S79135
- Alqahtani Z, Jamali F. Clinical outcomes of aspirin interaction with other non-steroidal anti-inflammatory drugs: a systematic review. J Pharm Pharm Sci. 2018;21(1s):29854. doi:10.18433/jpps29854
- Kurth T, Glynn RJ, Walker AM, et al. Inhibition of clinical benefits of aspirin on first myocardial infarction by nonsteroidal antiinflammatory drugs. Circulation. 2003;108(10):1191–1195. doi:10.1161/01.CIR.0000087593.07533.9B
- MacDonald TM, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet. 2003;361(9357):573–574. doi:10.1016/S0140-6736(03)12509-3
- Anglin R, Yuan Y, Moayyedi P, Tse F, Armstrong D, Leontiadis GI. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(6):811–819. doi:10.1038/ajg.2014.82
- Jiang HY, Chen HZ, Hu XJ, et al. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13(1):42–50. doi:10.1016/j.cgh.2014.06.021
- Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27(1):31–40. doi:10.1111/j.1365-2036.2007.03541.x
- Oka Y, Okamoto K, Kawashita N, Shirakuni Y, Takagi T. Meta-analysis of the risk of upper gastrointestinal hemorrhage with combination therapy of selective serotonin reuptake inhibitors and non-steroidal anti-inflammatory drugs. Biol Pharm Bull. 2014;37(6):947–953. doi:10.1248/bpb.b13-00885
- Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359(9300):14–22. doi:10.1016/S0140-6736(02)07273-2
- Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4(2):130–142. doi:10.1016/j.cgh.2005.10.006
- Chan FK, To KF, Wu JC, et al. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial. Lancet. 2002;359(9300):9–13. doi:10.1016/S0140-6736(02)07272-0
- Papatheodoridis GV, Archimandritis AJ. Role of Helicobacter pylori eradication in aspirin or non-steroidal anti-inflammatory drug users. World J Gastroenterol. 2005;11(25):3811–3816. doi:10.3748/wjg.v11.i25.3811
- Vergara M, Catalan M, Gisbert JP, Calvet X. Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther. 2005;21(12):1411–1418. doi:10.1111/j.1365-2036.2005.02444.x
- Excellence NIfHaC. Osteoarthritis: care and management (CG177). National Institute for Health and Care Excellence; 2014.
- Yeomans ND, Graham DY, Husni ME, et al. Randomised clinical trial: gastrointestinal events in arthritis patients treated with celecoxib, ibuprofen or naproxen in the PRECISION trial. Aliment Pharmacol Ther. 2018;47(11):1453–1463. doi:10.1111/apt.14610
- Solomon DH, Shao M, Wolski K, Nissen S, Husni ME, Paynter N. Derivation and validation of a major toxicity risk score among nonsteroidal antiinflammatory drug users based on data from a randomized controlled trial. Arthritis Rheumatol. 2019;71(8):1225–1231. doi:10.1002/art.40870
- Scarpignato C, Lanas A, Blandizi C, et al. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis--an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:55. 10.1186/s12916-015-0285-8
