Abstract
Background and Objective
Treatment of peripheral neuropathic pain (PNP) remains a challenge. In the absence of clear predictors of response, clinical decision-making involves trial and error. While many classes of pharmacological agent are used and have shown efficacy, one of the most commonly used first-line treatments is pregabalin. However, in the 60% of PNP cases in which the pain is localized, a local treatment may be more suitable. This article will summarize the evidence for the relative effectiveness and tolerability of the capsaicin 179 mg patch and pregabalin in the treatment of PNP and highlight the expert opinion of the authors based on their own clinical experiences.
Results
When compared in a head-to-head trial in patients with PNP, capsaicin 179 mg patch provided non-inferior pain relief compared with an optimized dose of pregabalin, as well as a reduction in dynamic mechanical allodynia, faster onset of action, fewer systemic side effects, and greater treatment satisfaction. Adverse events associated with capsaicin patch are mainly application site reactions, compared with systemic and central nervous system effects with pregabalin. Studies indicate that capsaicin 179 mg patch is associated with a lower burden of therapy than pregabalin in terms of improved tolerability, lack of a daily pill burden, lack of drug–drug interactions, and increased regimen flexibility.
Conclusion
In localized neuropathic pain, evidence supports a pragmatic approach of using a local treatment before considering a systemic treatment. For treatment selection, the patient profile (eg, concomitant medication use, age) and the treatments’ efficacy and tolerability profiles should be considered.
Plain Language Summary
Peripheral neuropathic pain (PNP) is pain caused by damage or disease of the peripheral somatosensory nervous system. In localized PNP, the pain can be located to a well-defined area of the body. Control of PNP is often challenging, as many patients' PNP does not respond to oral therapies. This expert opinion highlights the relevance of a local therapy, capsaicin 179 mg patch, for the treatment of localized PNP and shows that this treatment compares favorably with pregabalin, a well-established oral treatment. This expert opinion is based on analyses of both indirect (meta-analysis) and direct head-to-head comparisons between systemic and local treatments. In a randomized trial, capsaicin 179 mg patch offered comparable pain relief to pregabalin, with a faster onset of pain relief, fewer systemic side effects, a reduced burden of treatment, and a higher reported patient satisfaction. Capsaicin 179 mg patch is not associated with a daily pill burden and is unlikely to have drug–drug interactions, so it is appropriate for use in combination therapy. Patients who receive capsaicin 179 mg patch early are more likely to respond than those who receive it later. For localized PNP, it is logical to start with a local therapy such as capsaicin 179 mg patch before moving to an oral therapy if the local therapy does not work. Pregabalin is a more suitable option for facial pain or central neuropathic pain. This expert opinion lends support to recently published guidelines proposing that topical treatments should be considered first-line therapy of localized PNP.
Introduction
Pain control in patients with peripheral neuropathic pain (PNP) continues to be a challenge, with many patients receiving unsatisfactory treatment.Citation1 The efficacy of many currently available medications is unsatisfactory owing to their limited effect size and the low responder rate (<50%).Citation2 After diagnosis of PNP, a treatment focusing on the underlying disease could be a first step (eg glucose control for painful diabetic peripheral neuropathy [DPN] or interruption of chemotherapy when chemotherapy-induced neuropathy occurs), although this does not often lead to a successful reversal of the neuropathic pain.Citation3,Citation4 PNP is difficult to treat and often does not respond to conventional pain therapies because of the heterogeneity and complexity of the mechanisms underlying peripheral pain conditions, as well as the co-existence of psychological and emotional aspects of chronic pain.
Treatment of pain requires a multimodal and individualized approach. In the absence of clear predictors of treatment response, a stepwise approach is taken to identify which drugs or drug combinations offer the greatest pain relief with the fewest adverse effects.Citation5,Citation6 Pharmacotherapy is typically the first step and treatment classes often trialed include antidepressants (ie tricyclic antidepressants or selective serotonin and norepinephrine reuptake inhibitors [SSRIs/SNRIs]), antiarrhythmic medications, alpha-2-delta subunit ligands (gabapentin and pregabalin), N-methyl-D-aspartate (NMDA) receptor antagonists, sodium channel inhibitors, and synthetic opioids.Citation1,Citation7 Pregabalin (Lyrica®; Pfizer Inc., New York, NY, USA) is an orally administered calcium channel alpha-2-delta subunit ligand. It was one of the first pharmacotherapies introduced for the treatment of PNP (in 2004) and is approved in the USA and Europe for the treatment of pain from DPN and post-herpetic neuralgia (PHN) in adults.Citation8 Pregabalin was developed in follow-up to gabapentin.Citation9 While both have shown efficacy in neuropathic pain disorders, pregabalin has some pharmacological advantages, including more rapid absorption, linear pharmacokinetics, and greater bioavailability (≥90%) compared with gabapentin.Citation9 It is approximately 2.5-times more potent than gabapentin based on plasma concentrations. In a study from Sweden, the first prescription in 2220 patients with neuropathy was pregabalin in 25% of patients, gabapentin in 29%, and amitriptyline in 36%.Citation10 Nevertheless, a recent Cochrane review concluded that “some people will derive substantial benefit with pregabalin; more will have moderate benefit, but many will have no benefit or will discontinue treatment”.Citation11
In approximately 60% of cases, PNP is localized, affecting a specific and limited area of the body.Citation3 In the case of localized PNP, it is prudent to consider a local treatment such as a lidocaine 5% patch or capsaicin.Citation12
The aim of this article is to summarize the relative effectiveness and tolerability of the capsaicin 179 mg (also known as capsaicin 8%) patch compared with pregabalin in the treatment of PNP conditions, and to consider the place of each in clinical practice. Capsaicin 179 mg cutaneous patch (Qutenza®; Grünenthal GmbH, Aachen, Germany) delivers a high concentration (8%) of synthetic capsaicin, a highly selective agonist of the transient receptor potential vanilloid-1 (TRPV1). It is approved in Europe, alone or in combination, for the treatment of PNP in adults and in the USA for the treatment of PNP associated with PHN.Citation13,Citation14
Summary of Guideline Recommendations
summarizes the current guideline and consensus recommendations for the management of PNP. The first attempt to develop a specific and standardized approach to the diagnosis and treatment of localized PNP was made in 2015. An international group of pain specialists convened to generate an expert consensus on a treatment algorithm for localized PNP treatment, intended for use in the primary care and other non-specialist settings. This group agreed that first-line treatment should begin with a topical analgesic agent, owing to better benefit:risk ratios compared with systemic medications.Citation3 This stance has been corroborated by national expert consensus groups in France and Spain.Citation15,Citation16
Table 1 Summary of Key Guideline and Consensus Recommendations for Pharmacotherapy in Neuropathic Pain
In addition, a recent review of the literature to evaluate the evidence supporting the use of topical therapies for PNP is aligned with the expert consensus and indicates that first-line use of topical therapies may be particularly beneficial in those for which safety, tolerability, and compliance are a concern, and in those who are frail or elderly.Citation17 This review also points out, however, that it is important to take into account the practical aspects of each therapy, highlighting the need for the capsaicin 179 mg patch to be applied by a healthcare professional (HCP). Similarly, the UK’s National Institute for Health and Care Excellence guideline, which is focused on non-specialist settings, recommends that the capsaicin patch should be initiated in non-specialist settings only on the advice of a pain specialist.Citation18
Modes of Action and Pharmacodynamics of Capsaicin 179 mg Patch and Pregabalin
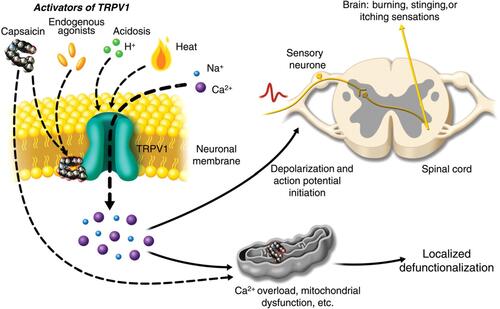
The active ingredient in the capsaicin 179 mg patch is chemically synthesized capsaicin, which is absorbed into the epidermal and dermal layers of the skin. It is applied for 30 minutes to the feet (eg in human immunodeficiency virus-associated neuropathy [HIV-AN], painful DPN) or 60 minutes to other locations (eg the trunk in PHN).Citation14 Capsaicin is a potent and highly selective exogenous agonist of the TRPV1 channel, a transmembrane receptor–ion channel complex distributed throughout the central nervous system (CNS) and peripheral nervous system.Citation13,Citation19–Citation21 TRPV1 is preferentially expressed on sensory (nociceptive) nerve fibers and is important in pain perception. When capsaicin comes into contact with these nociceptors, the initial excitation of primary sensory TRPV1-expressing neurons is experienced as a warming, burning, or stinging sensation with hyperalgesia.Citation20 With continued exposure, the peripheral nociceptors become less sensitive, leading to the “defunctionalization” and degeneration of nociceptors, causing them to recede from the epidermis ().Citation13,Citation19,Citation22,Citation23 In addition, secondary pharmacodynamic mechanisms in response to high doses of capsaicin have been implicated in the persistent effects of capsaicin on nociceptors, including the defunctionalization of mitochondria due to calcium overload leading to nerve terminal necrosis.Citation19,Citation20
Figure 1 Mechanism of action of capsaicin in treatment of localized peripheral neuropathic pain. Activation of transient receptor potential vanilloid-1 (TRPV1) by capsaicin results in sensory neuronal depolarization, and can induce local sensitization to activation by heat, acidosis, and endogenous agonists. Topical exposure to capsaicin leads to the sensations of heat, burning, stinging, or itching. High concentrations of capsaicin or repeated applications can produce a persistent local effect on cutaneous nociceptors, which is best described as “defunctionalization” and constituted by reduced spontaneous activity and a loss of responsiveness to a wide range of sensory stimuli. .Reproduced from Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentrationcapsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502, Copyright 2011, with permission from Elsevier.Citation19
The mechanism of action of pregabalin is not completely understood, but it is an antagonist of voltage-gated calcium ion channels and is thought to bind to the alpha-2-delta subunit.Citation24 This action is thought to reduce the release of several neurotransmitters, including glutamate, norepinephrine, and substance P, reducing neuronal excitability and alleviating allodynia and hyperalgesia.Citation24,Citation25 Pregabalin is administered orally and is rapidly absorbed from the intestines, with ~90% bioavailability.Citation26 It is excreted almost exclusively via the kidneys.Citation26 It is also known to be able to cross the blood–brain barrier and enter the CNS.Citation27
Clinical Comparisons Between Capsaicin 179 mg Patch and Pregabalin
To our knowledge, only one head-to-head comparative trial in patients with PNP has been completed to date and is therefore described here in more detail. ELEVATE, a Phase IV, 8-week, multicenter, open-label, randomized, non-inferiority study in 568 patients with PNP, was the first clinical trial to compare the capsaicin 179 mg patch with pregabalin.Citation28 The capsaicin 179 mg patch offered non-inferior pain relief compared with an optimized dose of pregabalin, as measured by the proportion of patients in each arm who achieved ≥30% decrease in the ‘average pain for the past 24 hours’ Numerical Pain Rating Scale (NPRS) score by week 8.Citation28 The capsaicin 179 mg patch also demonstrated a faster onset of action (median time to onset was 7.5 days vs 36.0 days with pregabalin [p<0.001]) and was associated with fewer systemic side effects and greater treatment satisfaction. Treatment-emergent adverse events (TEAEs) with the capsaicin 179 mg patch were mainly application site reactions versus CNS effects with pregabalin.
The ELEVATE study also demonstrated superiority of the capsaicin 179 mg patch over pregabalin in terms of reducing the intensity and area of dynamic mechanical allodynia (DMA).Citation29 From baseline to week 8, the mean change in intensity of DMA was –2.98 (95% confidence interval [CI] –3.36, –2.60; p<0.0001) with the capsaicin patch and –2.35 (95% CI –2.76, –1.93; p<0.0001) with pregabalin, with an estimated between-arm difference of –0.63 (95% CI –1.04, –0.23; p=0.002). The mean change in the area of DMA was –72.6 cm2 (95% CI –44.7, –100.6; p<0.0001) with the capsaicin patch and –33.1 cm2 (95% CI –2.7, –63.6; p=0.033) with pregabalin, with an estimated between-arm difference of –39.5 cm2 (95% CI –69.1, –10.0; p=0.009). In addition, the capsaicin patch was associated with a greater proportion of patients experiencing complete disappearance of DMA by week 8 (24.1% vs 12.3% with pregabalin; p=0.001).
The capsaicin 179 mg patch was compared with various oral medications, including pregabalin, for the treatment of DPN in a systematic review and network meta-analysis.Citation30 The capsaicin 179 mg patch was shown to be significantly more effective than placebo for ≥30% pain reduction (odds ratio [OR] 2.28 [95% CI 1.19–4.03]) and numerically better than pregabalin (OR 1.83 [95% CI 0.91–3.34]).
Tolerability Considerations
The differences in the tolerability profiles of the capsaicin 179 mg patch and pregabalin are based on their differing modes of administration. Pregabalin is considered to be generally well tolerated for an oral agent, with benign CNS and systemic effects, few unexpected adverse effects, and no major drug interactions.Citation26 The most common (≥10%) CNS and systemic side effects associated with pregabalin are dizziness, somnolence/sedation, peripheral edema, dry mouth, and weight gain.Citation26,Citation31 With the capsaicin 179 mg patch, the active agent is delivered directly to the site of pain, targeting the specific area affected and thus avoiding the systemic side effects associated with oral therapies. The typical side effects of the patch are burning and redness at the application site; these are transient and usually disappear without treatment.Citation13
The network meta-analysis mentioned above also analyzed capsaicin 179 mg patch and oral agents in terms of tolerability.Citation30 The capsaicin 179 mg patch could be included only for the analysis on headache as it is not associated with the other common systemic side effects of the other oral agents studied. However, no significant differences in the risk of headache were observed with any treatment versus placebo. Pregabalin, on the other hand, was associated with a significantly increased risk of somnolence (OR 4.14 [95% CI 3.00–5.60]) and dizziness (OR 4.63 [95% CI 3.44–6.16]), and numerically increased risk of nausea, diarrhea, constipation, headache, and fatigue, compared with placebo. Overall, this network meta-analysis suggests that the capsaicin 179 mg patch offers comparable efficacy to the oral agents in patients with DPN, but provides an improved tolerability profile.
Recently, a review and meta-analysis of Phase III randomized, placebo-controlled trials of pregabalin were conducted to assess the benefits and harms of pregabalin.Citation31 The rationale for the analysis was based on increasing evidence of pregabalin’s potential for abuse and reports of increased mortality, leading the UK government to reclassify the drug as a class C controlled substance.Citation32 While pregabalin was associated with significant benefits on pain reduction and sleep interference, it was also associated with a significant increase in the risk of adverse events (AEs) compared with placebo (relative risk 1.33 [95% CI 1.23–1.44]; p<0.00001), translating into an absolute effect of 145 more AEs per 1000 patients treated with pregabalin.Citation31 Although 23 of the 28 studies included in the meta-analysis examined the effectiveness of pregabalin for the treatment of PNP, there are still insufficient good-quality randomized controlled trials of pregabalin in PNP.Citation31
The Capsaicin 179 mg Patch is Associated with a Lower Burden of Therapy
The differing modes of administration between the capsaicin 179 mg patch and pregabalin also influence the overall burden of therapy. In a study designed to validate a novel standard methodology to evaluate treatment safety, the BURDEN OF THERAPY™© (BOTh™©) methodology was applied to the ELEVATE study results.Citation33 BOTh has been developed to quantify and assess the severity of TEAEs using patient-level safety data on each day of a study, rather than over the entire study period. The aim is to provide improved information on the tolerability and safety of a given drug.
In ELEVATE, patients receiving the capsaicin 179 mg patch had a greater incidence of TEAEs than those receiving pregabalin (74.5% vs 63.9%, respectively).Citation33 However, when assessed using BOTh, a different picture emerged: more TEAEs were experienced for more days with pregabalin compared with the capsaicin 179 mg patch. It is clear from that there was an initial peak followed by a rapid decline in the number and incidence of TEAEs for patients treated with the capsaicin 179 mg patch; these were primarily transient application site reactions (~3 days following application). After day 4, <15% of those in the capsaicin 179 mg patch group reported TEAEs for the remainder of the study. However, TEAEs with pregabalin increased during dose titration and generally persisted to the end of the study. The overall burden estimate was 23.5 for the capsaicin 179 mg patch and 61.2 for pregabalin (p<0.0001). also allows comparison of the severity of the TEAEs between the treatment arms, with more weighted moderate and severe TEAEs in the pregabalin arm.
Figure 2 BURDEN OF THERAPY™© in a peripheral neuropathic pain study. TEAE, treatment-emergent adverse event; TRPV1, transient receptor potential vanilloid-1. Reproduced from Abdulahad AK, Snijder RJ, Panni MK, Riaz FK, Karas AJ. A novelstandard to evaluate the impact of therapeutic agents on patientsafety – the BURDEN OF THERAPY™©. Contemp Clin Trials Commun. 2016;4:186–191, Copyright 2016, with permission from Elsevier.Citation33
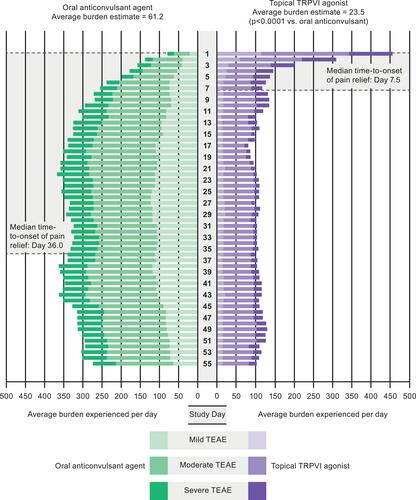
This BOTh study also examined the safety burden in the context of the median time to treatment response (defined as the first of three consecutive days in which the patient reported a ≥30% reduction in average daily pain score) in both arms during ELEVATE.Citation33 Patients receiving the capsaicin 179 mg patch achieved this treatment response in a median of 7.5 days, convergent with the period in which transient application site reactions were reported, while those receiving pregabalin achieved this treatment response in a median of 36 days, coinciding with dose titration and the increase in reported drug-related TEAEs.
These findings demonstrate that the capsaicin 179 mg patch is associated with a lower burden of therapy than pregabalin in terms of tolerability and the incidence of AEs, suggesting it may be an attractive option for doctors and patients. This was reflected in ELEVATE with patients reporting significantly greater treatment satisfaction with capsaicin 179 mg patch compared with pregabalin, and a greater proportion of patients willing to continue treatment with the capsaicin 179 mg patch, as assessed at the week 8 visit.Citation28 In other studies, capsaicin 179 mg was associated with improvements from baseline in quality of life (QoL) and patient satisfaction, as measured by the EuroQol 5-dimensions questionnaire index score and Patient Global Impression of Change (PGIC).Citation34,Citation35 Few studies have assessed the effect of pregabalin on QoL outcomes, and those that have report mixed results or no significant difference versus placebo. Thus, there is insufficient evidence to make definitive statements on pregabalin’s effects on QoL.Citation31
The capsaicin 179 mg patch is not associated with a daily pill burden, and adherence to treatment is determined by whether patients complete the intended application time. In phase III clinical trials, almost all (97–100%) of the patients completed ≥90% of the full duration of treatment (ie the patch was not removed early).Citation36–Citation40 In addition, it is difficult for patients to adjust their dose, whereas the authors have observed that those receiving oral medication frequently alter their own dose by taking a greater or fewer number of tablets than prescribed.
The capsaicin 179 mg patch is unlikely to have drug–drug interactions, and this means it is an appropriate candidate for combination therapy.Citation39 The patch has been evaluated in 21 trials and all but one permitted concomitant neuropathic pain medication, including non-steroidal anti-inflammatory drugs, antidepressants, analgesics, anticonvulsants, and anti-epileptics. No drug–drug interactions were observed with any treatment and the capsaicin patch was well tolerated in combination. In a pooled analysis of four double-blind, controlled trials involving patients with PHN receiving at least one systemic neuropathic pain medication (opioids, anticonvulsants, and non-SSRI antidepressants), the efficacy of the capsaicin patch was evident in terms of significantly greater reductions in NPRS scores compared with the control group, regardless of concomitant medication use.Citation41 In those receiving the capsaicin patch versus control, respectively, reductions of –26.1% versus –18.1% (p=0.0011) were seen in those receiving systemic medications and –36.5% versus –26.2% (p=0.0002) in those not using systemic medications, during weeks 2 to 8.
Nevertheless, while the capsaicin 179 mg patch can be combined effectively and safely with systemic therapies, it may also contribute to a lower burden of therapy by allowing concomitant systemic therapy to be reduced or stopped. In the QUEPP study, a large non-interventional, real-world study in 1044 patients with non-diabetic PNP, a reduction in the use of long-term concomitant PNP medication was shown, particularly among those with a shorter duration of pre-existing pain.Citation42 While over 73% of patients were receiving concomitant medications at baseline regardless of pain duration, 28% of those with pain for <6 months, 13.9% with pain for 6–24 months, 6.8% with pain for >2–10 years, and 2% with pain for >10 years discontinued concomitant medication.
Another aspect of treatment with the capsaicin patch that contributes to its reduced burden of treatment is the flexibility of the regimen and durability of response. It can be given based on individual patient need for a range of PNP subtypes.Citation34,Citation35,Citation43,Citation44 For example, ASCEND—an open-label, non-interventional, real-world study in patients with non-diabetic PNP—showed that administration of the capsaicin patch can provide pain relief that is sustained over time and with subsequent treatments. The median time to second treatment was >26 weeks and increased to >43 weeks from second to third treatment.Citation35 In the QAPSA study—a longitudinal, non-interventional, real-world study conducted in France in 684 patients with non-diabetic PNP,Citation43 patients were treated with up to five capsaicin 179 mg patches. The interval between successive patch applications ranged from 3.7 months between first and second applications to 3.2 months between third and fourth applications. Furthermore, a meta-analysis of seven double-blind, controlled studies in 1313 patients with PHN and 801 patients with HIV-AN found that >90% of those who responded to treatment continued to respond beyond 3 months and that the mean duration of response was >5 months.Citation44 Most long-term responders required just 1–3 patch treatments within a year to maintain their response. In these long-term responders, this limited number of treatments may have considerable implications for patient convenience, adherence, and cost-effectiveness.
Duration of PNP and Effect on Response
There appears to be a correlation between a shorter time from diagnosis to initiation of therapy with the capsaicin 179 mg patch and a better treatment response,Citation35,Citation42 as described below.
In the real-world QUEPP study, as described above, 1044 patients with non-diabetic PNP received a single application of the capsaicin 179 mg patch and were followed for up to 12 weeks.Citation42,Citation45 The mean duration of existing pain at baseline was 4.4 years and 9.4% of patients had suffered from pain for >10 years. The capsaicin 179 mg patch was associated with a consistent reduction in NPRS score versus baseline and, at week 12, 37.7% of patients experienced a ≥30% response and nearly a quarter (24.5%) achieved a ≥50% response. Changes in NPRS scores and responder rates were generally consistent across the different PNP subsets. When assessed according to the duration of pain reported at baseline, however, the greatest reduction (36.6%) was observed in those who had experienced pain for <6 months at baseline, compared with those who had pain for >6 months (). In addition, the smallest reduction (19.2%) was observed in those who had pain for >10 years at baseline. Responder rates followed a similar trend: ≥30% and ≥50% responder rates of 61.7% and 39.3% were observed among those with <6 months of pain, compared with 32.3% and 14.1%, respectively, in those with >10 years of pain.
Table 2 The Mean Changes of Pain Intensity Between 7 and 14 Days and 12 Weeks versus Baseline According to the Duration of Pre-Existing Neuropathic Pain in QUEPP
The ASCEND trial investigated repeated applications of the capsaicin 179 mg patch over 52 weeks in 420 patients with non-diabetic PNP.Citation35 It demonstrated an overall reduction in NPRS average daily pain score of 26.6% (95% CI 23.6–29.6) from baseline to weeks 2 and 8. This reduction was sustained with repeated applications, regardless of PNP condition, providing an overall pain reduction of 37% (95% CI 31.3–42.7) at week 52. Consistent with QUEPP, in ASCEND, those with the shortest duration of pain experienced the highest pain response from baseline to weeks 2 and 8 compared with patients with longer durations of pain (). Moreover, a greater proportion were ≥30% responders after the first treatment in the group with the shortest duration of pain (62.4% vs 39.4%, 40.4%, and 35.9% in the 0.72–2.1-year, >2.1–5.4-year, and >5.4-year PNP duration quartiles, respectively). In addition, greater pain reductions were observed in those treated with the capsaicin 179 mg patch as a first- or second-line treatment, compared with those in which it was at least a third-line treatment (changes in mean NPRS scores from baseline to weeks 2 and 8 were –30.5% [n=80], –28.1% [n=177], and –22.8% [n=155], respectively). Together, QUEPP and ASCEND suggest a benefit of early initiation of the capsaicin 179 mg patch for optimal pain outcomes.
Table 3 Reduction from Baseline to Weeks 2 and 8 in Mean NPRS Score According to PNP Duration in ASCEND
The observed benefit of early treatment is not well understood. While a spontaneous healing process cannot be ruled out, it has been suggested that it may be possible to reverse some of the underlying pathophysiological and psychological symptoms of PNP at an early stage of the condition, before the onset of chronicity.Citation42 Given that this phenomenon is so evident with the capsaicin 179 mg patch, it has been hypothesized that it may be associated with the particular components of pain that the capsaicin patch acts upon. Nevertheless, the response rate does not seem to be related to underlying pathology, intensity of pain at baseline, or with the presence of allodynic or hyperalgesia.Citation46
One possible explanation relates to the depletion of neuropeptides, calcitonin gene-related peptide (which has a key role in pain signal transmission by potentiating nociceptive signaling) and substance P. After application of capsaicin, there is a release of these substances from the depots and, when depleted, the inflammatory cascade stops.Citation47,Citation48 A further explanation considers that an interruption of peripheral input with a rapidly acting topical treatment may stop central sensitization, disrupting the complex psychological factors that contribute to pain, pain memory, and chronification. Clinical experience of the authors has shown that the onset of pain relief is slower with oral medication compared with topical therapy and it may be that, with oral medication, the cycle is not disrupted as quickly.
The phenomenon of the benefit of early therapy remains under investigation, and the authors note that in clinical practice, all patients who have been experiencing PNP for many years are refractory to multiple treatments; nevertheless, in our experience, patients with a long duration of PNP can benefit from treatment with the capsaicin 179 mg patch, possibly due to its distinct mechanism of action versus previously administered pharmacological therapies.
Other Determinants of Response to the Capsaicin 179 mg Patch
There have been several attempts to identify predictors of response to the capsaicin 179 mg patch. One study of 57 patients found that responders had a lower quantitative sensory testing (QST)-determined pressure pain threshold in the area of their PNP than non-responders, but both responders and non-responders experienced meaningful reductions in the size of the painful area following treatment.Citation49 Another study of 20 patients found that the presence of cold and pinprick hyperalgesia was predictive of response.Citation50 Research by the German Neuropathic Pain Research Network, EUROPAIN, and NEUROPAIN consortia suggests it may be possible to define clusters of patients according to QST, on the basis that those with different sensory profiles might exhibit different neurobiological mechanisms and therefore might respond differently to treatment.Citation51 This approach warrants further investigation in treatment trials. However, QST is time-consuming and this may not be the most efficient way to identify responders. Quantitative thermal testing,Citation52 efficacy of lidocaine pre-treatment, and high pre-treatment pain score variabilityCitation53 have also been suggested as predictive of response to the capsaicin 179 mg patch, but patterns of sensory symptoms as measured using the painDETECT questionnaire were not useful in this regard.Citation54
Emerging data suggest that TRPV1 polymorphisms could be useful in determining responders.Citation46 In a study of 38 patients treated with the capsaicin patch, the polymorphism T469I—related to increased TRPV1 function—was associated with a significantly lower value in the Pain Catastrophizing ScaleCitation55 and a non-significant but numerically greater reduction of pain, as measured using the Visual Analog Scale, than those without the polymorphism.Citation46
Conclusions
The capsaicin 179 mg patch is an effective and well-tolerated option for the treatment of localized PNP. The fact that it is associated with a low rate of systemic side effects makes it an appropriate option for those unable to tolerate the systemic side effects of commonly used oral treatments, such as pregabalin. The capsaicin 179 mg patch is equally effective when administered alone or in combination with systemic neuropathic pain medications. Given its lack of drug–drug interactions and lack of daily pill burden, the capsaicin 179 mg patch is particularly suitable for use in combination therapy. Real-world studies show particular benefit of early intervention with the capsaicin 179 mg patch, ie within 6 months of the onset of PNP. This highlights the need for an efficient process of referral to pain specialists, diagnosis, and initiation of appropriate treatment.
Pregabalin has demonstrated efficacy in the treatment of many PNP conditions, with benefits in terms of both pain reduction and sleep interference, and is recommended as the first-line option in many guidelines. Clinicians can be confident in their prescription of pregabalin given its manageable safety profile, lack of unexpected side effects, and drug–drug interactions.Citation26 Particularly when PNP is not localized and an oral option is warranted, pregabalin is a rational first choice.
Expert Opinion
In our clinical experience, for localized PNP on the trunk or extremities, the capsaicin 179 mg patch works at the site of pain and is effective quickly, with limited or no side effects. It also appears to be more effective in the relief of burning pain than in lancinating pain. In addition, although studies with QST have not conclusively demonstrated a relationship between response and dynamic allodynia, in our experience, the capsaicin 179 mg patch is especially useful in patients with dynamic and static allodynia, as well as hyperalgesia.
Pregabalin is an effective treatment and is positioned as a first-line option in many guidelines. From a practical point of view, for localized PNP, it is logical to start with the capsaicin 179 mg patch, which is rapid-acting and does not impose a considerable burden of therapy, before moving on to an oral pharmacological treatment if the patch does not work. It is our opinion that in most cases of localized PNP, it is worth a trial of the capsaicin 179 mg patch, even if the patient is already receiving pregabalin. The patient can be treated with the capsaicin patch, while the pregabalin dose is reduced over time. At the point at which the pain returns, the patient can continue on the last effective dose of pregabalin. On the other hand, pregabalin is a more suitable option for facial pain or central neuropathic pain, given that capsaicin 179 mg patch is contraindicated for use in the face/above the hairline and is not indicated for central neuropathic pain. Similarly, pregabalin is the more suitable option if there are doubts about the vascularization of an area (eg a poorly vascularized diabetic foot) or if the skin is not intact.
At times, it has been noted that patients do not experience an effect of the capsaicin 179 mg patch until its effect wears off. As a result, the authors recommend that HCPs and patients evaluate the effect over at least 12 weeks before trying a different treatment. As discussed, benefit manifests itself in various ways, not necessarily linked to a change in pain measures. Therefore, it is important to also assess patient-reported outcome measures (eg PGIC), reduction of area of pain or reduction in concomitant medication use.
A further key consideration in maximizing the potential for response to the capsaicin 179 mg patch is ensuring the correct application of the patch at the appropriate location. Well-trained clinical staff are paramount to ensure the patch is unfolded, applied, and removed correctly. In addition, an accurate assessment of the treatment area by physicians is needed to optimally position the patch. Inadequate response to the capsaicin patch may be linked to the inability of the patient to describe the painful area and/or inexperience of the clinician in identifying the location or type of pain. The best way to identify the treatment area is to use a von Frey hair to stroke the skin and identify the painful area. Once marked, this is where the patch should be applied. Training in this technique is necessary to enhance the accuracy and efficacy of capsaicin patch treatment.
Anxiety among patients about the use of the capsaicin 179 mg patch has been observed in clinical practice. It is the responsibility of the HCPs to address this by providing information on the AEs such as transient erythema. It is also key to remember that any approach to the treatment of PNP should be multimodal and that patient expectations should be managed. If a patient presents with a mixed pain condition with both nociceptive and neuropathic components, they should expect to see only a change in the neuropathic component with a PNP treatment. Thus, combination therapy is often warranted in such cases.
The treatment of PNP remains a challenge and the patient’s suffering is often high. In contrast to the treatment of purely nociceptive pain, in the treatment of PNP, we see that a pharmacological approach alone is often not effective. Research shows that numbers needed to treat vary from 4 to >10.Citation4 This variation is probably because different phenotypes are concealed under the term neuropathic pain. As long as we are unable to make a distinction between different phenotypes and likelihood of response to various therapies, pharmacological therapy will continue to be somewhat a “trial and error” exercise. Currently, treatment algorithms based on benefit:risk considerations are key, but it is also important to reduce the variation in clinical practice and to generate real-world data on whether current treatment choices are effective. In the meantime, one straightforward approach to the management of PNP is to first consider whether the pain is local versus more generalized and, for localized PNP, local treatment is preferred.
Overall Conclusion
Current expert opinion suggests that localized PNP should preferably be treated with a local treatment. Capsaicin 179 mg patch is a locally applied treatment that has been shown to be non-inferior in efficacy to the oral treatment pregabalin with a better systemic tolerability profile and lower patient burden. The greatest benefit from the capsaicin 179 mg patch can be derived when used early in the patient journey. Nevertheless, its benefit is sometimes not immediately obvious to patients as it may affect parameters other than pain scores, such as area of allodynia. It is advisable not to switch capsaicin 179 mg patch rapidly for another (eg oral) treatment as the effect of the capsaicin 179 mg patch may increase with an increasing number of applications. Capsaicin 179 mg patch is an appropriate approved treatment for localized PNP with a well-understood benefit:risk profile and is a valuable additional treatment for localized neuropathic pain.
Abbreviations
AE, adverse event; CI, confidence interval; CNS, central nervous system; DMA, dynamic mechanical allodynia; DPN, diabetic peripheral neuropathy; HCP, healthcare professional; HIV-AN, human immunodeficiency virus-associated neuropathy; NMDA, N-methyl-D-aspartate; NPRS, Numerical Pain Rating Scale; OR, odds ratio; PGIC, Patient Global Impression of Change; PHN, post-herpetic neuralgia; PNP, peripheral neuropathic pain; QoL, quality of life; QST, quantitative sensory testing; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TEAE, treatment-emergent adverse event; TRPV1, transient receptor potential vanilloid-1.
Data Sharing Statement
As this is a review article, all data are available in the references cited in this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
Medical writing and editorial assistance were provided by Arthur Smyth-Medina of NexGen Healthcare Communications (London, UK), funded by Grünenthal GmbH.
Disclosure
F.H. has been a member of advisory boards for Abbot and a member of the Change Pain Program, sponsored by Grünenthal. F.H reports grants, personal fees from Abbott, grants from Saluda, grants from Boston Scientific, outside the submitted work. K.-U.K. has worked in the past 3 years as a consultant and/or speaker for Berlin-Chemie, Boehringer Ingelheim, Betapharm, Grünenthal, Indivior, Lilly, Kyowa Kirin, Merck Sharp & Dohme, Sanofi, and Teva. C.P. has received in the past 3 years funding or speaker/consultancy fees from Grünenthal, Mundipharma, Boston Scientific, Kyowa Kirin, Aristo, and Teva. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Kessler TL, Brooks KG. Treatments for neuropathic pain. Clinical Pharmacist. 2017;9(12):1–16.
- Nagakura Y. The need for fundamental reforms in the pain research field to develop innovative drugs. Expert Opin Drug Discov. 2017;12(1):39–46. doi:10.1080/17460441.2017.1261108
- Allegri M, Baron R, Hans G, et al. A pharmacological treatment algorithm for localized neuropathic pain. Curr Med Res Opin. 2016;32(2):377–384. doi:10.1185/03007995.2015.1129321
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. doi:10.1016/j.pain.2007.08.033
- Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(Suppl 3):S3–S14. doi:10.4065/mcp.2009.0649
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–1123. doi:10.1111/j.1468-1331.2010.02999.x
- Pfizer Ltd, New York, USA. Lyrica® (Pregabalin) 100 Mg Hard Capsules [Summary of Product Characteristics]. 2019.
- Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49(10):661–669. doi:10.2165/11536200-000000000-00000
- Gustavsson A, Bjorkman J, Ljungcrantz C, et al. Pharmacological treatment patterns in neuropathic pain-- lessons from Swedish administrative registries. Pain Med. 2013;14(7):1072–1080. doi:10.1111/pme.12095
- Derry S, Bell RF, Straube S, et al. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1(1):CD007076. doi:10.1002/14651858.CD005619.pub3
- Bates D, Schultheis BC, Hanes MC, et al. A comprehensive algorithm for management of neuropathic pain. Pain Med. 2019;20(Suppl 1):S2–S12. doi:10.1093/pm/pnz075
- Blair HA. Capsaicin 8% dermal patch: A review in peripheral neuropathic pain. Drugs. 2018;78(14):1489–1500. doi:10.1007/s40265-018-0982-7
- Grünenthal GmbH, Aachen, Germany. Qutenza® (Capsaicin 179 Mg Cutaneous Patch) [Summary of Product Characteristics]. 2019.
- Pérez C, Rodríguez MJ, Guerrero A, et al. Consenso experto sobre el uso clínico de los tratamientos por vía tópica en el manejo del dolor neuropático periférico. Rev Soc Esp Dolor. 2013;20(6):308–323. doi:10.4321/S1134-80462013000600005
- Pickering G, Martin E, Tiberghien F, Delorme C, Mick G. Localized neuropathic pain: an expert consensus on local treatments. Drug Des Devel Ther. 2017;11:2709–2718. doi:10.2147/DDDT.S142630
- Sommer C, Cruccu G. Topical treatment of peripheral neuropathic pain: applying the evidence. J Pain Symptom Manage. 2017;53(3):614–629. doi:10.1016/j.jpainsymman.2016.09.015
- National Institute for Health and Care Excellence. CG173: the pharmacological management of neuropathic pain in adults in non-specialist settings. Published November 20, 2013. Updated July 19, 2019. Available from: https://www.nice.org.uk/guidance/cg173. Accessed April 9, 2020.
- Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502. doi:10.1093/bja/aer260
- Bley K. Effects of topical capsaicin on cutaneous innervation: implications for pain management. Open Pain J. 2013;6(1):81–94. doi:10.2174/1876386301306010081
- Moran MM, Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol. 2018;175(12):2185–2203. doi:10.1111/bph.14044
- Burness CB, McCormack PL. Capsaicin 8% patch: A review in peripheral neuropathic pain. Drugs. 2016;76(1):123–134. doi:10.1007/s40265-015-0520-9
- Premkumar LS, Sikand P. TRPV1: a target for next generation analgesics. Curr Neuropharmacol. 2008;6(2):151–163. doi:10.2174/157015908784533888
- Verma V, Singh N, Singh Jaggi A. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr Neuropharmacol. 2014;12:44–56. doi:10.2174/1570159X1201140117162802
- Coderre TJ, Kumar N, Laferriere A, Yu JSC, Leavitt A. Evidence that pregabalin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2010;113(2):552–561. doi:10.1111/j.1471-4159.2010.06625.x
- Toth C. Pregabalin: latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf. 2014;5(1):38–56. doi:10.1177/2042098613505614
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6(1):108–113. doi:10.1016/j.coph.2005.11.003
- Haanpää M, Cruccu G, Nurmikko TJ, et al. Capsaicin 8% patch versus oral pregabalin in patients with peripheral neuropathic pain. Eur J Pain. 2016;20(2):316–328. doi:10.1002/ejp.731
- Cruccu G, Nurmikko TJ, Ernault E, Riaz FK, McBride WT, Haanpää M. Superiority of capsaicin 8% patch versus oral pregabalin on dynamic mechanical allodynia in patients with peripheral neuropathic pain. Eur J Pain. 2018;22(4):700–706. doi:10.1002/ejp.1155
- van Nooten F, Treur M, Pantiri K, Stoker M, Charokopou M. Capsaicin 8% patch versus oral neuropathic pain medications for the treatment of painful diabetic peripheral neuropathy: A systematic literature review and network meta-analysis. Clin Ther. 2017;39(4):787–803.e18. doi:10.1016/j.clinthera.2017.02.010
- Onakpoya IJ, Thomas ET, Lee JJ, Goldacre B, Heneghan CJ. Benefits and harms of pregabalin in the management of neuropathic pain: A rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9(1):e023600. doi:10.1136/bmjopen-2018-023600
- Iacobucci G. UK government to reclassify pregabalin and gabapentin after rise in deaths. BMJ. 2017;358:j4441. doi:10.1136/bmj.j4441
- Abdulahad AK, Snijder RJ, Panni MK, Riaz FK, Karas AJ. A novel standard to evaluate the impact of therapeutic agents on patient safety – the BURDEN OF THERAPY™©. Contemp Clin Trials Commun. 2016;4:186–191. doi:10.1016/j.conctc.2016.09.003
- Hansson P, Jensen TS, Kvarstein G, Strömberg M. Pain-relieving effectiveness, quality of life and tolerability of repeated capsaicin 8% patch treatment of peripheral neuropathic pain in Scandinavian clinical practice. Eur J Pain. 2018;22(5):941–950. doi:10.1002/ejp.1180
- Mankowski C, Poole CD, Ernault E, et al. Effectiveness of the capsaicin 8% patch in the management of peripheral neuropathic pain in European clinical practice: the ASCEND study. BMC Neurol. 2017;17(1):1–11. doi:10.1186/s12883-017-0836-z
- Backonja M, Wallace MS, Blonsky ER, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7(12):1106–1112. doi:10.1016/S1474-4422(08)70228-X
- Brown S, Simpson DM, Moyle G, et al. NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two Phase III, randomized, controlled trials. AIDS Res Ther. 2013;10(1):5. doi:10.1186/1742-6405-10-5
- Clifford DB, Simpson DM, Brown S, et al. A randomized, double-blind, controlled study of NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. J Acquir Immune Defic Syndr. 2012;59(2):126–133. doi:10.1097/QAI.0b013e31823e31f7
- Irving GA, Backonja MM, Dunteman E, et al. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med. 2011;12:99–109. doi:10.1111/j.1526-4637.2010.01004.x
- Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70(24):2305–2313. doi:10.1212/01.wnl.0000314647.35825.9c
- Irving GA, Backonja M, Rauck R, et al. NGX-4010, a capsaicin 8% dermal patch, administered alone or in combination with systemic neuropathic pain medications, reduces pain in patients with postherpetic neuralgia. Clin J Pain. 2012;28(2):101–107. doi:10.1097/AJP.0b013e318227403d
- Maihöfner CG, Heskamp ML. Treatment of peripheral neuropathic pain by topical capsaicin: impact of pre-existing pain in the QUEPP-study. Eur J Pain. 2014;18(5):671–679. doi:10.1002/j.1532-2149.2013.00415.x
- Lantéri-Minet M, Perrot S. QAPSA: post-marketing surveillance of capsaicin 8% patch for long-term use in patients with peripheral neuropathic pain in France. Curr Med Res Opin. 2019;35(3):417–426. doi:10.1080/03007995.2018.1558850
- Mou J, Paillard F, Turnbull B, Trudeau J, Stoker M, Katz NP. Qutenza (capsaicin) 8% patch onset and duration of response and effects of multiple treatments in neuropathic pain patients. Clin J Pain. 2014;30(4):286–294. doi:10.1097/AJP.0b013e31829a4ced
- Maihöfner C, Heskamp ML. Prospective, non-interventional study on the tolerability and analgesic effectiveness over 12 weeks after a single application of capsaicin 8% cutaneous patch in 1044 patients with peripheral neuropathic pain: first results of the QUEPP study. Curr Med Res Opin. 2013;29(6):673–683. doi:10.1185/03007995.2013.792246
- Sánchez N, Sáiz M, Ochoa D, Muñoz M, Rojo E, Pérez C. Efecto de los polimorfismos de TRPV1 en la respuesta a capsaicina en pacientes con dolor neuropático. Rev Soc Esp Dolor. 2019;26(Suppl 1):67–68.
- Li Y, Liu G, Li H, Xu Y, Zhang H, Liu Z. Capsaicin-induced activation of ERK1/2 and its involvement in GAP-43 expression and CGRP depletion in organotypically cultured DRG neurons. Cell Mol Neurobiol. 2013;33(3):433–441. doi:10.1007/s10571-013-9909-8
- Sams-Nielsen A, Orskov C, Jansen-Olesen I. Pharmacological evidence for CGRP uptake into perivascular capsaicin sensitive nerve terminals. Br J Pharmacol. 2001;132(5):1145–1153. doi:10.1038/sj.bjp.0703910
- Gustorff B, Poole C, Kloimstein H, Hacker N, Likar R. Treatment of neuropathic pain with the capsaicin 8% patch: quantitative sensory testing (QST) in a prospective observational study identifies potential predictors of response to capsaicin 8% patch treatment. Scand J Pain. 2013;4(3):138–145. doi:10.1016/j.sjpain.2013.04.001
- Mainka T, Malewicz NMM, Baron R, Enax-Krumova EK, Treede RD, Maier C. Presence of hyperalgesia predicts analgesic efficacy of topically applied capsaicin 8% in patients with peripheral neuropathic pain. Eur J Pain. 2016;20(1):116–129. doi:10.1002/ejp.703
- Baron R, Maier C, Attal N, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158(2):261–272. doi:10.1097/j.pain.0000000000000753
- Serrano A, Torres D, Veciana M, Caro C, Montero J, Mayoral V. Quantitative thermal testing profiles as a predictor of treatment response to topical capsaicin in patients with localized neuropathic pain. Pain Res Treat. 2017;2017:7425907.
- Martini CH, Yassen A, Krebs-Brown A, et al. A novel approach to identify responder subgroups and predictors of response to low- and high-dose capsaicin patches in postherpetic neuralgia.. Eur J Pain. 2013;17(10):1491–1501. doi:10.1002/j.1532-2149.2013.00329.x
- Höper J, Helfert S, Heskamp ML, Maihöfner CG, Baron R. High concentration capsaicin for treatment of peripheral neuropathic pain: effect on somatosensory symptoms and identification of treatment responders. Curr Med Res Opin. 2014;30(4):565–574. doi:10.1185/03007995.2013.869491
- Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. doi:10.1037/1040-3590.7.4.524
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi:10.1016/j.jclinepi.2010.07.015
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi:10.1136/bmj.39489.470347.AD
- Deng Y, Luo L, Hu Y, Fang K, Liu J. Clinical practice guidelines for the management of neuropathic pain: a systematic review. BMC Anesthesiol. 2016;16(1):12. doi:10.1186/s12871-015-0150-5
- Legido-Quigley H, Panteli D, Brusamento S, et al. Clinical guidelines in the European Union: mapping the regulatory basis, development, quality control, implementation and evaluation across member states. Health Policy. 2012;107(2–3):146–156. doi:10.1016/j.healthpol.2012.08.004
