Abstract
Background
The aim of this study was to compare primary sensory neurons in controls and in an animal neuropathic pain model in order to understand which types of neurons undergo changes associated with peripheral neuropathy. On the basis of intracellular recordings in vivo from somata, L4 sensory dorsal root ganglion neurons were categorized according to action potential configuration, conduction velocity, and receptive field properties to mechanical stimuli.
Methods
Intracellular recordings were made from functionally identified dorsal root ganglion neurons in vivo in the Mosconi and Kruger animal model of peripheral neuropathic pain.
Results
In this peripheral neuropathy model, a specific population of Aβ-fiber low threshold mechanoreceptor neurons, which respond normally to innocuous mechanical stimuli, exhibited differences in action potential configuration and conduction velocity when compared with control animals. No abnormal conduction velocity, action potential shapes, or tactile sensitivity of C-fiber neurons were encountered.
Conclusion
This study provides evidence for defining a potential role of Aβ-fiber low threshold mechanoreceptor neurons that might contribute to peripheral neuropathic pain.
Introduction
Neuropathic pain is initiated or caused by a primary lesion or dysfunction in the nervous systemCitation1,Citation2 and includes postherpetic neuralgia, trigeminal neuralgia, diabetic neuropathy, spinal cord injury, cancer and chemotherapy, and stroke, as well as some degenerative neurological diseases.Citation3 In contrast to nociceptive pain, neuropathic pain is described as spontaneous burning pain with accompanying hyperalgesia and allodynia.Citation4 Tactile allodynia is a common stimulus-evoked response and is defined as pain resulting from a light touch that ordinarily does not elicit a painful response.
It has become evident that peripheral neuropathic pain is characterized by membrane ectopic activity that is thought to be generated in both damaged as well as neighboring intact/surviving fibers of primary sensory neurons.Citation5 Previous studies have shown that ectopic activity may arise from the dorsal root ganglion soma, along the axon, and in the peripheral nerve terminals,Citation6–Citation11 and prolonged responses to sensory inputs of dorsal horn neurons in neuropathic rats are reduced by local anesthetic application to the peripheral sensory nerve.Citation12 These abnormal activities of peripheral neurons are suggested to play a role as a pain signal and as an inducer of central sensitization observed in animal models of peripheral neuropathy.Citation7,Citation13–Citation15
However, it is still not clear which functional subgroup of afferent neurons is involved in altering nociceptive scores in various animal models of peripheral neuropathic pain, and particularly whether or not nociceptors are involved. The major concepts or hypotheses in this regard are clearly described in a recent review by Devor.Citation5 One theory is the “excitable nociceptor hypothesis”, which is based on a reduced response threshold in nociceptive afferents. Another hypothesis is that ectopic activity in low threshold mechanoreceptor afferents is abnormally “amplified” in the spinal cord.Citation16 Both hypotheses embrace the observation that C or Aβ fibers carry the ectopic activity in models of peripheral neuropathy. However, so far, most of the previous studies have been based on axotomized neurons in vitro that were anatomically and/or only partially functionally identified. Identifying axotomized dorsal root ganglion neurons as nociceptive or non-nociceptive is questionable because they are disconnected from their sensory receptors.
In our earlier studies using the Mosconi and Kruger rat model of neuropathic pain,Citation17 in which a polyethylene cuff is placed around a sciatic nerve unilaterally, we observed hypersensitivity to tactile stimuli, as assessed in the von Frey test; this is generally considered to be the animal equivalent of “allodynia” in humans.Citation18 Extracellular electrophysiological recordings made in this model showed an elevated discharge of wide dynamic range dorsal horn neurons in response to both noxious and innocuous mechanical stimulation of peripheral cutaneous receptive fields.Citation19,Citation20 Further, in acutely spinalized animals, the hyperactivity of dorsal horn neurons was blocked by application of lidocaine to the sciatic nerve.Citation20 Together, this evidence suggests that increased wide dynamic range neuron discharge may be maintained by increased or exaggerated input from primary afferents that can be directly stimulated from peripheral cutaneous receptive fields or are spontaneously active. Therefore, to understand the possible contribution of primary sensory neurons to tactile hypersensitivity in this model, we recorded intracellularly from dorsal root ganglion somata using in vivo electrophysiological experiments, and fully characterized each neuron on the basis of several parameters, including the configuration of the action potential, the conduction velocity of the axon, and activation of the respective sensory receptive field. Comparisons were then made with the same properties of dorsal root ganglion neurons recorded from naive control animals.
We report here that low threshold mechanoreceptor neurons associated with Aβ fibers in particular undergo changes in functional properties and thus might play a role as an essential trigger of tactile hypersensitivity in the Mosconi and Kruger model and possibly in tactile allodynia after peripheral nerve injury; small neurons and high threshold large neurons exhibited either no change or only minor changes in these properties.
Materials and methods
All experimental procedures conformed to the Guide to the Care and Use of Laboratory Animals, Volumes 1 and 2, of the Canadian Council on Animal Care, and all protocols were approved by the McMaster University Animal Review Ethics Board. At the end of the acute electrophysiological experiment, the animal was euthanized without recovery by an anesthetic overdose.
Experimental animals and neuropathic surgery
Young male Sprague-Dawley rats (obtained from Charles River Inc, St Constant, QC, Canada) weighing 170–200 g were used. The animals were divided into two groups, ie, naive control and neuropathic model groups. A peripheral neuropathy was induced according to a method previously described in detail.Citation17,Citation19,Citation21 Under anesthesia with a mixture of ketamine 5 mg/100 g (Ketamine®; Bimeda-MTC Animal Health Inc, Cambridge, ON, Canada), xylazine (Rompun®; 0.5 mg/100 g; Bayer HealthCare, Toronto, ON, Canada), and acepromazine (Atravet®; 0.1 mg/100 g; Ayerst Veterinary Laboratories, Guelph, ON, Canada) given intraperitoneally, the right sciatic nerve was exposed at the mid-thigh level. Two cuffs of 0.5 mm polyethylene tubing (Intramedic PE-90, Fisher Scientific Ltd, Whitby, Ontario, Canada) were inserted around the exposed nerve about 1 mm apart. The wound was then sutured in two layers, ie, muscle and skin. Antibiotic ointment (Furacin®; nitrofurazone 0.2%; Vetoquinol N-A Inc, Lavaltrie, QC, Canada) was applied over the wound, and 0.01 mL/100 g of injectable antibacterial solution (Bayer HealthCare) was injected subcutaneously. Animals were given 1 mL of saline subcutaneously and an ocular lubricant, and placed under a heating lamp until they recovered from the anesthetic, and were then returned to their home cages.
von Frey test of paw withdrawal threshold
In all cases, the von Frey test was run on the same day as the recording day before the rats were anesthetized for the acute electrophysiological experiment. This was done to confirm that each animal had developed the tactile hypersensitivity that characterizes this model. To quantify mechanical sensitivity of the foot, brisk foot withdrawal in response to normally innocuous mechanical stimuli was measured as described previously.Citation22 The rats were placed in a transparent Plexiglas box with a clear Plexiglas floor, containing 0.5 cm diameter holes spaced 1.5 cm apart to allowed full access to the paws.Citation12,Citation19,Citation21 The animals were allowed to habituate to the box for approximately 15 minutes, when cage exploration and major grooming activities had ceased.
von Frey filaments (Stoelting Co, Wood Dale, IL) were applied to the plantar surface of the hind paw to determine the withdrawal threshold. A von Frey filament was applied five times (for 3–4 seconds each, at 3-second intervals) to a different spot on each hind paw. For each rat, these filaments were applied in ascending order until a clear withdrawal response was observed. When this occurred, the next lightest filament was applied following the same procedure. A 50% withdrawal response threshold was derived according to responses to this testing regimen,Citation23 using the up-down method of Dixon.Citation24 Brisk foot withdrawal in response to these mechanical stimuli was interpreted as indicating mechanical hypersensitivity.
Intracellular recording in vivo
Details of the surgical preparation and intracellular recording approaches have been reported previously.Citation25,Citation26 In brief, the rat was initially anesthetized with the anesthetic mixture described above. The right jugular vein was cannulated for intravenous infusion of drugs. The rat was then fixed in a stereotaxic frame and the vertebral column rigidly clamped at the L2 and L6 vertebral levels. The right femur was fixed by a customized clamp onto the stereotaxic frame to minimize movement of the dorsal root ganglion during mechanical searching for receptive fields on the leg. The L4 dorsal root ganglion was selected for study because it contains large numbers of hind leg afferent somata. A laminectomy was performed to expose the ipsilateral L4 dorsal root ganglion. The L4 dorsal root was sectioned close to the spinal cord and placed on a bipolar electrode (FHC, Bowdoinham, ME) used for stimulation purposes. The exposed spinal cord and dorsal root ganglion were covered with warm paraffin oil at 37°C to prevent drying. Rectal temperature was maintained at about 37°C using a temperature-controlled infrared heating lamp.
For recording, each rat was anesthetized at a surgical level with sodium pentobarbital (20 mg/kg; Ceva Sante Animal, Libourne, France). The rat was mechanically ventilated via a tracheal cannula using a Harvard Ventilator (Model 683, Harvard apparatus, Quebec, Canada). The ventilation parameters were adjusted so that the end-tidal CO2 concentration was maintained at around 40–50 mmHg, as measured using a CapStar-100 end-tidal CO2 analyzer (CWE, Ardmore, PA). Immediately before the start of recording, an initial 1 mg/kg dose of pancuronium (Sandoz, Boucherville, QC, Canada) was given to eliminate muscle tone. The effects of pancuronium were allowed to wear off periodically to confirm a surgical level of anesthesia; this was monitored by observing pupil diameter and any response to noxious pinch of a forepaw. Supplements of pentobarbital and pancuronium were given at a dose of 1/3 the original dose approximately each hour via the jugular cannula.
Intracellular recordings from somata in the exposed dorsal root ganglion were made with borosilicate glass micropipettes (1.2 mm outside diameter, 0.68 mm inside diameter; Harvard Apparatus, Holliston MA). The electrodes were pulled using a Brown-Flaming puller (model p-87; Sutter Instrument Co, Novota, CA). These electrodes were filled with 3 M KCl (DC resistance 50–70 mΩ). Signals were recorded with a Multiclamp 700B amplifier (Molecular Devices, Union City CA) and digitized online via Digidata 1322A interface (Molecular Devices) with pClamp 9.2 software (Molecular Devices). The microelectrode was advanced using an EXFO IW-800 micromanipulator (EXFO, Montreal, QC, Canada) in 2 μm steps until a hyperpolarization of at least 40 mV suddenly appeared. Once a stable membrane potential had been confirmed, a single stimulus was applied to the dorsal roots to provoke an action potential; with the aid of the protocol editor function in pClamp 9.2 software, a somatic action potential was evoked by stimulation with a single rectangular voltage pulse.
Dorsal root ganglion neuron classification
The neurons were divided into three groups on the basis of dorsal root conduction velocity. The conduction velocity range was ≤0.8 m/sec for C-fiber neurons, 1.5–6.5 m/sec for Aδ-fiber neurons, and >6.5 m/sec for Aβ-fiber neurons, as defined elsewhere.Citation25–Citation29
The sensory receptive properties of each dorsal root ganglion neuron were examined using hand-held mechanical stimulators and classified as previously described.Citation25,Citation26,Citation29,Citation30 Differentiation between high threshold mechanoreceptor neurons and low threshold mechanoreceptor neurons was based on their sensory properties identified during receptive field searching. High threshold mechanoreceptor neurons responded to noxious stimuli including noxious pressure, pinch, probing with fine forceps, a sharp needle, coarse-toothed forceps, or coarse flat forceps, whereas low threshold mechanoreceptor neurons responded to innocuous stimuli such as a moving brush, light pressure with a blunt object, light manual tap, or vibration. Neurons that did not respond to any of the non-noxious or noxious mechanical stimuli were classified as unresponsive, as previously described.Citation29
In addition to the threshold of activation, the rate of adaption and tissue location of the receptive field were other major factors used to classify Aβ-fiber low threshold mechanoreceptor neurons further as guard/field hair neurons, glabrous skin neurons, Pacinian neurons, slowly adapting neurons, and muscle spindle neurons. Guard/field hair neurons were rapidly adapting cutaneous neurons. Glabrous and Pacinian neurons were both rapidly adapting non-hair neurons, and were named rapidly adapting neurons. Slowly adapting neurons were slowly adapting cutaneous neurons. Muscle spindle neurons were slowly adapting neurons with deep subcutaneous receptive fields activated by deep tissue manipulation of the muscle belly but not by cutaneous stimulation.
Action potential configuration
The first evoked action potential in each neuron was used to determine any differences in configuration between control and neuropathic animals. Criteria for acceptance of neurons in the analysis included a stable Vm more negative than about 40 mV, with a somatic spike evoked by dorsal root stimulation that was >40 mV.
Variables in action potential configuration included resting membrane potential (Vm), action potential amplitude, action potential duration at base, action potential rise time, action potential fall time, maximum action potential rising rate, maximum action potential falling rate, afterhyperpolarization amplitude, and afterhyperpolarization duration to 50% recovery (AHP50).
Conduction velocity
The distance from the stimulation site (cathode) to the recording site (center of the dorsal root ganglion) was measured at the end of the experiment to determine the conduction distance. This was then used to calculate the conduction velocity of the dorsal root axon associated with each neuron.
Mechanical sensitivity test during intracellular recording
The mechanical sensitivity of high threshold mechanoreceptor neurons was determined individually using calibrated von Frey filaments as described previously.Citation30–Citation32 Briefly, after functional classification of a neuron using the hand-held mechanical stimulators, von Frey filaments were applied to the identified receptive field, and the mechanical activation threshold of each neuron was expressed as the minimum force (g) necessary to evoke impulses on the most sensitive spot on the skin. The mechanical forces exerted with the calibrated von Frey filaments used in this study were a set of von Frey filaments exerting pressures of 0.008, 0.02, 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10, 15, 26, 60, 100, 180 and 300 g; tip diameters ranged from 1.65 mm to 6.65 mm.
Statistical analysis
Normality of electrophysiological data was done with the D’Agostino and Pearson omnibus test. Wherever appropriate, the Student’s t-test or the Mann-Whitney U-test was used for comparisons between neuropathic and control animals in various neuronal subtypes and for various parameters. All statistical tests and graphing was done using Prism4 software (Graphpad, La Jolla, CA). P < 0.05 was considered to indicate a statistically significant difference, as shown in the graphs.
Results
Withdrawal threshold in the von Frey test
Behavioral tests of tactile hypersensitivity were made from a total of 124 rats (60 control and 64 neuropathic rats). Stimulation of the plantar surface of the hind paw with von Frey filaments evoked a withdrawal response in control animals, with hairs exerting pressures of 10–100 g. Three weeks after cuff ligation of the sciatic nerve on the right side, model rats fully developed behavioral signs of neuropathic pain on the affected hind limb; filaments to which the control animals showed no withdrawal response, ie, 0.001–6.0 g, in neuropathic animals evoked a clear withdrawal of the nerve-injured hind limb. Furthermore, withdrawal was greatly exaggerated in amplitude and duration, and was frequently accompanied by licking of the paw. Withdrawal thresholds were 14.44 ± 0.221 g in control animals (n = 60) and 4.52 ± 0.69 g in neuropathic animals (n = 64); comparison of the data indicated P < 0.0001.
Electrophysiological recording
Intrasomal recordings in these rats were made from a total of 399 L4 dorsal root ganglion neurons (175 neurons in control animals and 224 neurons in neuropathic animals). In control rats, these included 33 C-fiber (21 high threshold mechanoreceptor, eight low threshold mechanoreceptor, and four unresponsive units), 22 Aδ-fiber (11 high threshold mechanoreceptor, seven low threshold mechanoreceptor, four unresponsive units), 120 Aβ-fiber (26 high threshold mechanoreceptor, 86 low threshold mechanoreceptor, and eight unresponsive) neurons. In neuropathic animals, these included 40 C-fiber (28 high threshold mechanoreceptor, eight low threshold mechanoreceptor, four unresponsive units), 20 Aδ-fiber (seven high threshold mechanoreceptor, nine low threshold mechanoreceptor, four unresponsive units), and 164 Aβ-fiber (39 high threshold mechanoreceptor, 116 low threshold mechanoreceptor and nine unresponsive units) neurons. All neurons included in these results met the inclusion criteria described above. Examples of action potentials recorded from individual neuron types are illustrated in . With respect to Aβ-fiber low threshold mechanoreceptor neurons, 20 guard/field hair neurons were recorded in control and 25 in neuropathic rats. Similarly, 20 rapidly adapting neurons were recorded in control and 24 in neuropathic rats, 10 slowly adapting neurons were recorded in control rats and 14 in neuropathic rats. Thirty-six muscle spindle neurons were recorded in control rats and 53 in neuropathic rats.
Figure 1 Examples of APs recorded from mechanoreceptive neurons. (A) Representative intracellular somatic action potential of an A-fiber neuron evoked by electrical stimulation of the dorsal root showing the electrophysiological parameters measured, including: 1, resting membrane potential; 2, action potential duration at base; 3, action potential rise time; 4, action potential fall time; 5, action potential amplitude; 6, AHP duration to 50% recovery; 7, and afterhyperpolarization amplitude below Vm. In addition, maximum rising and falling rates, (dV/dt) max, were measured from the differential trace of the action potential. (B) Somatic action potentials evoked by dorsal root stimulation and recorded intracellularly from 12 mechanoreceptive neurons selected to represent the mean action potential duration values for each of the different groups of neurons in control (upper) and neuropathic (lower) animals. The action potential duration and conduction velocity for each neuron are given below each record. The horizontal lines across the action potentials indicate zero membrane potential.
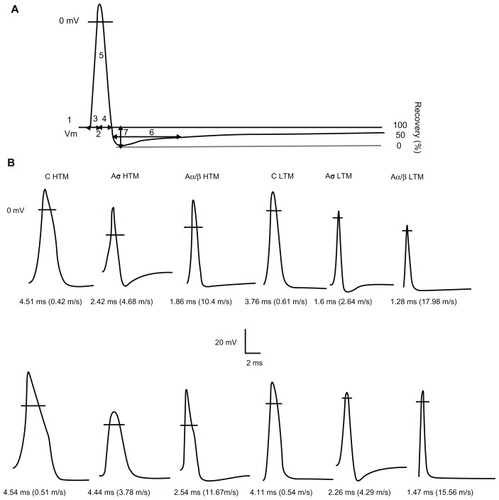
Action potential configuration
The various action potential configuration parameters of corresponding subclasses in each conduction velocity group were compared between control and neuropathic rats. All data are shown in and the scatter plots of , , and .
Figure 2 Comparison of action potential resting membrane potential and amplitude of dorsal root ganglion neurons between control and neuropathic rats. Scatter plots show the distribution of the variables with the median (horizontal line) superimposed in each case.
Abbreviations: HTM, high threshold mechanoreceptor neurons; LTM, low threshold mechanoreceptor neurons; UN, unresponsive neurons; CUT, Aβ LTM including guard/field hair neurons, rapidly adapting neurons and slowly adapting neurons; MS, Aβ LTM muscle spindle neurons.
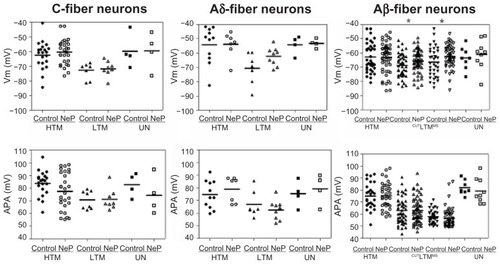
Figure 3 Comparison of action potential dynamic parameters of dorsal root ganglion neurons between control and neuropathic rats. Scatter plots show the distribution of the variables with the median (horizontal line) superimposed in each case.
Abbreviations: APdB, action potential duration at base; APRT, action potential rise time; APFT, action potential fall time; MRR, maximum AP rising rate; MFR, maximum AP falling rate; HTM, high threshold mechanoreceptor neurons; LTM, low threshold mechanoreceptor neurons; UN, unresponsive neurons.
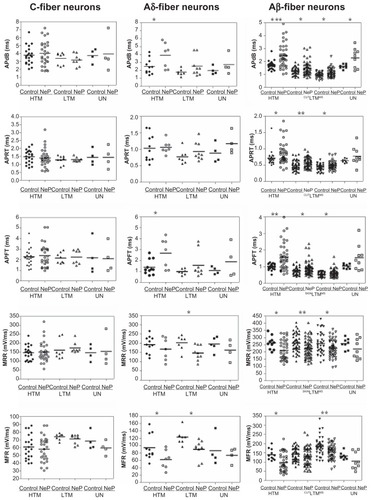
Figure 4 Comparison of AHP variables of DRG neurons between control and neuropathic rats. Scatter plots show the distribution of the variables with the median (horizontal line) superimposed in each case.
Abbreviations: AHPA, afterhyperpolarization amplitude; AHP50, afterhyperpolarization duration to 50% recovery; HTM, high threshold mechanoreceptor neurons; LTM, low threshold mechanoreceptor neurons; UN, unresponsive neurons.
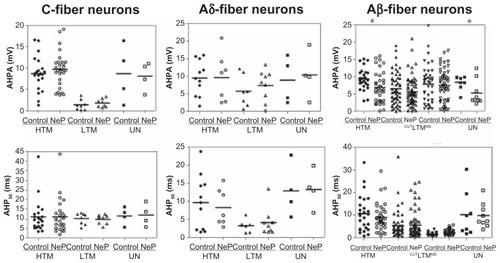
Table 1 Comparison of properties of high-threshold mechanoreceptive and low-threshold mechanoreceptive dorsal root ganglion neurons between control and neuropathic rats
Resting membrane potential
Values of Vm for all neurons included in this study are given in . Vm of all subtypes of C-fiber neurons was similar in control and neuropathic rats: this was the case for high threshold mechanoreceptor neurons, low threshold mechanoreceptor neurons, and unresponsive neurons. In all subtypes of Aδ-fiber neurons and Aβ-fiber high threshold mechanoreceptor neurons and unresponsive neurons, Vm was also similar between control and neuropathic rats. However, Vm in Aβ-fiber low threshold mechanoreceptor neurons in neuropathic rats was more depolarized than that in control rats, including both cutaneous and deep neurons, ie, cutaneous neurons (control, n = 50 versus neuropathic, n = 63; P = 0.0423) and muscle spindle neurons (control, n = 36 versus neuropathic, n = 53; P = 0.0442). These data are illustrated in .
Action potential amplitude
There were no significant differences in action potential amplitude between the control and neuropathic rats for any subtype of neurons in any conduction velocity group, as illustrated in . The data are shown in .
Action potential duration at base
The action potential duration at base differed between control and neuropathic rats in some neuron types, Aβ- and Aδ-fiber neurons in particular, as shown in . Thus, none of the subgroups of C-fiber neurons exhibited a difference between control and neuropathic rats. Of the Aδ-fiber neurons, only high threshold mechanoreceptor neurons exhibited a difference between control and neuropathic rats (control, n = 11 neurons versus neuropathic, n = 7 neurons; P = 0.0355). No difference was seen between control and neuropathic rats for low threshold mechanoreceptor or unresponsive neurons.
In marked contrast to C-fiber neurons, all Aβ-fiber neuron groups were different. Aβ-fiber neurons exhibited a longer action potential duration at base in neuropathic animals compared with controls, including Aβ-fiber high threshold mechanoreceptor neurons (control, n = 26 neurons versus neuropathic, n = 39 neurons; P = 0.0003), low threshold mechanoreceptor cutaneous neurons (control, n = 50 neurons versus neuropathic, n = 63 neurons; P = 0.0046), low threshold mechanoreceptor muscle spindle neurons (control, n = 36 neurons versus neuropathic, n = 53 neurons; P = 0.0229), and unresponsive neurons (control, n = 8 neurons versus neuropathic, n = 9 neurons; P = 0.0297). The data are shown in .
Action potential rise time
Action potential rise time did not differ between control and neuropathic rats in either C-fiber neurons or Aδ-fiber neurons. However, a longer action potential rise time was observed in the subgroups of Aβ-fiber neurons, with the exception of the unresponsive neurons. The data are presented in .
Types of Aβ-fiber neurons that exhibited a statistically significant difference between control and neuropathic rats included high threshold mechanoreceptor neurons (control, n = 26 versus neuropathic, n = 39; P = 0.0492), low threshold mechanoreceptor cutaneous neurons (control, n = 50 versus neuropathic, n = 63; P = 0.0027), and low threshold mechanoreceptor muscle spindle neurons (control, n = 36 versus neuropathic, n = 53; P = 0.0152).
Action potential fall time
The action potential fall time also showed a differential effect on different neuron types between control and neuropathic rats. The data are presented in . Thus, C-fiber neurons did not show a difference, whether high threshold mechanoreceptor neurons, low threshold mechanoreceptor neurons, or unresponsive neurons. In Aδ-fiber neurons, both high threshold mechanoreceptor and low threshold mechanoreceptor neurons exhibited longer action potential fall times in neuropathic than in control rats; high threshold mechanoreceptor (control, n = 11 versus neuropathic, n = 7; P = 0.0180) and low threshold mechanoreceptor (control, n = 7 versus neuropathic, n = 9; P = 0.0356). In contrast, unresponsive neurons were the same in both control and neuropathic rats. In Aβ-fiber neurons, all but the unresponsive neuron types displayed longer action potential fall times in neuropathic compared with control rats. Thus, longer action potential fall times were observed in neuropathic rats for high threshold mechanoreceptor neurons (control, n = 26 versus neuropathic, n = 39; P = 0.0012), low threshold mechanoreceptor cutaneous neurons (control, n = 50 versus neuropathic, n = 63; P = 0.0222), and low threshold mechanoreceptor muscle spindle neurons (control, n = 36 versus neuropathic, n = 53; P = 0.0467). These data are shown in .
Maximum rising rate
The action potential rising rate was slower in some but not all subtypes of neurons, as shown in . C-fiber neurons did not show a difference, whether high threshold mechanoreceptor neurons, low threshold mechanoreceptor neurons, or unresponsive neurons. Aδ-fiber low threshold mechanoreceptor neurons displayed a slower action potential rising rate in neuropathic rats compared with control rats (control, n = 7 versus neuropathic, n = 9; P = 0.0337). Neither high threshold mechanoreceptor neurons nor unresponsive neurons differed between neuropathic or control rats with respect to action potential rising rate. Of Aβ-fiber neurons, all showed a slower action potential rising rate in neuropathic rats compared with control rats, with the exception of the unresponsive neurons. Thus, a slower action potential rising rate was observed in high threshold mechanoreceptor neurons (control, n = 261 versus neuropathic, n = 39; P = 0.0281), low threshold mechanoreceptor cutaneous neurons (control, n = 50 versus neuropathic, n = 63; P = 0.0027), and low threshold mechanoreceptor muscle spindle neurons (control, n = 361 versus neuropathic, n = 53; P = 0.0239). The unresponsive neurons displayed a similar action potential rising rate in both groups of rats. The data are shown in .
Maximum falling rate
The maximum action potential falling rate was also slower in some neuron types in neuropathic rats compared with controls. shows the data. As above, such changes were not observed in C-fiber neurons, including high threshold mechanoreceptor neurons, low threshold mechanoreceptor neurons, and unresponsive neurons. Aδ-fiber neurons showed a different distribution of slowing of maximum action potential falling rate in the neuron types compared with that seen earlier with the action potential rising rate. For example, low threshold mechanoreceptor neurons displayed a slower maximum action potential falling rate in neuropathic rats compared with control rats (control, n = 7 versus neuropathic, n = 9; P = 0.0138). High threshold mechanoreceptor neurons also showed a slowing of maximum AP falling rate (control, n = 11 versus neuropathic, n = 7; P = 0.0444). However, unresponsive neurons did not show a difference in maximum action potential falling rate between rats. Aβ-neurons also displayed a neuron-type based grouping of differences in maximum action potential falling rate. The maximum action potential falling rate was different in high threshold mechanoreceptor neurons (control, n = 26 versus neuropathic, n = 39; P = 0.0118) and low threshold mechanoreceptor muscle spindle neurons (control, n = 36 versus neuropathic, n = 53; P = 0.0013), while there was no difference in maximum action potential falling rate in low threshold mechanoreceptor cutaneous neurons or in unresponsive neurons. The data are shown in .
AHP amplitude
A difference in AHP amplitude was seen only in two populations of Aβ-fiber neurons, comparing neuropathic versus control rats. These neuron types were Aβ-fiber high threshold mechanoreceptor neurons and unresponsive neurons. Thus, of the C-fiber neurons, none showed a difference between control and neuropathic animals, including the high threshold mechanoreceptor neurons, low threshold mechanoreceptor neurons, and unresponsive neurons. Similarly, of the neurons in the Aδ-fiber range, no subtypes showed a difference between control and neuropathic rats. In the Aβ-fiber range, AHP amplitude differed in high threshold mechanoreceptor neurons (control, n = 26 versus neuropathic, n = 39; P = 0.0101) and unresponsive neurons (control, n = 8 versus neuropathic, n = 9; P = 0.0487), but did not change in low threshold mechanoreceptor cutaneous or low threshold mechanoreceptor muscle spindle neurons. The data are shown in .
AHP50 was different between control and neuropathic rats only in low threshold mechanoreceptor muscle spindle neurons. None of the C-fiber and Aδ-fiber neurons showed a difference between control and neuropathic animals, including the high threshold mechanoreceptor, low threshold mechanoreceptor, and unresponsive neurons. Of the neurons in the Aβ-fiber range, the AHP50 was longer only in the low threshold mechanoreceptor muscle spindle group (control, n = 36 versus neuropathic, n = 53; P = 0.0030). There was no difference between control and neuropathic animals in the other types of neurons in the Aβ-fiber range, including high threshold mechanoreceptor neurons, low threshold mechanoreceptor cutaneous neurons, and unresponsive neurons. The data are shown in .
Conduction velocity
The conduction velocity was studied because it reflects properties of the axon rather than of the soma, as pertains to the data presented above. illustrates the distributions of conduction velocities for individual neurons in each neuron type in control and neuropathic rats. Comparison between these groups of rats for each type of C-fiber and Aδ-fiber neurons did not show a significant difference. However, in the Aβ-fiber range, low threshold mechanoreceptor neurons showed significantly slower conduction velocity in neuropathic animals.
Figure 5 Comparison of dorsal root conduction velocity of dorsal root ganglion neurons between control and neuropathic rats. Scatter plots show the distribution of variables with the median (horizontal line) superimposed in each case. Details are the same as in .
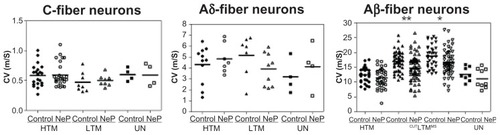
Thus, in C-fiber neurons, the conduction velocity was 0.59 ± 0.040 m/sec (n = 21) and 0.60 ± 0.036 m/sec (n = 28) in control and neuropathic high threshold mechanoreceptor neurons, respectively (P = 0.8355). In low threshold mechanoreceptor neurons, the conduction velocity was 0.47 ± 0.058 m/sec (n = 8) and 0.50 ± 0.033 m/sec (n = 8) in control and neuropathic rats, respectively (P = 0.6855), and conduction velocity was 0.60 ± 0.054 m/sec (n = 4) and 0.61 ± 0.094 m/sec (n = 4) in control and neuropathic rats, respectively (P = 0.9469).
In Aδ-fiber neurons, conduction velocity was 4.32 ± 0.514 m/sec (n = 11) and 4.86 ± 0.505 m/sec (n = 7) in control and neuropathic high threshold mechanoreceptor neurons, respectively (P = 0.4871), while it was 5.17 ± 0.705 m/sec (n = 7) and 3.91 ± 0.500 m/sec (n = 9) in control and neuropathic low threshold mechanoreceptor neurons, respectively (P = 0.1548) and was 3.21 ± 0.766 m/sec (n = 3) and 4.14 ± 1.041 m/sec (n = 3) in control and neuropathic unresponsive neurons, respectively (P = 0.4966).
In Aβ-fiber neurons, conduction velocity was 12.42 ± 0.548 m/sec (n = 26) in control high threshold mechanoreceptor neurons and 11.38 ± 0.504 m/sec (n = 39) in neuropathic high threshold mechanoreceptor neurons (P = 0.1794), 17.02 ± 0.469 m/sec (n = 50) and 14.70 ± 0.548 m/sec (n = 63) in control and neuropathic cutaneous low threshold mechanoreceptor neurons, respectively (P = 0.0023), 18.76 ± 0.638 m/sec (n = 36) and 16.69 ± 0.599 m/sec (n = 53) in control and neuropathic low threshold mechanoreceptor muscle spindle neurons, respectively (P = 0.0235), and 12.67 ± 0.831 m/sec (n = 8) in control versus 11.15 ± 1.088 m/sec (n = 9) in neuropathic unresponsive neurons (P = 0.2922).
Activation of sensory receptors
The activation threshold of sensory receptors associated with different neuron types was studied throughout the entire hind leg. This was done to complement the data reported above regarding properties of the soma and the axon. Almost all of the neurons studied with identifiable receptive fields could be classified into one of the categories according to the type of stimulus; shows the locations of the receptive fields of the neurons recorded.
Table 2 Locations of receptive fields of high-threshold mechanoreceptors and low-threshold mechanoreceptors in controls and neuropathic rats
C-fiber neurons in neuropathic rats did not show differences in the threshold of activation of the receptive field compared with those in control rats. C-fiber low threshold mechanoreceptor neurons (n = 8 in control; n = 8 in neuropathic rats) were activated by slow brushing on the receptive field or lightly stretching of the skin surrounding the receptive field. Neither stimulus produced any discharge in C-fiber high threshold mechanoreceptor neurons (n = 21 in control rats, n = 28 in neuropathic rats), which was activated only by stimuli in the noxious range. The distributions of C-fiber high threshold mechanoreceptor and low threshold mechanoreceptor neuron receptive fields were relatively evenly distributed over the hind leg, each with relatively small receptive field sizes.
Similarly, none of the Aδ-fiber neurons in neuropathic rats showed any difference in activation of the receptive field when data from control and neuropathic rats were compared. For Aδ-fiber high threshold mechanoreceptor neurons (n = 11 in control, n = 7 in neuropathic rats), the receptive fields usually consisted of several small spots and were found on the foot, calf, and thigh. All responded to noxious stimuli.
Aδ-fiber low threshold mechanoreceptor neurons tested in this way were D-hair neurons (n = 7 in control; n = 9 in neuropathic rats). They were activated by von Frey filaments (≤0.16 g) and were found on the foot, calf, and thigh.
For Aβ-fiber high threshold mechanoreceptor neurons (n = 26 in control rats, n = 39 in neuropathic rats), the distribution of receptive fields was also over the entire hind leg. All responded to the noxious stimuli, including noxious stimulation deeply toward the bone. Aβ-fiber low threshold mechanoreceptor neurons and guard/field hair neurons (n = 20 in control rats, n = 25 in neuropathic rats) were distributed entirely over hairy skin and were activated by lightly stimulating the tip of the hair/moving a group of hairs. Rapidly adapting glabrous neurons (n = 6 in control rats, n = 6 in neuropathic rats) were activated by lightly stimulating the glabrous skin of the rat foot with blunt objects; these neurons responded to these stimuli with a brief, quickly adapting discharge. Pacinian neurons (n = 14 in control rats, n = 18 in neuropathic rats) were activated by gently tapping on the experiment table and were found over the entire hind leg. Slowly adapting neurons (n = 10 in control rats, n = 14 in neuropathic rats) were activated by lightly stimulating narrow skin strips surrounding the nails in the foot, except for one control neuron with a receptive field on the ankle joint. muscle spindle neurons were activated by touching along the muscle belly or changing joint position. One particular abnormality observed was found in two Aβ-fiber neurons in neuropathic rats; in response to current injection into the cell body, the response of these neurons (n = 2) resembled that of muscle spindle neurons or slowly adapting neurons in terms of relatively slow adaptation, but the receptive-field characteristics of these neurons were similar to other hair neurons with low thresholds to stimulation of hairs (≤0.008 g von Frey filament stimulation; these neurons are not included in this study).
Previous studies have shown that the threshold of activation of most nociceptors in control rats is higher than 14 mN (1.43 g).Citation30 To identify whether high threshold mechanoreceptor neurons showed any differences in mechanical sensitivity in this model, we also measured the mechanical threshold of a selected number of high threshold mechanoreceptor neurons in control and neuropathic rats. The mechanical threshold for C-fiber high threshold mechanoreceptor neurons was within the range of 2–100 g in control rats (n = 14) versus 6–100 g in neuropathic rats (n = 15). Mechanical thresholds for Aδ-fiber high threshold mechanoreceptor neurons were in the range 4–60 g in control rats (n = 8) versus 4–60 g in neuropathic rats (n = 6). Mechanical thresholds for Aβ-fiber high threshold mechanoreceptor neurons were in the range of 4–60 g in control rats (n = 8) versus 2–100 g in neuropathic rats (n = 6). None of the high threshold mechanoreceptor neurons in neuropathic rats showed a mechanical threshold of activation below 1.4 g.
Discussion
Classification of dorsal root ganglion neurons
The aim of the present study was to compare primary sensory neurons in control and neuropathic rats in order to identify changes in peripheral neurons associated with tactile hypersensitivity. Our interest was particularly in the type of neuron exhibiting change. To achieve this aim, dorsal root ganglion sensory neurons were classified during intracellular in vivo electrophysiological experiments according to parameters reported previously by other laboratories to distinguish dorsal root ganglion neuron types, including the configuration of the action potential, conduction velocity, and response properties to application of natural stimuli to peripheral receptive fields, such as threshold of activation and adaptation.Citation25–Citation30 With this classification, each dorsal root ganglion neuron could be functionally classified and each could be distinguished as nociceptive (high threshold mechanoreceptor), non-nociceptive (low threshold mechanoreceptor), or unresponsive. The conduction velocity criteria for classification in the present study were based on those described previously by Lawson et al.Citation27–Citation29 These criteria were followed because the experimental approach in the present study most closely approximated those in Lawson’s previous reports, including similar surgical procedures, recording techniques, and recording setups.
Although differentiation of Aβ-fiber, Aδ-fiber, and C-fiber neurons was based on the conduction velocity ranges in this study, as reported by others,Citation25–Citation29 there is a potential overlap between these three main neuron subtypes if the conduction velocity changes as a result of nerve injury. The concern here is that an increase in excitability could lead to an increase in conduction velocity, so that some Aδ-fiber and C-fiber neurons would conduct faster and thus be classified as Aβ neurons. However, our current study and a previous study from another groupCitation33 showed that conduction velocity was slower in the neuropathic model studied. As a result, faster conducting neurons might exhibit slower conduction velocities and thus be classified as Aδ-fiber or C-fiber neurons. It should be noted, then, that Aδ-fiber and C-fiber neurons would not be classified as Aβ-fiber neurons. Furthermore, our data showed that Aβ-fiber, Aδ-fiber, and C-fiber neurons in the cuff model of neuropathic pain still conducted in three clearly separate ranges.
The differentiation between the high threshold mechanoreceptor neurons and low threshold mechanoreceptor neurons in control rats was also clearly maintained in this neuropathic animal model. In neuropathic rats, high threshold mechanoreceptor neurons in the C-fiber, Aδ-fiber, and Aβ-fiber neuron ranges exhibited a relatively depolarized resting membrane potential, a relatively higher action potential amplitude, relatively slower action potential kinetics (ie, longer action potential duration, action potential rise and fall times, and slower action potential maximum rising and falling rates), a relatively higher AHP amplitude, and a longer 50% AHP recovery time than that exhibited by low threshold mechanoreceptor neurons. Thus, other than the few neurons that are described in the results and excluded in this classification, all neurons were clearly classified in both control and neuropathic rats.
Differences in properties of neuron types
In the present study, when comparing control and neuropathic animals, none of the small diameter C-fiber dorsal root ganglion neurons with identifiable receptive fields exhibited any differences in any of the properties investigated here, including both low threshold mechanoreceptor and high threshold mechanoreceptor neurons. In contrast, in the present study, dorsal root ganglion A-type neurons, in particular Aβ-fiber low threshold mechanoreceptor neurons in neuropathic rats, showed significant differences in electrophysiological properties compared with those in control animals, manifesting as decreased conduction velocity, a more depolarized Vm, and slower action potential kinetics.
Our results, demonstrating changes mainly in large diameter primary afferent neurons, are compatible with those of several groups investigating other types of rat model, which showed that most ectopic discharge is generated in large-diameter, fast-conducting myelinated Aβ-fiber neurons after nerve injury.Citation9,Citation34–Citation37 In fact, some features such as the longer action potential duration, rise time and fall time, and lack of AHP changes in A-fiber neurons in our neuropathic rats are similar to the well described differences in A-fiber neurons after peripheral axotomyCitation36,Citation38–Citation41 and neighboring intact dorsal root ganglion neurons recorded in L5 neurons in the spinal nerve ligation model of peripheral neuropathy.Citation36 The decreased conduction velocity of A-fiber neurons is similar to that reported in A-fiber neurons after chronic constriction injury of the sciatic nerve.Citation33 Somewhat different magnitudes of change in these parameters in the different reports might be attributable to many factors, such as different sensory neurons targeted (“injured” versus “intact” neurons) and to different animal models employed (eg, nerve-section axotomy model, spinal nerve ligation model, peripheral nerve section model, chronic constriction injury model, and compression of the dorsal root ganglion model). Different recording techniques, such as sharp microelectrodes in vivo or in vitro in some experiments versus patch electrodes to study dissociated cells in others, and different animal species chosen, strain, age, gender, and time between the initial model induction and electrophysiological experiment may have also contributed to differences in the magnitude of the changes in different reports.Citation38
Conduction velocities and action potential properties of unresponsive neurons were not significantly different from those of high threshold mechanoreceptor units in the C-fiber, Aδ-fiber, and Aβ-fiber ranges, both in control and neuropathic animals. These types of neuron were thus probably high threshold mechanoreceptor neurons with inaccessible receptive fields or were very high threshold mechanoreceptor units, as described earlier by Lawson et al.Citation29 However, although unresponsive neurons in neuropathic animals undergo electrophysiological changes compared with control animals, measurement of mechanical sensitivity was not possible because these neurons were not responsive to any mechanical stimulation of the cutaneous receptive field, and thus they might not be related to tactile allodynia.
Novel contribution to the literature
One point of differentiation between the present study and previous literature is that we used the Mosconi and Kruger model, whereas previous reports were based on the chronic constrictive injury model, Chung model, peripheral nerve cut model, and dorsal root ganglion compression model. In the Mosconi and Kruger model, there is no peripheral axotomy, no dorsal root axotomy, no physical damage to the dorsal root ganglion through compression, and all neurons are classified on the basis of their response to activation of their respective receptive fields using natural stimuli. The Mosconi and Kruger model most closely resembles the chronic constrictive injury model in these respects.
As pointed out earlier, many previous studies were based on axotomized neurons that were anatomically and/or only partially functionally identified. Identifying axotomized dorsal root ganglion neurons as nociceptive or nonnociceptiveCitation39,Citation42,Citation43 on the basis of conduction velocity alone is less valid than activating neurons from their respective receptive fields because some slowly conducting sensory neurons are non-nociceptive and some fast conducting sensory neurons are nociceptive.Citation29 Koerber et alCitation44 cut peripheral sensory nerves and allowed time for regeneration to peripheral tissues; however, although they activated sensory neurons by peripheral stimulation, it is unclear whether the neurons retained their original nociceptive or non-nociceptive properties after the regeneration process, and the focus of the study was on plasticity of central projections of sensory neurons after axotomy, and there was no attempt to focus specifically on the possible participation of Aβ-fiber neurons in nociception following peripheral neuropathy.
Another point of differentiation between the present study and several of the earlier studies is that our data were derived from in vivo recordings made from dorsal root ganglion sensory neurons. Some previous studiesCitation45–Citation48 were based on in vitro recording raising a similar concern about accurately classifying neurons as nociceptive versus non-nociceptive. Yet other studies were run in vivo in the anesthetized rat model of peripheral neuropathy based their classification of neurons on the basis of conduction velocity alone.Citation9,Citation34 Xie et alCitation49 recorded from dorsal root ganglion neurons in vivo and reported functional changes in the chronic constrictive injury model. However, while they studied responses of neurons to thermal stimuli, there was no classification based on responses to low or high threshold mechanical stimulation, and neurons were not classified as nociceptive versus non-nociceptive. Zhao et alCitation50 recorded from sensory axons in the chronic constrictive injury model in vivo and reported spontaneous ectopic discharge in these neurons. However, the neurons were not functionally classified and therefore no information was provided on the neuron type that displayed such changes.
Therefore, in the present study, a number of methodological approaches were used that differed from those reported previously. Further, a novel contribution is that this is the first to report changes in fully classified sensory neurons on the basis of their responses to natural stimuli to the receptive fields in vivo in an animal model in which axons were not cut.
Correlation of changes in Aβ-type low threshold mechanoreceptors with tactile hypersensitivity
While this evidence argues against C-fiber neurons contributing to tactile allodynia after induction of the model, this has sometimes been assumed to involve high threshold mechanoreceptor units as a possible contribution. Similarly, while there is evidence for Aβ-fiber neurons contributing to tactile allodynia after nerve or tissue injury, this has sometimes been assumed to involve low threshold mechanoreceptor units as a possible contribution.Citation5
Action potential configuration, conduction velocity, and peripheral activation threshold of all subtypes of dorsal root ganglion neuron were systematically evaluated in this study, because each of these parameters might reflect changed electrophysiological properties in various parts of the primary sensory neuron, such as the soma, the axon, and the receptive field, respectively. These features of peripheral neurons have been previously described and have been thought to play a role in generating the pain signal in several animal models of peripheral neuropathy.Citation5,Citation7,Citation14,Citation15
Although it is widely believed that afferent C-fiber input is a necessary condition for the induction and maintenance of central sensitization, none of the C-fiber neurons in this study showed any difference in electrophysiological properties. Perhaps most important, no C-fiber high threshold mechanoreceptor neurons showed any decrease in mechanical sensitivity when tested with von Frey filaments.
On the other hand, A-type neurons, especially Aβ-fiber low threshold mechanoreceptor neurons in neuropathic rats, showed significant differences in electrophysiological properties. One of the changes was a decrease in conduction velocity, possibly due to demyelination. It has been suggested that in models of peripheral neuropathic pain, demyelination leads to crosstalk whereby non-nociceptive afferents can activate nociceptive afferents.Citation51
Our previous studies in this same model showed nociceptive responses to innocuous mechanical stimulation in a wide dynamic range of spinal neurons,Citation19 and it was further proposed that ectopic activity recorded from these neurons was mediated via myelinated afferents.Citation20 Thus, the present results provide further support for a possible role of Aβ-fiber low threshold mechanoreceptors in the behavioral tactile hypersensitivity exhibited in this model, because these neurons remained connected to their normal impulse generating site and respond to normal innocuous mechanical stimuli, which are essential for defining tactile sensitivity.
Our finding adds to the body of evidence that C-fiber dorsal root ganglion neurons are likely not related to tactile allodynia,Citation52,Citation53 and raise the possibility that low threshold mechanoreceptor neurons of Aβ-fiber dorsal root ganglion neurons fulfill this role. The mechanisms underlying the electrophysiological changes in Aβ-type low threshold mechanoreceptor neurons that could induce neuropathic pain or allodynia remain to be determined. One possible explanation is that some Aβ-type low threshold mechanoreceptor neurons undergo phenotypic changes and take up a new role in nociception, and began to convey signals along novel pathways leading to activation of spinal nociceptive mechanisms. Citation5 There is evidence that mediators released, such as substance P, calcitonin gene-related peptide, and brain-derived neurotrophic factor, as well as neuropeptide Y released by A-type neurons, might trigger changes in the responsiveness of postsynaptic neurons and rewiring of sensory pathway at the first sensory synapse in the spinal cord.Citation54–Citation60
We speculate that there may be an altered supply of such factors in the neurons that have undergone the changes reported here. These changes may therefore constitute a mechanism leading to the pain, dysesthesia, and allodynia that commonly accompany peripheral neuropathy.
Cellular mechanisms of underlying differences in A-type neurons
These differences in neurons in neuropathic animals might be due to membrane remodeling, thus altering the intrinsic electrogenic properties of the neuronal membrane in those neuron types exhibiting changes. There are three major ion channels, ie, Na+, Ca2+ and K+, which play major roles in determining electrogenic properties of neurons. For example, Na+ channels can modulate the resting membrane potential in dorsal root ganglion neurons, and Na+ channels are also likely to have a major influence on the action potential rising phase and therefore on the duration of the action potential.Citation61 Ca+ inward currents likely contribute to the falling phase inflections in high threshold mechanoreceptor neurons.Citation27,Citation62 K+ channels have been reported to contribute to the repolarization phase in low threshold mechanoreceptor neurons.Citation27 Differences in expression and/or activation of both voltage-gated and Ca2+-activated K+ channels have been reported as likely to contribute to the AHP.Citation27 Thus, alterations in the level of expression, cellular localization, and distribution or activation/kinetics of each of these ion channel types might lead to the changes in action potential configuration in neuropathic rats.
Changes in the activity of sodium channels and the consequences of these changes have been reported in various neuropathic pain models. Immunohistochemical studies have demonstrated a reorganization of the levels of expression and distribution of various sodium channels in neuropathic animal models.Citation14,Citation63,Citation64 The expression of some sodium channel subtypes in dorsal root ganglion cell bodies is diminished following nerve injury, while others appear de novo and yet others are distributed to different parts of the neuron.Citation65 Changes in the activity of calcium channels and potassium channels have also been reported.Citation66 For example, voltage- clamp studies of isolated currents in dissociated axotomized dorsal root ganglion cells have revealed an upregulation of a tetrodotoxin-sensitive Na+ current and a downregulation of a tetrodotoxin-resistant Na+ current,Citation61,Citation67–Citation69 together with a reduction of the K+ and Ca2+ currents.Citation70–Citation72
Explanations for the mechanisms underlying these changes in membrane channel expression might be based on many factors, such as nerve growth factor or glial cell line-derived neurotrophic factor, cytokines, or other inflammatory mediators released by immune cells and Schwann cells.Citation73–Citation79 Nerve growth factor and glial cell line-derived neurotrophic factor are important neurotrophic factors for maintaining normal function of sensory neurons and may influence action potential electrogenesis and neuronal excitability in dorsal root ganglion neurons via regulating ionic currents.
Demyelination, which has been proposed to result in membrane remodeling, should also be considered as a possible driving force of change, because demyelination has been reported to lead to ion channel redistribution.Citation80 In normal conditions, newly synthesized Na+ channels are transported in endocytoplasmic vesicles along the axons to be expressed only at specific target sites, such as nodes of Ranvier and nerve terminal endings. As a consequence of the neuronal damage in this model, this target-specific transfer may be altered and the channels in transit may be redistributed in any remaining part of the membrane, particularly in dorsal root ganglions and demyelinated patches.Citation66 In fact, our data show that the decreased conduction velocity of Aβ low threshold mechanoreceptor neurons might be due to demyelination so as to be compatible with the change in action potential shape in these neurons following peripheral neuropathy.
Some of these findings or other factors might be contributing in various ways to the changes we have observed in the shape of the action potential of different A-type neurons. In fact, the more heavily myelinated Aβ-fiber low threshold mechanoreceptors in this neuropathic model might be more affected by such comprehensive factors and thus showed significant changes in functional properties.
Conclusion
The purpose of this study was to examine the electrophysiological properties and mechanical sensitivity of the different types of dorsal root ganglion neurons in control versus neuropathic animals. Comparing previous reports, this is the first study providing evidence showing changes in functional properties of sensory dorsal root ganglion neurons with identifiable receptive fields. In this model, there were no differences in C-fiber neurons between control and model animals. However, there were significant and possibly important differences in dorsal root ganglion neurons associated with Aβ-fibers and Aδ-fibers, especially Aβ low threshold mechanoreceptor neurons. These findings are unique and unexpected because Aβ low threshold mechanoreceptor neurons are normally considered to be non-nociceptive neurons. We interpret these data to suggest that A-type but not C-type primary sensory neurons in this model of peripheral neuropathy may be involved in generating the tactile hypersensitivity seen in these animals in the von Frey behavioral test.
Disclosure
The authors report no conflicts of interest in this work.
References
- FinnerupNBSindrupSHJensenTSChronic neuropathic pain: mechanisms, drug targets and measurementFundam Clin Pharmacol200721212913617391285
- HawksleyHManaging pain after shingles: a nursing perspectiveBr J Nurs2006151581481816936604
- RoLSChangKHNeuropathic pain: mechanisms and treatmentsChang Gung Med J200528959760516323550
- RichardsRLCausalgia. A centennial reviewArch Neurol19671643393505336504
- DevorMEctopic discharge in A-beta afferents as a source of neuropathic painExp Brain Res2009196111512819242687
- AmayaFWangHCostiganMThe voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivityJ Neurosci20062650128521286017167076
- CampbellJNMeyerRAMechanisms of neuropathic painNeuron2006521779217015228
- KatzEJGoldMSInflammatory hyperalgesia: a role for the C-fiber sensory neuron cell body?J Pain20067317017816516822
- LiuCNWallPDBen-DorEMichaelisMAmirRDevorMTactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injuryPain200085350352110781925
- SchaibleHGPeripheral and central mechanisms of pain generationHandb Exp Pharmacol200717732817087118
- YangRHXingJLDuanJHHuSJEffects of gabapentin on spontaneous discharges and subthreshold membrane potential oscillation of type A neurons in injured DRGPain2005116318719315935557
- PitcherGMHenryJLSecond phase of formalin-induced excitation of spinal dorsal horn neurons in spinalized rats is reversed by sciatic nerve blockEur J Neurosci20021591509151512028361
- DevorMNerve pathophysiology and mechanisms of pain in causalgiaJ Auton Nerv Syst198373–43713846192166
- DevorMSodium channels and mechanisms of neuropathic painJ Pain200671 Suppl 1S3S1216426998
- LangPMSchoberGMRolkeRSensory neuropathy and signs of central sensitization in patients with peripheral arterial diseasePain20061241–219020016716518
- CampbellJNRajaSNMeyerRAMackinnonSEMyelinated afferents signal the hyperalgesia associated with nerve injuryPain198832189943340426
- MosconiTKrugerLFixed-diameter polyethylene cuffs applied to the rat sciatic nerve induce a painful neuropathy: ultrastructural morphometric analysis of axonal alterationsPain199664137578867246
- MerskeyHLindblomUMumfordJMNathanPWSunderlandSPain terms. A current list with definitions and notes on usageMerskeyHBoldukNClassification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of PainPart III2nd edSeattle, WAInternational Association for the Study of Pain Press1994
- PitcherGMHenryJLNociceptive response to innocuous mechanical stimulation is mediated via myelinated afferents and NK-1 receptor activation in a rat model of neuropathic painExp Neurol2004186217319715026255
- PitcherGMHenryJLGoverning role of primary afferent drive in increased excitation of spinal nociceptive neurons in a model of sciatic neuropathyExp Neurol2008214221922818773893
- PitcherGMRitchieJHenryJLNerve constriction in the rat: model of neuropathic, surgical and central painPain1999831374610506670
- KimSHChungJMAn experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the ratPain19925033553631333581
- ChaplanSRBachFWPogrelJWChungJMYakshTLQuantitative assessment of tactile allodynia in the rat pawJ Neurosci Methods199453155637990513
- DixonWJEfficient analysis of experimental observationsAnnu Rev Pharmacol Toxicol1980204414627387124
- WuQHenryJLDelayed onset of changes in soma action potential genesis in nociceptive A-beta DRG neurons in vivo in a rat model of osteoarthritisMol Pain200955719785765
- WuQHenryJLChanges in A-beta non-nociceptive primary sensory neurons in a rat model of osteoarthritis painMol Pain201063720594346
- DjouhriLBleazardLLawsonSNAssociation of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neuronesJ Physiol1998513Pt 38578729824723
- FangXMcMullanSLawsonSNDjouhriLElectrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivoJ Physiol2005565Pt 392794315831536
- LawsonSNCreppsBAPerlERRelationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pigJ Physiol1997505Pt 11771919409481
- LeemJWWillisWDChungJMCutaneous sensory receptors in the rat footJ Neurophysiol1993695168416998509832
- BoadaMDWoodburyCJPhysiological properties of mouse skin sensory neurons recorded intracellularly in vivo: temperature effects on somal membrane propertiesJ Neurophysiol200798266868017537905
- ShimBKimDWKimBHNamTSLeemJWChungJMMechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathyNeuroscience2005132119320115780478
- GabayETalMPain behavior and nerve electrophysiology in the CCI model of neuropathic painPain20041101–235436015275786
- HanHCLeeDHChungJMCharacteristics of ectopic discharges in a rat neuropathic pain modelPain2000842–325326110666530
- KhanGMChenSRPanHLRole of primary afferent nerves in allodynia caused by diabetic neuropathy in ratsNeuroscience2002114229129912204199
- MaCShuYZhengZSimilar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neuronsJ Neurophysiol20038931588160212612024
- TalMWallPDDevorMMyelinated afferent fiber types that become spontaneously active and mechanosensitive following nerve transection in the ratBrain Res1999824221822310196451
- AbdullaFASmithPAAxotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neuronsJ Neurophysiol200185263064311160499
- KimYINaHSKimSHCell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neuropathic painNeuroscience19988613013099692763
- LiuBEisenachJCHyperexcitability of axotomized and neighboring unaxotomized sensory neurons is reduced days after perineural clonidine at the site of injuryJ Neurophysiol20059453159316716222073
- StebbingMJEschenfelderSHablerHJAcostaMCJanigWMcLachlanEMChanges in the action potential in sensory neurones after peripheral axotomy in vivoNeuroreport199910220120610203309
- LiuXEschenfelderSBlenkKHJanigWHablerHSpontaneous activity of axotomized afferent neurons after L5 spinal nerve injury in ratsPain2000842–330931810666536
- Omana-ZapataIKhabbazMAHunterJCClarkeDEBleyKRTetrodotoxin inhibits neuropathic ectopic activity in neuromas, dorsal root ganglia and dorsal horn neuronsPain1997721–241499272786
- KoerberHRMirnicsKMendellLMProperties of regenerated primary afferents and their functional connectionsJ Neurophysiol19957326937027760128
- SongXJHuSJGreenquistKWZhangJMLaMotteRHMechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root gangliaJ Neurophysiol19998263347335810601466
- SongXJVizcarraCXuDSRupertRLWongZNHyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neuronsJ Neurophysiol20038942185219312612043
- ZhangJMSongXJLaMotteRHAn in vitro study of ectopic discharge generation and adrenergic sensitivity in the intact, nerve-injured rat dorsal root ganglionPain1997721–251579272787
- ZhangJMSongXJLaMotteRHEnhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglionJ Neurophysiol19998263359336610601467
- XieYZhangJPetersenMLaMotteRHFunctional changes in dorsal root ganglion cells after chronic nerve constriction in the ratJ Neurophysiol1995735181118207623082
- ZhaoFYSpanswickDMartindaleJCReeveAJChessellIPGW406381, a novel COX-2 inhibitor, attenuates spontaneous ectopic discharge in sural nerves of rats following chronic constriction injuryPain20071281–2788717055166
- UedaHPeripheral mechanisms of neuropathic pain – involvement of lysophosphatidic acid receptor-mediated demyelinationMol Pain200841118377664
- AndersenOKGracelyRHArendt-NielsenLFacilitation of the human nociceptive reflex by stimulation of A beta-fibres in a secondary hyperalgesic area sustained by nociceptive input from the primary hyperalgesic areaActa Physiol Scand1995155187978553881
- GracelyRHLynchSABennettGJPainful neuropathy: altered central processing maintained dynamically by peripheral inputPain19925121751941484715
- LeverIJBradburyEJCunninghamJRBrain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulationJ Neurosci200121124469447711404434
- MaWBisbyMAIncrease of calcitonin gene-related peptide immunoreactivity in the axonal fibers of the gracile nuclei of adult and aged rats after complete and partial sciatic nerve injuriesExp Neurol199815211371499682021
- MalcangioMRamerMSJonesMGMcMahonSBAbnormal substance P release from the spinal cord following injury to primary sensory neuronsEur J Neurosci200012139739910651897
- MichaelGJAverillSShortlandPJYanQPriestleyJVAxotomy results in major changes in BDNF expression by dorsal root ganglion cells: BDNF expression in large trkB and trkC cells, in pericellular baskets, and in projections to deep dorsal horn and dorsal column nucleiEur J Neurosci199911103539355110564362
- MikiKFukuokaTTokunagaANoguchiKCalcitonin generelated peptide increase in the rat spinal dorsal horn and dorsal column nucleus following peripheral nerve injury: up-regulation in a subpopulation of primary afferent sensory neuronsNeuroscience1998824124312529466443
- NoguchiKKawaiYFukuokaTSenbaEMikiKSubstance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neuronsJ Neurosci19951511763376437472514
- WeissnerWWintersonBJStuart-TilleyADevorMBoveGMTime course of substance P expression in dorsal root ganglia following complete spinal nerve transectionJ Comp Neurol20064971788716680762
- WaxmanSGDib-HajjSCumminsTRBlackJASodium channels and painProc Natl Acad Sci U S A199996147635763910393872
- YoshidaSMatsudaYSamejimaATetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouseJ Neurophysiol197841510961106702189
- CumminsTRSheetsPLWaxmanSGThe roles of sodium channels in nociception: Implications for mechanisms of painPain2007131324325717766042
- RogersMTangLMadgeDJStevensEBThe role of sodium channels in neuropathic painSemin Cell Dev Biol200617557158117123847
- HainsBCKleinJPSaabCYCranerMJBlackJAWaxmanSGUpregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injuryJ Neurosci200323268881889214523090
- AurilioCPotaVPaceMCPassavantiMBBarbarisiMIonic channels and neuropathic pain: physiopathology and applicationsJ Cell Physiol2008215181418205177
- BlackJACumminsTRPlumptonCUpregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neuronsJ Neurophysiol19998252776278510561444
- RizzoMAKocsisJDWaxmanSGSelective loss of slow and enhancement of fast Na+ currents in cutaneous afferent dorsal root ganglion neurones following axotomyNeurobiol Dis19952287968980012
- SleeperAACumminsTRDib-HajjSDChanges in expression of two tetrodotoxin-resistant sodium channels and their currents in dorsal root ganglion neurons after sciatic nerve injury but not rhizotomyJ Neurosci200020197279728911007885
- AbdullaFAStebbingMJSmithPAEffects of substance P on excitability and ionic currents of normal and axotomized rat dorsal root ganglion neuronsEur J Neurosci200113354555211168562
- BacceiMLKocsisJDVoltage-gated calcium currents in axotomized adult rat cutaneous afferent neuronsJ Neurophysiol20008342227223810758131
- EverillBKocsisJDReduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomyJ Neurophysiol199982270070810444667
- CuiJGHolminSMathiesenTMeyersonBALinderothBPossible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathyPain200088323924811068111
- GherardiniGLundebergTCuiJGErikssonSVTrubekSLinderothBSpinal cord stimulation improves survival in ischemic skin flaps: an experimental study of the possible mediation by calcitonin gene-related peptidePlast Reconstr Surg199910341221122810088510
- LiYDorsiMJMeyerRABelzbergAJMechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibersPain200085349350210781924
- RamerMSFrenchGDBisbyMAWallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRGPain1997721–271789272789
- ShamashSReichertFRotshenkerSThe cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1betaJ Neurosci20022283052306011943808
- SommerCSchafersMPainful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivityBrain Res19987841–21541629518588
- WagnerRMyersRRSchwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nervesNeuroscience19967336256298809782
- KretschmerTHappelLTEnglandJDAccumulation of PN1 and PN3 sodium channels in painful human neuroma – evidence from immunocytochemistryActa Neurochir (Wien)2002144880381012181690