Abstract
Background
Ketamine, an N-methyl-D-aspartate receptor antagonist, can suppress hyperalgesia and allodynia. The purpose of the present study was to evaluate the clinical efficacy of preincisional intravenous or subcutaneous infiltration of ketamine for postoperative pain relief after appendectomy.
Methods
Ninety patients, aged 18–60 years, scheduled for appendectomy was enrolled in this study. Patients were divided into three groups of 30 each and received subcutaneous infiltration of ketamine 0.5 mg/kg (KS), intravenous ketamine 0.5 mg/kg (KI), or subcutaneous infiltration of normal saline 3 mL (C) before surgery. Visual analog scale (VAS) values and analgesic consumption were evaluated for 24 hours after surgery.
Results
VAS scores were significantly lower at the time of arrival in the recovery room, and at 10, 20, and 30 minutes thereafter in group KI and group KS compared with group C (P < 0.05). VAS scores were not significantly different between group KI and group KS at these intervals. Postoperative VAS scores were significantly lower at 6, 12, 18, and 24 hours in group KI compared with group C (P < 0.05). In group KS, the postoperative VAS score was significantly lower at 6 hours (P < 0.05). VAS scores were significantly lower at 12, 18, and 24 hours after surgery in group KI compared with group KS (P < 0.05).
Conclusion
A 0.5 mg/kg dose of ketamine given at approximately 15 minutes before surgery by the intravenous route provided analgesia for 24 hours after surgery in patients undergoing appendectomy.
Introduction
The most important factor related to patient discomfort after surgery is pain. Although many studiesCitation1,Citation2 have shown that effective analgesia reduces postoperative complications, pain is often overlooked and not adequately controlled.Citation3 It has been shown that many excitatory substances are released by painful impulses which stimulate receptors to cause pain.Citation3,Citation4 The principal phenomenon in inflammatory pain transmission is sensitization of the spinal cord, with an active contribution from glutamate and aspartate amino acids at the N-methyl-dimethyl-aspartate (NMDA) receptor.Citation4
Tan et alCitation5 showed that preincisional treatment with subcutaneous infiltration of ketamine decreased the pain score after circumcision surgery. It seems reasonable to assume that peripheral pretreatment with ketamine may be a useful method for preventing postoperative pain in some other minor surgeries, such as appendectomy. Therefore, we designed this randomized, double-blind, prospective, placebo-controlled study to evaluate the analgesic effect of preincisional subcutaneous infiltration of ketamine after appendectomy. To investigate the central and peripheral effects of subcutaneous infiltration of ketamine, it was necessary to include an additional group of patients who were administered intravenous ketamine at a similar dose. The analgesic effect of subcutaneous ketamine was also compared with that of intravenous ketamine.
Materials and methods
Ninety patients with American Society of Anesthesiologists physical status I–II, aged 18–60 years, and scheduled for appendectomy, gave their written informed consent to participate in this study, which was approved by our institutional ethics committee. All patients were candidates for appendectomy because appendicitis was not resolved by conservative treatment. No patients had abscess or perforation.
Patients with a known history of hypertension, hyperthyroidism, psychiatric disorders, chronic pain syndrome, allergy to ketamine, renal or hepatic insufficiency, history of seizure or intracranial hypertension, or drug or alcohol abuse in the preceding 6 months were excluded.
Patients were informed about all the study methods and educated on the use of the visual analog scale (VAS) before surgery. Each patients knew that he/she belonged to one of three treatment groups (KI, KS, control). Patients had no awareness about which study drug was administered. No information about route of drug administration or its type (ketamine or placebo) was provided. Patients were informed that one drug was being administered to them and that it would not have any significant side effects.
Systolic, diastolic, and mean arterial pressure, heart rate, respiratory rate, and pulse oximeter oxygen saturation level were monitored noninvasively in the operating theater.
The patients were randomized to one of three experimental groups using random selection of sealed envelopes, with 30 patients in each group. No premedication was administered because the patients were emergency rather than elective cases. This does not mean that we stopped all medication from a specific time point. An anesthesiologist prepared the syringes containing either normal saline or the study medications for each subject. All medications were 3 mL in volume. Patients received either subcutaneous infiltration of ketamine (KS) 0.5 mg/kg, intravenous ketamine (KI) 0.5 mg/kg, or subcutaneous infiltration of normal saline (C) 3 mL before surgery. The subcutaneous injection was given at the incision site.
After induction of anesthesia using 5 mg/kg of thiopental sodium, 3 μg/kg fentanyl, and 1.5 mg/kg of succinylcholine, the study drugs were administered by a surgeon who was not involved in the test sessions. All the surgical incisions were started after 15 minutes of ketamine or saline injection. Time to onset following the intravenous bolus (dosage 2 mg/kg) was about 30–60 seconds, with the effect lasting 10–15 minutes. We used a lower dosage of ketamine (0.5 mg/kg) so it was probable that the onset of effect was delayed. Time of onset of effect for subcutaneous ketamine is 5–15 minutes. Therefore, we considered 15 minutes to be the time point of achieving the ketamine effect.Citation6
The study drugs were prepared by an anesthesiologist who was not involved in data collection. The prepared syringes were similar in shape and volume. Subcutaneous injection of ketamine or saline in group KS and group C was performed by a surgeon who was not aware of the study drug. Intravenous administration of ketamine in group KI was administered by a resident in anesthesiology who was not involved in data collection, which was done by a nurse who was not aware of group allocation.
Anesthesia was maintained with 1.2% isoflurane and nitrous oxide 50% in oxygen, with atracurium 0.6 mg/kg for intraoperative neuromuscular block. Morphine 0.1 mg/kg was administered for intraoperative analgesia. At the end of surgery, neuromuscular blockade was reversed by intravenous neostigmine 0.04 mg/kg and intravenous atropine 0.02 mg/kg. Consequently, anesthesia was discontinued and the tracheal tube removed in the operating room when airway reflexes had returned.
Heart rate, pulse oximeter oxygen saturation, systolic arterial pressure, diastolic arterial pressure, and mean arterial pressure were recorded at 10-minute intervals throughout surgery, at the time of arrival in the post-anesthesia care unit (PACU), at 10, 20, and 30 minutes thereafter, and 6, 12, 18, and 24 hours postoperatively.
After extubation, patients were transferred to the PACU where an anesthetist and nurse unaware of the study drug administered observed the patients. Pain scores were evaluated by the blinded observer physician at the time of arrival in the PACU, and 10, 20, and 30 minutes thereafter using a VAS (0–10 cm: 0 = no pain, 10 = the worst pain possible).
During the postoperative period, pain scores were evaluated at hours 6, 12, 18, and 24 by an investigator who was blinded to treatment assignment. Sedation state was also assessed using a sedation scale (0: awake, 1: drowsy but responsive to verbal orders, 2: drowsy but responsive to physical stimulus, 3: sleepy but responsive to pain stimulus) at the all above-mentioned times.
If the VAS score was >4 cm, 0.4 mg/kg meperidine was given intravenously and if the score did not decrease within 10 minutes, an additional 0.2 mg/kg meperidine was administered. The total meperidine dose did not exceed a maximum of 2 mg/kg in any 4 hours. Time to first need for additional meperidine and total meperidine consumption were recorded.
Adverse effects (dizziness, allergic reactions, nausea, vomiting, and hallucinations) were recorded in the postoperative period. Intravenous metoclopramide 0.1 mg/kg was administered to patients with vomiting or nausea lasting more than 10 minutes. If required, metoclopramide was readministered one hour after the previous injection. Analgesia administration and adverse effects were recorded by a physician blinded to the study medication.
Time from induction of anesthesia to discontinuation of anesthetic drugs was regarded as the anesthesia duration, and the time between discontinuation of nitrous oxide and extubation was considered to be the extubation time. Time from the first surgical incision to the last skin suture was considered to be the operation time. Duration of PACU stay was recorded using the modified Aldrete scoring system.Citation7
The sample size estimation was based on a power calculation showing that 30 patients per group were necessary to achieve 80% power for detecting a 20% difference in VAS scoring between group C with groups KS and KI with α = 0.05. Data are presented as the mean ± standard deviation or as numbers. Differences among group means were compared using one-way analysis of variance and post hoc comparisons at various points in time using Bonferroni’s type I error rate correction for multiple tests of significance. Gender, American Society of Anesthesiologists physical status, and complication rates were analyzed by Pearson’s Chi-square test and by Fisher’s Exact test when the anticipated number was less than five. P < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS software (v 16.0 for Windows; SPSS Inc, Chicago, IL).
Results
Ninety patients were enrolled in the study. No patient was excluded from the study due to any problem. There was no significant difference between the three groups with respect to demographic data, duration of surgery, or anesthesia ().
Table 1 Patient characteristics and duration of surgery and anesthesia in three groups
VAS scores were significantly lower upon arrival to the PACU, at 10, 20, and 30 minutes thereafter in the KI and KS groups compared with group C (P < 0.05, , ). VAS scores were not significantly different between group KI and group KS at these time points. Postoperative VAS scores were significantly lower at 6, 12, 18, and 24 hours in group KI compared with group C (P < 0.05, , ). VAS scores were significantly lower at 12, 18, and 24 hours after surgery in group KI compared with group KS (P < 0.05, ). In group KS, postoperative VAS score was significantly lower at 6 hours (P < 0.05, ). There was no significant difference between the three groups in pulse oximeter oxygen saturation, heart rate, and systolic, diastolic, and mean arterial pressure at any time points during surgery or in the postoperative period.
Figure 1 Postoperative visual analog scale scores at 0, 10, 20, and 30 minutes after arrival in the post-anesthesia care unit.
Abbreviations: KI, intravenous ketamine; KS, subcutaneous ketamine group; C, control.
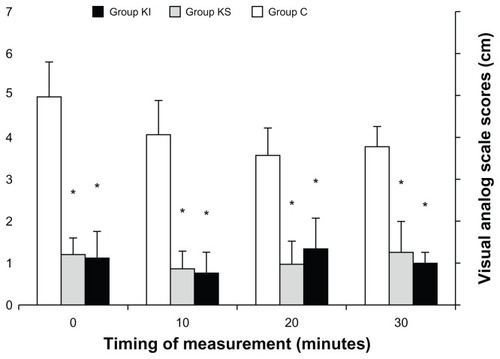
Figure 2 Visual analog scale scores at 6, 12, 18, and 24 hours after surgery.
Abbreviations: KI, intravenous ketamine; KS, subcutaneous ketamine group; C, control.
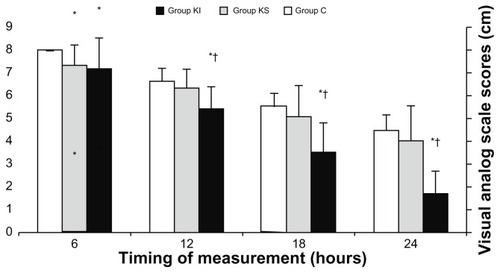
Table 4 Postoperative visual analog scale scores at different time intervals in the three groups
Median sedation values at any time postoperatively were not significantly different between the three groups. Time to first rescue analgesia in the postoperative period was significantly longer in group KI and group KS compared with group C (P < 0.05, ). Postoperative analgesic requirement was also significantly lower in group KI and group KS compared with group C (P < 0.05, ). There were no significant differences in the duration of stay in the PACU, time to tracheal extubation, and postoperative antiemetic requirements between the three groups ().
Table 2 Postoperative analgesics and antiemetic use and time to tracheal extubation and PACU stay in three groups
The incidence of adverse effects was not significantly different between the three groups (). There was no patient with emergent delirium, hallucinations, or nightmares in any of the groups.
Table 3 Incidence of adverse effects in the three groups
No significant hypotension (systolic blood pressure less than 90 mmHg) or bradycardia (heart rate less than 50 beats per minute) was noted throughout surgery or postoperatively.
Discussion
Our results showed that preincisional treatment with intravenous or subcutaneous infiltration of ketamine, a NMDA receptor antagonist, reduced postoperative pain scores compared with preincisional infiltration of normal saline solution in patients undergoing appendectomy, and had no side effects. Our results also confirmed that a single small dose of ketamine administered intravenously delays the first request for analgesic and produces a significant pethidine-sparing effect during the first 24 hours after appendectomy, while subcutaneous ketamine had this effect until 6 hours.
All drugs used to induce and maintain anesthesia were similar between the three groups, so any significant difference in some variables, such as the VAS score, could be due to our intervention, ie, administering intravenous or subcutaneous ketamine. Our results indicate that NMDA receptors in the skin and subcutaneous tissue may contribute to induction of postoperative hyperalgesia. This conclusion is also consistent with the results of previous studies showing that pretreatment with intraplantar injection of NMDA and non-NMDA glutamate receptor antagonists attenuates the magnitude and time course of lifting and licking behavior produced by formalin injection in the rat paw,Citation8 and that peripheral administration of ketamine, an NMDA receptor antagonist, inhibits the long-lasting secondary hyperalgesia generated by burn injury in humans.Citation9
Elia et alCitation10 showed that when ketamine was administered intravenously during anesthesia in adults, it decreased postoperative pain intensity for up to 48 hours, decreased cumulative 24-hour morphine use, and delayed the time to first demand for rescue analgesic. Hazama et alCitation11 also showed that preincisional administration of intravenous ketamine significantly reduced the postoperative VAS score in patients undergoing abdominal hysterectomy compared with the control group.
Recently, Sonbaty et alCitation12 showed that peritonsillar infiltration of a combination of bupivacaine 10 mg and ketamine 0.5 mg/kg provided efficient postoperative analgesia after adenotonsillectomy compared with using each drug alone. Also, Safavi et alCitation13 showed that a 2 mg/kg dose of subcutaneous infiltration ketamine or 1 mg/kg dose of intravenous ketamine given at approximately 15 minutes before surgery provided adjunctive analgesia for 24 hours after surgery in patients undergoing cholecystectomy surgery. In our study, we studied the efficacy of subcutaneous infiltration of ketamine 0.5 mg/kg or intravenous ketamine 0.5 mg/kg on postoperative pain relief after appendectomy and showed that a lower dosage of intravenous ketamine can also significantly decrease analgesic use until 24 hours. Using subcutaneous ketamine 0.5 mg/kg in our study had a significant pain-reducing effect until 6 hours after surgery that could be due to use of a low dosage of ketamine.
It seems probable that the antinociceptive effects of ketamine occur as a result of blockage of NMDA receptors located on primary afferent axons in the skin. Carlton and CoggeshallCitation14 showed that NMDA and non-NMDA glutamate receptors are present on primary afferent axons and are increased after onset of inflammation. Moreover, release of glutamate into peripheral tissues is increased after injury and inflammation.Citation15
The stimulation of NMDA and non-NMDA glutamate receptors by glutamate may induce hyperalgesia and allodynia which is interpreted as pain.Citation16,Citation17 Therefore, an explanation for our results may be that ketamine binding to the NMDA receptors might inhibit glutamate-induced activation of NMDA receptors on primary afferents in the skin, which consequently decreases peripheral nociceptive input into the spinal cord and central sensitization of the dorsal horn.
The objectives of pre-emptive analgesia are, firstly, to inhibit or lessen the development of any “memory” of pain stimulus in the central nervous system and, secondly, to lessen analgesic requirements as a consequence.Citation18 The pre-emptive effect of preincisional administration of intravenous ketamine on the inflammatory response to surgery could explain the long-lasting effect of ketamine on decreasing postoperative pain observed in group KI at 24 hours.Citation19,Citation20
The local anesthetic properties of ketamine have been shown in a previous report.Citation21 Tverskoy et alCitation22 investigated the analgesic efficacy of bupivacaine with ketamine infiltration during inguinal hernia incision, and concluded that the duration of local anesthesia was doubled via peripheral mechanisms. Moreover, it was shown that intravenous ketamine produced adequate regional anesthesia in a sample of volunteers.Citation22,Citation23 The pre-emptive blockade of initial nociceptive afferent input to the spinal cord may inhibit the development of long-term changes in the excitability of central neurons and consequently produce long-lasting antinociceptive effects.Citation24
The analgesic effect of NMDA antagonism has been demonstrated to be relatively brief when administered after pain stimulation. This is probably due to failure to inhibit the maintenance of pain behavior (because peripheral or central sensitization has developed earlier).Citation24 Therefore, it is likely that prevention of the original neural activity by local infiltration of ketamine during induction of pain behavior may inhibit the preservation of pain behavior.
An additional theory to describe the analgesic effect of small doses of ketamine may be the synergistic or additive interaction among opioids, which reveals activation of the NMDA receptorsCitation25 and NMDA antagonists. One of the mechanisms of severe postoperative pain which leads to the need for increasingly higher doses of opioids in postoperative patients is tolerance. It seems that small doses of ketamine work specifically to inhibit tolerance and pain sensitization.Citation26,Citation27
In conclusion, preincisional intravenous administration of low-dose ketamine provides analgesia for 24 hours after surgery without significant side effects in patients undergoing appendectomy. This conclusion holds true for subcutaneous administration of ketamine for the first 6 hours after surgery. Whether this is due to antagonism of peripheral NMDA receptors, local anesthesia, or other unknown effects of ketamine, remains unidentified. It is unknown if a higher dosage of ketamine can be advantageous or not. However, our results are interesting in light of the potential clinical application of this agent in appendectomy and potentially other types of surgery.
Disclosure
The authors report no conflicts of interests in this work.
References
- Arroyo-NovoaCMFigueroa-RamosMIMiaskowskiCEfficacy of small doses of ketamine with morphine to decrease procedural pain responses during open wound careClin J Pain201127756156621436683
- Benítez-RosarioMASalinas-MartínAGonzález-GuillermoTFeriaMA strategy for conversion from subcutaneous to oral ketamine in cancer pain patients: Effect of a 1:1 ratioJ Pain Symptom Manage20114161098110521398087
- PokelaMLPain relief can reduce hypoxemia in distressed neonates during routine treatment proceduresPediatrics19949333793838115195
- HwangINohJIKimSIPrevention of pain with the injection of microemulsion propofol: a comparison of a combination of lidocaine and ketamine with lidocaine or ketamine aloneKorean J Anesthesiol201059423323721057611
- TanPHChengJTKuoCHPreincisional subcutaneous infiltration of ketamine suppresses postoperative pain after circumcision surgeryClin J Pain200723321421817314579
- CorreiaMADrug biotransformationKatzungBGBasic and Clinical PharmacologyNorwalk, CTAppleton-Lange1998
- AldreteJAThe post-anesthesia recovery score revisitedJ Clin Anesth19957189917772368
- DavidsonEMCoggeshallRECarltonSMPeripheral NMDA and non-NMDA glutamate receptors contribute to nociceptive behaviors in the rat formalin testNeuroreport1997849419469141069
- WarnckeTJorumEStubhaugALocal treatment with the N-methyl-D-aspartate receptor antagonist ketamine, inhibits development of secondary hyperalgesia in man by a peripheral actionNeurosci Lett19972271149178844
- EliaNTramerMRKetamine and postoperative pain-a quantitative systematic review of randomized trialsPain20051131–2617015621365
- HazamaKNakaoMKawaguchiRNakataniKNakagawaMUnetaniHPre-incisional administration of ketamine reduced the postoperative painMasui199948121302130710658408
- El SonbatyMIAbo el DahabHMostafaAAbo ShanabOPreemptive peritonsillar ketamine infiltration: postoperative analgesic efficacy versus meperidineMiddle East J Anesthesiol2011211435121991732
- SafaviMRHonarmandANematollahyZPre-incisional analgesia with intravenous or subcutaneous infiltration of ketamine reduces postoperative pain in patients after open cholecystectomy: A randomized, double-blind, placebo-controlled studyPain Med20111291418142621812910
- CarltonSMCoggeshallREInflammation-induced changes in peripheral glutamate receptor populationsBrain Res19998201–2637010023031
- CarstensenMMøllerAMAdding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: a qualitative review of randomized trialsBr J Anaesth2010104440140620207747
- SenHSizlanAYanaratesOA comparison of gabapentin and ketamine in acute and chronic pain after hysterectomyAnesth Analg200910951645165019843803
- DuJKoltzenburgMCarltonSMGlutamate-induced excitation and sensitization of nociceptors in rat glabrous skinPain2001892–318719811166475
- ZahnPKBrennanTJIntrathecal metabotropic glutamate receptor antagonists do not decrease mechanical hyperalgesia in a rat model of postoperative painAnesth Analg1998876135413599842826
- LoftusRWYeagerMPClarkJAIntraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgeryAnesthesiology2010113363964620693876
- CousinsMJPowerISmithG1996 Labat lecture: pain – a persistent problemReg Anesth Pain Med200025162110660235
- DowdyEGKayaKGochoYSome pharmacologic similarities of ketamine, lidocaine, and procaineAnesth Analg19735258398424738209
- TverskoyMOrenMVaskovichMDashkovskyIKissinIKetamine enhances local anesthetic and analgesic effects of bupivacaine by peripheral mechanism: a study in postoperative patientsNeurosci Lett19962151588880740
- DurraniZWinnieAPZsigmondEKBurnettMLKetamine for intravenous regional anesthesiaAnesth Analg19896833283322486181
- ElmetwalyKFHegazyNAAboelseoudAAAlshaerAADoes the use of ketamine or nitroglycerin as an adjuvant to lidocaine improve the quality of intravenous regional anesthesia?Saudi J Anaesth201042556220927263
- LawandNBWillisWDWestlundKNExcitatory amino acid receptor involvement in peripheral nociceptive transmission in ratsEur J Pharm19973242–3169177
- MaoJPriceDDMayerDJMechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactionsPain19956232592748657426
- RaghavendraVTangaFYDeLeoJAAttenuation of morphine tolerance, withdrawal-induced hyperalgesia, and associated spinal inflammatory immune responses by propentofylline in ratsNeuropsychopharmacology200429232733414532913